Combining the Optimized Maximum Entropy Model to Detect Key Factors in the Occurrence of Oedaleus decorus asiaticus in the Typical Grasslands of Central and Eastern Inner Mongolia
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Sources
2.2.1. Characteristics of the Oedaleus decorus asiaticus
2.2.2. Data on Grasshoppers Occurrence
2.2.3. Remote Sensing Data
2.2.4. Meteorological Data
2.2.5. Other Data
2.3. Variable Filtering
2.4. MaxEnt Model and Evaluation
3. Results
3.1. Modelling Optimization and Model Performance for Potential Distribution and Environmental Variables
3.2. Spatial Distribution of Grasshopper Occurrence in Typical Central and Eastern Inner Mongolian Steppes
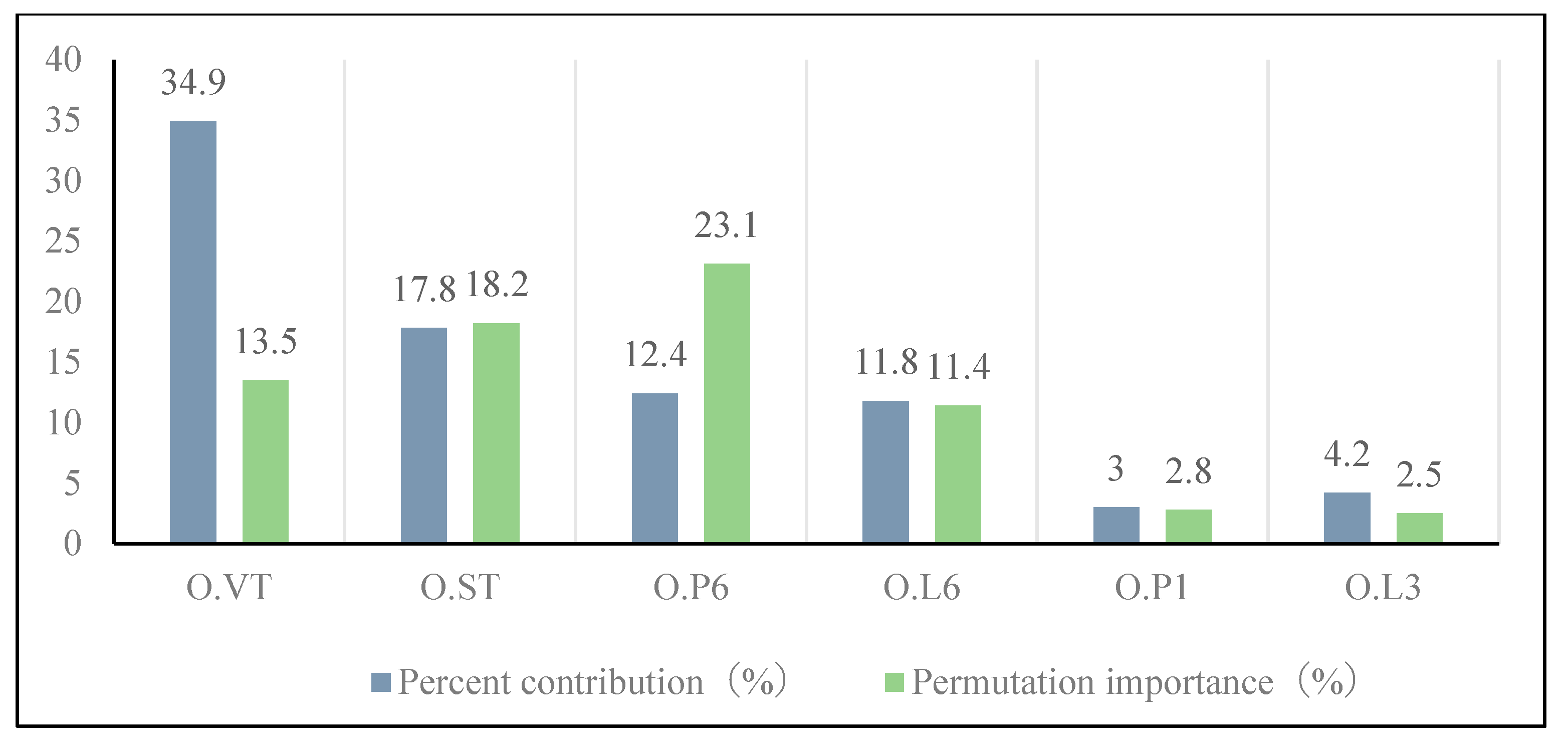
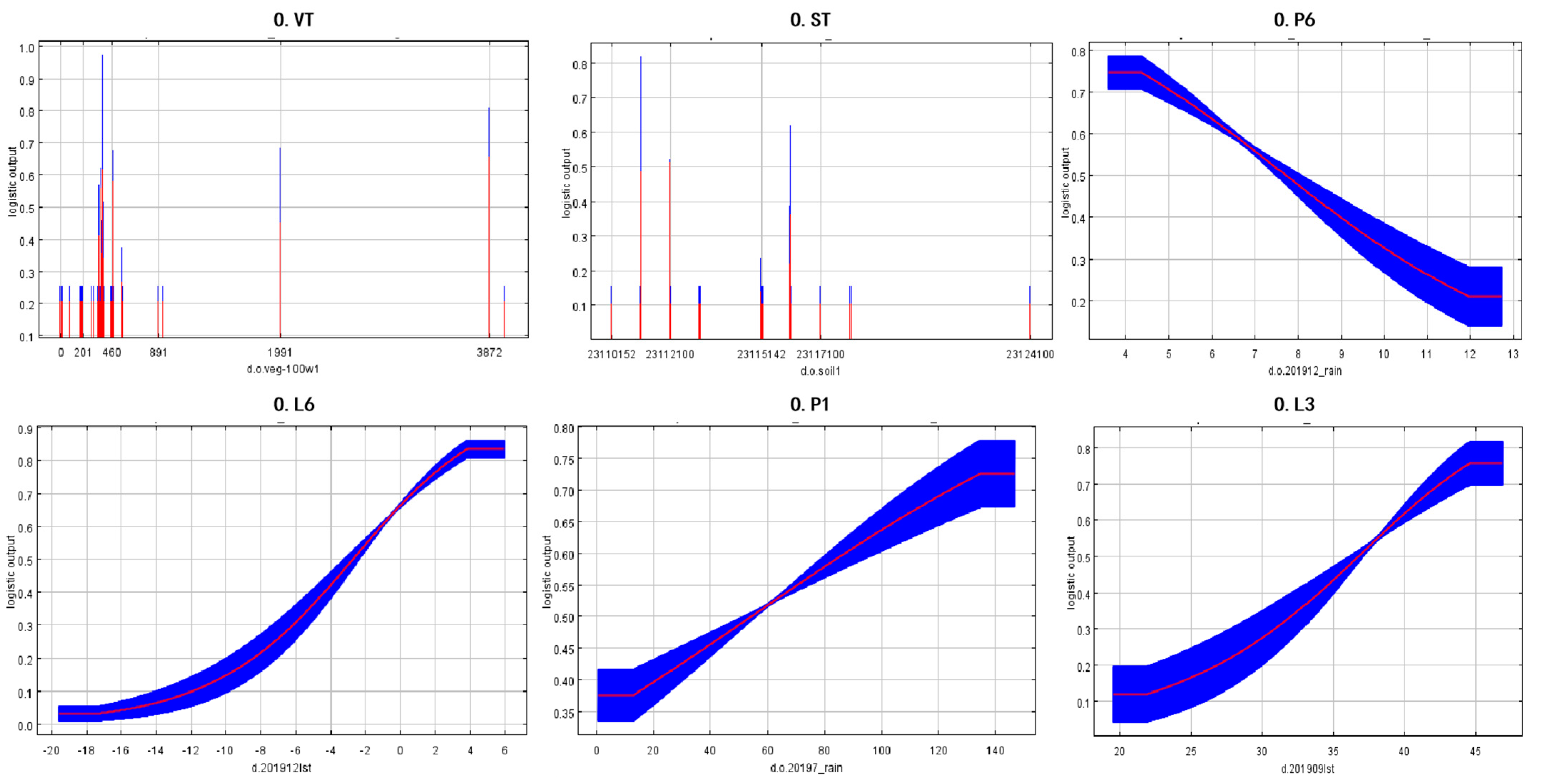
3.3. Key Factors in Grasshopper Occurrence in Typical Central and Eastern Inner Mongolian Steppes
4. Discussion
4.1. Evaluation of MaxEnt Output Performance
4.2. Potential Distribution of Oedaleus asiaticus
4.3. Environmental Variables Affecting the Distribution of Oedaleus asiaticus
4.4. Management Implications
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- He, K.; Huang, J.F. Remote sensing of locust and grasshopper plague in China: A review. In Proceedings of the 5th International Conference on Agro-Geoinformatics (Agro-Geoinformatics), Tianjin, China, 18–20 July 2016. [Google Scholar] [CrossRef]
- Ibanez, S.; Manneville, O.; Miquel, C. Plant functional traits reveal the relative contribution of habitat and food preferences to the diet of grasshoppers. Oecologia 2013, 173, 1459–1470. [Google Scholar] [CrossRef] [PubMed]
- Klein, I.; Uereyen, S.; Eisfelder, C.; Pankov, V.; Oppelt, N.; Kuenzer, C. Application of Geospatial and Remote Sensing Data to Support Locust Management. Int. J. Appl. Earth Obs. Geoinf. 2023, 117, 103212. [Google Scholar] [CrossRef]
- Peng, W.; Ma, N.L.; Zhang, D.; Zhou, Q.; Yue, X.; Khoo, S.C.; Yang, H.; Guan, R.; Chen, H.; Zhang, X.; et al. A Review of Historical and Recent Locust Outbreaks: Links to Global Warming, Food Security and Mitigation Strategies. Environ. Res. 2020, 191, 110046. [Google Scholar] [CrossRef]
- Ma, J.; Han, X.; Hasibagan; Wang, C.; Zhang, Y.; Tang, J.; Xie, Z.; Deveson, T. Monitoring East Asian Migratory Locust Plagues Using Remote Sensing Data and Field Investigations. Int. J. Remote Sens. 2005, 26, 629–634. [Google Scholar] [CrossRef]
- Ji, R.; Bintao, X.; Li, D.-M.; Li, Z.-Z.; Zhang, X. Use of MODIS Data to Monitor the Oriental Migratory Locust Plague. Agric. Ecosyst. Environ. 2004, 104, 615–620. [Google Scholar] [CrossRef]
- Nature. A Lack of Locust Preparedness Will Cost Lives. Available online: https://www.nature.com/articles/d41586-020-00692-3 (accessed on 1 May 2024).
- Kimathi, E.; Tonnang, H.E.Z.; Subramanian, S.; Cressman, K.; Abdel-Rahman, E.M.; Tesfayohannes, M.; Niassy, S.; Torto, B.; Dubois, T.; Tanga, C.M.; et al. Prediction of Breeding Regions for the Desert Locust Schistocerca gregaria in East Africa. Sci. Rep. 2020, 10, 11937. [Google Scholar] [CrossRef]
- Wettstein, W.; Schmid, B. Conservation of Arthropod Diversity in Montane Wetlands: Effect of Altitude, Habitat Quality and Habitat Fragmentation on Butterflies and Grasshoppers. J. Appl. Ecol. 1999, 36, 363–373. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, K.; Zhao, C.; Zhang, Q. Analysis of Spatial Pattern among Grasshopper and Vegetation in Heihe Based on GIS. Phys. Procedia 2012, 33, 1261–1268. [Google Scholar] [CrossRef]
- Ozment, K.A.; Welti, E.A.R.; Shaffer, M.; Kaspari, M. Tracking Nutrients in Space and Time: Interactions between Grazing Lawns and Drought Drive Abundances of Tallgrass Prairie Grasshoppers. Ecol. Evol. 2021, 11, 5413–5423. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, X.; Warner, E.; Ge, Y.; Yu, D.; Ni, S.; Wang, H. Relationship between Oriental Migratory Locust Plague and Soil Moisture Extracted from MODIS Data. Int. J. Appl. Earth Obs. Geoinf. 2008, 10, 84–91. [Google Scholar] [CrossRef]
- Lu, L.; Kong, W.; Eerdengqimuge; Ye, H.; Sun, Z.; Wang, N.; Du, B.; Zhou, Y.; Weijun; Huang, W. Detecting Key Factors of Grasshopper Occurrence in Typical Steppe and Meadow Steppe by Integrating Machine Learning Model and Remote Sensing Data. Insects 2022, 13, 894. [Google Scholar] [CrossRef]
- Essl, F.; Dirnbck, T. What Determines Orthoptera Species Distribution and Richness in Temperate Semi-Natural Dry Grassland Remnants? Biodivers. Conserv. 2012, 21, 2525–2537. [Google Scholar] [CrossRef]
- Ni, S.; Lockwood, J.A.; Wei, Y.; Jiang, J.; Zha, Y.; Zhang, H. Spatial Clustering of Rangeland Grasshoppers (Orthoptera: Acrididae) in the Qinghai Lake Region of Northwestern China. Agric. Ecosyst. Environ. 2003, 95, 61–68. [Google Scholar] [CrossRef]
- Matenaar, D.; Bazelet, C.S.; Hochkirch, A. Simple tools for the evaluation of protected areas for the conservation of grasshoppers. Biol. Conserv. 2015, 192, 192–199. [Google Scholar] [CrossRef]
- Yadav, S.; Stow, A.; Dudaniec, R.Y. Elevational Partitioning in Species Distribution, Abundance and Body Size of Australian Alpine Grasshoppers (Kosciuscola): Elevation Partitioning in Alpine Grasshoppers. Austral Ecol. 2020, 45, 609–620. [Google Scholar] [CrossRef]
- Buckley, L.B.; Graham, S.I.; Nufio, C.R. Grasshopper Species’ Seasonal Timing Underlies Shifts in Phenological Overlap in Response to Climate Gradients, Variability and Change. J. Anim. Ecol. 2021, 90, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Theron, K.J.; Pryke, J.S.; Samways, M.J. Identifying Managerial Legacies within Conservation Corridors Using Remote Sensing and Grasshoppers as Bioindicators. Ecol. Appl. 2022, 32, e02496. [Google Scholar] [CrossRef]
- Escorihuela, M.J.; Merlin, O.; Stefan, V.; Moyano, G.; Eweys, O.A.; Zribi, M.; Kamara, S.; Benahi, A.S.; Ebbe, M.A.B.; Chihrane, J.; et al. SMOS Based High Resolution Soil Moisture Estimates for Desert Locust Preventive Management. Remote Sens. Appl. Soc. Environ. 2018, 11, 140–150. [Google Scholar] [CrossRef]
- Miao, H.-T.; Liu, Y.; Shan, L.-Y.; Wu, G.-L. Linkages of Plant-Soil Interface Habitat and Grasshopper Occurrence of Typical Grassland Ecosystem. Ecol. Indic. 2018, 90, 324–333. [Google Scholar] [CrossRef]
- Ni, S.-X.; Wang, J.-C.; Jiang, J.-J.; Zha, Y. Rangeland Grasshoppers in Relation to Soils in the Qinghai Lake Region, China1 1Project Supported by the National Natural Science Foundation of China (No. 40371081). Pedosphere 2007, 17, 84–89. [Google Scholar] [CrossRef]
- Wu, T.; Hao, S.; Kang, L. Effects of Soil Temperature and Moisture on the Development and Survival of Grasshopper Eggs in Inner Mongolian Grasslands. Front. Ecol. Evol. 2021, 9, 727911. [Google Scholar] [CrossRef]
- Guo, J.; Lu, L.; Dong, Y.; Huang, W.; Zhang, B.; Du, B.; Ding, C.; Ye, H.; Wang, K.; Huang, Y.; et al. Spatiotemporal Distribution and Main Influencing Factors of Grasshopper Potential Habitats in Two Steppe Types of Inner Mongolia, China. Remote Sens. 2023, 15, 866. [Google Scholar] [CrossRef]
- Gobeyn, S.; Mouton, A.M.; Cord, A.F.; Kaim, A.; Volk, M.; Goethals, P.L.M. Evolutionary Algorithms for Species Distribution Modelling: A Review in the Context of Machine Learning. Ecol. Model. 2019, 392, 179–195. [Google Scholar] [CrossRef]
- Hao, T.; Elith, J.; Guillera-Arroita, G.; Lahoz-Monfort, J.J. A Review of Evidence about Use and Performance of Species Distribution Modelling Ensembles like BIOMOD. Divers. Distrib. 2019, 25, 839–852. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Rebelo, H.; Jones, G. Ground Validation of Presence-Only Modelling with Rare Species: A Case Study on Barbastelles Barbastella Barbastellus (Chiroptera: Vespertilionidae). J. Appl. Ecol. 2010, 47, 410–420. [Google Scholar] [CrossRef]
- Graham, C.H.; Elith, J.; Hijmans, R.J.; Guisan, A.; Townsend Peterson, A.; Loiselle, B.A.; The Nceas Predicting Species Distributions Working Group. The Influence of Spatial Errors in Species Occurrence Data Used in Distribution Models: Spatial Error in Occurrence Data for Predictive Modelling. J. Appl. Ecol. 2007, 45, 239–247. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.; Zou, X.; Chai, G.; Wu, M.; Wang, Z. Estimating the Fractional Cover of Photosynthetic Vegetation, Non-Photosynthetic Vegetation and Bare Soil from MODIS Data: Assessing the Applicability of the NDVI-DFI Model in the Typical Xilingol Grasslands. Int. J. Appl. Earth Obs. Geoinf. 2019, 76, 154–166. [Google Scholar] [CrossRef]
- Yin, H.; Pflugmacher, D.; Li, A.; Li, Z.; Hostert, P. Land Use and Land Cover Change in Inner Mongolia—Understanding the Effects of China’s Re-Vegetation Programs. Remote Sens. Environ. 2018, 204, 918–930. [Google Scholar] [CrossRef]
- Le, Z.; Bai, Y.; Liu, L. The effect of temperature on hatching of Asiatic migratory locust in the grassland of northeast China. J. Meteorol. Environ. 2013, 29, 144–147. [Google Scholar]
- Chen, S.H.; Wulangbater; Wu, X.D. Mongolia Inner in change climatic to breeding and subsistence grasshoppers’ of Response; Inner Mongolia Ecological and Agrometeorological Centre: Hohhot, China, 2007. [Google Scholar]
- Dong, W.; Zhang, X.; Zhang, X.; Wu, H.; Zhang, M.; Ma, E.; Zhang, J. Susceptibility and Potential Biochemical Mechanism of Oedaleus decorus to Beta-Cypermethrin and Deltamethrin in the Inner Mongolia, China. Pestic. Biochem. Physiol. 2016, 132, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, G.; Liang, Y.; Wang, H. Impacts of Locust Feeding on Interspecific Relationships and Niche of the Major Plants in Inner Mongolia Grasslands. Glob. Ecol. Conserv. 2024, 51, e02913. [Google Scholar] [CrossRef]
- Kang, L.; Chen, Y. Studies on the trophic ecological niche of the grassland locust. Acta Entomol. Sin. 1994, 37, 178–189. [Google Scholar] [CrossRef]
- GB/T 25875-2010; Guideline for Segmenting and Monitoring the Inhabitable areas of for Locusts and Grasshoppers in Grasslands. Standardization Administration of the People’s Republic of China: Beijing, China, 2011.
- Ni, W.; Li, X. A Coupled Vegetation-Soil Bidirectional Reflectance Model for a Semiarid Landscape. Remote Sens. Environ. 2000, 74, 113–124. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, H.-Y.; He, B.; Gexigeduren; Xin, Z.-Y.; Lin, H. Spatiotemporal Heterogeneity of the Potential Occurrence of Oedaleus Decorus Asiaticus in Inner Mongolia Steppe Habitats. J. Arid. Environ. 2015, 116, 33–43. [Google Scholar] [CrossRef]
- Word, M.L.; Hall, S.J.; Robinson, B.E.; Manneh, B.; Beye, A.; Cease, A.J. Soil-Targeted Interventions Could Alleviate Locust and Grasshopper Pest Pressure in West Africa. Sci. Total Environ. 2019, 663, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wang, Q. Spatial Distribution and Influencing Factors of Settlements in the Farming–Pastoral Ecotone of Inner Mongolia, China. Ecosyst. Health Sustain. 2020, 6, 1771213. [Google Scholar] [CrossRef]
- Dampc, J.; Kula-Maximenko, M.; Molon, M.; Durak, R. Enzymatic Defense Response of Apple Aphid Aphis Pomi to Increased Temperature. Insects 2020, 11, 436. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.-L.; Wang, X.; Xu, H.; Kang, L. Influence of Soil Moisture on Egg Cold Hardiness in the Migratory Locust Locusta migratoria (Orthoptera: Acridiidae). Physiol. Entomol. 2007, 32, 219–224. [Google Scholar] [CrossRef]
- Whitman, D.W. Function and Evolution of Thermoregulation in the Desert Grasshopper Taeniopoda Eques. J. Anim. Ecol. 1988, 57, 369. [Google Scholar] [CrossRef]
- Lactin, D.J.; Johnson, D.L. Behavioural Optimization of Body Temperature by Nymphal Grasshoppers (Melanoplus sanguinipes, Orthoptera: Acrididae) in Temperature Gradients Established Using Incandescent Bulbs. J. Therm. Biol. 1996, 21, 231–238. [Google Scholar] [CrossRef]
- Tu, X.; Zhang, Z.; Johnson, D.L.; Cao, G.; Li, Z.; Gao, S.; Nong, X.; Wang, G. Growth, Development and Daily Change in Body Weight of Locusta migratoria manilensis (Orthoptera: Acrididae) Nymphs at Different Temperatures. J. Orthoptera Res. 2012, 21, 133–140. [Google Scholar] [CrossRef]
- Wan, H.; Bai, Y.; Hooper, D.U.; Schönbach, P.; Gierus, M.; Schiborra, A.; Taube, F. Selective Grazing and Seasonal Precipitation Play Key Roles in Shaping Plant Community Structure of Semi-Arid Grasslands. Landsc. Ecol 2015, 30, 1767–1782. [Google Scholar] [CrossRef]
- White, S.R.; Bork, E.W.; Cahill, J.F. Direct and Indirect Drivers of Plant Diversity Responses to Climate and Clipping across Northern Temperate Grassland. Ecology 2014, 95, 3093–3103. [Google Scholar] [CrossRef]
- Cleland, E.E.; Collins, S.L.; Dickson, T.L.; Farrer, E.C.; Gross, K.L.; Gherardi, L.A.; Hallett, L.M.; Hobbs, R.J.; Hsu, J.S.; Turnbull, L.; et al. Sensitivity of Grassland Plant Community Composition to Spatial vs. Temporal Variation in Precipitation. Ecology 2013, 94, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Lenhart, P.A.; Eubanks, M.D.; Behmer, S.T. Water Stress in Grasslands: Dynamic Responses of Plants and Insect Herbivores. Oikos 2015, 124, 381–390. [Google Scholar] [CrossRef]
- Lu, L.; Sun, Z.; Qimuge, E.; Ye, H.; Huang, W.; Nie, C.; Wang, K.; Zhou, Y. Using Remote Sensing Data and Species–Environmental Matching Model to Predict the Potential Distribution of Grassland Rodents in the Northern China. Remote Sens. 2022, 14, 2168. [Google Scholar] [CrossRef]
- Wang, H. Spatial-Temporal Change of Climate and Driving Mechanisms of Vegetation Cover Change in Xilinguole Grasslands; Inner Mongolia Agricultural University: Hohhot, China, 2009. [Google Scholar]






| Category | Factors | Description | |
|---|---|---|---|
| LST | O.L1 | 201907LST | Land Surface Temperature of July in 2019 |
| O.L2 | 201908LST | Land Surface Temperature of August in 2019 | |
| O.L3 | 201909LST | Land Surface Temperature of September in 2019 | |
| O.L4 | 201910LST | Land Surface Temperature of October in 2019 | |
| O.L6 | 201912LST | Land Surface Temperature of December in 2019 | |
| O.L9 | 202003LST | Land Surface Temperature of March in 2020 | |
| O.L10 | 202004LST | Land Surface Temperature of April in 2020 | |
| O.L11 | 202005LST | Land Surface Temperature of May in 2020 | |
| O.L12 | 202006LST | Land Surface Temperature of June in 2020 | |
| FVC | O.F1 | 201907fvc | Fraction of Vegetation Cover of July in 2019 |
| O.F4 | 201910fvc | Fraction of Vegetation Cover of October in 2019 | |
| O.F6 | 202006fvc | Fraction of Vegetation Cover of June in 2020 | |
| DEM | O.DEM | DEM | Digital Elevation Model |
| Rain | O.P1 | 20197_rain | Precipitation of Wettest Month of July in 2019 |
| O.P2 | 20198_rain | Precipitation of Wettest Month of August in 2019 | |
| O.P6 | 201912_rain | Precipitation of Wettest Month of December in 2019 | |
| O.P8 | 20202_rain | Precipitation of Wettest Month of February in 2020 | |
| Veg | O.VT | veg | Vegetation Type |
| Soil Type | O.ST | Soil Type | Soil Type |
| Temp | O.T2 | 20198_temp | Average Temperature of August in 2019 |
| O.T5 | 20204_temp | Average Temperature of April in 2020 | |
| O.T7 | 20206_temp | Average Temperature of June in 2020 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, X.; Du, B.; Lu, L.; Lin, K.; Sa, R.; Gao, Y.; Guo, J.; Wang, N.; Huang, W. Combining the Optimized Maximum Entropy Model to Detect Key Factors in the Occurrence of Oedaleus decorus asiaticus in the Typical Grasslands of Central and Eastern Inner Mongolia. Insects 2024, 15, 488. https://doi.org/10.3390/insects15070488
Ding X, Du B, Lu L, Lin K, Sa R, Gao Y, Guo J, Wang N, Huang W. Combining the Optimized Maximum Entropy Model to Detect Key Factors in the Occurrence of Oedaleus decorus asiaticus in the Typical Grasslands of Central and Eastern Inner Mongolia. Insects. 2024; 15(7):488. https://doi.org/10.3390/insects15070488
Chicago/Turabian StyleDing, Xiaolong, Bobo Du, Longhui Lu, Kejian Lin, Rina Sa, Yang Gao, Jing Guo, Ning Wang, and Wenjiang Huang. 2024. "Combining the Optimized Maximum Entropy Model to Detect Key Factors in the Occurrence of Oedaleus decorus asiaticus in the Typical Grasslands of Central and Eastern Inner Mongolia" Insects 15, no. 7: 488. https://doi.org/10.3390/insects15070488
APA StyleDing, X., Du, B., Lu, L., Lin, K., Sa, R., Gao, Y., Guo, J., Wang, N., & Huang, W. (2024). Combining the Optimized Maximum Entropy Model to Detect Key Factors in the Occurrence of Oedaleus decorus asiaticus in the Typical Grasslands of Central and Eastern Inner Mongolia. Insects, 15(7), 488. https://doi.org/10.3390/insects15070488







