Pesticide Contamination in Native North American Crops, Part I—Development of a Baseline and Comparison of Honey Bee Exposure to Residues in Lowbush Blueberry and Cranberry
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Maine Lowbush Blueberry Sites
2.1.1. Sample Collection
2.1.2. Storage of Samples
2.1.3. Grower Pesticide Application Records
2.2. Massachusetts Cranberry Sites
2.2.1. Sample Collection
2.2.2. Grower Pesticide Application Records
2.3. Storing, Shipping, and Analytical Methods Applied for Sample Handling and Residue Analysis
2.4. Statistical Methods
3. Results
3.1. Pesticide Use and Honey Bee Exposure in Blueberry and Cranberry Farms
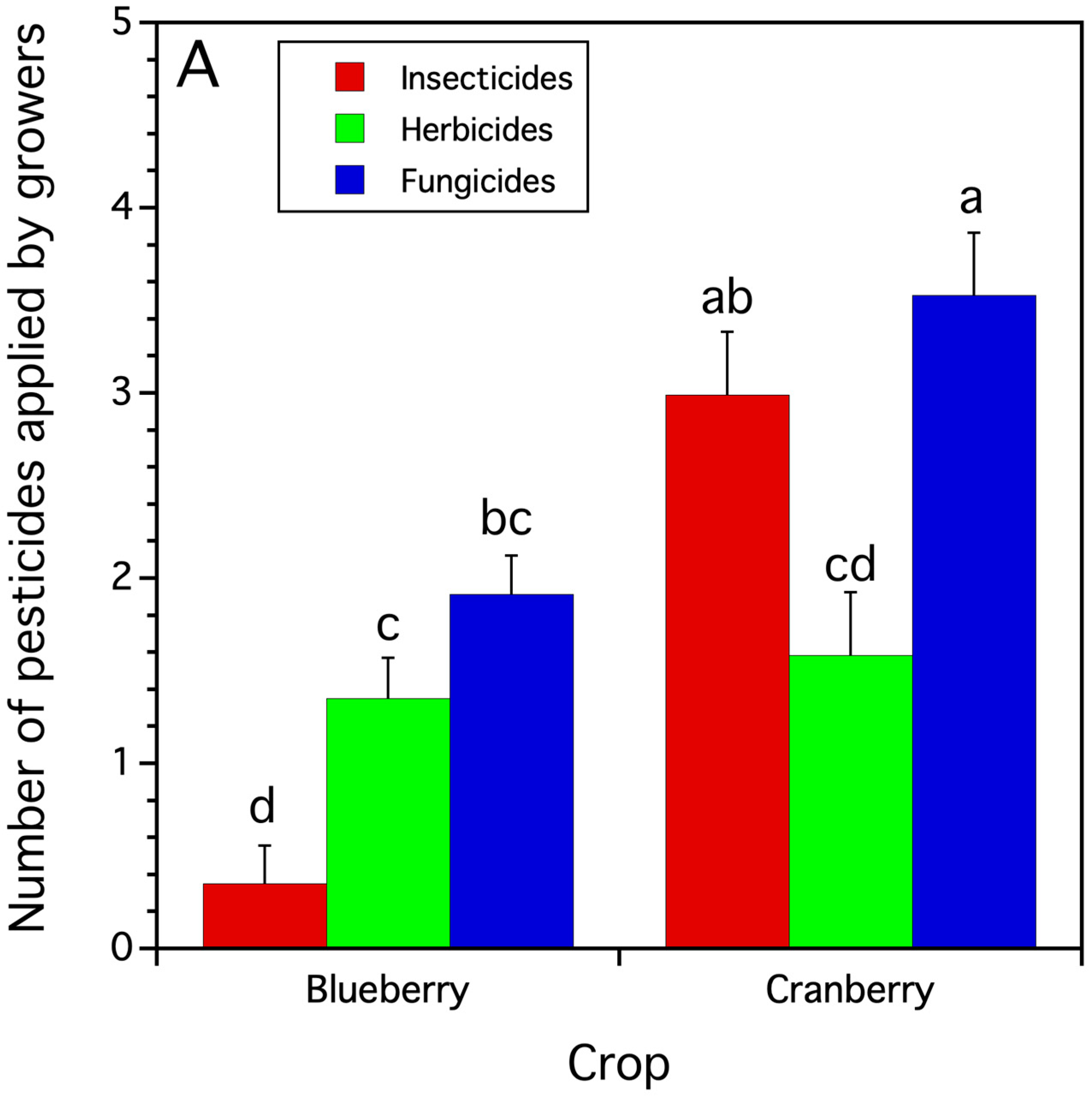
3.1.1. The Number of Different Pesticides Applied by Growers in Blueberry and Cranberry Crops
3.1.2. Pesticide Residues Detected in Trapped Pollen in Blueberry and Cranberry Crops
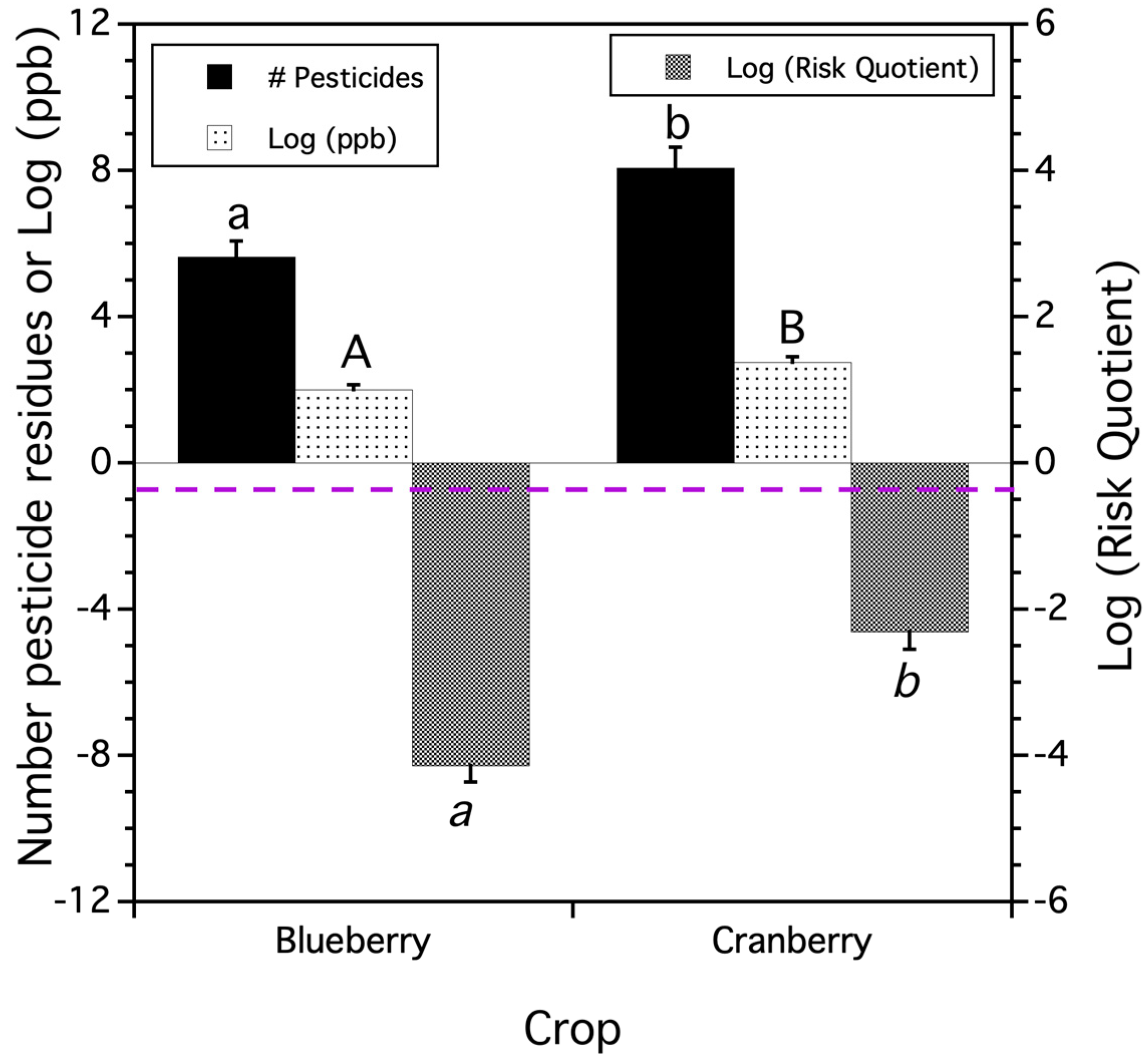
3.2. Total Residue Pesticide Concentrations and Risk of Honey Bees to Provisioned Pollen
3.2.1. Concentrations and Risk from Total Residues Detected in Trapped Pollen
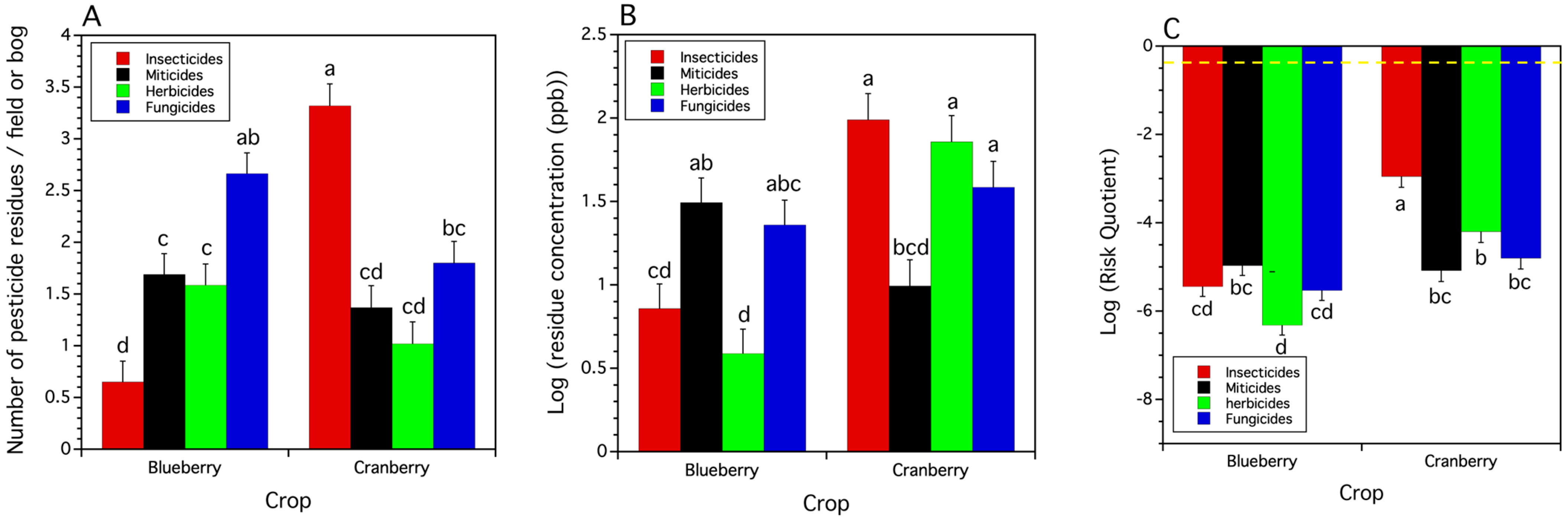
3.2.2. Pesticide Use Class Total Residues
3.2.3. Varroacide Residues in Trapped Pollen
3.3. Proportion of Total Residues in Trapped Pollen That Matched Grower Application Compounds
3.3.1. Residues That Matched Pesticides Applied by Growers, a Proportion of Total Residues Detected in Trapped Pollen
3.3.2. Concentrations and Risk Quotients of Grower Applications Relative to Total Residues
3.3.3. Residues Matched to Grower Applications—Pesticide Use Classes
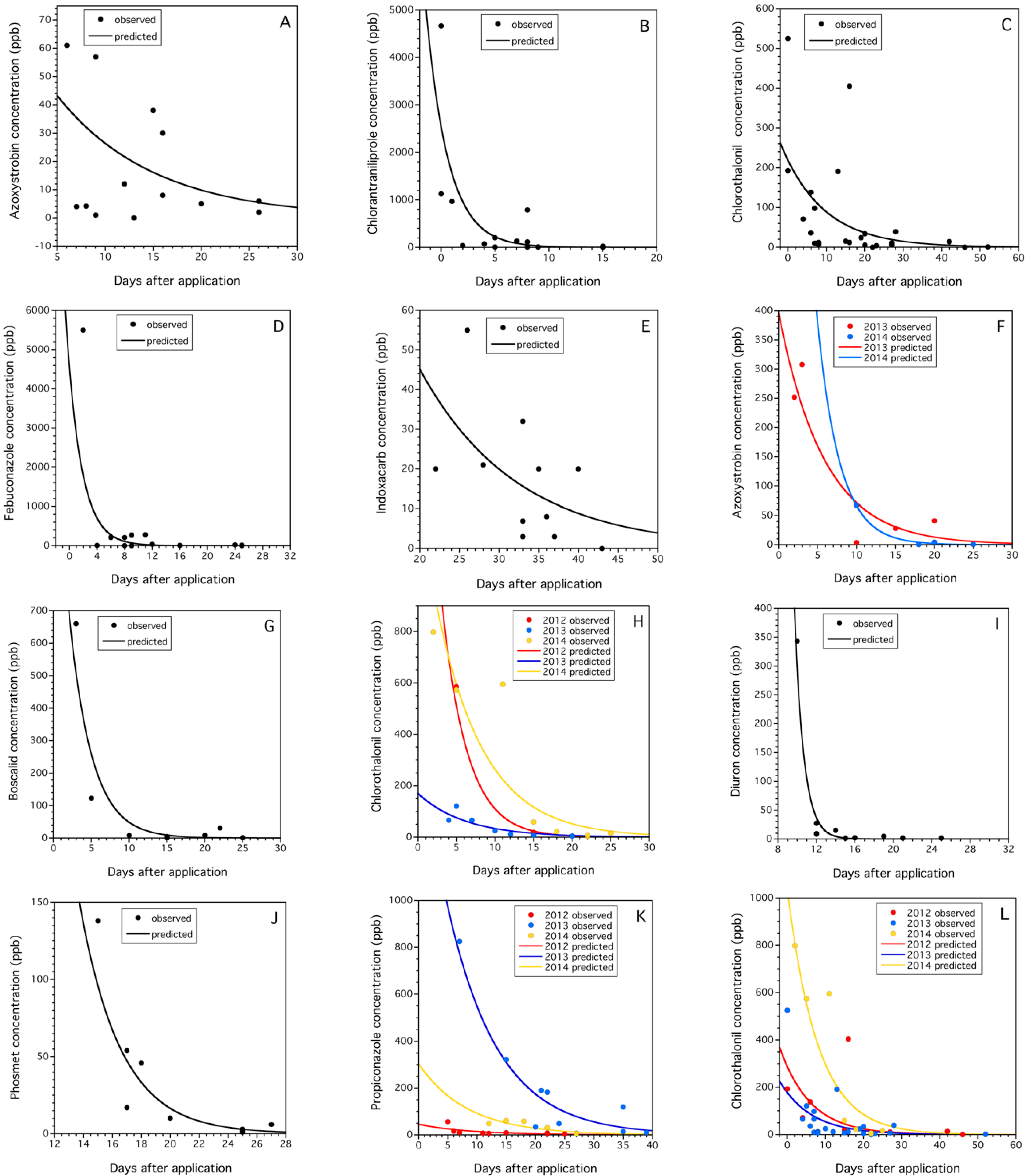
3.3.4. Field Decay Rates of Pesticide Residues in Cranberry Bogs and Blueberry Fields
| Crop | Pesticide 1,2 | Predictors 3,4 | Coefficients 5 | p Value | Proportion Variance Explained 6 | Mean Concentration 7 (ppb ± s.e.) |
|---|---|---|---|---|---|---|
| Cranberry | azoxystrobin | Intercept | 3.977 ± 0.154 | <0.0001 | 0.327 | 16.4 5.7 |
| Days 4 | −0.090 ± 0.012 | <0.0001 | ||||
| chlorantrani-liprole | Intercept | 8.172 ± 0.0155 | <0.0001 | 0.841 | 481.4 276.4 | |
| Days | −0.484 ± 0.005 | 0.001 | ||||
| chlorothalonil | Intercept | 5.388 ± 0.392 | <0.0001 | 0.289 | 74.5 26.3 | |
| Days | −0.089 ± 0.037 | 0.016 | ||||
| fenbuconazole 2 | Intercept | 9.595 ± 0.185 | <0.0001 | 0.907 | 467.9 388.0 | |
| Days | −0.525 ± 0.074 | <0.0001 | ||||
| indoxacarb 2 | Intercept | 5.443 ± 1.215 | <0.0001 | 0.267 | 17.2 4.8 | |
| Days | −0.082 ± 0.038 | 0.031 | ||||
| Blueberry | azoxystrobin 2 | Intercept | 7.684 ± 0.699 | <0.0001 | 0.899 | 78.3 39.1 |
| Year | 0.733 ± 0.669 | 0.273 | ||||
| Days | −0.349 ± 0.070 | <0.0001 | ||||
| Year x Days | 0.178 ± 0.089 | 0.046 | ||||
| boscalid | Intercept | 7.235 ± 0.552 | <0.0001 | 0.896 | 104.7 80.6 | |
| Days | −0.335 ± 0.093 | 0.004 | ||||
| 4-hydroxy- chlorothalonil | Intercept | 5.836 ± 0.629 | <0.0001 | 0.837 | 133.4 57.9 | |
| Year (2012–14) | −1.609 ± 0.398 | <0.0001 | ||||
| Year (2013–14) | −2.458 ± 0.423 | <0.0001 | ||||
| Days | −0.159 ± 0.030 | <0.0001 | ||||
| diuron 2 | Intercept | 16.151 ± 2.139 | <0.0001 | 0.978 | 41.4 33.6 | |
| Days | −1.046 ± 0.206 | <0.0001 | ||||
| phosmet | Intercept | 10.769 ± 1.065 | <0.0001 | 0.888 | 30.8 13.9 | |
| Days | −0.401 ± 0.064 | <0.0001 | ||||
| propiconazole | Intercept | 5.641 ± 0.355 | <0.0001 | 0.945 | 80.5 33.2 | |
| Year (2012–14) | −1.828 ± 0.348 | <0.0001 | ||||
| Year (2013–14) | 1.816 ± 0.226 | <0.0001 | ||||
| Days | −0.115 ± 0.017 | <0.0001 | ||||
| Both crops | 4-hydroxy-chlorothalonil | Intercept | 7.667 ± 0.419 | <0.0001 | 0.603 | 89.4 21.3 |
| Crop (cran-blue) | −0.724 ± 0.371 | 0.051 | ||||
| Year (2012–14) | −1.853 ± 0.561 | 0.001 | ||||
| Year (2013–14) | −2.147 ± 0.295 | <0.0001 | ||||
| Days | −0.125 ± 0.028 | <0.0001 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Delaplane, K.; Mayer, D.F. Crop Pollination by Bees; CABI Publishing: Wallingford, UK, 2000. [Google Scholar]
- James, R.; Pitts-Singer, T.L. (Eds.) Bee Pollination in Agricultural Ecosystems; Oxford Univ. Press: Oxford, UK, 2008. [Google Scholar]
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and control of Varroa destructor. J. Invert. Path. 2010, 103, S96–S119. [Google Scholar] [CrossRef]
- Pettis, J.S.; Lichtenberg, E.M.; Andree, M.; Stitzinger, J.; Rose, R.; van Engelsdorp, D. Crop pollination exposes honey bees which alters their susceptibility to the gut pathogen Nosema ceranae. PLoS ONE 2013, 8, e70182. [Google Scholar] [CrossRef] [PubMed]
- Traynor, K.S.; Pettis, J.S.; Tarpy, D.R.; Mullin, C.A.; Frazier, J.L.; Frazier, M.; van Engelsdorp, D. In-hive pesticide exposome: Assessing risks to migratory honey bees from in-hive pesticide contamination in the Eastern United States. Sci. Rep. 2016, 6, 33207. [Google Scholar] [CrossRef]
- Goulson, D.; Nicholls, E.; Botias, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 6229. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z. Pollen nutrition affects honey bee resistance. Terr. Arthropod Rev. 2012, 5, 175–189. [Google Scholar] [CrossRef]
- Colwell, M.J.; Williams, G.R.; Evans, R.C.; Shutler, D. Honey bee-collected pollen in agro-ecosystems reveals diet diversity, diet quality, and pesticide exposure. Ecol. Evol. 2017, 7, 7243–7253. [Google Scholar] [CrossRef]
- Alburaki, M.; Chen, D.; Skinner, J.A.; Meikle, W.G.; Tarpy, D.R.; Adamczyk, J.; Stewart, S.D. Honey bee survival and pathogen prevalence: From the perspective of landscape and exposure to pesticides. Insects 2018, 9, 65. [Google Scholar] [CrossRef]
- Hritov, P.; Shumkova, R.; Palova, N.; Neov, B. Factors associated with honey bee colony losses: A mini-review. Vet. Sci. 2020, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Traynor, K.S.; Tosi, S.; Rennich, K.; Steinhauer, N.; Forsgren, E.; Rose, R.; Kunkel, G.; Madella, S.; Lopez, D.; Eversole, H.; et al. Pesticides in honey bee colonies: Establishing a baseline for real world exposure over seven years in the USA. Environ. Poll. 2021, 279, 116566. [Google Scholar] [CrossRef]
- Frazier, M.T.; Mullin, C.A.; Frazier, J.L.; Ashcraft, S.A.; Leslie, T.W.; Mussen, E.C.; Drummond, F.A. Assessing honey bee (Hymenoptera: Apidae) foraging populations and the potential impact of pesticides on eight U.S. crops. J. Econ. Entomol. 2015, 108, 2141–2152. [Google Scholar] [CrossRef]
- Sanchez-Bayo, F.; Goka, K. Pesticide residues and bees—A risk assessment. PLoS ONE 2014, 9, e94482. [Google Scholar] [CrossRef]
- Connolly, C. The risk of insecticides to pollinating insects. Comm. Integr. Biol. 2013, 6, e25074. [Google Scholar] [CrossRef]
- Goulson, D.; Thompson, J.; Croomb, A. Rapid rise in toxic load for bees revealed by analysis of pesticide use in Great Britain. PeerJ 2018, 6, e5255. [Google Scholar] [CrossRef]
- Douglas, M.R.; Sponsler, D.B.; Lonsdorf, E.V.; Grozinger, C.M. County-level analysis reveals a rapidly shifting landscape of insecticide hazard to honey bees (Apis mellifera) on US farmland. Sci. Rep. 2020, 10, 797. [Google Scholar] [CrossRef] [PubMed]
- Stoner, K.A.; Eitzer, B.D. Using a hazard quotient to evaluate pesticide residues detected in pollen trapped from honey bees (Apis mellifera) in Connecticut. PLoS ONE 2013, 8, e77550. [Google Scholar] [CrossRef]
- Yarborough, D.E. Wild Blueberry Culture in Maine. Wild Blueberry Fact Sheet 220. Bulletin No. 2088. Available online: https://www.wildblueberries.com/wp-content/uploads/2017/04/UMaineWildBlueberryFact-Sheet.pdf (accessed on 28 June 2024).
- Sandler, H.S.; DeMoranville, C.J. Cranberry Production: A Guide for Massachusetts. UMASS Publ. CP-08. Available online: https://www.umass.edu/cranberry/downloads/CP-08.pdf (accessed on 28 June 2024).
- US-EPA. Guidance for Assessing Pesticide Risks to Bees; Environmental Protection Agency: Washington, DC, USA, 2014; Volume 59, p. 20460. Available online: https://www.epa.gov/sites/default/files/2014-06/documents/pollinator_risk_assessment_guidance_06_19_14.pdf (accessed on 28 June 2024).
- Thompson, H.M. The use of the hazard quotient approach to assess the potential risk to honeybees (Apis mellifera) posed by pesticide residues detected in bee-relevant matrices is not appropriate. Pest Manag. Sci. 2021, 77, 3934–3941. [Google Scholar] [CrossRef] [PubMed]
- Carlson, E.A.; Melathopoulos, A.; Sagili, R. The value of hazard quotients in honey bee (Apis mellifera) ecotoxicology: A review. Front. Ecol. Evol. 2022, 10, 824992. [Google Scholar]
- Hester, K.P.; Stoner, K.A.; Eitzer, B.D.; Koethe, R.W.; Lehmann, D.M. Pesticide residues in honey bee (Apis mellifera) pollen collected in two ornamental plant nurseries in Connecticut: Implications for bee health and risk assessment. Environ. Poll. 2023, 333, 122037. [Google Scholar]
- Sylvia, M.M.; Gauvin, D. (Eds.) Cranberry Chart Book 2012: Management Guide for Massachusetts; UMass Extension: Center for Agriculture: Boston, MA, USA, 2012. [Google Scholar]
- Drummond, F.A.; Lund, J.; Eitzer, B. A two-year survey of honey bee health on Maine’s blueberry barrens. Insects 2021, 12, 523. [Google Scholar] [CrossRef]
- Beekman, M.; Ratnieks, F.L.W. Long-range foraging by the honey-bee, Apis mellifera L. Funct. Ecol. 2000, 14, 490–496. [Google Scholar] [CrossRef]
- Osborne, J.L.; Williams, I.H.; Marshall, A.H.; Michaelson-Yeates, T.P.T. Pollination and gene flow in white clover, growing in a patchy habitat. Acta Hortic. 2001, 561, 35–40. [Google Scholar] [CrossRef]
- Drummond, F.A.; Smagula, J.; Annis, S.; Yarborough, D. Organic Lowbush Blueberry Production. Univ. Maine Agric. For. Exp. Stn. Tech. Bull. 2009, 852, 43. [Google Scholar]
- Yarborough, D.E. Fertilizing with Nitrogen and Phosphorus. Univ. Maine Coop. Ext. Fact Sheet No. 225, UMaine Extension. 2013. No. 2094. p. 4. Available online: https://extension.umaine.edu/blueberries/factsheets/production/fertilizing-with-nitrogen-phosphorus/ (accessed on 28 June 2024).
- Yarborough, D.E.; Drummond, F.A.; Collins, J.A. Insect Control Guide for Lowbush Blueberries. Univ. Maine Coop. Ext. Fact Sheet No. 209, UMaine Extension. 2018. No. 2001. p. 14. Available online: https://extension.umaine.edu/blueberries/factsheets/insects/ (accessed on 28 June 2024).
- Disease Management Guide: 2014 Pest Management Charts. Fact Sheet No. 219. Cooperative Extension: Maine Wild Blueberries. Available online: https://extension.umaine.edu/blueberries/factsheets/disease-2/disease-control-for-wild-blueberries/ (accessed on 28 June 2024).
- Calderwood, L.; Tooley, B. 2024 Pest Management Guide: Weeds. Available online: https://extension.umaine.edu/blueberries/wp-content/uploads/sites/41/2024/02/2024-Herbicide-Chart-Final.pdf (accessed on 28 June 2024).
- Ghantous, K. (Ed.) Cranberry Chart Book 2024–2025: Management Guide for Massachusetts; UMass Extension: Center for Agriculture: Amherst, MA, USA, 2024. [Google Scholar]
- Mullin, C.A.; Frazier, M.; Frazier, J.L.; Ashcraft, S.; Simonds, R.; van Engelsdorp, D.; Pettis, J. High levels of miticides and agrochemicals in North American apiaries: Implications for honey bee health. PLoS ONE 2010, 5, E9754. [Google Scholar] [CrossRef] [PubMed]
- SAS Institute. JMP(R), Version 16; SAS Institute Inc.: Cary, NC, USA, 2021.
- US-EPA Office of Pesticide Programs. BeeREX; United States Environmental Protection Agency: Washington, DC, USA, 2015.
- Ostiguy, N.; Drummond, F.A.; Aronstein, K.; Eitzer, B.; Ellis, J.D.; Spivak, M.; Shepherd, W.S. Pesticide exposure to honey bees in a four-year nationwide study. Insects 2019, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Olker, J.H.; Elonen, C.M.; Pilli, A.; Anderson, A.; Kinziger, B.; Erickson, S.; Skopinski, M.; Pomplun, A.; LaLone, C.A.; Russom, C.L.; et al. The ECOTOXicology knowledgebase: A curated database of ecologically relevant toxicity tests to support environmental research and risk assessment. Environ. Tox. Chem. 2022, 41, 1520–1539. [Google Scholar] [CrossRef] [PubMed]
- Kruskal, W. Ordinal measures of association. Am. Stat Assoc. 1958, 53, 814–861. [Google Scholar] [CrossRef]
- Shapiro, S.S. How to Test Normality and Other Distributional Assumptions; ASQ: Milwaukee, WI, USA, 1980. [Google Scholar]
- Kuznetsova, A.; Brockhoff, P.V.; Christensen, R.H.B. ImerTest Package: Tests in linear mixed effects models. J. Stat. Software 2017, 88, 13. [Google Scholar]
- Montgomery, D.C. Design and Analysis of Experiments, 8th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; p. 753. [Google Scholar]
- Hummel, R.M.; Claassen, E.A.; Wolfinger, R.D. JMP for Mixed Models; SAS Institute: Cary, NC, USA, 2021; p. 245. [Google Scholar]
- Zou, H. The adaptive lasso and its oracle properties. J. Am. Stat. Assoc. 2006, 101, 1418–1429. [Google Scholar] [CrossRef]
- Nagelkerke, N.J.D. A note on a general definition of the coefficient of determination. Biometrika 1991, 78, 691–692. [Google Scholar] [CrossRef]
- Drummond, F.A. Honey bee exposure to the fungicide propiconazole in lowbush blueberry production. Agronomy 2022, 12, 3081. [Google Scholar] [CrossRef]
- Drummond, F.A. Commercial bumblebee pollination of lowbush blueberry. Int. J. Fruit Sci. 2012, 12, 54–64. [Google Scholar] [CrossRef]
- Graham, K.K.; Milbrath, M.O.; Zhang, Y.; Soehnlen, A.; Baert, N.; McArt, S.; Isaacs, R. Identities, concentrations, and sources of pesticide exposure in pollen collected by managed bees during blueberry pollination. Sci. Rep. 2021, 11, 16857. [Google Scholar] [CrossRef] [PubMed]
- McArt, S.H.; Fersch, A.A.; Milano, N.J.; Truitt, L.L.; Borozky, K. High pesticide risk to honey bees despite low focal crop pollen collection during pollination of a mass blooming crop. Sci. Rep. 2017, 7, 46554. [Google Scholar] [CrossRef] [PubMed]
- Zioga, E.; Kelly, R.; White, B.; Stout, J. Plant protection product residues in plant pollen and nectar: A review of current knowledge. Environ. Res. 2020, 189, 109873. [Google Scholar] [CrossRef] [PubMed]
- Cappellari, A.; Malagnini, V.; Fontana, P.; Zanotelli, L.; Tonidandel, L.; Angeli, G.; Ioriatti, C.; Marini, L. Impacts of landscape composition on honey bee pollen contamination by pesticide: A multi-residue analysis. Chemosphere 2024, 349, 140829. [Google Scholar] [CrossRef]
- Lu, Y.; Gao, J.; Wu, T.; Han, B.; Qian, B.; Shi, M.; Yang, S.; Diao, Q.; Bu, C.; Dai, P. Expoure of chlorothalonil and acetamiprid reduce the survival and cause multiple internal disturbances in Apis mellifera larvae in vitro. Front. Physiol. 2023, 14, 1114403. [Google Scholar] [CrossRef] [PubMed]
- Rondeau, S.; Raine, N.E. Fungicides and bees: A review of exposure and risk. Environ. Int. 2022, 165, 107311. [Google Scholar] [CrossRef]
- Abbassy, M.A.; Nasr, H.M.; Abo-Yousef, H.M.; Dawood, R.R. Acute toxicity of selected insecticides and their safety to honeybee (Apis mellifera L.) workers under laboratory conditions. Austin Environ. Sci 2020, 5, 1046. [Google Scholar]
- Bergero, M.; Bosco, L.; Giacomelli, A.; Angelozzi, G.; Perugini, M.; Merola, C. Agrochemical contamination of honey and bee bread collected in the Peidmont region, Italy. Environments 2021, 8, 62. [Google Scholar] [CrossRef]
- Asare, E.; Hoshide, A.K.; Drummond, F.A.; Chen, X.; Criner, G.K. Economic risk of bee pollination in Maine lowbush blueberry, Vaccinium angustifolium Aiton. J. Econ. Entomol. 2017, 110, 1980–1992. [Google Scholar] [CrossRef]
- Gierer, F.; Vaughan, S.; Slater, M.; Thompson, H.M.; Elmore, J.S.; Girling, R.D. A review of the factors that influence pesticide residues in pollen and nectar: Future research requirements for optimizing the estimation of pollinator exposure. Environ. Poll. 2019, 249, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Dively, G.P.; Kamel, A. Insecticide residue in pollen and nectar of a cucurbit crop and their potential exposure to pollinators. J. Agric. Food Chem. 2012, 60, 4449–4456. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.S.; Vanhanen, H.; Peltola, R.; Drummond, F.A. A global review of arthropod-mediated ecosystem-services in Vaccinium berry agroecosystems. Terr. Arthropod Rev. 2014, 7, 41–78. [Google Scholar] [CrossRef]
- Graham, K.K.; Milbrath, M.O.; Zhang, Y.; Baert, N.; McArt, S.; Isaacs, R. Pesticide risk to managed bees during blueberry pollination is primarily driven by off-farm exposures. Sci. Rep. 2022, 12, 7189. [Google Scholar] [CrossRef] [PubMed]
- Greenleaf, S.S.; Williams, N.M.; Winfree, R.; Kremen, C. Bee foraging ranges and their relationship to body size. Oecologia 2007, 153, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Zurbuchen, A.; Landert, L.; Klaiber, J.; Müller, A.; Hein, S.; Dorn, S. Maximum foraging ranges in solitary bees: Only few individuals have the capability to cover long foraging distances. Biol. Conserv. 2010, 143, 669–676. [Google Scholar] [CrossRef]
- Wade, A.; Lin, C.-H.; Kurkul, C.; Regan, E.R.; Johnson, R.M. Combined toxicity of insecticides and fungicides applied to California almond orchard to honey bee larvae and adults. Insects 2019, 10, 20. [Google Scholar] [CrossRef]
- Walker, E.K.; Brock, G.N.; Arvidson, R.S.; Johnson, R.M. Acute toxicity of fungicide-insecticide-adjuvant combinations applied to almonds during bloom on adult honey bees. Environ. Toxicol. Chem. 2022, 41, 1043–1053. [Google Scholar] [CrossRef]
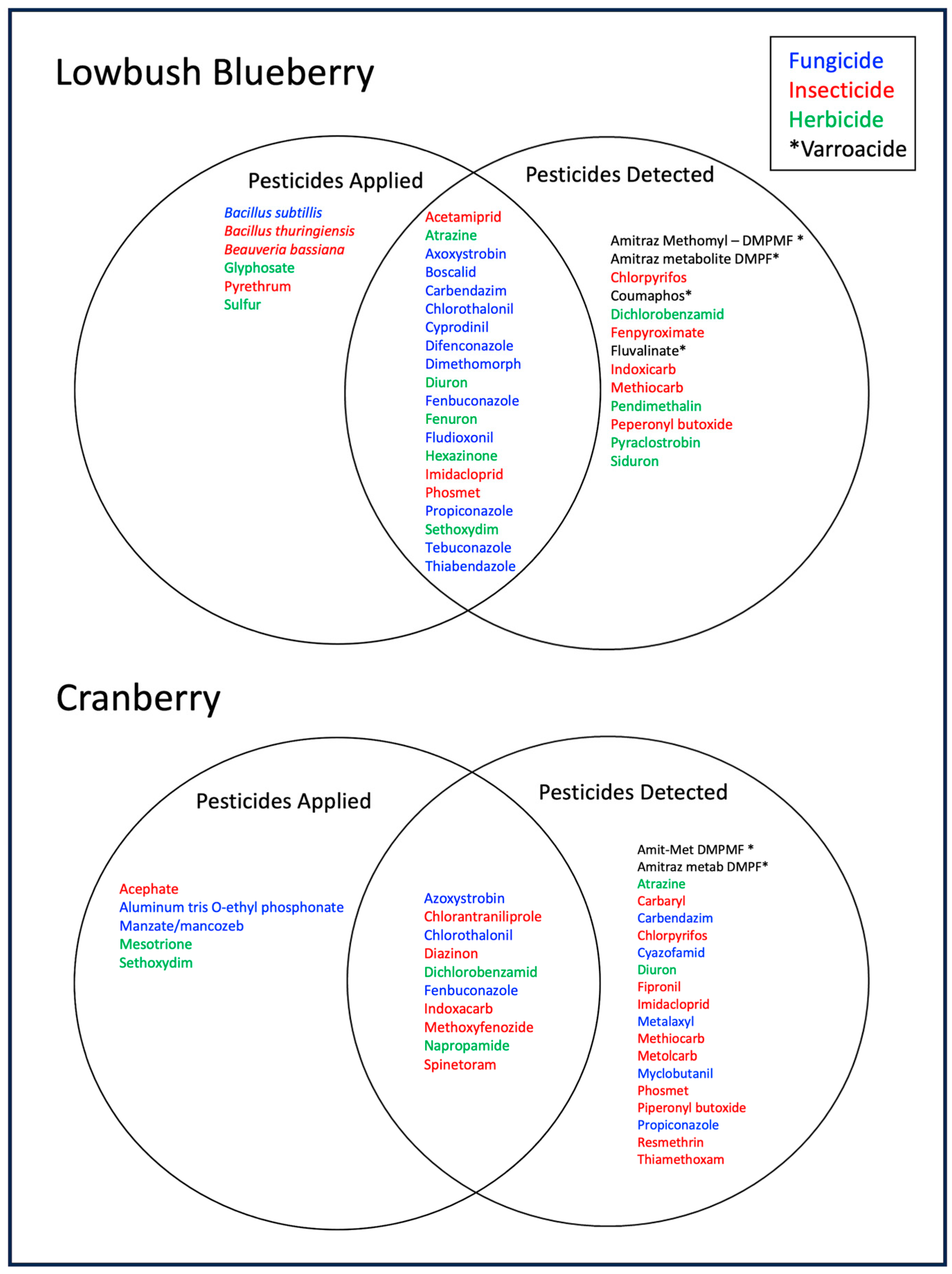
| Cranberry Residues *,1,2 | Frequency 3 | Mean ppb 4 | Blueberry Residues *,1,2 | Frequency 5 | Mean ppb 4 |
|---|---|---|---|---|---|
| 4-hydroxy- chlorothalonil F | 0.90 | 61.1 (17.4) | propiconazole F | 0.82 | 56.8 (22.7) |
| dichlorobenzamid H | 0.88 | 350.8 (104.2) | Amitraz metabolite DMPF *,M | 0.72 | 811.6 (632.8) |
| chlorantraniliproleI | 0.65 | 505.9 (260.2) | Amit-met DMPMF *,M (methomyl coelutes) | 0.67 | 226.0 (79.3) |
| Amit-met DMPMF *,M (methomyl coelutes) | 0.63 | 43.2 (43.2) | hexazinone H | 0.62 | 2.1 (1.1) |
| Amitraz metabolite DMPF *,M | 0.63 | 99.1 (48.5) | 4-hydroxy- chlorothalonil F | 0.54 | 61.7 (28.5) |
| azoxystrobin F | 0.58 | 10.7 (4.6) | atrazine H | 0.31 | 0.13 (0.1) |
| diazinon I | 0.55 | 8.4 (4.0) | carbendazim F | 0.28 | 0.6 (0.3) |
| fenbuconazole F | 0.50 | 165.2 (137.2) | diuron H | 0.26 | 10.6 (8.8) |
| indoxacarb I | 0.30 | 6.2 (1.9) | phosmet I | 0.23 | 24.9 (18.0) |
| methoxyfenozide I | 0.25 | 22.5 (16.7) | azoxystrobin F | 0.23 | 18.1 (10.1) |
| boscalid F | 0.23 | 21.5 (17.1) | |||
| pyraclostrobin *,F | 0.21 | 3.4 (2.6) |
| Test Description | Dependent Variable | F-Statistic 2 | p-Value | Treatment Means |
|---|---|---|---|---|
| grower total applications | total pesticide applications 3/year/farm | crop: F(1,42.8) = 10.045 | p = 0.003 | cranberry = 6.3 0.7 blueberry = 3.7 0.4 |
| proportion pesticide applications 4/year/farm | crop: F(1,53.0) = 14.459 | p = 0.0004 | cranberry = 0.87 blueberry = 0.54 | |
| proportion pesticide applications 5 detected/year/farm | crop: F(1,43.1) = 0.036 | p = 0.851 | cranberry = 0.50 0.09 blueberry = 0.52 0.05 | |
| proportion pesticide applications 6 detected/year/farm | crop: F(1,45.0) = 6.906 | p = 0.012 | cranberry = 0.55 blueberry = 0.90 | |
| proportion residue concentrations 7/year/farm | crop: F(1,40.9) = 7.566 | p = 0.008 | cranberry = 0.61 0.09 blueberry = 0.40 | |
| proportion residue RQ 8/year/farm | crop: F(1,36.7) = 0.123 | p = 0.728 | cranberry = 0.35 blueberry = 0.40 | |
| beekeeper applied varroacides | proportion varroacide compounds 9 applied by beekeepers/year/farm | crop F(1,46.1) = 19.972 | p < 0.0001 | cranberry = 0.02 blueberry = 0.27 |
| proportion of varroacide concentrations 7/year/farm | crop F(1,51.0) = 18.421 | p < 0.0001 | cranberry = 0.02 0.05 blueberry = 0.39 0.03 | |
| proportion of varroacide RQ 8/year/farm | crop F(1,51.0) = 2.577 | p = 0.115 | cranberry = 0.16 blueberry = 0.33 |
| Dependent Variable | F-Statistic 2 | p-Value | Blueberry Treatment Means | Cranberry Treatment Means |
|---|---|---|---|---|
| number pesticide use class applications 3/year/farm | crop: | fungicide: | fungicide: | |
| F(1,41.6) = 24.130 | p < 0.0001 | 1.91 ± 0.21 bc | 3.53 ± 0.34 a | |
| pesticide use class: | herbicide: | herbicide: | ||
| F(2,118.8) = 17.161 | p < 0.0001 | 1.35 ± 0.21 c | 1.58 ± 0.34 c | |
| interaction: | insecticide: | insecticide: | ||
| F(2,118.8) = 13.920 | p < 0.0001 | 0.35 ± 0.21 d | 2.99 ± 0.34 ab | |
| proportion pesticide use class applications 4/year/farm | crop: | fungicide: | fungicide: | |
| F(1,34.0) = 19.091 | p < 0.0001 | 0.27 ± 0.03 bc | 0.50 ± 0.06 a | |
| pesticide use class: | herbicide: | herbicide: | ||
| F(2,118.8) = 10.744 | p < 0.0001 | 0.21 ± 0.03 c | 0.25 ± 0.06 bc | |
| interaction: | insecticide: | insecticide: | ||
| F(2,118.9) = 8.979 | p < 0.0001 | 0.04 ± 0.03 d | 0.41 ± 0.06 ab | |
| proportion pesticide use class residue con-centration 5/year/farm | crop: | fungicide: | fungicide: | |
| F(1,161.0) = 1.989 | p = 0.160 | 0.27 ± 0.03 a | 0.13 ± 0.05 abc | |
| pesticide use class: | herbicide: | herbicide: | ||
| F(2,161.0) = 2.706 | p = 0.069 | 0.09 ± 0.03 c | 0.09 ± 0.05 bc | |
| interaction: | insecticide: | insecticide: | ||
| F(2,161.0) = 9.216 | p = 0.0002 | 0.03 ± 0.03 c | 0.31 ± 0.05 ab | |
| proportion pesticide use class risk quotients 6/year/farm | crop: | fungicide: | fungicide: | |
| F(1,16.8) = 3.578 | p = 0.076 | 0.18 ± 0.3 | 0.08 ± 0.05 | |
| pesticide use class: | herbicide: | herbicide: | ||
| F(2,123.6) = 2.062 | p = 0.132 | 0.66 ± 0.03 | 0.024 ± 0.05 | |
| interaction: | insecticide: | insecticide: | ||
| F(2,123.6) = 0.142 | p = 0.867 | 0.17 ± 0.03 | 0.10 ± 0.05 |
| Crop | Pesticide Residue Model 1 | Model Predicted RT50 (Days) 2 | Published Mean and Range RT50 (Days) 3 | Number of Published Studies 4 | Model Agreement with Published 5 |
|---|---|---|---|---|---|
| Cranberry | azoxystrobin | 13.0 (11.6–14.7) | 8.0 (0.4–17.5) | 17 | yes |
| chlorantrani-liprole | 3.5 (1.9–7.8) | 4.3 (2.2–9.9) | 7 | yes | |
| 4-hydroxy-chlorothalonil | 11.9 (5.5–36.9) | 5.0 (1.7–12.7) | 21 | yes | |
| fenbuconazole | 2.2 (−2.0–6.3) | 10.2 (5.7–14.8) | 2 | no | |
| indoxacarb | 31.8 (19.9–45.7) | 1.6 (0.8–2.4) | 2 | no | |
| Blueberry | azoxystrobin | 9.5 (7.8–15.4) | 8.0 (0.4–17.5) | 17 | yes |
| boscalid | 7.7 (5.9–15.6) | 5.5 (1.3–11) | 8 | yes | |
| 4-hydroxy-chlorothalonil | 8.9 (6.4–12.3) | 5.0 (1.7–12.7) | 21 | yes | |
| diuron | 11.9 (11.4–12.9) | na | 0 | no published data | |
| phosmet | 18.3 (17.5–19.5) | 2.7 (1.6–4.6) | 5 | no | |
| propiconazole | 12.5 (14.6–16.2) | 7.4 (1.0–16.9) | 10 | yes | |
| Both crops | 4-hydroxy-chlorothalonil | 10.2 (8.5–16.3) | 5.0 (1.7–12.7) | 21 | yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Averill, A.L.; Eitzer, B.D.; Drummond, F.A. Pesticide Contamination in Native North American Crops, Part I—Development of a Baseline and Comparison of Honey Bee Exposure to Residues in Lowbush Blueberry and Cranberry. Insects 2024, 15, 489. https://doi.org/10.3390/insects15070489
Averill AL, Eitzer BD, Drummond FA. Pesticide Contamination in Native North American Crops, Part I—Development of a Baseline and Comparison of Honey Bee Exposure to Residues in Lowbush Blueberry and Cranberry. Insects. 2024; 15(7):489. https://doi.org/10.3390/insects15070489
Chicago/Turabian StyleAverill, Anne L., Brian D. Eitzer, and Francis A. Drummond. 2024. "Pesticide Contamination in Native North American Crops, Part I—Development of a Baseline and Comparison of Honey Bee Exposure to Residues in Lowbush Blueberry and Cranberry" Insects 15, no. 7: 489. https://doi.org/10.3390/insects15070489
APA StyleAverill, A. L., Eitzer, B. D., & Drummond, F. A. (2024). Pesticide Contamination in Native North American Crops, Part I—Development of a Baseline and Comparison of Honey Bee Exposure to Residues in Lowbush Blueberry and Cranberry. Insects, 15(7), 489. https://doi.org/10.3390/insects15070489








