An Effective Fluorescent Marker for Tracking the Dispersal of Small Insects with Field Evidence of Mark–Release–Recapture of Trissolcus japonicus
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Marking Procedure
2.3. Experiment 1: Marking Persistence
2.4. Experiment 2: Longevity Experiment
2.5. Experiment 3: Parasitism
2.6. Experiment 4: Flight Ability
2.7. Experiment 5: Activity Monitor
2.8. Field Mark–Release–Recapture
2.9. Statistical Analysis
3. Results
3.1. Experiment 1: Marking Persistence
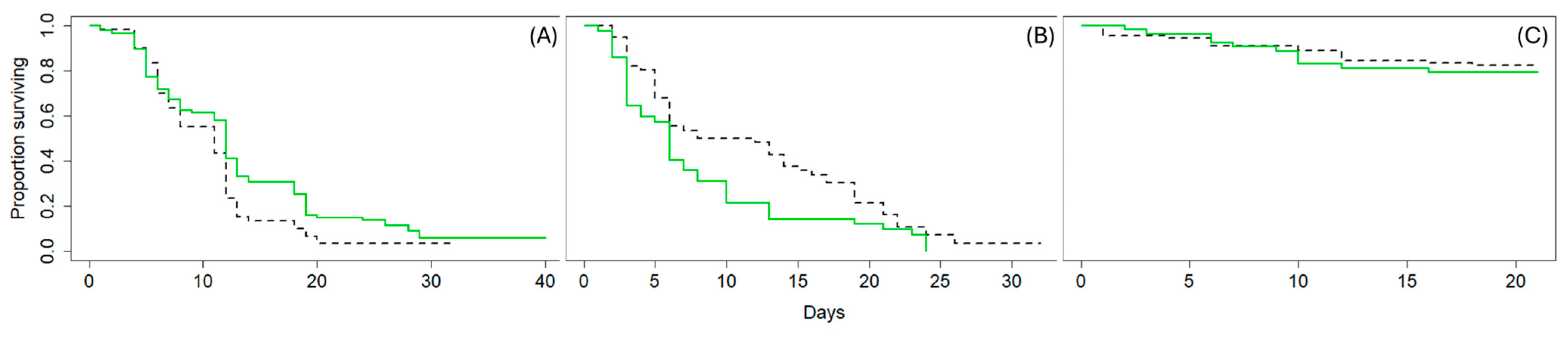
3.2. Experiment 2: Survival
3.3. Experiment 3: Flight Ability
3.4. Experiment 4: Parasitism
3.5. Experiment 5: Locomotor Activity
3.6. Field Mark–Release–Recapture of T. japonicus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kean, J.M.; Barlow, N.D. Effects of dispersal on local population increase. Ecol. Lett. 2000, 3, 479–482. [Google Scholar] [CrossRef]
- Phillips, B.L.; Brown, G.P.; Shine, R. Life-history evolution in range-shifting populations. Ecology 2010, 91, 1617–1627. [Google Scholar] [CrossRef] [PubMed]
- Heimpel, G.E.; Asplen, M.K. A ‘Goldilocks’ hypothesis for dispersal of biological control agents. BioControl 2011, 56, 441–450. [Google Scholar] [CrossRef]
- Clobert, J. Dispersal Ecology and Evolution; Oxford University Press: Oxford, UK, 2012; ISBN 978-0-19-960889-8. [Google Scholar]
- Blackmer, J.L.; Hagler, J.R.; Simmons, G.S.; Henneberry, T.J. Dispersal of Homalodisca vitripennis (Homoptera: Cicacellidae) from a point release site in citrus. Environ. Entomol. 2006, 35, 1617–1625. [Google Scholar] [CrossRef]
- Robinet, C.; David, G.; Jactel, H. Modeling the distances traveled by flying insects based on the combination of flight mill and mark-release-recapture experiments. Ecol. Model. 2019, 402, 85–92. [Google Scholar] [CrossRef]
- Petit, J.N.; Hoddle, M.S.; Grandgirard, J.; Roderick, G.K.; Davies, N. Short-distance dispersal behavior and establishment of the parasitoid Gonatocerus ashmeadi (Hymenoptera: Mymaridae) in Tahiti: Implications for its use as a biological control agent against Homalodisca vitripennis (Hemiptera: Cicadellidae). Biol. Control 2008, 45, 344–352. [Google Scholar] [CrossRef]
- Goode, A.B.C.; Minteer, C.R.; Tipping, P.W.; Knowles, B.K.; Valmonte, R.J.; Foley, J.R.; Gettys, L.A. Small-scale dispersal of a biological control agent—Implications for more effective releases. Biol. Control 2019, 132, 89–94. [Google Scholar] [CrossRef]
- Plouvier, W.N.; Wajnberg, E. Improving the efficiency of augmentative biological control with arthropod natural enemies: A modeling approach. Biol. Control 2018, 125, 121–130. [Google Scholar] [CrossRef]
- Martí-Campoy, A.; Ávalos, J.A.; Soto, A.; Rodríguez-Ballester, F.; Martínez-Blay, V.; Malumbres, M.P. Design of a computerised flight mill device to measure the flight potential of different insects. Sensors 2016, 16, 485. [Google Scholar] [CrossRef]
- Hagler, J.R.; Cohen, A.C.; Bradley-Dunlop, D.; Enriquez, F.J. New approach to mark insects for feeding and dispersal studies. Environ. Entomol. 1992, 21, 20–25. [Google Scholar] [CrossRef]
- Hagler, J.R.; Hull, A.M.; Casey, M.T.; Machtley, S.A. Use of a fluorophore to tag arthropods for mark-release-recapture type research. J. Insect Sci. 2021, 21, 20. [Google Scholar] [CrossRef] [PubMed]
- Hagler, J.R.; Jackson, C.G. Methods for marking insects: Current techniques and future prospects. Annu. Rev. Entomol. 2001, 46, 511–543. [Google Scholar] [CrossRef]
- Schellhorn, N.A.; Siekmann, G.; Paull, C.; Furness, G.; Baker, G. The use of dyes to mark populations of beneficial insects in the field. Int. J. Pest Manag. 2004, 50, 153–159. [Google Scholar] [CrossRef]
- Whitehead, M.R.; Peakall, R. Microdot technology for individual marking of small arthropods. Agric. For. Entomol. 2012, 14, 171–175. [Google Scholar] [CrossRef]
- Hagler, J.R. Super mark it! A review of the protein immunomarking technique. Ann. Entomol. Soc. Am. 2019, 112, 200–210. [Google Scholar] [CrossRef]
- White, A.; Minch, R.; Bidder, L.; Gaff, H. A simple, inexpensive method for mark-recapture of Ixodid ticks. J. Insect Sci. 2020, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- Hagler, J.R.; Jackson, C.G. An immunomarking technique for labeling minute parasitoids. Environ. Entomol. 1998, 27, 1010–1016. [Google Scholar] [CrossRef]
- Hagler, J.R.; Casey, M.T.; Hull, A.M.; Machtley, S.A. A labor-saving marking and sampling technique for mark-release-recapture research. Entomol. Exp. Appl. 2023, 171, 138–145. [Google Scholar] [CrossRef]
- Hagler, J.R.; Jackson, C.G.; Henneberry, T.J.; Gould, J.R. Parasitoid mark-release-recapture techniques-- II. development and application of a protein marking technique for Eretmocerus spp., parasitoids of Bemisia argentifolii. Biocontrol Sci. Technol. 2002, 12, 661–675. [Google Scholar] [CrossRef]
- Hagler, J.R.; Casey, M.T.; Hull, A.M.; Machtley, S.A. Liquid fluorophore taggants for mark-release-recapture research: A survey of potential arthropod targets. Entomol. Exp. Appl. 2022, 170, 821–830. [Google Scholar] [CrossRef]
- Faiman, R.; Krajacich, B.J.; Graber, L.; Dao, A.; Yaro, A.S.; Yossi, O.; Sanogo, Z.L.; Diallo, M.; Samaké, D.; Sylla, D.; et al. A novel fluorescence and DNA combination for versatile, long-term marking of mosquitoes. Methods Ecol. Evol. 2021, 12, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Calvo, J.; Forshage, M.; Buffington, M.L. Circumscription of the Ganaspis brasiliensis (Ihering, 1905) species complex (Hymenoptera, Figitidae), and the description of two new species parasitizing the spotted wing drosophila, Drosophila suzukii Matsumura, 1931 (Diptera, Drosophilidae). J. Hymenopt. Res. 2024, 97, 441–470. [Google Scholar] [CrossRef]
- Hogg, B.N.; Lee, J.C.; Rogers, M.A.; Worth, L.; Nieto, D.J.; Stahl, J.M.; Daane, K.M. Releases of the parasitoid Pachycrepoideus vindemmiae for augmentative biological control of spotted wing drosophila, Drosophila suzukii. Biol. Control 2022, 168, 104865. [Google Scholar] [CrossRef]
- Sriram, A.; Voyvot, S.; Johnson, B.C.; Chowdhury, S.M.; Fanning, P.D.; Lee, J.C. Mesh covers on sentinel parasitoid traps prevent Drosophila suzukii movement and impact parasitism by Ganaspis brasiliensis and Pachycrepoideus vindemiae. Biocontrol Sci. Technol. 2023, 33, 1030–1040. [Google Scholar] [CrossRef]
- Rossi-Stacconi, M.V.; Wang, X.; Stout, A.; Fellin, L.; Daane, K.M.; Biondi, A.; Stahl, J.M.; Buffington, M.L.; Anfora, G.; Hoelmer, K.A. Methods for rearing the parasitoid Ganaspis brasiliensis, a promising biological control agent for the invasive Drosophila suzukii. J. Vis. Exp. 2022, 63898. [Google Scholar] [CrossRef]
- Woltz, J.M.; Donahue, K.M.; Bruck, D.J.; Lee, J.C. Efficacy of commercially available predators, nematodes and fungal entomopathogens for augmentative control of Drosophila suzukii. J. Appl. Entomol. 2015, 139, 759–770. [Google Scholar] [CrossRef]
- Hagler, J.R. Field retention of a novel mark-release-recapture method. Environ. Entomol. 1997, 26, 1079–1086. [Google Scholar] [CrossRef]
- Paul, R.L.; Abram, P.K.; Lee, J.C. Host patch quality increases parasitoid locomotor activity despite risk of egg limitation. Ecol. Entomol. 2022, 47, 810–821. [Google Scholar] [CrossRef]
- Geissmann, Q.; Garcia Rodriguez, L.; Beckwith, E.J.; Gilestro, G.F. Rethomics: An R framework to analyse high-throughput behavioural data. PLoS ONE 2019, 14, e0209331. [Google Scholar] [CrossRef]
- Rosser, E.; Willden, S.A.; Loeb, G.M. Effects of SmartWater, a fluorescent mark, on the dispersal, behavior, and biocontrol efficacy of Phytoseiulus persimilis. Exp. Appl. Acarol. 2022, 87, 163–174. [Google Scholar] [CrossRef]
- Miksanek, J.R.; Heimpel, G.E. Density-dependent lifespan and estimation of life expectancy for a parasitoid with implications for population dynamics. Oecologia 2020, 194, 311–320. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, H.R.; Skillman, V.P.; Galindo, G.; Lee, J.C. Floral resources for Trissolcus japonicus, a parasitoid of Halyomorpha halys. Insects 2020, 11, 413. [Google Scholar] [CrossRef]
- Sabbatini-Peverieri, G.; Dieckhoff, C.; Giovannini, L.; Marianelli, L.; Roversi, P.F.; Hoelmer, K. Rearing Trissolcus japonicus and Trissolcus mitsukurii for biological control of Halyomorpha halys. Insects 2020, 11, 787. [Google Scholar] [CrossRef] [PubMed]
- van Alphen, J.J.M.; Bernstein, C.; Driessen, G. Information acquisition and time allocation in insect parasitoids. Trends Ecol. Evol. 2003, 18, 81–87. [Google Scholar] [CrossRef]
- Quinn, N.F.; Talamas, E.J.; Leskey, T.C.; Bergh, J.C. Sampling methods for adventive Trissolcus japonicus (Hymenoptera: Scelionidae) in a wild tree host of Halyomorpha halys (Hemiptera: Pentatomidae). J. Econ. Entomol. 2019, 112, 1997–2000. [Google Scholar] [CrossRef] [PubMed]
- Quinn, N.F.; Talamas, E.J.; Leskey, T.C.; Bergh, J.C. Seasonal captures of Trissolcus japonicus (Ashmead) (Hymenoptera: Scelionidae) and the effects of habitat type and tree species on detection frequency. Insects 2021, 12, 118. [Google Scholar] [CrossRef] [PubMed]
- Lowenstein, D.M.; Andrews, H.; Hilton, R.J.; Kaiser, C.; Wiman, N.G. Establishment in an introduced range: Dispersal capacity and winter survival of Trissolcus japonicus, an adventive egg parasitoid. Insects 2019, 10, 443. [Google Scholar] [CrossRef]
- Richardson, K.V.; Alston, D.G.; Spears, L.R. Adventive population of Trissolcus japonicus (Hymenoptera: Scelionidae), parasitoid of Halyomorpha halys (Hemiptera: Pentatomidae), discovered in southwestern Idaho. J. Integr. Pest Manag. 2023, 14, 6. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paul, R.L.; Hagler, J.R.; Janasov, E.G.; McDonald, N.S.; Voyvot, S.; Lee, J.C. An Effective Fluorescent Marker for Tracking the Dispersal of Small Insects with Field Evidence of Mark–Release–Recapture of Trissolcus japonicus. Insects 2024, 15, 487. https://doi.org/10.3390/insects15070487
Paul RL, Hagler JR, Janasov EG, McDonald NS, Voyvot S, Lee JC. An Effective Fluorescent Marker for Tracking the Dispersal of Small Insects with Field Evidence of Mark–Release–Recapture of Trissolcus japonicus. Insects. 2024; 15(7):487. https://doi.org/10.3390/insects15070487
Chicago/Turabian StylePaul, Ryan L., James R. Hagler, Eric G. Janasov, Nicholas S. McDonald, Saliha Voyvot, and Jana C. Lee. 2024. "An Effective Fluorescent Marker for Tracking the Dispersal of Small Insects with Field Evidence of Mark–Release–Recapture of Trissolcus japonicus" Insects 15, no. 7: 487. https://doi.org/10.3390/insects15070487





