Simple Summary
Food security and environmental sustainability are two hot topics nowadays. Chemical pesticides used with no discretion threaten human health and ecosystem functioning and, thus, ecological alternatives for herbivorous pest control are required. An environmentally friendly alternative to chemical pesticides is biological control—the use of natural enemies to control pest populations. Ladybirds (known as ladybugs in North America) are iconic biological control agents used against several herbivorous pests, such as aphids and scale insects, among others. However, small-sized ladybirds such as Scymnus are still poorly studied, and there is only one case of a biological control program using species in this genus. In the Azores archipelago (Portugal), 30% of ladybird species are small, but present in high population densities; therefore, despite their size, their ability to help to regulate prey population densities is expected to be important. In this work, we studied the life cycle of Scymnus nubilus on two new prey species: aphids that attack agricultural and forestry plants. The biological performance of the ladybird predator on the new prey tested was the best obtained to date. Our results agree with other studies focusing on the use of Scymnus ladybirds as biocontrol agents and suggest that the predator can contribute to the control of aphid pests.
Abstract
Life tables are an important tool to forecast the performance of biological control agents used in pest management programs, and they are often assessed in terms of population growth. In the present study, the suitability of the aphids Aphis fabae Scopoli and Myzus persicae (Sulzer) for the ladybird predator Scymnus nubilus Mulsant was assessed for the first time. For this, we evaluated and compared the life history traits of immature individuals and adults of the predator fed single-aphid diets and the consequences of the single-aphid diets for the demographic parameters. Scymnus nubilus that were fed A. fabae were significantly more fecund and presented a shorter immature development time than those fed M. persicae. The predators fed A. fabae had a significantly higher net reproductive rate, an intrinsic and finite rate of increase, while their doubling time was significantly lower than that of those fed M. persicae. The aphid species used in this study are new additions to the essential prey list of the ladybird, with the predator presenting a better biological performance than that found on the previously known essential prey species.
1. Introduction
Aphid pest management in orchards relies heavily on chemical control [1,2,3]. Some efforts to promote biological control have been made, but the reduced impacts found on aphid populations have made the task quite challenging [4,5]. As a main pilar of integrated pest management (IPM) programs, biological control can contribute greatly to reduce the quantity of pesticides applied. Concerned with the detrimental effects of such products on human health and the environment, the European Union has funded programs to promote biological control using ladybirds in orchards precisely with the objective of reducing the use of phytopharmaceutical products [6]. Biological control as a component of IPM for crop protection plays an important role for several Sustainable Development Goals of the Food and Agriculture Organization of the United Nations, for instance, to achieve food security, promote sustainable agriculture, and halt biodiversity loss.
Ladybirds (Coleoptera: Coccinellidae) act as natural biological control agents of herbivorous pests; indeed, this is the primary ecosystem service provided by these predator insects [7]. In 1989, the ‘Vedalia Symposium of Biological Control: A Century of Success’ was held in Riverside, California (United States of America), to commemorate 100 years of the outstanding success of the biological control program that saved the citrus industry. Specifically, it commemorated the most successful case of classical biological control, which was the importation of the vedalia ladybird, Novius cardinalis (Mulsant), from Australia to control the cottony cushion scale, Icerya purchasi Maskell (Hemiptera: Margarodidae), in the citrus orchards of California.
Among Coccinellidae, most research has focused on the biology and ecology of large species. In Mediterranean areas and habitats of small insular regions, however, an important proportion of the ladybird community (30%) is composed of small species weighing less than 5 mg [8,9,10]. Thus, small ladybirds are expected to make an important contribution to the natural control of insect pests. Despite this, small ladybirds, such as most Scymnini, sensu [11], have often been overlooked, and their potential for aphid control remains to be assessed (for details, see [12]).
In Portugal, Scymnini are the most abundant ladybirds in citrus [13], olive, and chestnut groves [14]. This group is also common in almond groves [14,15]. In Spain, Scymnini dominate olive groves [16]. Most Scymnini are very small, often measuring 2–3 mm long, with domed pubescent bodies and dull colorations. They also have diverse food preferences, with predators of aphids, scale insects, mealybugs, adelgids, and whiteflies. They may, therefore, serve as biological control agents against a wide range of fruit orchard and forest pests.
The type genus of Scymini, Scymnus, is also the largest genus in the tribe, with over eight hundred species [17]. To our knowledge, only one attempt has been made to use Scymnus spp. as biological control agents in pest management programs [18]. The attempt aimed to control the hemlock woolly adelgid, Adelges tsugae (Annand) (Hemiptera: Adelgidae), but the ladybirds failed to establish after their release [18]. In contrast, aphidophagous Scymnini have yet to be used in a biological control program; however, recent studies on aphidophagous Scymnus species indicate that they are efficient predators of aphids present in orchards [19,20,21,22,23]. For instance, Scymnus subvillosus (Goeze) and Scymnus interruptus (Goeze) show promise in the biological control of the aphids Aphis spiraecola Patch (Hemiptera: Aphididae) and Aphis gossypii Glover in clementine orchards (Citrus × clementina; Sapindales: Rutaceae) when they are present early [21]. In the Portuguese archipelago of the Azores, the potential of Scymnus nubilus Mulsant to control the aphid pests of endemic plants is being studied, and they have shown promise for the control of A. spiraecola on Azorean laurustinus (Viburnum treleasei Gand.; Dipsacales: Adroxaceae) [22] and Aphis frangulae Kaltenbach on Azores buckthorn (Frangula azorica Grubov; Rosales: Rhamnaceae) [23].
However, some aspects of the biology and ecology of aphidophagous Scymnus are still poorly understood, including their food relationships and the effects of food suitability on population dynamics. For a biological control agent to be successful, it must quickly achieve high population densities to be able to suppress the pest effectively, which occurs when the predators have favorable environmental conditions, such as high food suitability [24]. A high predation rate and high predator population growth rate are then expected to be drivers of effective pest control [25,26].
In the present study, we assessed the suitability of two aphid pests—the black bean aphid, Aphis fabae Scopoli (Hemiptera: Aphididae), and the green peach aphid, Myzus persicae (Sulzer) (Hemiptera: Aphididae)—as food sources for the ladybird predator S. nubilus. To do this, we reared the ladybirds on single-aphid diets of either species, compared measurements of the life history traits of their immature and adult stages, and evaluated the consequences of their diets on their demographic parameters.
2. Materials and Methods
2.1. Biological Traits
The ladybirds were collected on São Miguel Island, Azores, Portugal, in a coastal prairie area (Santa Clara: 37°44′17.65″ N, 25°42′1.14″ W) using sweep nets. To obtain eggs, field-collected adults of S. nubilus were paired in plastic boxes measuring 5 cm diameter × 2 cm height, with a 2.5 cm diameter mesh-covered hole in the lid for ventilation. All of the experiments were performed at 25° ± 1° C, with 75 ± 5% relative humidity, and a 16L:8D light regime in climatized rooms. Every day, the adults were transferred to new boxes, allowing the eggs laid in the old boxes to be monitored to determine the hatching time. Newly hatched larvae were transferred into their own plastic boxes and provided with a single-aphid diet ad libitum (approximately 20–30 aphids) of either A. fabae or M. persicae, with a mix of aphid developmental stages. Both aphid species were mass-reared in the laboratory at 15° ± 1° C, 75 ± 5% relative humidity, with a 16L:8D light regime in a climatized room using fava bean (Vicia faba L.; Fabales: Fabaceae) as the host plant.
To monitor larval development and survival, the boxes were checked daily and the developmental stage was noted, using exuviae as an indicator of molting, until the emergence of the adults. At emergence, the adults’ fresh weight was recorded, and they were sexed using simple external morphological characteristics, as follows: males have a light-colored head (yellowish), while females have a dark-colored head (dark brown). Pairs (N = 10 per treatment) were formed and kept in separate boxes to study how the two single-aphid diets of A. fabae or M. persicae affected their reproductive performances. Every day, these lab-reared adults were transferred to new boxes with ad libitum aphids of the same species that they were fed as larvae, and the boxes from the previous day were checked for the presence of eggs to determine the pre-oviposition time. After the females started laying, the number of eggs laid per female was counted daily. Four days after being laid, the eggs were inspected to determine the number of hatched larvae, embryonated eggs (fertile eggs with unsuccessful hatching), and infertile eggs. The females were followed until their death. The males that died were replaced with unpaired lab-reared males. While it would have been ideal to obtain the sex ratios of the progeny to use in life table calculations, rearing over fourteen thousand larvae until they completed development to determine their sex was not practical. Therefore, we used the sex ratios obtained from the parental population instead.
2.2. Demographic Parameters
To calculate the intrinsic rate of increase (), the Euler–Lotka equation was iteratively solved, according to the following equation [27]:
where is time (days), is age-specific survival, and is age-specific female offspring (hatched larvae). The following mathematical formulas were used to calculate the net reproductive rate (, defined as female offspring per female), mean generation time (τ), finite rate of increase (λ), and doubling time (DT):
Considering that population growth parameters are calculated with the data of several females, it is not possible to statistically compare them directly, because only one value is obtained. Jackknife analysis was, therefore, used to obtain the means and standard error (SE) of the demographic parameters. To obtain jackknife estimates, a series of pseudo-values of the variables () was obtained by eliminating one female at a time from the dataset. The pseudo-values () were calculated as follows:
The population growth parameters were then compared statistically.
When obtaining jackknife estimates, the omission of a male, of an individual that died as an immature individual, or of an infertile female can result in an estimate of the net reproductive rate (R0, j) of zero for groups of individuals for which the number of (female) offspring per female is clearly non-zero [28,29,30]. Since we used only fertile females, our use of jackknifing is robust to these potential issues. Jackknifing can also result in biologically inconsistent life table values [31], however, the values we obtained were all consistent.
2.3. Statistical and Survival Analyses
The egg development time and immature development time of S. nubilus were not normally distributed for either single-aphid diet; therefore, the non-parametric Mann–Whitney (MW) test was used to compare these variables. The immature survival of the predators was compared using a chi-square test. Data on the pre-oviposition time of the predators fed A. fabae and the male weight of the predators fed M. persicae could not be normalized and were compared using the MW test. T-tests were performed to compare female weight, fecundity, fertility, oviposition rate, and oviposition period of S. nubilus fed either A. fabae or M. persicae. Homogeneity of variance was confirmed with Levene’s test for all variables except mean generation time, . As we were unable to normalize the distribution of for the females fed on A. fabae, and due to the heterogeneity of variances of , the non-parametric MW test was used to compare these variables. T-tests were used for the other population growth parameters after confirming data normality and homogeneity of variances. The aforementioned statistical analyses were performed with SPSS v. 25 [32], and analysis of sex ratio was performed using exact binomial tests in R v. 4.3.2 [33].
Survival analysis was performed using the Online Application for Survival Analysis 2 [34]. For both single-aphid diets, the predator mean lifespan was determined and compared using the Log-Rank test. Kaplan-Meier survival curves and 95% confidence intervals were generated using the package survival 3.5-7 [35,36] in R v. 4.3.2 [33].
3. Results
3.1. Biological Traits
No difference in incubation time was found between the diets (MW test: U = 291.000; N A. fabae = 30, N M. persicae = 22; p = 0.336), but the ladybird larvae developed significantly faster on the A. fabae diet (MW test: U = 247.000; N A. fabae = 37, N M. persicae = 35; p < 0.001). Both diets allowed most of the immature individuals to complete development with no significant differences in survival rate (Chi-square test: χ2 = 0.3756; df = 1; p = 0.539955). The adult weight was not influenced by diet both for females (t-test: t = −1.415; df = 42; p = 0.164) and males (MW test: U = −76.500; N A. fabae = 11, N M. persicae = 16; p = 0.570). The data indicate that the sex ratio is female-biased on an A. fabae diet (32% male, 95% CI [18–50%], p = 0.0470), whereas it is even on an M. persicae diet (48% male, 95% CI [29–63%], p = 0.7359). The females took the same time to reach sexual maturity (MW test: U = −59.500; N A. fabae = 13, N M. persicae = 12; p = 0.288), but a significantly larger number of eggs were laid when the females were fed A. fabae (t-test: t = 2.747; df = 18; p = 0.013). However, the number of hatched larvae did not differ between the diets (t-test: t = 1.907; df = 18; p = 0.073). The data indicate that the oviposition rate (t-test: t = 1.438; df = 18; p = 0.168) and oviposition period (t-test: t = 1.644; df = 18; p = 0.118) are similar under both food regimes (Table 1).

Table 1.
Biological traits of Scymnus nubilus fed either Aphis fabae or Myzus persicae (25° ± 1 °C, 75 ± 5% relative humidity, and 16L:8D light regime).
3.2. Demographic Parameters
Concerning population growth parameters, we found that the intrinsic rate of increase (rm; t-test: t = 3.524; df = 18; p = 0.002), the finite rate of increase (λ; t-test: t = 3.519; df = 18; p = 0.002), and the net reproductive rate (; MW test: U = 15.00; N A. fabae = 10, N M. persicae = 10; p = 0.008) were significantly higher when the ladybirds were fed A. fabae. The doubling time (DT) was significantly lower when the adults were fed A. fabae (t-test: t = −3.549; df = 9; p = 0.002). No significant differences were found in the mean generation time (τ) between the two diets (MW test: U = 35.00; N A. fabae = 10, N M. persicae = 10; p = 0.257) (Table 2).

Table 2.
Population growth parameters (mean ± SE) (rm: intrinsic rate of increase; λ: finite rate of increase; DT: doubling time; τ: mean generation time; R0: net reproductive rate) of Scymnus nubilus fed single-aphid diets of Aphis fabae or Myzus persicae (25° ± 1° C, 75 ± 5% relative humidity, and 16L:8D light regime).
The oviposition pace of the females is similar under the different diets and mirrors the oviposition pattern of other aphidophagous species; moreover, it shows typical triangular fecundity, that is, a rapid increase in the number of eggs laid, with a single oviposition peak, followed by a slow decrease in the rate of oviposition (Figure 1). The reproductive lifespan, that is, the oviposition period, was similar under the A. fabae and M. persicae diets. Most of the eggs were laid within 90 days, but the females continued laying until their death (Figure 1).
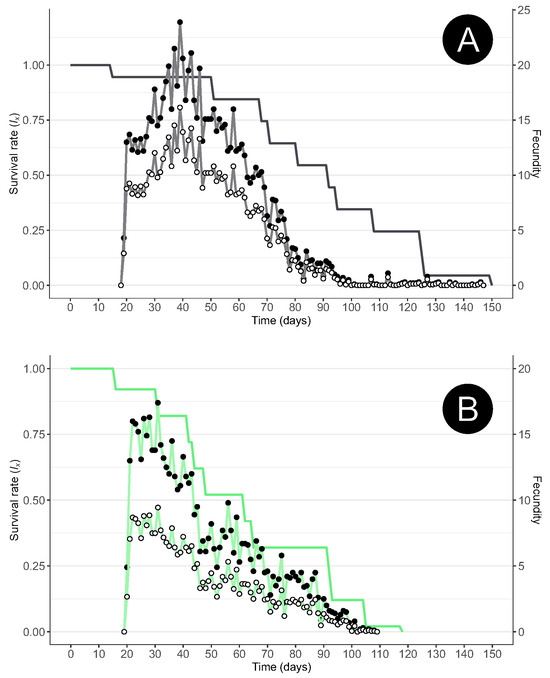
Figure 1.
Age-specific survival (lx; line only), age-specific mean number of offspring (line with full circles), and age-specific mean number of female offspring (mx; line with white circles) of Scymnus nubilus fed (A) black bean aphids (Aphis fabae) or (B) green peach aphids (Myzus persicae).
On the A. fabae diet, the mean lifespan was significantly longer than on M. persicae (Log-Rank test: χ2 = 398.77, p < 0.001; Figure 2). Across the entire life cycle, the predator showed a higher survival rate when fed A. fabae.
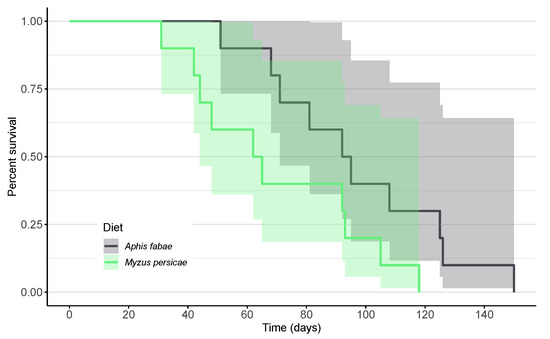
Figure 2.
Kaplan–Meier survival curves of Scymnus nubilus fed single-aphid diets of black bean aphids (Aphis fabae; dark gray) or green peach aphids (Myzus persicae; pale green) and 95% confidence intervals.
4. Discussion
Population growth parameters provide important information about biological traits, which is essential for sound decision making in pest management. Most often, a high intrinsic rate of increase (rm) and net reproductive rate (R0) are used as indicators of good candidates for biological control agents, because they represent fast predator population growth and high reproductive indices. Following this rationale, our results indicate that S. nubilus would be able to control A. fabae more efficiently than M. persicae.
Moreover, both aphid species meet the criteria to be essential prey species for S. nubilus. Essential prey species, under the criteria of [37], are those of high enough quality to support the growth and development of predator larvae and the reproduction by adults. Until the last decade, the essential prey list of S. nubilus was limited to A. gossypii [38] and Rhopalosiphum padi L. (Hemiptera: Aphididae) [19], but recent results indicate that A. spiraecola and Cinara juniperi (De Geer) (Hemiptera: Aphididae) allow the predator to successfully develop [22] and reproduce (unpublished data [22]), as well as A. frangulae [23]. In the Azores archipelago, S. nubilus can be found in many types of habitats, such as the following: agroecosystems, greenhouses, coastal prairies, city gardens, and orchards. Because it is present in diverse habitats, it is reasonable to assume that its prey range covers many more species. Indeed, A. fabae and M. persicae allowed the ladybird predator to successfully develop and reproduce. Therefore, these two species are new additions to its essential prey list.
However, even species on the essential prey list vary in food quality for predators. Food quality is an important determinant of the physiological processes in coccinellids [12], with direct impacts on their developmental rate, survival, and reproductive performance, such as fecundity and fertility (e.g., [39,40,41,42,43,44]). Of all of the aphid species on S. nubilus’ prey list, A. fabae is the best food resource, followed by M. persicae. Conversely, S. nubilus performs at its worst when fed C. juniperi, an aphid that specializes on cedar (this study, [22,23,38]). A congener of the predator, S. subvillosus, also has a good biological performance on A. fabae and M. persicae as prey, and, similarly, has a lower biological performance when fed M. persicae [20]. These differences in performance may represent inherent differences in the quality of the aphid species as food items (e.g., [45]), a tri-trophic interaction between aphids, their host plants, and the predator—where the predators that consume the aphids that are well-suited to their host plant do better themselves [46,47]—or both. Notably, A. fabae performs much better on V. faba than on M. persicae [48]; moreover, both this study and [20] used this host plant to rear both aphid species.
Recent research considers the use of S. nubilus under an augmentation approach to control aphids in the Azores (e.g., [22,23]) in light of their life history and demographic traits. Scymnus species, particularly S. nubilus and S. interruptus, are the most abundant aphidophagous ladybird predator in the Azores [10]. Scymnus nubilus can singly lay up to 30 eggs per day, scattering the eggs over many aphid colonies—a behavior that may contribute to more effective pest control [19]. The results of [22,23] indicate that S. nubilus has the potential to control S. spiraecola and A. frangulae. The potential of S. nubilus to control the aphid species infesting Azorean endemic plants is being further investigated, specifically for the control of the black citrus aphid, Toxoptera aurantii (Boyer de Fonscolombe) (Hemiptera: Aphididae). The life table data suggest that S. interruptus and S. subvillosus perform well on A. gossypii and A. spiraecola, which are both aphid pests in clementine orchards [21]. We believe that S. nubilus could similarly be used to control aphids in citrus orchards.
One downside to using small ladybirds for biological control rather than larger ones is their lower food demands. However, these lower food demands also allow small ladybirds to start feeding earlier on aphid colonies and to feed for longer on senescent prey colonies. Compared to larger species, small ladybirds have an extended oviposition window, because their food requirements are met for longer. Additionally, the higher abundance of small-sized predators could compensate for their lower voracity. After assessing the suitability of aphid species for Scymnus ladybirds in the laboratory, field tests are required to assess their effectiveness as biological control agents of aphids in different habitats and field conditions.
Author Contributions
I.B.: Conceptualization, data curation, formal analysis, investigation, methodology, validation, and writing—original draft and review and editing. G.J.D.: Investigation, formal analysis, validation, visualization, and writing—review and editing. A.O.S.: Funding acquisition, project administration, supervision, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
During the investigation portion of this work, G.J.D. was funded by an internship through the Office Québec-Amériques pour la Jeunesse and Les Offices Jeunesse Internationaux du Québec (OQAJ–LOJIQ), and, during revisions and validation, was funded by the Stengl-Wyer Postdoctoral Scholars Program at the University of Texas at Austin.
Data Availability Statement
The original data presented in the study are openly available in Mendeley Data at DOI 10.17632/c5stf3cnyp.1.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Penvern, S.; Bellon, S.; Fauriel, J.; Sauphanor, B. Peach orchard protection strategies and aphid communities: Towards an integrated agroecosystem approach. Crop Prot. 2010, 10, 1148–1156. [Google Scholar] [CrossRef]
- Codron, J.M.; Habib, R.; Jacquet, F.; Sauphanor, B. Bilan et perspectives environnementales de la filière arboriculture fruitière. In Agriculture, Territoire, Environnement dans les Politiques Européennes. Dossier de L’environnement de l’INRA 23; Dron, D., Ed.; INRA: Paris, France, 2003; pp. 31–67. [Google Scholar]
- Niu, J.-Z.; Hull-Sanders, H.; Zhang, Y.-X.; Lin, J.-Z.; Dou, W.; Wang, J.-J. Biological control of arthropod pests in citrus orchards in China. Biol. Control 2014, 68, 15–22. [Google Scholar] [CrossRef]
- Biological Control of Aphids in Orchards to Produce “Greener” Fruits. Available online: https://cordis.europa.eu/project/id/QLK5-CT-2000-40898 (accessed on 15 March 2024).
- Miñarro, M.; Hemptinne, J.-L.; Dapena, E. Colonization of apple orchards by predators of Dysaphis plantaginea: Sequential arrival, response to prey abundance and consequences for biological control. BioControl 2005, 50, 403–414. [Google Scholar] [CrossRef]
- Fréchette, B.; Cormier, D.; Chouinard, G.; Vanoosthuyse, F.; Lucas, É. Apple aphid, Aphis spp. (Hemiptera: Aphididae), and predator populations in an apple orchard at the non-bearing stage: The impact of ground cover and cultivar. Eur. J. Entomol. 2008, 105, 521–529. [Google Scholar] [CrossRef]
- Ameixa, O.M.C.C.; Soares, A.O.; Soares, A.M.V.M.; Lillebø, A.I. Ecosystem Services Provided by the Little Things That Run the World. In Selected Studies in Biodiversity; Ṣen, B., Grillo, O., Eds.; IntechOpen Limited: London, UK, 2018; pp. 267–302. [Google Scholar]
- Soares, A.O.; Honěk, A.; Martinkova, Z.; Skuhrovec, J.; Cardoso, P.; Borges, I. Harmonia axyridis failed to establish in the Azores: The role of species richness, intraguild interactions and resource availability. BioControl 2017, 62, 423–434. [Google Scholar] [CrossRef]
- Honěk, A.; Dixon, A.F.G.; Soares, A.O.; Skuhrovec, J.; Martinkova, Z. Spatial and temporal changes in the abundance and composition of ladybird (Coleoptera: Coccinellidae) communities. Curr. Opin. Insect Sci. 2017, 20, 61–67. [Google Scholar] [CrossRef]
- Soares, A.O.; Honěk, A.; Martinkova, Z.; Brown, P.M.J.; Borges, I. Can Native Geographical Range, Dispersal Ability and Development Rates Predict the Successful Establishment of Alien Ladybird (Coleoptera: Coccinellidae) Species in Europe? Front. Ecol. Evol. 2018, 6, 57. [Google Scholar] [CrossRef]
- Che, L.; Zhang, P.; Deng, S.; Escalona, H.E.; Wang, X.; Li, Y.; Pang, H.; Vandenberg, N.; Ślipiński, A.; Tomaszewska, W.; et al. New insights into the phylogeny and evolution of lady beetles (Coleoptera: Coccinellidae) by extensive sampling of genes and species. Mol. Phylogenet. Evol. 2021, 156, 107045. [Google Scholar] [CrossRef]
- Hodek, I.; van Emden, H.F.; Honek, A. Ecology and Behaviour of the Ladybird Beetles (Coccinellidae); Wiley-Blackwell: Chichester, UK, 2012. [Google Scholar]
- Magro, A.; Hemptinne, J.-L. The pool of Coccinellids (Coleoptera: Coccinellidae) to control Coccids (Homoptera: Coccoidea) in Portuguese citrus groves. Bol. San. Veg. Plagas 1999, 25, 311–320. [Google Scholar]
- Santos, S.A.P.; Raimundo, A.; Bento, A.; Pereira, J.A. Species abundance patterns of coccinellid communities associated with olive, chestnut and almond crops in north-eastern Portugal. Agric. Forest Entomol. 2012, 14, 376–382. [Google Scholar] [CrossRef]
- Benhadi-Marin, J.; Pereira, J.A.; Barrientos, J.A.; Bento, A.; Santos, S.A.P. Diversity of predaceous arthropods in the almond tree canopy in northeastern Portugal: A methodological approach. Entomol. Sci. 2011, 14, 347–358. [Google Scholar] [CrossRef]
- Cotes, B.; Campos, M.; Pascual, F.; Ruano, F. The Ladybeetle Community (Coleoptera: Coccinellidae) in Southern Olive Agroecosystems of Spain. Environ. Entomol. 2010, 39, 79–87. [Google Scholar] [CrossRef]
- Chen, X.; Huo, L.; Wang, X.; Ren, S. The Subgenus Pullus of Scymnus from China (Coleoptera, Coccinellidae). Part I. The Hingstoni and Subvillosus Groups. Annales Zoologici 2015, 65, 187–237. [Google Scholar] [CrossRef]
- Mayfield, A.E., III; Bittner, T.D.; Dietschler, N.J.; Elkinton, J.S.; Havill, N.P.; Keena, M.A.; Mausel, D.L.; Rhea, J.R.; Salom, S.M.; Whitmore, M.C. Biological control of hemlock woolly adelgid in North America: History, status, and Outlook. Biol. Control 2023, 185, 105308. [Google Scholar] [CrossRef]
- Borges, I.; Soares, A.O.; Hemptinne, J.-L. Contrasting population growth parameters of the aphidophagous Scymnus nubilus and the coccidophagous Nephus reunioni. BioControl 2013, 58, 351–357. [Google Scholar] [CrossRef]
- Sebastião, D.; Borges, I.; Soares, A.O. Effect of temperature and prey in the biology of Scymnus subvillosus. BioControl 2015, 60, 241–249. [Google Scholar] [CrossRef]
- Bouvet, J.P.R.; Urbaneja, A.; Monzó, C. Life history traits of the coccinellids Scymnus subvillosus and S. interruptus on their prey Aphis spiraecola and A. gossypii: Implications for biological control of aphids in clementine citrus. Biol. Control 2019, 132, 49–56. [Google Scholar] [CrossRef]
- Rosagro, R.; Borges, I.; Vieira, V.; Pons, G.; Soares, A.O. Evaluation of Scymnus nubilus (Coleoptera: Coccinellidae) as a biological control agent against Aphis spiraecola and Cinara juniperi. Pest Manag. Sci. 2020, 76, 818–826. [Google Scholar] [CrossRef]
- Borges, I.; Arruda, P.; Meseguer, R.; Vieira, V.; Pons-Solé, G.; Soares, A.O. Can the ladybird predator Scymnus nubilus contribute to control of the aphid Aphis frangulae, a pest threatening the Macaronesia endemic Frangula azorica? BioControl 2022, 67, 523–531. [Google Scholar] [CrossRef]
- Berryman, A.A.; Kindlmann, P. Population Systems: A General Introduction; Springer: New York, NY, USA, 2008; 222p. [Google Scholar]
- Yu, J.Z.; Chi, H.; Chen, B.H. Comparison of the life tables and predation rates of Harmonia dimidiata (F.) (Coleoptera: Coccinellidae) fed on Aphis gossypii Glover (Hemiptera: Aphididae) at different temperatures. Biol. Control 2013, 64, 1–9. [Google Scholar] [CrossRef]
- Ismail, M.; van Baaren, J.; Briand, V.; Pierre, J.S.; Vernon, P.; Hance, T. Fitness consequences of low temperature storage of Aphidius ervi. Biocontrol 2014, 59, 139–178. [Google Scholar] [CrossRef][Green Version]
- Carey, J.R. Applied Demography for Biologists: With Special Emphasis on Insects; Oxford University Press: New York, NY, USA, 1993. [Google Scholar]
- Huang, Y.B.; Chi, H. Life Tables of Bactrocera cucurbitae (Diptera: Tephritidae): With an Invalidation of the Jackknife Technique. J. App. Entomol. 2013, 137, 327–339. [Google Scholar] [CrossRef]
- Huang, Y.-B.; Chi, H. Assessing the Application of the Jackknife and Bootstrap Techniques to the Estimation of the Variability of the Net Reproductive Rate and Gross Reproductive Rate: A Case Study in Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae). J. Agri. Forest. 2012, 61, 37–45. [Google Scholar]
- Yu, L.-Y.; Chen, Z.-Z.; Zheng, F.-Q.; Shi, A.-J.; Guo, T.-T.; Yeh, B.-H.; Chi, H.; Xu, Y.-Y. Demographic Analysis, a Comparison of the Jackknife and Bootstrap Methods, and Predation Projection: A Case Study of Chrysopa pallens (Neuroptera: Chrysopidae). J. Econ. Entomol. 2013, 106, 1–9. [Google Scholar] [CrossRef]
- Chi, H.; Kavousi, A.; Gharekhani, G.; Atlihan, R.; Özgökçe, M.S.; Güncan, A.; Gökçe, A.; Smith, C.L.; Benelli, G.; Guedes, R.N.C.; et al. Advances in Theory, Data Analysis, and Application of the Age-Stage, Two-Sex Life Table for Demographic Research, Biological Control, and Pest Management. Entomol. Gen. 2023, 43, 705–732. [Google Scholar] [CrossRef]
- IBM Corp. SPSS Statistics for Windows, Version 25.0; IBM Corp.: Armonk, NY, USA, 2017. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 23 June 2024).
- Han, S.K.; Lee, D.; Lee, H.; Kim, D.; Son, H.G.; Yang, J.; Lee, S.V.; Kim, S. OASIS 2: Online application for survival analysis 2 with features for the analysis of maximal lifespan and healthspan in aging research. Oncotarget 2016, 7, 56147–56152. [Google Scholar] [CrossRef]
- Therneau, T. A Package for Survival Analysis in R. R Package Version 3.5-7. 2023. Available online: https://CRAN.R-project.org/package=survival (accessed on 23 June 2024).
- Therneau, T.M.; Grambsch, P.M. Modeling Survival Data: Extending the Cox Model; Springer: New York, NY, USA, 2000; 350p. [Google Scholar]
- Hodek, I.; Evans, E.W. Food relationship. In Ecology and Behavior of the Ladybird Beetles (Coccinellidae); Hodek, I., Van Emden, H.F., Honěk, A., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2012. [Google Scholar]
- Uygun, N.; Atlihan, R. The effect of temperature on development and fecundity of Scymnus levaillanti. BioControl 2000, 45, 453–462. [Google Scholar] [CrossRef]
- Hodek, I. Habitat and food specificity in aphidophagous predators. Biocontrol Sci. Technol. 1993, 3, 91–100. [Google Scholar] [CrossRef]
- Işıkber, A.A.; Copland, M.J.W. Effects of various aphid foods on Cycloneda sanguinea. Entomol. Exp. Appl. 2002, 102, 93–97. [Google Scholar] [CrossRef][Green Version]
- Soares, A.O.; Coderre, D.; Schanderl, H. Dietary self-selection behavior by the adults of the aphidophagous ladybeetle Harmonia axyridis (Coleoptera: Coccinellidae). J. Anim. Ecol. 2004, 73, 478–486. [Google Scholar] [CrossRef]
- Michaud, J.P. On the assessment of prey suitability in aphidophagous Coccinelidae. Eur. J. Entomol. 2005, 102, 385–390. [Google Scholar] [CrossRef]
- Soares, A.O.; Coderre, D.; Schanderl, H. Influence of prey quality on the fitness of two phenotypes of the adults of Harmonia axyridis. Entomol. Exp. Appl. 2005, 114, 227–232. [Google Scholar] [CrossRef]
- Ungerová, D.; Kalushkov, P.; Nedvěd, O. Suitability of diverse prey species for development of Harmonia axyridis and the effect of container size. IOBC WPRS Bull. 2010, 58, 165–174. [Google Scholar]
- Bista, M.; Omkar. Effects of Body Size and Prey Quality on the Reproductive Attributes of Two Aphidophagous Coccinellidae (Coleoptera) Species. Can. Entomol. 2013, 145, 566–576. [Google Scholar] [CrossRef]
- Sentis, A.; Bertram, R.; Dardenne, N.; Simon, J.-C.; Magro, A.; Pujol, B.; Danchin, E.; Hemptinne, J.-L. Intraspecific Difference among Herbivore Lineages and Their Host-Plant Specialization Drive the Strength of Trophic Cascades. Ecol. Lett. 2020, 23, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Frederic, F.; Haubruge, E.; Hastir, P.; Gaspar, C. Effect of Aphid Host Plant on Development and Reproduction of the Third Trophic Level, the Predator Adalia bipunctata (Coleoptera: Coccinellidae). Environ. Entomol. 2001, 30, 947–952. [Google Scholar]
- Fernandez-Quintanilla, C.; Fereres, A.; Godfrey, L.; Norris, R.F. Development and Reproduction of Myzus persicae and Aphis fabae (Hom., Aphididae) on Selected Weed Species Surrounding Sugar Beet Fields. J. Appl. Entomol. 2002, 126, 198–202. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).