Geometric Morphometrics and Genetic Diversity Analysis of Chalcidoidea (Diglyphus and Pachyneuron) at Various Elevations
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Collection of Samples
2.2. Morphological Analysis
2.2.1. Morphological Identification
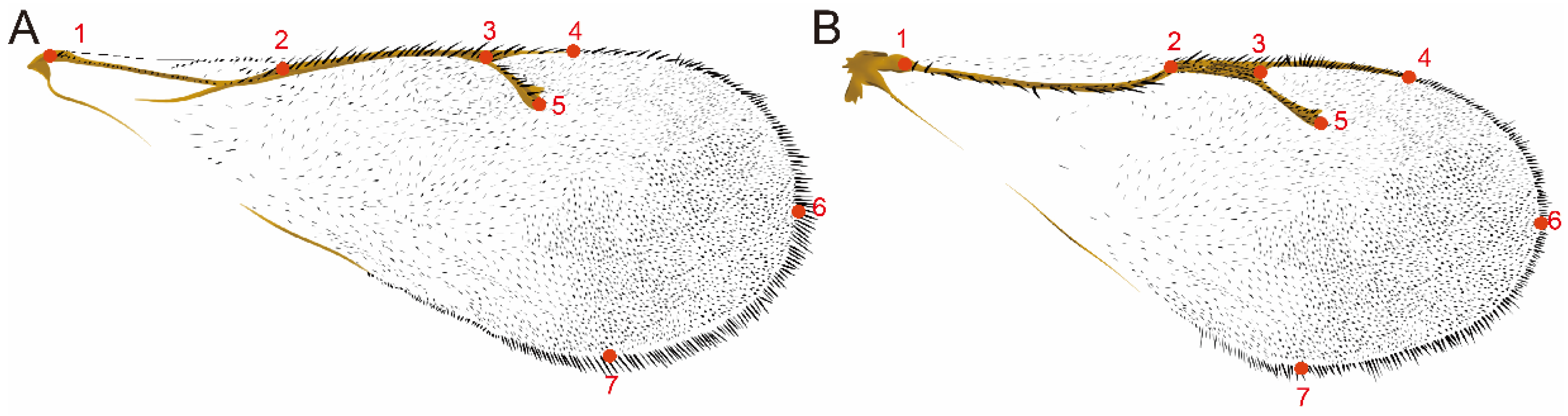
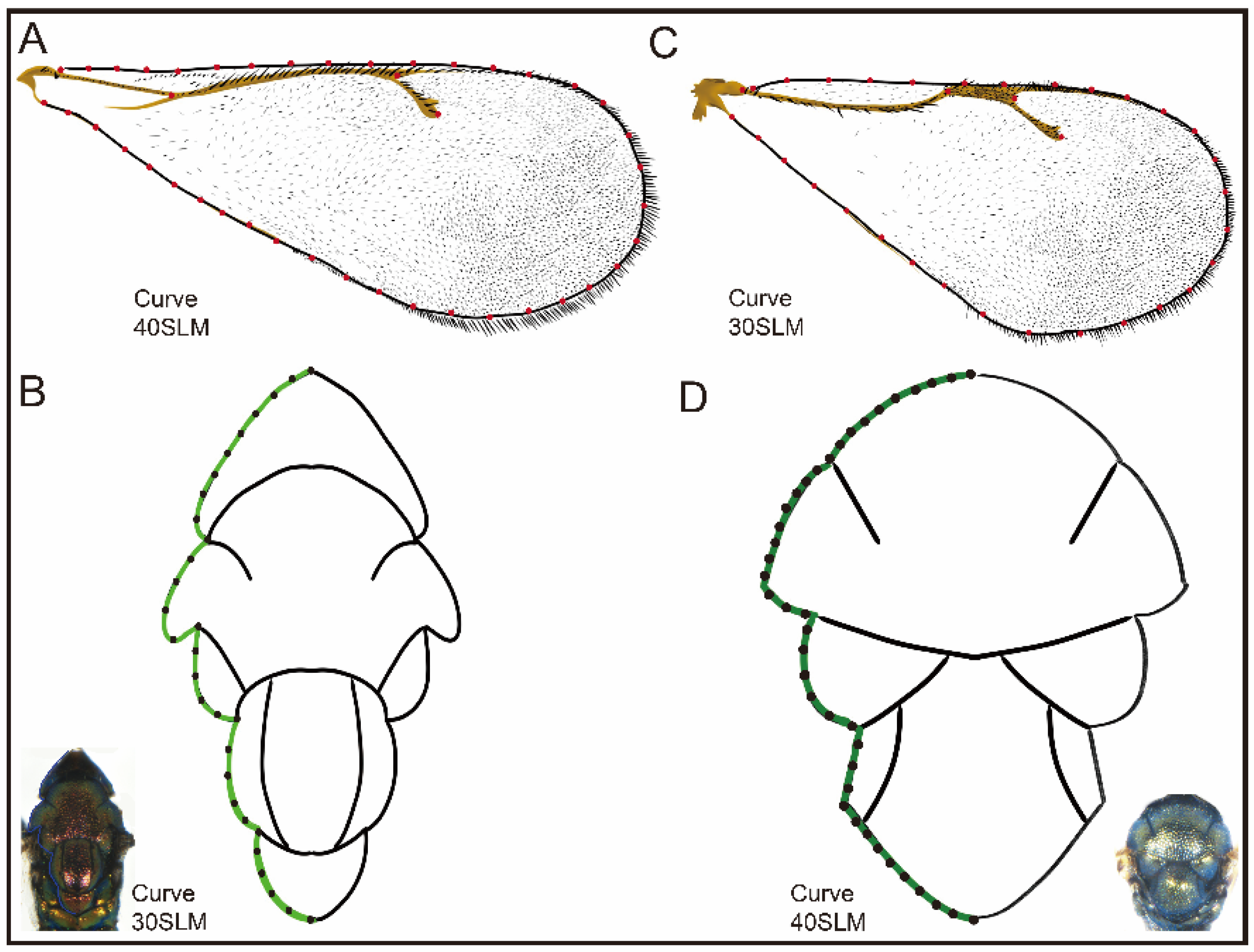
2.2.2. Morphometric Analysis
2.3. Molecular Analysis
2.4. Analysis of Morphology of the Two Genera at Different Altitudes
2.5. Analysis of D. isaea Haplotypes at Different Altitudes
3. Results
3.1. Morphological Identification
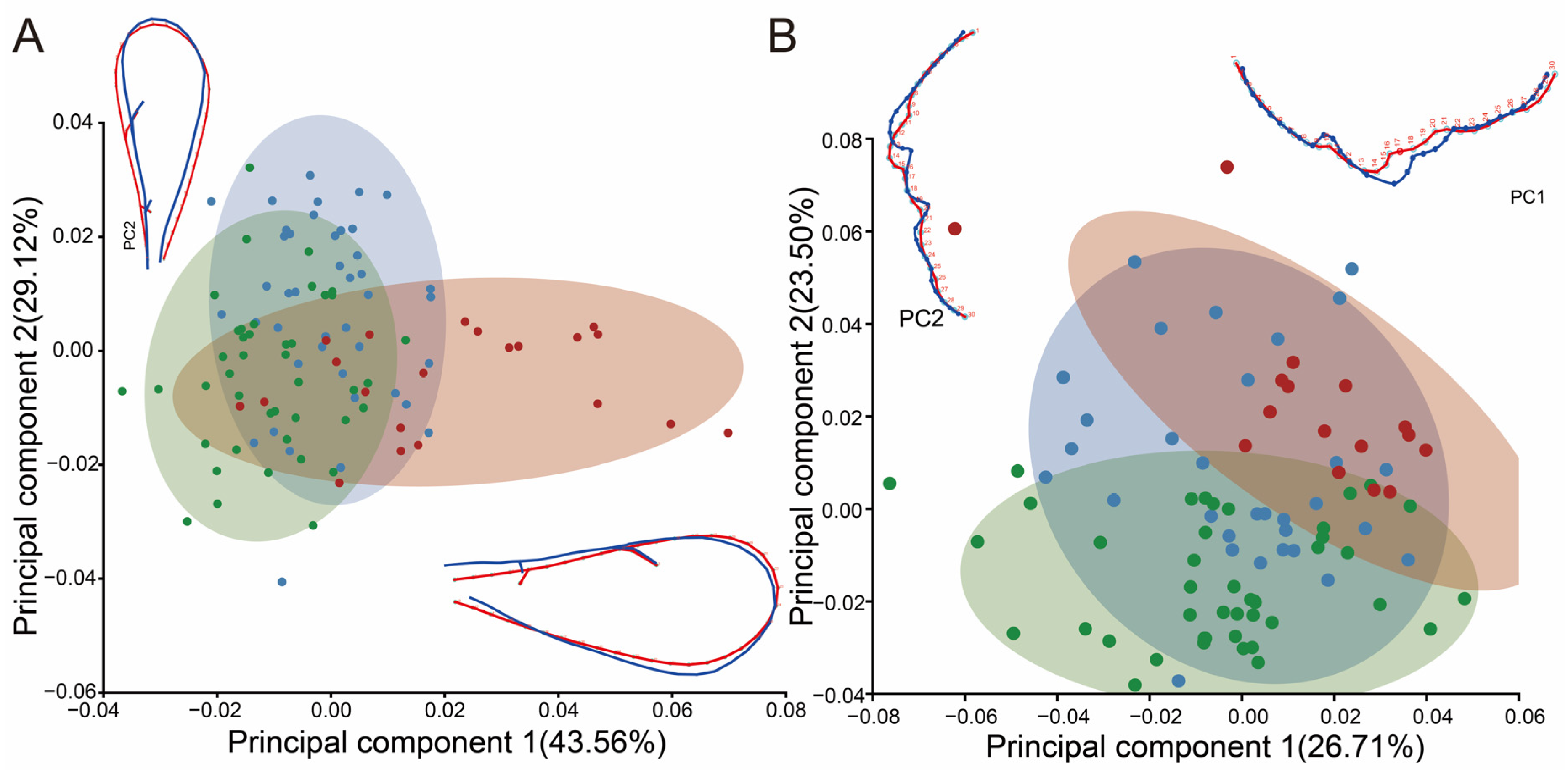
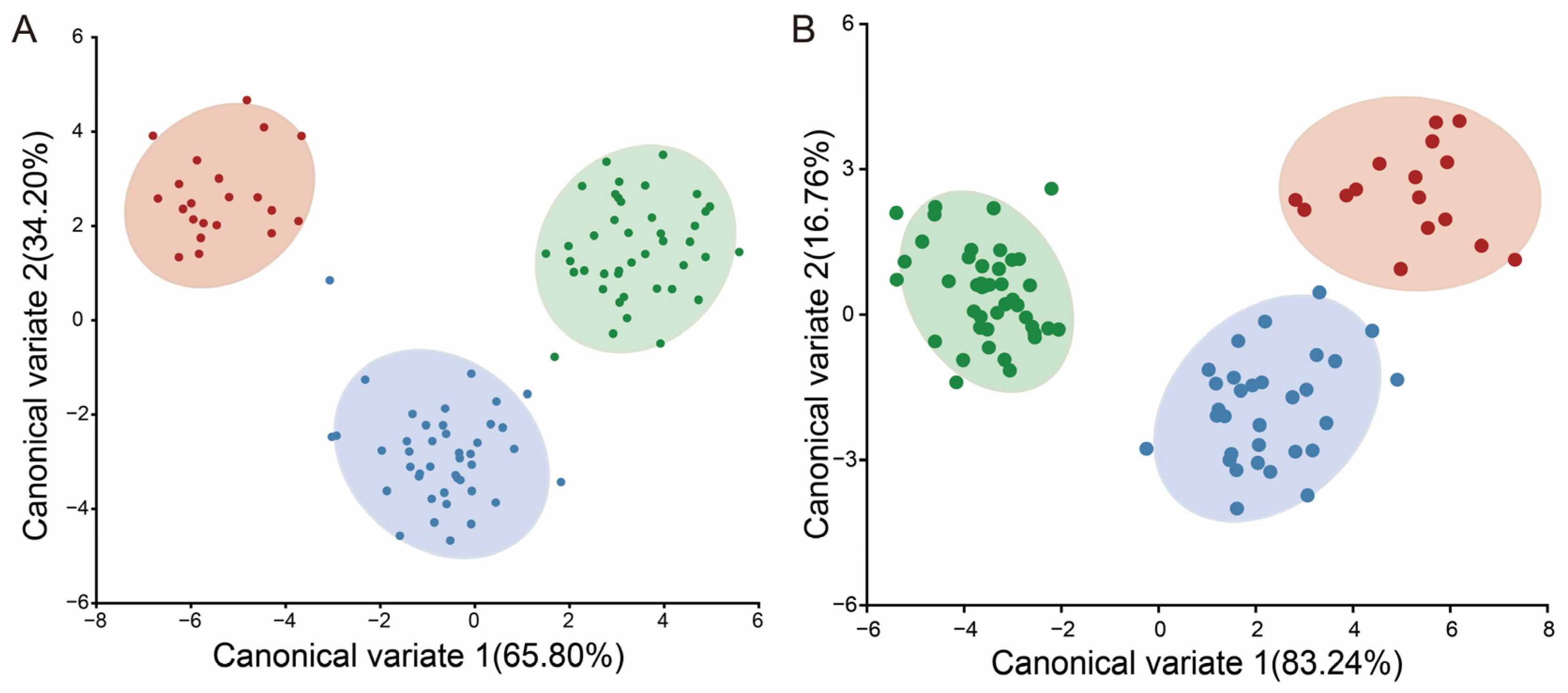
3.1.1. Morphological Classification
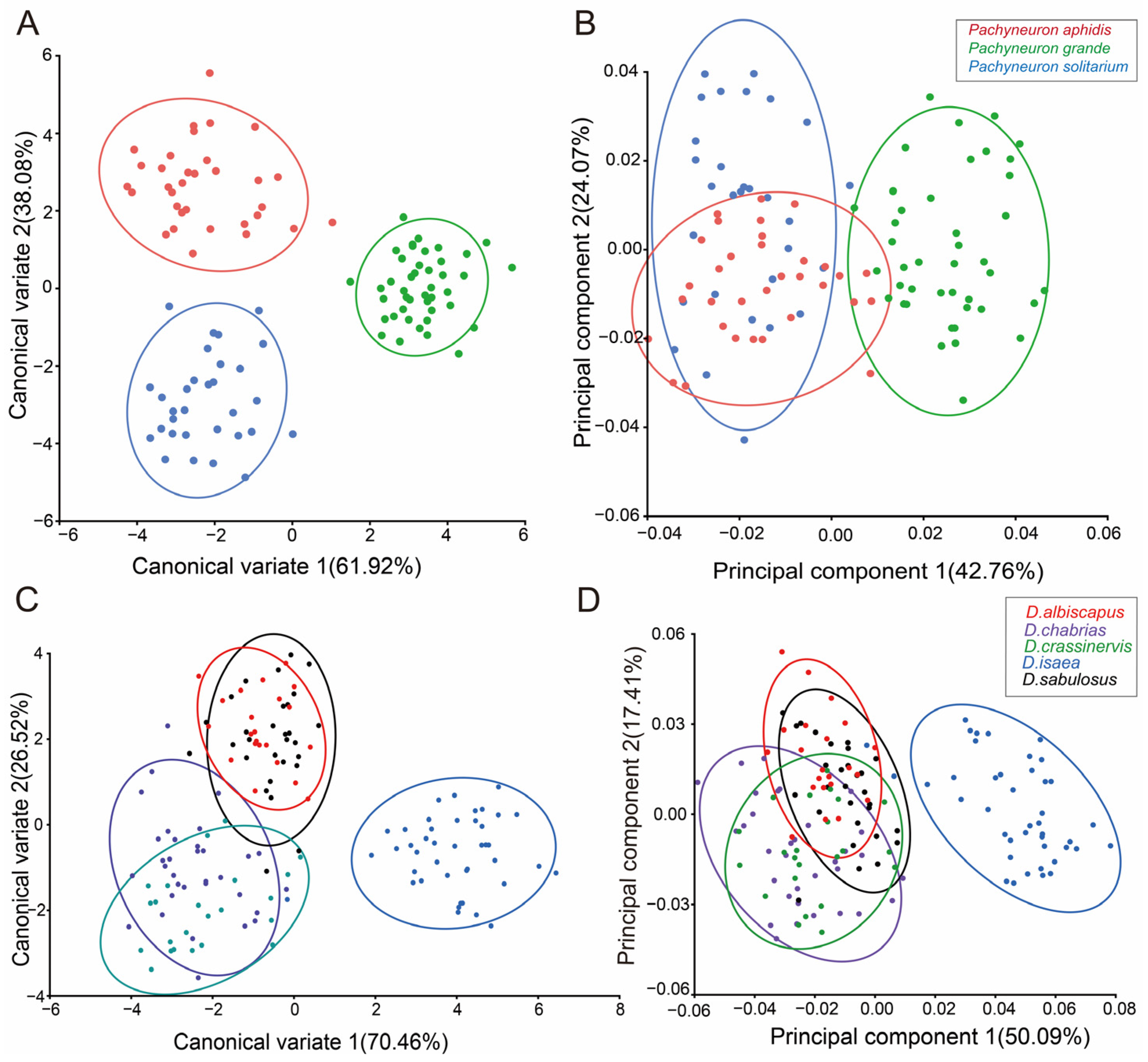
3.1.2. Classification of Geometric Morphometrics
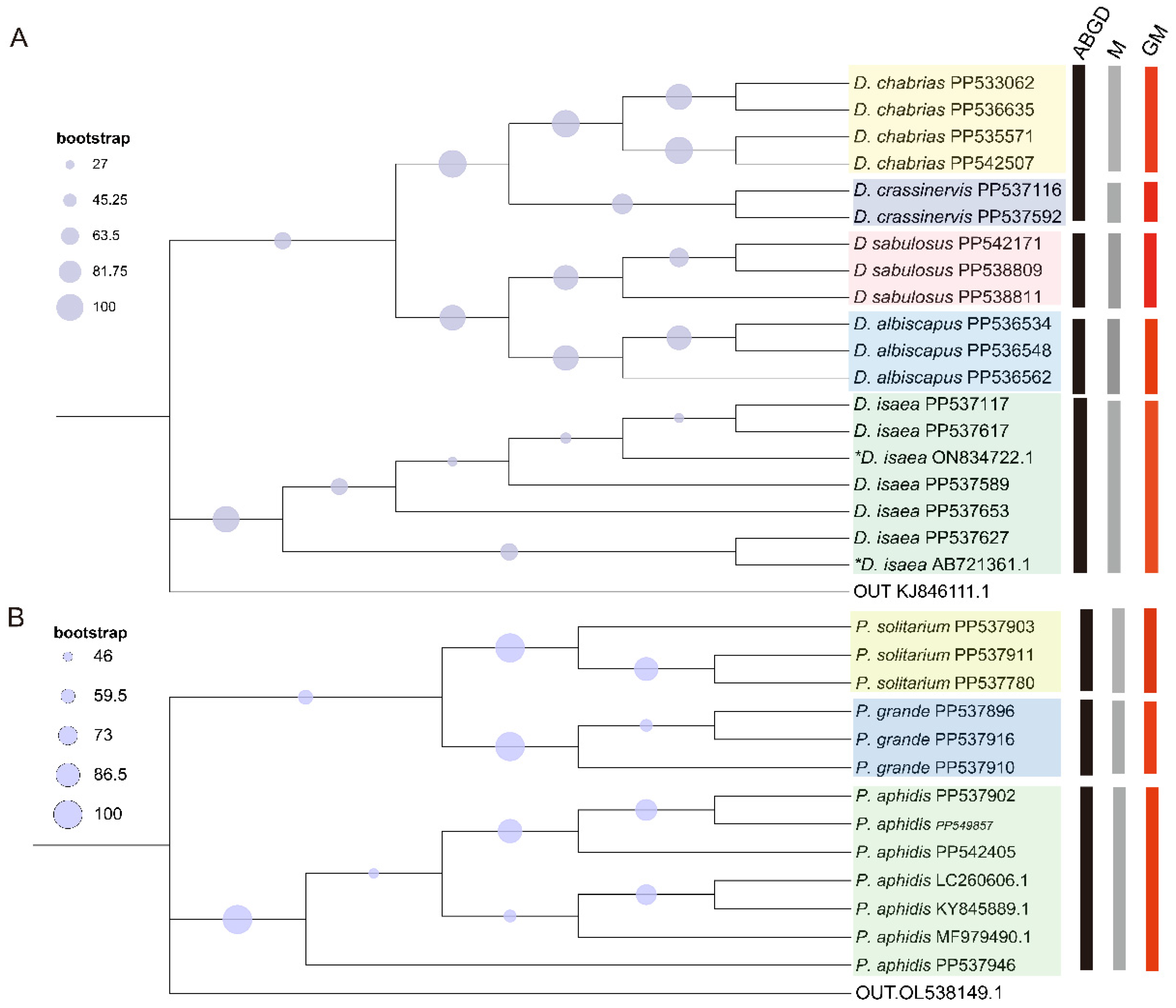
3.1.3. Phylogenetic Analysis
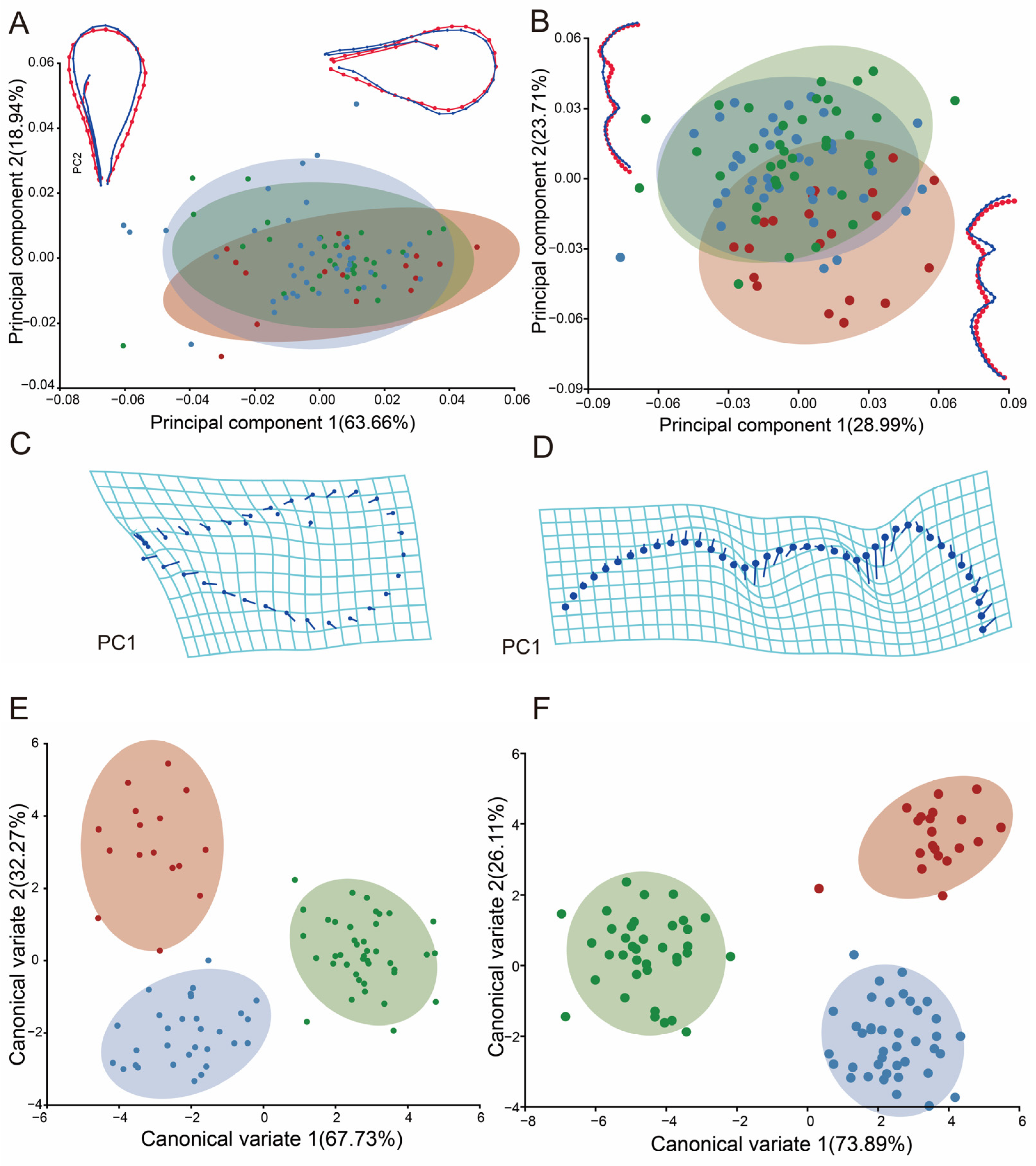
3.2. Studying Variations in Shape of Diglyphus isaea at Different Altitudes
3.3. Analysis of Shape Variability at Different Elevations in Pachyneuron aphidis
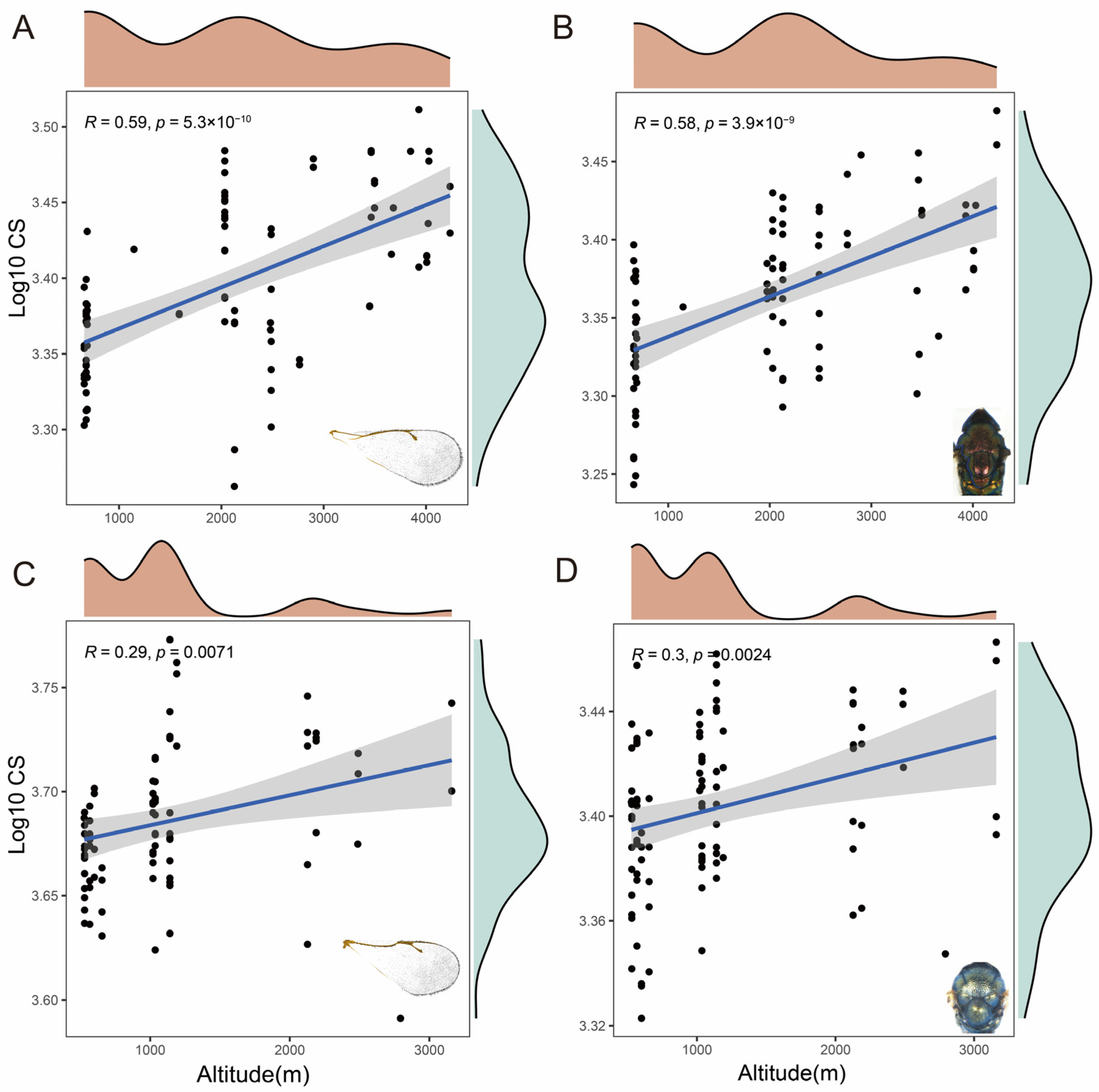
3.4. Analysis of Size Variation by Centroid Sizes
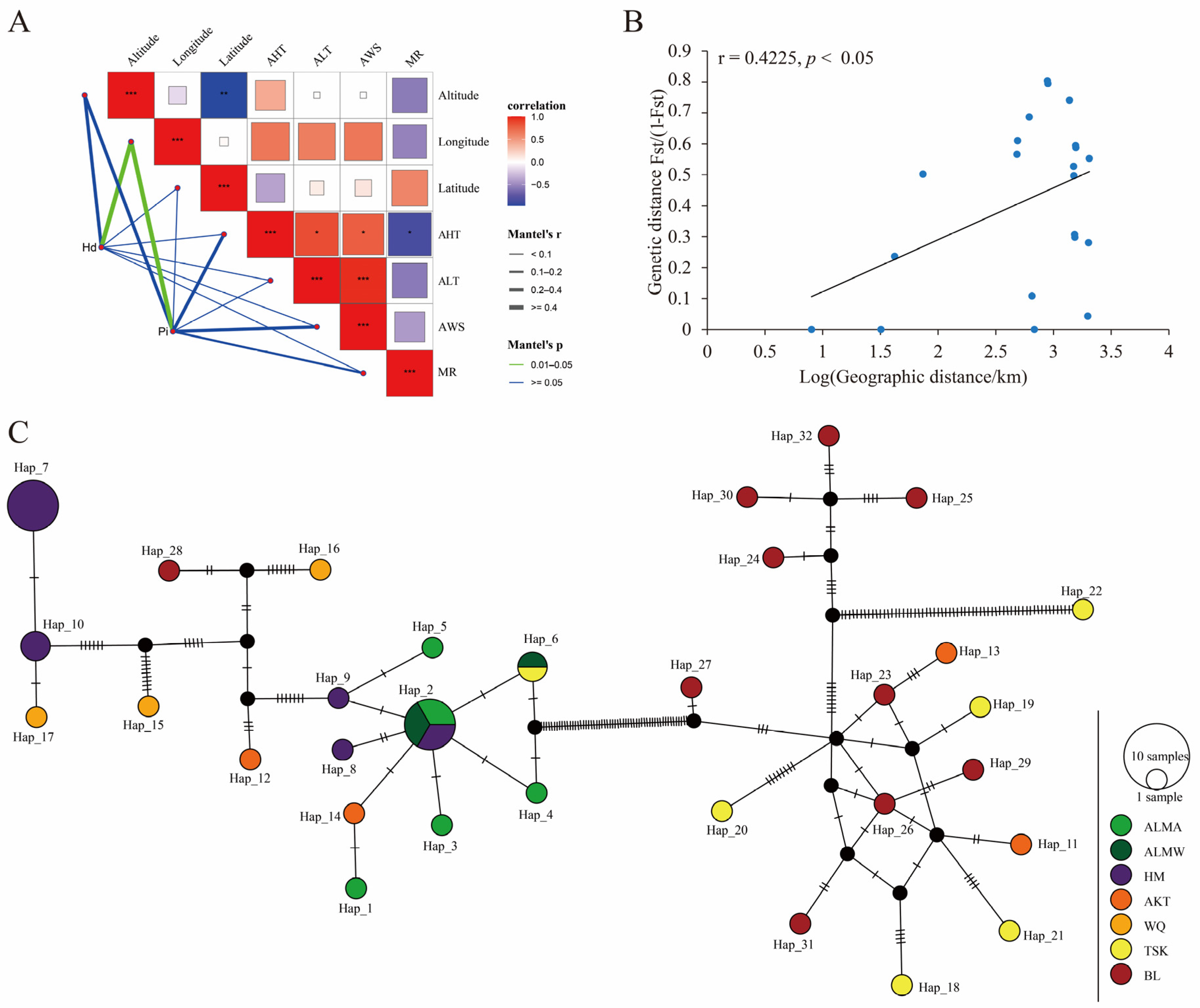
3.5. COI Gene Haplotype Analysis of D. isaea
3.5.1. Analysis of Genetic Diversity
3.5.2. Haplotype Analysis of D. isaea Populations
4. Discussions
4.1. Integrating Taxonomy to Define Diglyphus and Pachyneuron
4.2. Forewing and Thorax Shape Adaptation at Different Altitudes
4.3. Utility of the COI Gene in Population Genetic Differentiation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Slice, D.E. Geometric Morphometrics. Annu. Rev. Anthropol. 2007, 36, 261–281. [Google Scholar] [CrossRef]
- DemİRcİ, B. Size and shape variations in wing morphology of Anopheles maculipennis s.s. Meigen, 1818 (Diptera: Culicidae) from northeastern Turkey. Turk. J. Entomol. 2022, 45, 499–510. [Google Scholar] [CrossRef]
- Martinet, J.P.; Ferte, H.; Sientzoff, P.; Krupa, E.; Mathieu, B.; Depaquit, J. Wing Morphometrics of Aedes Mosquitoes from North-Eastern France. Insects 2021, 12, 341. [Google Scholar] [CrossRef] [PubMed]
- Simoes, R.F.; Wilke, A.B.B.; Chagas, C.R.F.; Menezes, R.M.T.; Suesdek, L.; Multini, L.C.; Silva, F.S.; Grech, M.G.; Marrelli, M.T.; Kirchgatter, K. Wing Geometric Morphometrics as a Tool for the Identification of Culex Subgenus Mosquitoes of Culex (Diptera: Culicidae). Insects 2020, 11, 567. [Google Scholar] [CrossRef] [PubMed]
- Lao, J.S.; Chang, B.J.; Chaiphongpachara, T. Evaluation of modern techniques for species identification of Lutzia Mosquitoes (Diptera: Culicidae) in Thailand: Geometric morphometrics and DNA barcoding. Insects 2023, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.J.; Xu, Z.; Huang, R.; Wang, H.; Yue, Y.; Ren, D. Systematic significance of wing morphology in extinct Prophalangopsidae (Insecta, Ensifera) revealed by geometric morphometrics and description of two new species. J. Syst. Palaeontol. 2022, 19, 1587–1599. [Google Scholar] [CrossRef]
- Osorio-Canadas, S.; Arnan, X.; Rodrigo, A.; Torné-Noguera, A.; Molowny, R.; Bosch, J.; Gómez, J.M. Body size phenology in a regional bee fauna: A temporal extension of Bergmann’s rule. Ecol. Lett. 2016, 19, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- Nowrouzi, S.; Andersen, A.N.; Bishop, T.R.; Robson, S.K.A. Is thermal limitation the primary driver of elevational distributions? Not for montane rainforest ants in the Australian Wet Tropics. Oecologia 2018, 188, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.E.; Sheehan, M.J. Ecogeographical patterns of body size differ among North American paper wasp species. Insectes Sociaux 2021, 68, 109–122. [Google Scholar] [CrossRef]
- Wu, C.H.; Holloway, J.D.; Hill, J.K.; Thomas, C.D.; Chen, I.C.; Ho, C.K. Reduced body sizes in climate-impacted Borneo moth assemblages are primarily explained by range shifts. Nat. Commun. 2019, 10, 4612. [Google Scholar] [CrossRef]
- Acquah-Lamptey, D.; Brandle, M.; Brandl, R.; Pinkert, S. Temperature-driven color lightness and body size variation scale to local assemblages of European Odonata but are modified by propensity for dispersal. Ecol. Evol. 2020, 10, 8936–8948. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Suárez, S.; Villalobos-Leiva, A.; Fabres, A.; Órdenes-Clavería, R.; Cruz-Jofré, F.; Laroze, D.; Correa, M.; Valladares, M.A.; Cáceres, J.S.D.; Benítez, H.A. A geometric morphometrics and genetics characterization of Vanessa carye in an extreme elevational gradient in the Chilean Altiplano. Zool. Anz. 2023, 304, 105–112. [Google Scholar] [CrossRef]
- Kang, N.; Hu, H.Y.; Huang, Z.Q.; Luo, S.G.; Guo, S.H. Environmental factors drive Chalcid body size increases with altitudinal gradients for two hyper-diverse taxa. Insects 2023, 14, 67. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, A.È.; Martel, V.; Boivin, G. Does the temperature–size rule apply to idiobiont parasitoids? Entomol. Exp. Appl. 2024, 172, 544–552. [Google Scholar] [CrossRef]
- Farsi, F.; Nozari, J. Morphometric variation in the wing of four populations of Trichogramma brassicae Bezdenko (Hymenoptera: Trichogrammatidae) in Iran. Munis Entomol. Zool. 2020, 15, 595–603. [Google Scholar]
- Giacomini, G.; Herrel, A.; Chaverri, G.; Brown, R.P.; Russo, D.; Scaravelli, D.; Meloro, C. Functional correlates of skull shape in Chiroptera: Feeding and echolocation adaptations. Integr. Zool. 2022, 17, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Dong, J.J.; Guan, D.L.; Xie, J.Y.; Xu, S.Q. Geographic variation in wing size and shape of the grasshopper Trilophidia annulata (Orthoptera: Oedipodidae): Morphological trait variations follow an ecogeographical rule. Sci. Rep. 2016, 6, 32680. [Google Scholar] [CrossRef] [PubMed]
- Nan, N.L.; Hua, M.L.; Yan, J.; Xin, G.L.; Yang, L.; Tian, J.W.; Jin, M.H.; Qing, S.N.; Xiu, M.X. Geometric morphology and population genomics provide insights into the adaptive evolution of Apis cerana in Changbai Mountain. BMC Genom. 2022, 23, 64. [Google Scholar]
- Clarke, B.C. The evolution of genetic diversity. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1997, 205, 453–474. [Google Scholar]
- Seyahooei, M.A.; Alphen, J.J.; Kraaijeveld, K. Genetic structure of Leptopilina boulardi populations from different climatic zones of Iran. BMC Ecol. 2011, 11, 4. [Google Scholar] [CrossRef]
- Yu, X.; Chen, F.; Chen, Z.; Wei, P.; Song, X.; Liu, C.; Liu, T.; Li, X.; Liu, X. Genetic diversity and gene expression diversity shape the adaptive pattern of the aquatic plant Batrachium bungei along an altitudinal gradient on the Qinghai–Tibet plateau. Plant Mol. Biol. 2022, 111, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhang, D.K.; Jiang, L.L.; Guo, J.; Hu, H.Y. Globular galls on Haloxylon (Centrospermae: Chenopodiaceae) induced by gall midges, and the corresponding parasitoids in China. Appl. Entomol. Zool. 2023, 58, 345–356. [Google Scholar] [CrossRef]
- Sha, Z.L.; Zhu, C.D.; Murphy, R.W.; Salle, J.L.; Huang, D.W. Mitochondrial phylogeography of a leafminer parasitoid, Diglyphus isaea (Hymenoptera: Eulophidae) in China. Biol. Control 2006, 38, 380–389. [Google Scholar] [CrossRef]
- Xiao, H.; Jiao, T.Y.; Huang, D.W. Pachyneuron (Hymenoptera: Pteromalidae) from China. Orient. Insects 2009, 43, 341–359. [Google Scholar] [CrossRef]
- DeSalle, R.; Munro, J.B.; Heraty, J.M.; Burks, R.A.; Hawks, D.; Mottern, J.; Cruaud, A.; Rasplus, J.Y.; Jansta, P. A Molecular Phylogeny of the Chalcidoidea (Hymenoptera). PLoS ONE 2011, 6, e27023. [Google Scholar]
- Gibson, G.A.P. The Australian species of Pachyneuron Walker (Hymenoptera: Chalcidoidea: Pteromalidae). J. Hymenopt. Res. 2001, 10, 29–54. [Google Scholar]
- Wu, Y.F.; Yang, H.L.; Feng, Z.B.; Li, B.Y.; Zhou, W.B.; Song, F.; Li, H.; Zhang, L.M.; Cai, W.Z. Novel gene rearrangement in the mitochondrial genome of Pachyneuron aphidis (Hymenoptera: Pteromalidae). Int. J. Biol. Macromol. 2020, 149, 1207–1212. [Google Scholar] [CrossRef]
- Li, J.H.; Wu, Y.K.; Zhang, Q.; Li, H.Q.; Pan, H.S.; Lu, W.; Wang, D.M.; Zhang, J.P.; Lu, Y.H. Aphid parasitism and parasitoid diversity in cotton fields in Xinjiang, China. PLoS ONE 2018, 13, e0207034. [Google Scholar] [CrossRef]
- Shen, S.F.; Wu, W.; Zhang, Z.; Wang, Y.M.; Kong, X.B.; Liu, F.; Zhang, S.F. Morphological analysis of sensilla on different organs in Pachyneuron aphidis, a hyperparasitoid of Myzus persicae. Microsc. Res. Tech. 2019, 82, 1810–1818. [Google Scholar] [CrossRef]
- Yeates, D.K.; Seago, A.; Nelson, L.; Cameron, S.L.; Joseph, L.E.O.; Trueman, J.W.H. Integrative taxonomy, or iterative taxonomy? Syst. Entomol. 2010, 36, 209–217. [Google Scholar] [CrossRef]
- Takahashi, T.A.; Nishimura, G.; Querino, R.B.; Foerster, L.A. An Integrative Taxonomy of a New Species of Trichogramma Westwood (Hymenoptera: Trichogrammatidae) with High Reproductive Capacity. Neotrop. Entomol. 2021, 50, 90–99. [Google Scholar] [CrossRef]
- Padial, J.M.; Miralles, A.; De la Riva, I.; Vences, M. The integrative future of taxonomy. Front. Zool. 2010, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Chen, Y.; Guan, X.; Zhao, Y.; Chen, J.; Mao, W. Recent climate and hydrological changes in a mountain–basin system in Xinjiang, China. Earth-Sci. Rev. 2022, 226, 103957. [Google Scholar] [CrossRef]
- Luo, M.; Liu, T.; Meng, F.H.; Duan, Y.C.; Bao, A.M.; Xing, W.; Feng, X.W.; Maeyer, P.D.; Frankl, A. Identifying climate change impacts on water resources in Xinjiang, China. Sci. Total Environ. 2019, 676, 613–626. [Google Scholar] [CrossRef]
- Graham, M.W.R. A Reclassfication of the European Tetrastichinae (Hymenoptera: Eulophidae), with a Revision of Certain Genera; British Museum Press: London, UK, 1987; pp. 14–192. [Google Scholar]
- Gibson, G.A.P.; Huber, J.T.; Woolley, J.B. Annotated Keys to the Genera of Nearctic Chalcidoidea (Hymenoptera); NRC Research Press: Ottawa, ON, Canada, 1997; pp. 1–726. [Google Scholar]
- Zhu, C.D.; Lasalle, J.; Huang, D.W. A review of the Chinese Diglyphus Walker (Hymenoptera: Eulophidae). Orient. Insects 2000, 34, 263–288. [Google Scholar] [CrossRef]
- Sha, Z.L.; Zhu, C.D.; Murphy, R.W.; Huang, D.W. Diglyphus isaea (Hymenoptera: Eulophidae): A probable complex of cryptic species that forms an important biological control agent of agromyzid leaf miners. J. Zool. Syst. Evol. Res. 2007, 45, 128–135. [Google Scholar] [CrossRef]
- Dzhanokmen, K.A. Review of Kazakhstan species of the genus Pachyneuron Walker, 1833 (Hymenoptera: Chalcidoidea: Pteromalidae). Zoosyst. Ross. 2009, 18, 73–82. [Google Scholar] [CrossRef]
- Hansson, C.; Navone, P. Review of the European species of Diglyphus Walker (Hymenoptera: Eulophidae) including the description of a new species. Zootaxa 2017, 4269, 197–229. [Google Scholar] [CrossRef]
- Rohlf, F.J. The tps series of software. Hystrix Ital. J. Mammal. 2015, 26, 9–12. [Google Scholar]
- Klingenberg, C.P. MorphoJ: An integrated software package for geometric morphometrics. Mol. Ecol. Resour. 2010, 11, 353–357. [Google Scholar] [CrossRef]
- Baur, H.; Kranz-baltensperger, Y.V.O.N.N.E.; Cruaud, A.; Rasplus, J.Y.; Timokhov, A.V.; Gokhman, V.E. Morphometric analysis and taxonomic revision of Anisopteromalus Ruschka (Hymenoptera: Chalcidoidea: Pteromalidae)—An integrative approach. Syst. Entomol. 2014, 39, 691–709. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. Clustal w: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 1673–1680. [Google Scholar] [CrossRef]
- Xiang, C.Y.; Gao, F.; Jakovlić, I.; Lei, H.P.; Hu, Y.; Zhang, H.; Zou, H.; Wang, G.T.; Zhang, D. Using PhyloSuite for molecular phylogeny and tree-based analyses. iMeta 2023, 16, e87. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; Haeseler, A.V.; Jermiin, L.S. Modelfinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; Von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.T.; Vinh, L.S.; Flouri, T.; Stamatakis, A.; von Haeseler, A.; Minh, B.Q. MPBoot: Fast phylogenetic maximum parsimony tree inference and bootstrap approximation. BMC Evol. Biol. 2018, 18, 11. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Puillandre, N.; Lambert, A.; Brouillet, S.; Achaz, G. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol. Ecol. 2012, 21, 1864–1877. [Google Scholar] [CrossRef] [PubMed]
- Nishimaki, T.; Sato, K. An extension of the Kimura two-parameter model to the natural evolutionary process. J. Mol. Evol. 2019, 87, 60–67. [Google Scholar] [CrossRef]
- Adams, D.C.; Otárola-Castillo, E.; Paradis, E. Geomorph: An r package for the collection and analysis of geometric morphometric shape data. Methods Ecol. Evol. 2013, 4, 393–399. [Google Scholar] [CrossRef]
- Diniz-Filho, J.A.; Soares, T.N.; Lima, J.S.; Dobrovolski, R.; Landeiro, V.L.; Campos, T.M.P.; Rangel, T.F.; Bini, L.M. Mantel test in population genetics. Genet. Mol. Biol. 2013, 36, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Brock, J.; Dyer, J.M.; Kutchan, T.; Schachtman, D.; Augustin, M.; Ge, Y.; Fahlgren, N.; Abdel-Haleem, H. Genetic Diversity and Population Structure of a Camelina sativa Spring Panel. Front. Plant Sci. 2019, 10, 425924. [Google Scholar] [CrossRef]
- Bohonak, A.J. IBD (isolation by distance): A program for analyses of isolation by distance. J. Hered. 2002, 93, 153–154. [Google Scholar] [CrossRef]
- Rasplus, J.Y.; Blaimer, B.B.; Brady, S.G.; Burks, R.A.; Delvare, G.; Fisher, N.; Gates, M.; Gauthier, N.; Gumovsky, A.V.; Hansson, C.; et al. A first phylogenomic hypothesis for Eulophidae (Hymenoptera, Chalcidoidea). J. Nat. Hist. 2020, 54, 597–609. [Google Scholar] [CrossRef]
- Yefremova, Z.; Civelek, H.S.; Boyadzhiyev, P.; Dursun, O.; Eskin, A. A review of Turkish DiglyphusWalker (Hymenoptera: Eulophidae), with description of a new species. Ann. Soc. Entomol. Fr. (NS) 2013, 47, 273–279. [Google Scholar] [CrossRef]
- Barour, C.; Baylac, M. Geometric morphometric discrimination of the three African honeybee subspecies Apis mellifera intermissa, A. m. sahariensis and A. m. capensis (Hymenoptera, Apidae): Fore wing and hind wing landmark configurations. J. Hymenopt. Res. 2016, 52, 61–70. [Google Scholar] [CrossRef]
- Santoso, M.A.D.; Juliandi, B.; Raffiudin, R. Honey bees species differentiation using geometric morphometric on wing venations. IOP Conf. Ser. Earth Environ. Sci. 2018, 197, 01205. [Google Scholar] [CrossRef]
- Peter, K.C.; Monteiro, L.R. Distances and directions in multidimensional shape spaces: Implications for morphometric applications. Syst. Biol. 2005, 54, 678–688. [Google Scholar]
- Klingenberg, C.P. Size, shape, and form: Concepts of allometry in geometric morphometrics. Dev. Genes Evol. 2016, 226, 113–137. [Google Scholar] [CrossRef]
- Sontigun, N.; Sukontason, K.L.; Zajac, B.K.; Sukontason, K.; Wannasan, A.; Amendt, J. Wing morphometrics as a tool in species identification of forensically important blow flies of Thailand. Parasites Vectors 2017, 10, 229. [Google Scholar] [CrossRef]
- Ma, Y.; Guo, Z.; Wang, L.; Wang, B.; Huang, T.; Tang, B.; Zhang, G.; Zhou, Q. The genome of the rice planthopper egg parasitoid wasps Anagrus nilaparvatae casts light on the chemo- and mechanosensation in parasitism. BMC Genom. 2022, 23, 541. [Google Scholar] [CrossRef]
- Tang, L.D.; Sun, J.W.; Dai, P.; Mu, M.Y.; Nkunika, P.O.Y.; Desneux, N.; Zang, L.S. Performance of two dominant Trichogrammatid species of fall armyworm from China and Africa under contrasted temperature and humidity regimes. Biol. Control 2023, 179, 105179. [Google Scholar] [CrossRef]
- Önder, B.Ş.; Aksoy, C.F. Seasonal variation in wing size and shape of Drosophila melanogaster reveals rapid adaptation to environmental changes. Sci. Rep. 2022, 12, 14622. [Google Scholar] [CrossRef] [PubMed]
- Wallberg, A.; Schoning, C.; Webster, M.T.; Hasselmann, M. Two extended haplotype blocks are associated with adaptation to high altitude habitats in East African honey bees. PLoS Genet. 2017, 13, e1006792. [Google Scholar] [CrossRef] [PubMed]
- Ray, R.P.; Nakata, T.; Henningsson, P.; Bomphrey, R.J. Enhanced flight performance by genetic manipulation of wing shape in Drosophila. Nat. Commun. 2016, 7, 10851. [Google Scholar] [CrossRef]
- Camargo-Martinez, N.D.; Camacho-Erazo, M.; Amarillo-Suarez, A.R.; Herrera, H.W.; Sarmiento, C.E. Morphologic differentiation of the exotic parasitoid Eupelmus pulchriceps (Hymenoptera: Eupelmidae) in the Galapagos Archipelago. Neotrop. Entomol. 2024, 53, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.P.; Seena, S.; Girish, K.P.; Shibu, V.Y. Species morphospace boundary revisited through wing phenotypic variations of Antodynerus species (Hymenoptera: Vespidae: Eumeninae) from the Indian subcontinent. Front. Ecol. Evol. 2023, 10, 965577. [Google Scholar] [CrossRef]
- Benitez, H.A.; Munoz-Ramirez, C.; Correa, M.; Acuna-Rodriguez, I.S.; Villalobos-Leiva, A.; Contador, T.; Velasquez, N.A.; Suazo, M.J. Breaking the law: Is it correct to use the converse Bergmann Rule in ceroglossus chilensis? An overview using geometric morphometrics. Insects 2024, 15, 97. [Google Scholar] [CrossRef]
- Wonglersak, R.; Fenberg, P.B.; Langdon, P.G.; Brooks, S.J.; Price, B.W. Temperature-body size responses in insects: A case study of British Odonata. Ecol. Entomol. 2020, 45, 795–805. [Google Scholar] [CrossRef]
- Lozier, J.D.; Parsons, Z.M.; Rachoki, L.; Jackson, J.M.; Pimsler, M.L.; Oyen, K.J.; Strange, J.; Dillon, M.E.; Hines, H. Divergence in body mass, wing loading, and population structure reveals species-specific and potentially adaptive trait variation across elevations in montane bumble bees. Insect Syst. Divers. 2021, 5, 1–15. [Google Scholar] [CrossRef]
- Mazon, M.; Nunez-Penichet, C.; Cobos, M.E. Relationship between body mass and forewing length in neotropical Ichneumonidae (Insecta: Hymenoptera). Neotrop. Entomol. 2020, 49, 713–721. [Google Scholar] [CrossRef]
- Peters, R.S.; Krogmann, L.; Mayer, C.; Donath, A.; Gunkel, S.; Meusemann, K.; Kozlov, A.; Podsiadlowski, L.; Petersen, M.; Lanfear, R.; et al. Evolutionary history of the hymenoptera. Curr. Biol. 2017, 27, 1013–1018. [Google Scholar] [CrossRef]
- Sarmiento, A.R.; Jace, J.A. Altitudinal distribution and body resource allocation in a high mountain social wasp (Hymenoptera: Vespidae). Ecol. Behav. Bionomics 2008, 37, 1–7. [Google Scholar]
- Tatsuta, H.; Takahashi, K.H.; Sakamaki, Y. Geometric morphometrics in entomology: Basics and applications: Geometric morphometrics in entomology. Entomol. Sci. 2017, 21, 164–184. [Google Scholar] [CrossRef]
- Bullard, B.; Pastore, A. Regulating the contraction of insect flight muscle. J. Muscle Res. Cell Motil. 2011, 32, 303–313. [Google Scholar] [CrossRef]
- Deora, T.; Gundiah, N.; Sane, S.P. Mechanics of the thorax in flies. J. Exp. Biol. 2017, 220, 1382–1395. [Google Scholar] [CrossRef]
- Fartyal, R.S.; Sarswat, M.; Dewan, S.; Fartyal, P. Morphometric analysis of wild-caught flies of Drosophila (Diptera: Drosophilidae) species: Altitudinal pattern of body size traits, wing morphology and sexual dimorphism. Turk. J. Entomol. 2018, 41, 367–382. [Google Scholar] [CrossRef]
- Cao, T.; Jin, J.P. Evolution of flight muscle contractility and energetic efficiency. Front. Physiol. 2020, 11, 1038. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.P.; Badea, C.T. Advances in micro-CT imaging of small animals. Phys. Med. 2021, 88, 175–192. [Google Scholar] [CrossRef]
- Badea, C.T.; Drangova, M.; Holdsworth, D.W.; Johnson, G.A. In vivo small-animal imaging using micro-CT and digital subtraction angiography. Phys. Med. Biol. 2008, 53, 319–350. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.D.; Chhakchhuak, L.; Gurusubramanian, G.; Kumar, N.S. Mitochondrial markers for identification and phylogenetic studies in insects—A Review. DNA Barcodes 2014, 2, 1–9. [Google Scholar] [CrossRef]
- Tougeron, K.; Brodeur, J.; Lann, C.; Baaren, J.V. How climate change affects the seasonal ecology of insect parasitoids. Ecol. Entomol. 2020, 45, 167–181. [Google Scholar] [CrossRef]
| Region | Location | Coordinates | Altitude | Number |
|---|---|---|---|---|
| Pamir plateau | Aketo county | 38°53′54″ 75°12′5″ | 2764 m | 55 |
| Pamir plateau | Ulugqat county | 39°52′17″ 75°32′41″ | 2127 m | 40 |
| Pamir plateau | Artush city | 39°39′40″ 75°31′23″ | 2899 m | 35 |
| Pamir plateau | Tashkurgan county | 37°42′24″ 75°17′30″ | 3001 m | 48 |
| Altun mountain | Ruoqiang county—1 | 37°58′15″ 88°57′5″ | 3930 m | 23 |
| Altun mountain | Ruoqiang county—2 | 37°48′14″ 89°54′41″ | 3449 m | 18 |
| Altun mountain | Ruoqiang county—3 | 37°39′45″ 89°48′26″ | 3941 m | 22 |
| Basin | Hami city | 42°47′58″ 93°21′9″ | 679 m | 72 |
| Plain | Emin county | 46°37′49″ 83°48′30″ | 567 m | 85 |
| Plain | Habahe county | 48°03′36″ 86°23′10″ | 530 m | 48 |
| Basin | Shache county | 38°25′10″ 77°7′32″ | 980 m | 61 |
| Region | Population | h | Hd | Pi | K |
|---|---|---|---|---|---|
| Ruoqiang county (Altun mountain) | ALMA | 5 | 0.9333 | 0.0028 | 2.0000 |
| ALMW | 2 | 0.6667 | 0.0001 | 0.6667 | |
| Hami city | HM | 5 | 0.7424 | 0.0134 | 9.5303 |
| Kashi region (Pamir plateau) | AKT | 4 | 1.0000 | 0.0671 | 47.8333 |
| WQ | 3 | 1.0000 | 0.2338 | 16.6667 | |
| TSK | 6 | 1.0000 | 0.0624 | 44.4667 | |
| Bole city | BL | 10 | 1.0000 | 0.0321 | 22.8889 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, O.; Zhang, S.; Li, J.; Hu, H.; Bai, M. Geometric Morphometrics and Genetic Diversity Analysis of Chalcidoidea (Diglyphus and Pachyneuron) at Various Elevations. Insects 2024, 15, 497. https://doi.org/10.3390/insects15070497
Xi O, Zhang S, Li J, Hu H, Bai M. Geometric Morphometrics and Genetic Diversity Analysis of Chalcidoidea (Diglyphus and Pachyneuron) at Various Elevations. Insects. 2024; 15(7):497. https://doi.org/10.3390/insects15070497
Chicago/Turabian StyleXi, Ouyan, Shuli Zhang, Jinzhe Li, Hongying Hu, and Ming Bai. 2024. "Geometric Morphometrics and Genetic Diversity Analysis of Chalcidoidea (Diglyphus and Pachyneuron) at Various Elevations" Insects 15, no. 7: 497. https://doi.org/10.3390/insects15070497






