Comparative Toxicological Analyses of Traditional Matrices and Blow Fly Larvae in Four Cases of Highly Decomposed Human Cadavers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
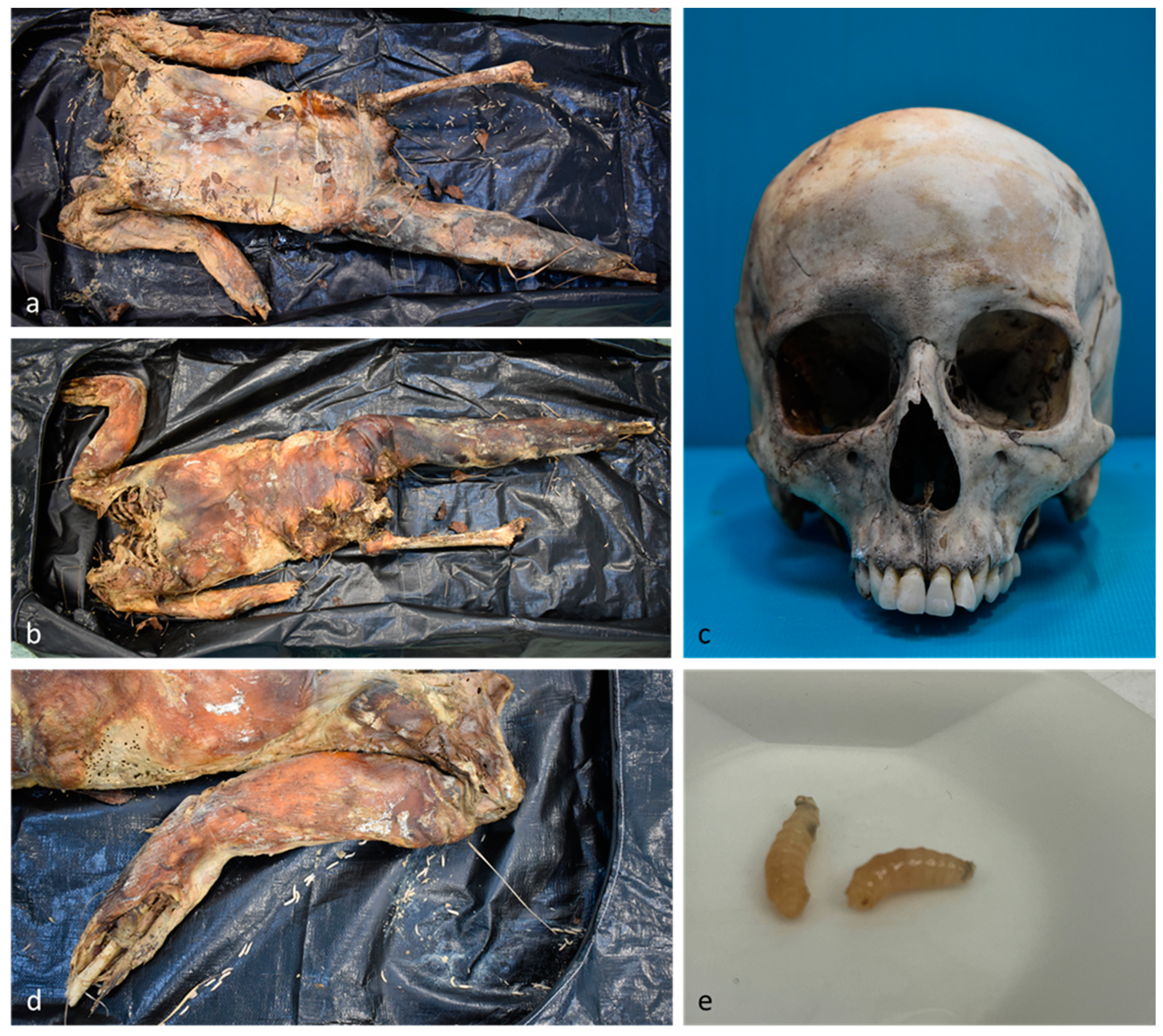
2.1. Case Histories
2.1.1. Case 1
2.1.2. Case 2
2.1.3. Case 3
2.1.4. Case 4
2.2. Biological Samples for Toxicological Analyses
2.3. Chemicals and Reagents
2.4. Sample Preparation
2.5. Forensic Toxicology Screening
2.6. Forensic Toxicology Confirmation
2.7. Data Processing and Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wells, J.D. A Forensic Entomological Analysis Can Yield an Estimate of Postmortem Interval, and Not Just a Minimum Postmortem Interval: An Explanation and Illustration Using a Case. J. Forensic Sci. 2019, 64, 634–637. [Google Scholar] [CrossRef] [PubMed]
- Hodecek, J. Revisiting the Concept of Entomotoxicology. Forensic Sci. Int. Synergy 2020, 2, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Nuorteva, P.; Häsänen, E. Transfer of Mercury from Fishes to Sarcosaprophagous Flies. Ann. Zool. Fenn. 1972, 9, 23–27. [Google Scholar]
- Beyer, J.C.; Enos, W.F.; Stajić, M. Drug Identification through Analysis of Maggots. J. Forensic Sci. 1980, 25, 411–412. [Google Scholar] [CrossRef] [PubMed]
- Chophi, R.; Sharma, S.; Sharma, S.; Singh, R. Forensic Entomotoxicology: Current Concepts, Trends and Challenges. J. Forensic Leg. Med. 2019, 67, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, M.; Wille, S.M.R.; Fernandez, M.D.M.R.; Di Fazio, V.; Samyn, N.; De Boeck, G.; Bourel, B. Entomotoxicology, Experimental Set-up and Interpretation for Forensic Toxicologists. Forensic Sci. Int. 2011, 208, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Campobasso, C.P.; Bugelli, V.; Carfora, A.; Borriello, R.; Villet, M.H. Forensic Entomology—The Utility of Arthropods in Legal Investigation, 3rd ed.; Byrd, J.H., Tomberlin, J.K., Eds.; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Da Silva, E.I.T.; Wilhelmi, B.; Villet, M.H. Forensic Entomotoxicology Revisited—Towards Professional Standardisation of Study Designs. Int. J. Legal Med. 2017, 131, 1399–1412. [Google Scholar] [CrossRef] [PubMed]
- Parry, S.; Linton, S.M.; Francis, P.S.; O’Donnell, M.J.; Toop, T. Accumulation and Excretion of Morphine by Calliphora stygia, an Australian Blow Fly Species of Forensic Importance. J. Insect Physiol. 2011, 57, 62–73. [Google Scholar] [CrossRef]
- Butzbach, D.M. The Influence of Putrefaction and Sample Storage on Post-Mortem Toxicology Results. Forensic Sci. Med. Pathol. 2010, 6, 35–45. [Google Scholar] [CrossRef]
- Pien, K.; Laloup, M.; Pipeleers-Marichal, M.; Grootaert, P.; De Boeck, G.; Samyn, N.; Boonen, T.; Vits, K.; Wood, M. Toxicological Data and Growth Characteristics of Single Post-Feeding Larvae and Puparia of Calliphora vicina (Diptera: Calliphoridae) Obtained from a Controlled Nordiazepam Study. Int. J. Legal Med. 2004, 118, 190–193. [Google Scholar] [CrossRef]
- Magni, P.A.; Voss, S.C.; Testi, R.; Borrini, M.; Dadour, I.R. A Biological and Procedural Review of Forensically Significant Dermestes Species (Coleoptera: Dermestidae). J. Med. Entomol. 2015, 52, 755–769. [Google Scholar] [CrossRef]
- Voss, S.C.; Magni, P.; Dadour, I.; Nansen, C. Reflectance-Based Determination of Age and Species of Blowfly Puparia. Int. J. Legal Med. 2017, 131, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Magni, P.A.; Harvey, A.D.; Guareschi, E.E. Insects Associated with Ancient Human Remains: How Archaeoentomology Can Provide Additional Information in Archaeological Studies. Heritage 2023, 6, 435–465. [Google Scholar] [CrossRef]
- Bushby, S.K.; Thomas, N.; Priemel, P.A.; Coulter, C.V.; Rades, T.; Kieser, J.A. Determination of Methylphenidate in Calliphorid Larvae by Liquid–Liquid Extraction and Liquid Chromatography Mass Spectrometry—Forensic Entomotoxicology Using an in Vivo Rat Brain Model. J. Pharm. Biomed. Anal. 2012, 70, 456–461. [Google Scholar] [CrossRef]
- Gunn, J.A.; Shelley, C.; Lewis, S.W.; Toop, T.; Archer, M. The Determination of Morphine in the Larvae of Calliphora stygia Using Flow Injection Analysis and HPLC with Chemiluminescence Detection. J. Anal. Toxicol. 2006, 30, 519–523. [Google Scholar] [CrossRef]
- Tracqui, A.; Keyser-Tracqui, C.; Kintz, P.; Ludes, B. Entomotoxicology for the Forensic Toxicologist: Much Ado about Nothing? Int. J. Legal Med. 2004, 118, 194–196. [Google Scholar] [CrossRef]
- Sadler, D.W.; Fuke, C.; Court, F.; Pounder, D.J. Drug Accumulation and Elimination in Calliphora vicina Larvae. Forensic Sci. Int. 1995, 71, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, M.; Fernandez, M.D.M.R.; Wille, S.M.R.; Samyn, N.; De Boeck, G.; Bourel, B. Quantification of Methadone and Its Metabolite 2-Ethylidene-1,5-Dimethyl-3,3-Diphenylpyrrolidine in Third Instar Larvae of Lucilia sericata (Diptera: Calliphoridae) Using Liquid Chromatography-Tandem Mass Spectrometry. J. Anal. Toxicol. 2010, 34, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.J.R. The Relationship between Research and Casework in Forensic Entomology. Insects 2021, 12, 174. [Google Scholar] [CrossRef]
- Magni, P.A.; Senigaglia, V.; Robinson, S.C.; Dadour, I.R. The Effect of Submersion in Different Types of Water on the Survival and Eclosion of Blow-Fly Intra-Puparial Forms (Diptera: Calliphoridae). Forensic Sci. Int. 2021, 319, 110663. [Google Scholar] [CrossRef]
- Bambaradeniya, T.B.; Magni, P.A.; Dadour, I.R. A Summary of Concepts, Procedures and Techniques Used by Forensic Entomologists and Proxies. Insects 2023, 14, 536. [Google Scholar] [CrossRef] [PubMed]
- Benecke, M. Six Forensic Entomology Cases: Description and Commentary. J. Forensic Sci. 1998, 43, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Brockbals, L.; Staeheli, S.N.; Gascho, D.; Ebert, L.C.; Kraemer, T.; Steuer, A.E. Time-Dependent Postmortem Redistribution of Opioids in Blood and Alternative Matrices. J. Anal. Toxicol. 2018, 42, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.L.; Nielsen, M.K.K.; Linnet, K.; Rasmussen, B.S. Simple Implementation of Muscle Tissue into Routine Workflow of Blood Analysis in Forensic Cases—A Validated Method for Quantification of 29 Drugs in Postmortem Blood and Muscle Samples by UHPLC–MS/MS. Forensic Sci. Int. 2021, 325, 110901. [Google Scholar] [CrossRef] [PubMed]
- Baselt, R.C. Disposition of Toxic Drugs and Chemicals in Man, 12th ed.; Biomedical Publications: Seal Beach, CA, USA, 2020; ISBN 978-0-578-57749-4. [Google Scholar]
- Manning, T.; Bidanset, J.H.; Cohen, S.; Lukash, L. Evaluation of the Abuscreen for Methadone. J. Forensic Sci. 1976, 21, 112–120. [Google Scholar] [CrossRef]
- Robinson, A.E.; Williams, F.M. The Distribution of Methadone in Man. J. Pharm. Pharmacol. 2011, 23, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Buratti, E.; Mietti, G.; Cippitelli, M.; Cerioni, A.; Froldi, R.; Cingolani, M.; Scendoni, R. Detection of Three Opioids (Morphine, Codeine and Methadone) and Their Metabolites (6-Monoacetylmorphine and 2-Ethylidene-1,5-Dimethyl-3,3-Diphenylpyrrolidine) in Larvae of Lucilia sericata Species by UHPLC-TF-MS and Validation. Molecules 2023, 28, 4649. [Google Scholar] [CrossRef] [PubMed]
- Keshavarzi, D.; Rassi, Y.; Oshaghi, M.A.; Azizi, K.; Rafizadeh, S.; Alimohammadi, A.; Parkhideh, S.Z. Effects of Ante-Mortem Use of Methadone on Insect Succession Patterns. Egypt. J. Forensic Sci. 2021, 11, 17. [Google Scholar] [CrossRef]
- Groth, O.; Franz, S.; Fels, H.; Krueger, J.; Roider, G.; Dame, T.; Musshoff, F.; Graw, M. Unexpected Results Found in Larvae Samples from Two Postmortem Forensic Cases. Forensic Toxicol. 2022, 40, 144–155. [Google Scholar] [CrossRef]
- Wood, T.; Pyper, K.; Casali, F. Effects of Cocaine and Heroin, and Their Combination, on the Development Rate of Calliphora vomitoria (Diptera: Calliphoridae). Sci. Justice 2022, 62, 471–475. [Google Scholar] [CrossRef]
- Boulkenafet, F.; Dob, Y.; Karroui, R.; Al-Khalifa, M.; Boumrah, Y.; Toumi, M.; Mashaly, A. Detection of Benzodiazepines in Decomposing Rabbit Tissues and Certain Necrophagic Dipteran Species of Forensic Importance. Saudi J. Biol. Sci. 2020, 27, 1691–1698. [Google Scholar] [CrossRef]
- Carvalho, L.M.L.; Linhares, A.X.; Trigo, J.R. Determination of Drug Levels and the Effect of Diazepam on the Growth of Necrophagous Flies of Forensic Importance in Southeastern Brazil. Forensic Sci. Int. 2001, 120, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Annasaheb Bansode, S.; Ramrao More, V. Effect of Lorazepam on the Development of the Hairy Maggot Blow Fly, Chrysomya rufifacies (Macquart): Implication for Forensic Entomology. J. Toxicol. 2023, 2023, 1051736. [Google Scholar] [CrossRef] [PubMed]
- Aly, S.M.; Gish, A.; Hakim, F.; Guelmi, D.; Mesli, V.; Hédouin, V.; Allorge, D.; Gaulier, J. In the Case of Extensively Putrefied Bodies, the Analysis of Entomological Samples May Support and Complement the Toxicological Results Obtained with Other Alternative Matrices. Legal Med. 2023, 63, 102261. [Google Scholar] [CrossRef] [PubMed]
- Bugelli, V.; Papi, L.; Fornaro, S.; Stefanelli, F.; Chericoni, S.; Giusiani, M.; Vanin, S.; Campobasso, C.P. Entomotoxicology in Burnt Bodies: A Case of Maternal Filicide-Suicide by Fire. Int. J. Legal Med. 2017, 131, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Cerioni, A.; Buratti, E.; Mietti, G.; Cippitelli, M.; Cingolani, M.; Froldi, R.; Scendoni, R. Validation of a New Method for the Detection of Ethyl Glucuronide in Larvae of Lucilia sericata as a Marker of Ante-Mortem Alcohol Consumption. Heliyon 2023, 9, e20802. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chu, J.; Wang, Y.; Li, F.; Liao, M.; Shi, H.; Zhang, Y.; Hu, G.; Wang, J. Forensic Entomology Application in China: Four Case Reports. J. Forensic Leg. Med. 2019, 63, 40–47. [Google Scholar] [CrossRef]
- Bessa, B.G.D.O.; Silva-Neto, H.D.A.; Coltro, W.K.T.; Rocha, T.L.; Lopes, W.R. Lead Toxicity in Lucilia cuprina and Electrochemical Analysis: A Simple and Low-Cost Alternative for Forensic Investigation. Anal. Bioanal. Chem. 2021, 413, 3201–3208. [Google Scholar] [CrossRef]
- Magni, P.A.; Pacini, T.; Pazzi, M.; Vincenti, M.; Dadour, I.R. Development of a GC–MS Method for Methamphetamine Detection in Calliphora vomitoria L. (Diptera: Calliphoridae). Forensic Sci. Int. 2014, 241, 96–101. [Google Scholar] [CrossRef]
- Magni, P.A.; Pazzi, M.; Vincenti, M.; Alladio, E.; Brandimarte, M.; Dadour, I.R. Development and Validation of a GC–MS Method for Nicotine Detection in Calliphora vomitoria (L.) (Diptera: Calliphoridae). Forensic Sci. Int. 2016, 261, 53–60. [Google Scholar] [CrossRef]
- Magni, P.A.; Pazzi, M.; Vincenti, M.; Converso, V.; Dadour, I.R. Development and Validation of a Method for the Detection of α- and β-Endosulfan (Organochlorine Insecticide) in Calliphora vomitoria (Diptera: Calliphoridae). J. Med. Entomol. 2018, 55, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Salimi, M.; Rassi, Y.; Chatrabgoun, O.; Kamali, A.; Oshaghi, M.A.; Shiri-Ghaleh, V.; Moradi, M.; Rafizadeh, S.; Akbarzadeh, K.; Parkhideh, S.Z. Toxicological Analysis of Insects on the Corpse: A Valuable Source of Information in Forensic Investigations. J. Arthropod-Borne Dis. 2018, 12, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.K.T.D.A.; Barbosa, T.M.; Santos, M.C.D.; Jales, J.T.; De Araújo, A.M.U.; Morais, C.L.M.; De Lima, L.A.S.; Bicudo, T.C.; Gama, R.A.; Marinho, P.A.; et al. Detection of Terbufos in Cases of Intoxication by Means of Entomotoxicological Analysis Using ATR-FTIR Spectroscopy Combined with Chemometrics. Acta Tropica 2023, 238, 106779. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, N.I.; Ferrero, A.A.; Centeno, N.D. Determination of Fluoxetine in Dermestes maculatus (Coleoptera: Dermestidae) by a Spectrophotometric Method. Sci. Justice 2016, 56, 464–467. [Google Scholar] [CrossRef]



| Case # | Gender, Age | Location | Circumstantial Data | Decomposition Changes | Estimated PMI Based on Decomposition Changes and Last Known Sighting | Toxicological Analyses Performed on | ||
|---|---|---|---|---|---|---|---|---|
| Death Scenario | Presence of Drugs | Human Specimens | Entomological Samples | |||||
| 1 | Male, 59 | Home | Presence of a plastic bag around the cadaver’s head, also containing a butane can. In the house, open kitchen gas knobs. | Numerous blister packs of zolpidem, gabapentin, duloxetine, levosulpiride, propantheline bromide/bromazepam, paracetamol/codeine phosphate. | Advance putrefactive phenomena in the chromatic-colliquative phase. Skeletonization of the upper part of the body. | 20–30 days | Hair, muscle | Ch. albiceps L3, PF |
| 2 | Female, 58 | Home | Subject in substitutive treatment with methadone. | Next to the cadaver presence of a blister of quetiapine and three bottles of methadone. | Advanced putrefactive phenomena in the chromatic-colliquative phase and skeletonization. Partial mummification. | Approximately 3 weeks | Spleen | Ch. albiceps L3 |
| 3 | Male, 54 | Suburban area | Homeless hanged by a rope at the parapet of a road. No signs of violence. | None associated with the body or the surrounding environment. | Advanced putrefactive phenomena in the chromatic-colliquative phase. Corification. | 6–8 days | Blood | Calliphorids L3 |
| 4 | Female, 67 | Wood | Woman disappeared approximately 3 months before. Reported to be in treatment with flurazepam, quetiapine and lorazepam. | None associated with the body or the surrounding environment. | Advanced putrefactive phenomena- skeletonization. Special transformative phenomena: mummification and saponification. | 2–3 months | n.a. | Calliphorids L3 |
| Matrix | Results | Drugs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Coc. | Cod. | Norcod. | Morph. | Brom. | Diaz. | Nordiaz. | Temaz. | Oxaz. | Zolp. | ||
| Skeletal muscle | Screening | n.d. | + | + | + | + | + | + | + | n.d. | + |
| Concentration (ng/mg) | / | 3820 | 1311 | 331 | 434 | 6824 | 9654 | 781 | / | 6276 | |
| Hair | Screening | + | + | n.d. | + | n.d. | n.d. | n.d. | n.d. | n.d. | + |
| Concentration (ng/mg) | 0.03 | 0.91 | / | 0.09 | / | / | / | / | / | 0.41 | |
| Larvae | Screening | n.d. | + | + | + | + | + | + | + | + | + |
| Concentration (ng/g) | / | 235 | 113 | 28 | 3 | 58 | 24 | 7 | 12 | 18 | |
| Matrix | Results | Drugs | ||||
|---|---|---|---|---|---|---|
| 7-Aminoclonazepam | BEG | Methadone | EDDP | Quetiapine | ||
| Spleen | Screening | + | n.d. | + | + | + |
| Concentrations (ng/g) | 706 | / | 27,699 | 1990 | 3693 | |
| Larvae | Screening | + | + | + | + | + |
| Concentrations (ng/g) | 4 | 5 | 172 | 101 | 13 | |
| Matrix | Results | Drugs | ||
|---|---|---|---|---|
| Desalkyl Flurazepam | Pregabalin | Quetiapine | ||
| Larvae | Screening | + | + | + |
| Concentrations (ng/g) | 86.8 | 456.0 | 14,623.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peruch, M.; Buffon, M.; Jakovski, Z.; Spiliopoulou, C.; Addobbati, R.; Franzin, M.; Magni, P.A.; D’Errico, S. Comparative Toxicological Analyses of Traditional Matrices and Blow Fly Larvae in Four Cases of Highly Decomposed Human Cadavers. Insects 2024, 15, 500. https://doi.org/10.3390/insects15070500
Peruch M, Buffon M, Jakovski Z, Spiliopoulou C, Addobbati R, Franzin M, Magni PA, D’Errico S. Comparative Toxicological Analyses of Traditional Matrices and Blow Fly Larvae in Four Cases of Highly Decomposed Human Cadavers. Insects. 2024; 15(7):500. https://doi.org/10.3390/insects15070500
Chicago/Turabian StylePeruch, Michela, Maria Buffon, Zlatko Jakovski, Chara Spiliopoulou, Riccardo Addobbati, Martina Franzin, Paola A. Magni, and Stefano D’Errico. 2024. "Comparative Toxicological Analyses of Traditional Matrices and Blow Fly Larvae in Four Cases of Highly Decomposed Human Cadavers" Insects 15, no. 7: 500. https://doi.org/10.3390/insects15070500
APA StylePeruch, M., Buffon, M., Jakovski, Z., Spiliopoulou, C., Addobbati, R., Franzin, M., Magni, P. A., & D’Errico, S. (2024). Comparative Toxicological Analyses of Traditional Matrices and Blow Fly Larvae in Four Cases of Highly Decomposed Human Cadavers. Insects, 15(7), 500. https://doi.org/10.3390/insects15070500






