Lysine Methylation and Histone Modifications during Cold Stress of Insects: Freeze-Tolerant Eurosta solidaginis and Freeze-Avoiding Epiblema scudderiana
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Collection and Cold Stress
2.2. Total Protein Isolation
2.3. Western Immunoblotting
2.4. Statistical Analysis
3. Results
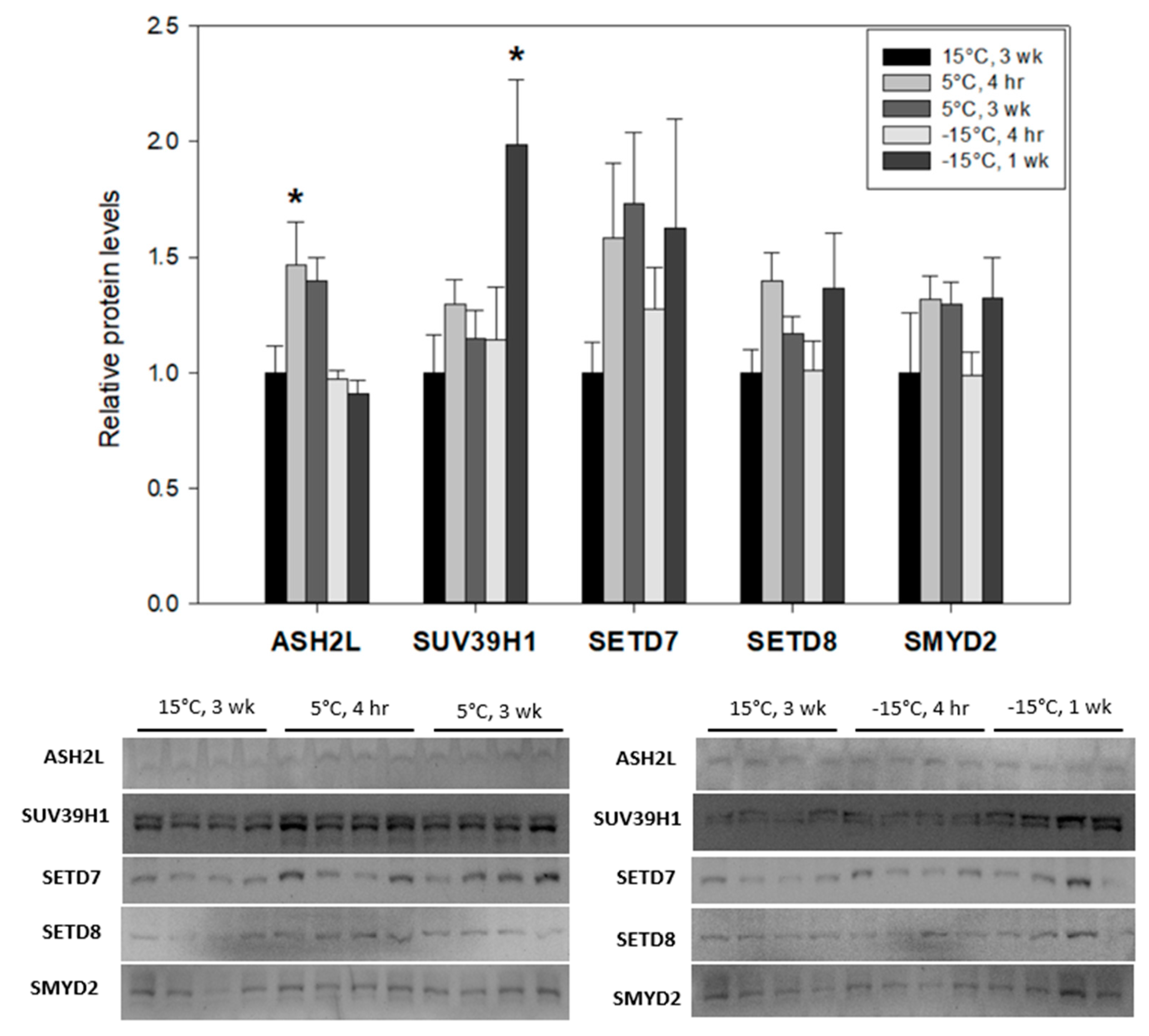
3.1. Marked Differences in the Expression of Lysine Methyltransferases in Ep. scudderiana and E. solidaginis in Response to Low Temperature
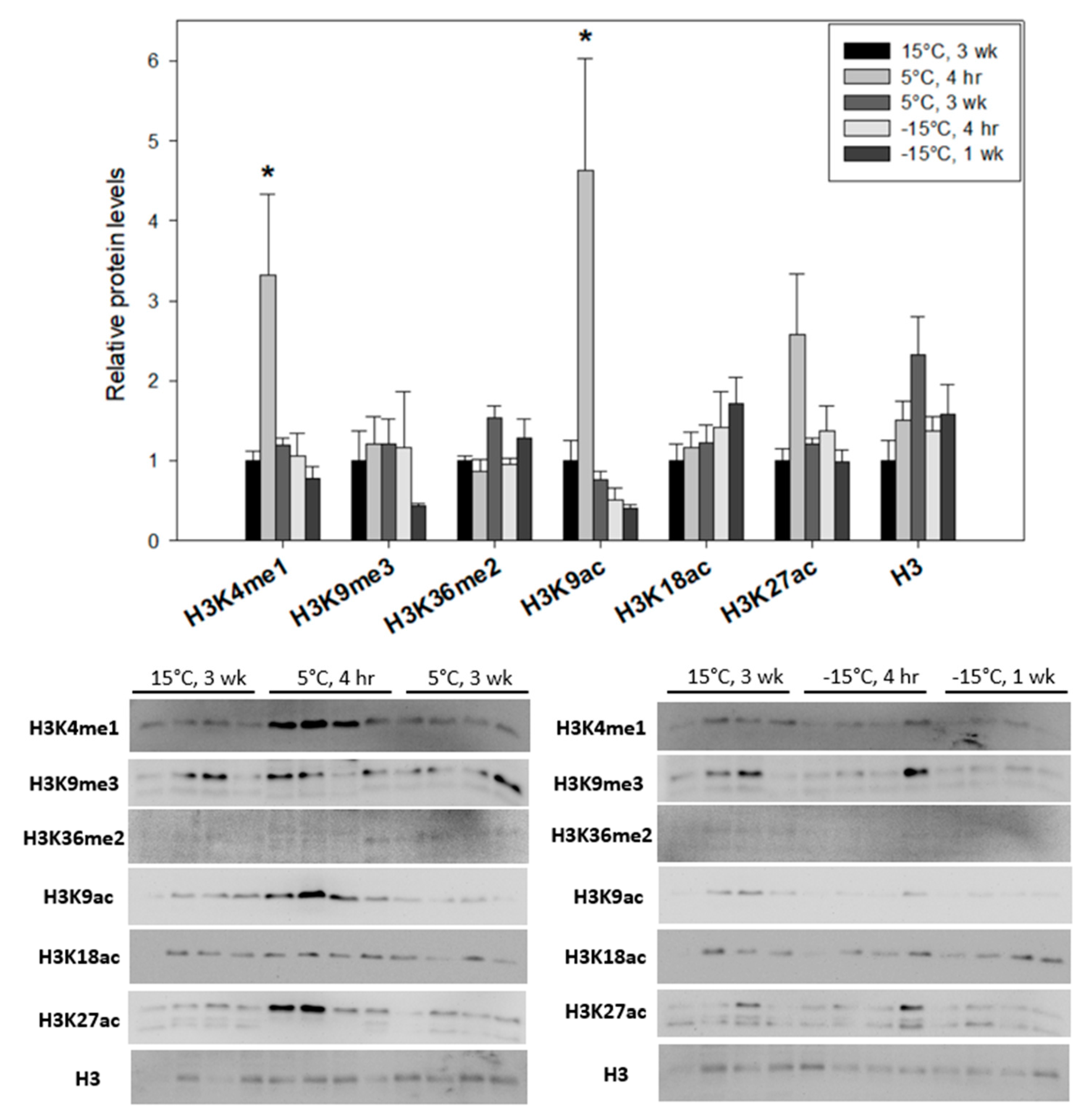
3.2. The Trends of Histone Methylation Correlate with Methyltransferase Expression and Suggest Distinctions between the Responses of Freeze-Tolerant E. solidaginis and Freeze-Avoiding Ep. scudderiana to Low Temperature
3.3. Opposing Levels of Histone Acetylation in Larval E. solidaginis and Ep. scudderiana in Response to Low-Temperature Exposure
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bollati, V.; Baccarelli, A. Environmental epigenetics. Heredity 2010, 105, 105–112. [Google Scholar] [CrossRef]
- Jaenisch, R.; Bird, A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat. Genet. 2003, 33, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, S.; Rajakumar, R.; Abouheif, E.; Szyf, M. Epigenetic variation in the Egfr gene generates quantitative variation in a complex trait in ants. Nat. Commun. 2015, 6, 6513. [Google Scholar] [CrossRef]
- He, X.J.; Chen, T.; Zhu, J.K. Regulation and function of DNA methylation in plants and animals. Cell Res. 2011, 21, 442–465. [Google Scholar] [CrossRef]
- Hunt, B.G.; Glastad, K.M.; Yi, S.V.; Goodisman, M.A.D. The function of intragenic DNA methylation: Insights from insect epigenomes. Integ. Comp. Biol. 2013, 53, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Nasrullah, H.A.; Ahmed, S.; Rasool, M.; Shah, A.J. DNA methylation across the tree of life, from micro to macro-organism. Bioengineered 2022, 13, 1666–1685. [Google Scholar] [CrossRef]
- Peterson, C.L.; Laniel, M.A. Histones and histone modifications. Curr. Biol. 2004, 14, R546–R551. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, C.; Saiardi, A. Why always lysine? The ongoing tale of one of the most modified amino acids. Adv. Biol. Reg. 2016, 60, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Chinnusamy, V.; Zhu, J.K. Epigenetic regulation of stress responses in plants. Curr. Opin. Plant Biol. 2009, 12, 133–139. [Google Scholar] [CrossRef]
- Roth, S.Y.; Denu, J.M.; Allis, C.D. Histone acetyltransferases. Ann. Rev. Biochem. 2001, 70, 81–120. [Google Scholar] [CrossRef]
- Mosammaparast, N.; Shi, Y. Reversal of histone methylation: Biochemical and molecular mechanisms of histone demethylases. Ann. Rev. Biochem. 2010, 79, 155–179. [Google Scholar] [CrossRef]
- Palli, S.R. Epigenetic regulation of post-embryonic development. Curr. Opin. Insect Sci. 2021, 43, 63–69. [Google Scholar] [CrossRef]
- Wojewodzic, M.W.; Beaton, M.J. The future of environmental epigenetics: Insights using the clonal water flea model. Adv. Insect Physiol. 2017, 53, 287–312. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Sun, Y.; Li, R.; Deng, J.; Deng, J. DNA methylation and histone deacetylation regulating insulin sensitivity due to chronic cold exposure. Cryobiology 2017, 74, 36–42. [Google Scholar] [CrossRef]
- Geisler, S.J.; Paro, R. Trithorax and Polycomb group-dependent regulation: A tale of opposing activities. Development 2015, 142, 2876–2887. [Google Scholar] [CrossRef] [PubMed]
- Verlinden, H. Introduction to advances in insect physiology: Insect epigenetics. Adv. Insect Physiol. 2017, 53, 13–17. [Google Scholar] [CrossRef]
- Bale, J.S. Insects at Low Temperature: A Predictable Relationship? Funct. Ecol. 1991, 5, 291. [Google Scholar] [CrossRef]
- Duman, J.G. Antifreeze and ice nucleator proteins in terrestrial arthropods. Ann. Rev. Physiol. 2001, 63, 327–357. [Google Scholar] [CrossRef] [PubMed]
- Storey, K.B.; Storey, J.M. Insect cold hardiness: Metabolic, gene, and protein adaptation. Can. J. Zool. 2012, 90, 456–475. [Google Scholar] [CrossRef]
- Ullah, F.; Abbas, A.; Gul, H.; Güncan, A.; Hafeez, M.; Gadratagi, B.G.; Cicero, L.; Ramirez-Romero, R.; Desneux, N.; Li, Z. Insect resilience: Unraveling responses and adaptations to cold temperatures. J. Pest Sci. 2024, 22, 1–7. [Google Scholar] [CrossRef]
- Reynolds, J.A.; Bautista-Jimenez, R.; Denlinger, D.L. Changes in histone acetylation as potential mediators of pupal diapause in the flesh fly, Sarcophaga bullata. Insect Biochem. Mol. Biol. 2016, 76, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.A.; Clark, J.; Diakoff, S.J.; Denlinger, D.L. Transcriptional evidence for small RNA regulation of pupal diapause in the flesh fly, Sarcophaga bullata. Insect Biochem. Mol. Biol. 2013, 43, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Poupardin, R.; Schöttner, K.; Korbelová, J.; Provazník, J.; Doležel, D.; Pavlinic, D.; Beneš, V.; Koštál, V. Early transcriptional events linked to induction of diapause revealed by RNAseq in larvae of drosophilid fly, Chymomyza costata. BMC Genom. 2015, 16, 720. [Google Scholar] [CrossRef] [PubMed]
- Kreß, A.; Oppold, A.M.; Kuch, U.; Oehlmann, J.; Müller, R. Cold tolerance of the Asian tiger mosquito Aedes albopictus and its response to epigenetic alterations. J. Insect Physiol. 2017, 99, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Martel, J. Performance of Eurosta solidaginis (Diptera: Tephritidae) and Epiblema scudderiana (Lepidoptera: Tortricidae), two gall-formers of goldenrod, in roadside environments. Environ. Entomol. 1995, 24, 697–706. [Google Scholar] [CrossRef]
- Williamson, S.; Storey, K. Changes in DNA methyltransferase expression in Epiblema sccuderiana and Eurosta solidaginis. Cryobiology 2016, 3, 435. [Google Scholar] [CrossRef]
- Joanisse, D.R.; Storey, K.B. Temperature acclimation and seasonal responses by enzymes in cold-hardy gall insects. Arch. Insect Biochem. Physiol. 1995, 28, 339–349. [Google Scholar] [CrossRef]
- Williamson, S.M.; Ingelson-Filpula, W.A.; Hadj-Moussa, H.; Storey, K.B. Epigenetic underpinnings of freeze avoidance in the goldenrod gall moth, Epiblema scudderiana. J. Insect Physiol. 2021, 134, 104298. [Google Scholar] [CrossRef] [PubMed]
- Bale, J.S.; Hayward, S.A. Insect overwintering in a changing climate. J. Exp. Biol. 2010, 213, 980–994. [Google Scholar] [CrossRef]
- Denlinger, D.L. Diapause. In Encyclopedia of Insects, 2nd ed.; Resh, V.H., Cardé, R.T., Eds.; Academic Press: London, UK, 2009; pp. 267–271. [Google Scholar]
- Hahn, D.A.; Denlinger, D.L. Energetics of insect diapause. Ann. Rev. Entomol. 2011, 56, 103–121. [Google Scholar] [CrossRef]
- Storey, K.B.; Storey, J.M. Metabolic rate depression in animals: Transcriptional and translational controls. Biol. Rev. Cam. Philosoph. Soc. 2004, 79, 207–233. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Armstrong, R.L.; Duronio, R.J.; MacAlpine, D.M. Methylation of histone H4 lysine 20 by PR-Set7 ensures the integrity of late replicating sequence domains in Drosophila. Nucleic Acids Res. 2016, 44, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Calpena, E.; Palau, F.; Espinos, C.; Galindo, M.I. Evolutionary history of the Smyd gene family in metazoans: A framework to identify the orthologs of human Smyd genes in Drosophila and other animal species. PLoS ONE 2015, 10, e0134106. [Google Scholar] [CrossRef] [PubMed]
- Ingelson-Filpula, W.A.; Bloskie, T.; Storey, K.B. Epigenetics and the extreme stress response. In Epigenetics, Development, Ecology and Evolution; Springer: Cham, Switzerland, 2022; pp. 177–213. [Google Scholar] [CrossRef]
- Nishioka, K.; Chuikov, S.; Sarma, K.; Erdjument-Bromage, H.; Allis, C.D.; Tempst, P.; Reinberg, D. Set9, a novel histone H3 methyltransferase that facilitates transcription by precluding histone tail modifications required for heterochromatin formation. Genes Dev. 2002, 16, 479–489. Available online: http://www.genesdev.org/cgi/doi/10.1101/gad.967202 (accessed on 15 June 2017).
- Hyun, K.; Jeon, J.; Park, K.; Kim, J. Writing, erasing and reading histone lysine methylations. Exp. Mol. Med. 2017, 49, e324. [Google Scholar] [CrossRef] [PubMed]
- Rea, S.; Eisenhaber, F.; O’Carroll, D.; Strahl, B.D.; Sun, Z.W.; Schmid, M.; Opravil, S.; Mechtier, K.; Ponting, C.P.; Allis, C.D.; et al. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature 2000, 406, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.S.; Zurita-Lopez, C.I.; Clarke, S.G.; Bedford, M.T.; Dent, S.Y. Protein-arginine methyltransferase 1 (PRMT1) methylates Ash2L, a shared component of mammalian histone H3K4 methyltransferase complexes. J. Biol. Chem. 2011, 286, 12234–12244. [Google Scholar] [CrossRef]
- Mohan, M.; Herz, H.M.; Smith, E.R.; Zhang, Y.; Jackson, J.; Washburn, M.P.; Florens, L.; Eissenberg, J.C.; Shilatifard, A. The COMPASS family of H3K4 methylases in Drosophila. Mol. Cell. Biol. 2011, 31, 4310–4318. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.L.; Yerbanga, R.S.; Lefèvre, T.; Ouedraogo, J.B.; Corces, V.G.; Gómez-Díaz, E. Chromatin changes in Anopheles gambiae induced by Plasmodium falciparum infection. Epigenet. Chromatin 2019, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- DiVito, E.A.; Fairbanks, R.A.; Schmidt, P.; Levine, M.T. Histone methylation regulates reproductive diapause in Drosophila melanogaster. PLoS Genet. 2023, 19, e1010906. [Google Scholar] [CrossRef]
- Shilatifard, A. The COMPASS family of histone H3K4 methylases: Mechanisms of regulation in development and disease pathogenesis. Ann. Rev. Biochem. 2012, 81, 65–95. [Google Scholar] [CrossRef]
- Yocum, G.D.; Rinehart, J.P.; Larson, M.L. Down-regulation of gene expression between the diapause initiation and maintenance phases of the Colorado potato beetle, Leptinotarsa decemlineata (Coleoptera: Chrysomelidae). Eur. J. Entomol. 2009, 106, 471–476. [Google Scholar] [CrossRef]
- Wang, Z.; Zang, C.; Rosenfeld, J.A.; Schones, D.E.; Barski, A.; Cuddapah, S.; Cui, K.; Roh, T.Y.; Peng, W.; Zhang, M.Q.; et al. Combinatorial patterns of histone acetylations and methylations in the human genome. Nat. Genet. 2008, 40, 897. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Li, Y.; Liao, J.; Ni, Z.; Xia, S.; Yang, M.; Li, H.Y.; Guo, J. Histone acetylation is associated with pupal diapause in cotton bollworm, Helicoverpa armigera. Pest Manag. Sci. 2024, 80, 1400–1411. [Google Scholar] [CrossRef]
- Tie, F.; Banerjee, R.; Stratton, C.A.; Prasad-Sinha, J.; Stepanik, V.; Zlobin, A.; Diaz, M.O.; Scacheri, P.C.; Harte, P.J. CBP-mediated acetylation of histone H3 lysine 27 antagonizes Drosophila Polycomb silencing. Development 2009, 136, 3131–3141. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Z.; Pei, T.; Wang, H.; Wang, C.; Liu, J.; Storey, K.B. Lysine Methylation and Histone Modifications during Cold Stress of Insects: Freeze-Tolerant Eurosta solidaginis and Freeze-Avoiding Epiblema scudderiana. Insects 2024, 15, 498. https://doi.org/10.3390/insects15070498
Yu Z, Pei T, Wang H, Wang C, Liu J, Storey KB. Lysine Methylation and Histone Modifications during Cold Stress of Insects: Freeze-Tolerant Eurosta solidaginis and Freeze-Avoiding Epiblema scudderiana. Insects. 2024; 15(7):498. https://doi.org/10.3390/insects15070498
Chicago/Turabian StyleYu, Zhijun, Tingwei Pei, Han Wang, Chunyuan Wang, Jingze Liu, and Kenneth B. Storey. 2024. "Lysine Methylation and Histone Modifications during Cold Stress of Insects: Freeze-Tolerant Eurosta solidaginis and Freeze-Avoiding Epiblema scudderiana" Insects 15, no. 7: 498. https://doi.org/10.3390/insects15070498




