Larvicidal Activity of Hemp Extracts and Cannabidiol against the Yellow Fever Mosquito Aedes aegypti
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Hemp Plants
2.2. Hemp Extract
2.3. Methanol and Hexane Partitioning
2.4. Gas Chromatography–Mass Spectrometry (GC-MS) and Nuclear Magnetic Resonance (NMR)
2.5. Aedes Aegypti Colonies and Strains
2.6. Larval Bioassay
2.7. Statistical Analysis
3. Results
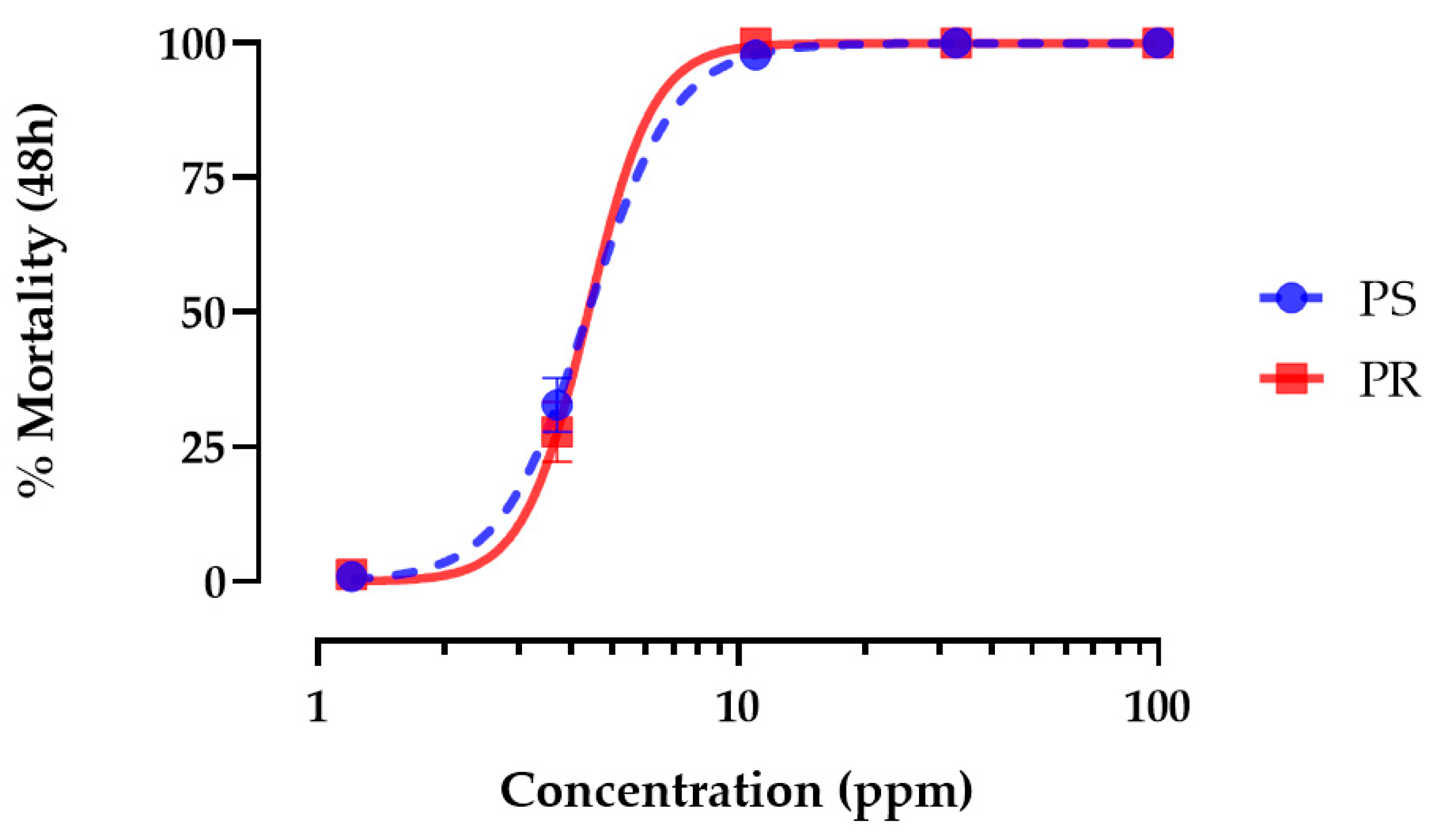
3.1. Hemp Leaf Extract Toxicity against Larvae
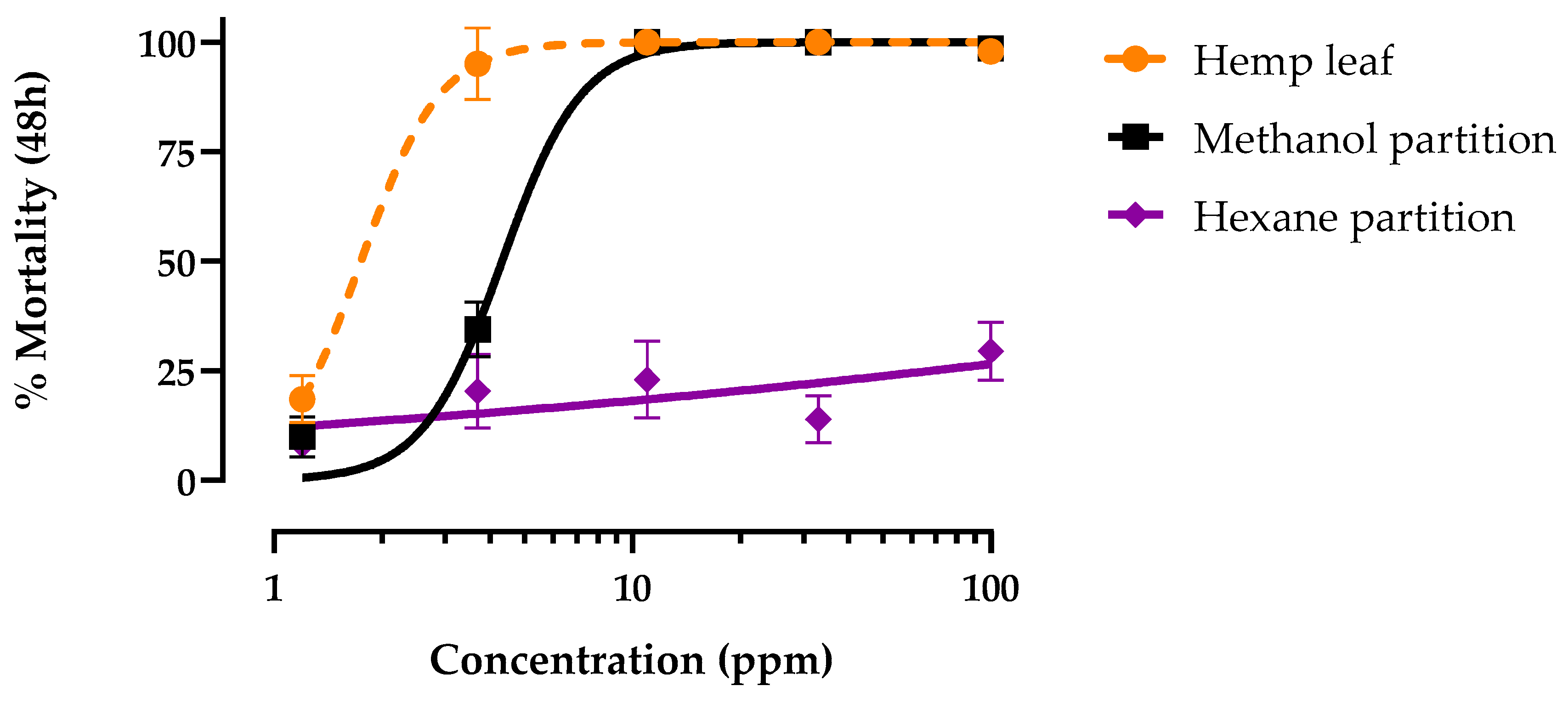
3.2. Methanol and Hexane Partition Toxicity against Larvae
3.3. GC-MS and NMR Analysis
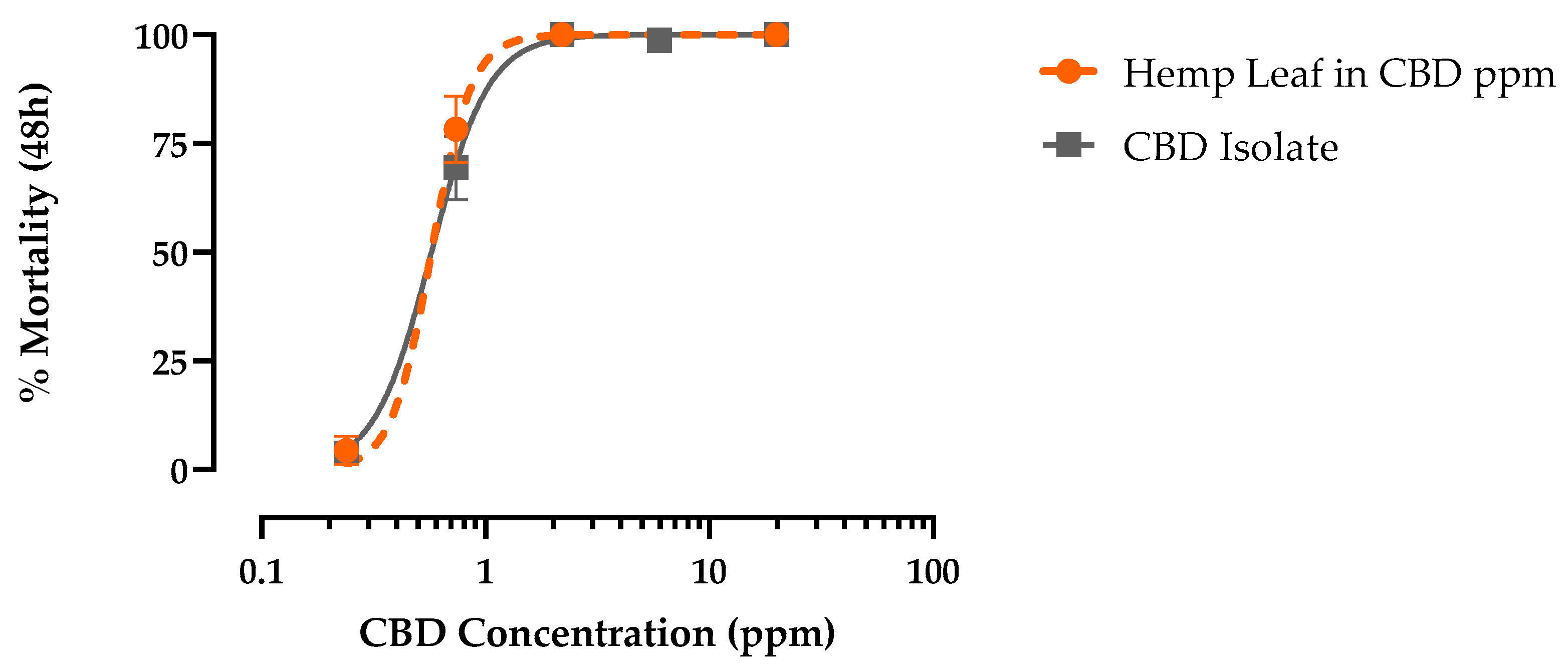
3.4. CBD Toxicity against First-Instar Larvae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Unlu, I.; Mackay, A.J.; Roy, A.; Yates, M.M.; Foil, L.D. Evidence of Vertical Transmission of West Nile Virus in Field-Collected Mosquitoes. J. Vector Ecol. 2010, 35, 95–99. [Google Scholar] [CrossRef]
- Karema, C.; Wen, S.; Sidibe, A.; Smith, J.L.; Gosling, R.; Hakizimana, E.; Tanner, M.; Noor, A.M.; Tatarsky, A. History of Malaria Control in Rwanda: Implications for Future Elimination in Rwanda and Other Malaria-Endemic Countries. Malar. J. 2020, 19, 356. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Gubler, D.J.; Izquierdo, A.; Martinez, E.; Halstead, S.B. Dengue Infection. Nat. Rev. Dis. Primers. 2016, 2, 16055. [Google Scholar] [CrossRef] [PubMed]
- Spence Beaulieu, M.R.; Federico, J.L.; Reiskind, M.H. Mosquito Diversity and Dog Heartworm Prevalence in Suburban Areas. Parasites Vectors 2020, 13, 12. [Google Scholar] [CrossRef]
- Estep, A.S.; Sanscrainte, N.D.; Waits, C.M.; Louton, J.E.; Becnel, J.J. Resistance Status and Resistance Mechanisms in a Strain of Aedes Aegypti (Diptera: Culicidae) From Puerto Rico. J. Med. Entomol. 2017, 54, 1643–1648. [Google Scholar] [CrossRef]
- Gan, S.J.; Leong, Y.Q.; Bin Barhanuddin, M.F.H.; Wong, S.T.; Wong, S.F.; Mak, J.W.; Ahmad, R.B. Dengue Fever and Insecticide Resistance in Aedes Mosquitoes in Southeast Asia: A Review. Parasites Vectors 2021, 14, 315. [Google Scholar] [CrossRef]
- Vontas, J.; Kioulos, E.; Pavlidi, N.; Morou, E.; della Torre, A.; Ranson, H. Insecticide Resistance in the Major Dengue Vectors Aedes albopictus and Aedes aegypti. Pestic. Biochem. Physiol. 2012, 104, 126–131. [Google Scholar] [CrossRef]
- Malik, A.; Grohmann, E.; Akhtar, R. (Eds.) Environmental Deterioration and Human Health: Natural and Anthropogenic Determinants; Springer: Dordrecht, The Netherlands, 2014; ISBN 978-94-007-7889-4. [Google Scholar]
- Ashraf, M.A.; Iqbal, M.; Rasheed, R.; Hussain, I.; Riaz, M.; Arif, M.S. Environmental Stress and Secondary Metabolites in Plants. In Plant Metabolites and Regulation Under Environmental Stress; Elsevier: Amsterdam, The Netherlands, 2018; pp. 153–167. ISBN 978-0-12-812689-9. [Google Scholar]
- Rattan, R.S. Mechanism of Action of Insecticidal Secondary Metabolites of Plant Origin. Crop Prot. 2010, 29, 913–920. [Google Scholar] [CrossRef]
- Górski, R.; Sobieralski, K.; Siwulski, M. The Effect of Hemp Essential Oil on Mortality Aulacorthum solani Kalt. And Tetranychus Urticae Koch. Ecol. Chem. Eng. S 2016, 23, 505–511. [Google Scholar] [CrossRef]
- Fang, X.; Yang, C.; Wei, Y.; Ma, Q.; Yang, L.; Chen, X. Genomics Grand for Diversified Plant Secondary Metabolites. Plant Divers. Resour. 2011, 33, 53–64. [Google Scholar] [CrossRef]
- Jin, D.; Dai, K.; Xie, Z.; Chen, J. Secondary Metabolites Profiled in Cannabis Inflorescences, Leaves, Stem Barks, and Roots for Medicinal Purposes. Sci. Rep. 2020, 10, 3309. [Google Scholar] [CrossRef] [PubMed]
- Flores Guillermo, A.; Nahuel, F.; María, T.; Andrés, M.; Sara, M. Adulticidal Effect of Seven Terpenes and a Binary Combination against Aedes aegypti. J. Vector Borne Dis. 2020, 57, 356. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-H.; Kim, M.-K.; Noh, D.-J.; Yoon, C.; Kim, G.-H. Spray Adulticidal Effects of Plant Oils against House Mosquito, Culex pipiens Pallens (Diptera: Culicidae). J. Pestic. Sci. 2009, 34, 100–106. [Google Scholar] [CrossRef]
- Ramzi, A.; El Ouali Lalami, A.; Annemer, S.; Ez zoubi, Y.; Assouguem, A.; Almutairi, M.H.; Kamel, M.; Peluso, I.; Ercisli, S.; Farah, A. Synergistic Effect of Bioactive Monoterpenes against the Mosquito, Culex pipiens (Diptera: Culicidae). Molecules 2022, 27, 4182. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Staples, S.K.; Gostin, E.L.; Smith, J.P.; Vigil, J.J.; Seifried, D.; Kinney, C.; Pauli, C.S.; Heuvel, B.D.V. Contrasting Roles of Cannabidiol as an Insecticide and Rescuing Agent for Ethanol–Induced Death in the Tobacco Hornworm Manduca Sexta. Sci. Rep. 2019, 9, 10481. [Google Scholar] [CrossRef] [PubMed]
- Stack, G.M.; Snyder, S.I.; Toth, J.A.; Quade, M.A.; Crawford, J.L.; McKay, J.K.; Jackowetz, J.N.; Wang, P.; Philippe, G.; Hansen, J.L.; et al. Cannabinoids Function in Defense against Chewing Herbivores in Cannabis sativa L. Hortic. Res. 2023, 10, uhad207. [Google Scholar] [CrossRef]
- Mantzoukas, S.; Ntoukas, A.; Lagogiannis, I.; Kalyvas, N.; Eliopoulos, P.; Poulas, K. Larvicidal Action of Cannabidiol Oil and Neem Oil against Three Stored Product Insect Pests: Effect on Survival Time and in Progeny. Biology 2020, 9, 321. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R. Larvicidal Property of Essential Oils against Culex quinquefasciatus Say (Diptera: Culicidae). Ind. Crops Prod. 2009, 30, 311–315. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Lupidi, G.; Nabissi, M.; Petrelli, R.; Ngahang Kamte, S.L.; Cappellacci, L.; Fiorini, D.; Sut, S.; Dall’Acqua, S.; et al. The Crop-Residue of Fiber Hemp Cv. Futura 75: From a Waste Product to a Source of Botanical Insecticides. Environ. Sci. Pollut. Res. 2018, 25, 10515–10525. [Google Scholar] [CrossRef]
- Bedini, S.; Flamini, G.; Cosci, F.; Ascrizzi, R.; Benelli, G.; Conti, B. Cannabis sativa and Humulus lupulus Essential Oils as Novel Control Tools against the Invasive Mosquito Aedes albopictus and Fresh Water Snail Physella acuta. Ind. Crops Prod. 2016, 85, 318–323. [Google Scholar] [CrossRef]
- Maurya, P.; Mohan, L.; Sharma, P.; Srivastava, C.N. Larval Susceptibility of Aloe barbadensis and Cannabis sativa against Culex quinquefasciatus, the Filariasis Vector. J. Environ. Biol. 2008, 29, 941–943. [Google Scholar] [PubMed]
- McPartland, J.M.; Sheikh, Z. A Review of Cannabis sativa-Based Insecticides, Miticides, and Repellents. J. Entomol. Zool. Stud. 2018, 6, 1288–1299. [Google Scholar]
- Shaalan, E.A.-S.; Canyon, D.; Younes, M.W.F.; Abdel-Wahab, H.; Mansour, A.-H. A Review of Botanical Phytochemicals with Mosquitocidal Potential. Environ. Int. 2005, 31, 1149–1166. [Google Scholar] [CrossRef] [PubMed]
- Barthlott, I.; Scharinger, A.; Golombek, P.; Kuballa, T.; Lachenmeier, D. A Quantitative 1H NMR Method for Screening Cannabinoids in CBD Oils. Toxics 2021, 9, 136. [Google Scholar] [CrossRef] [PubMed]
- Inocente, E.A.; Shaya, M.; Acosta, N.; Rakotondraibe, L.H.; Piermarini, P.M. A Natural Agonist of Mosquito TRPA1 from the Medicinal Plant Cinnamosma fragrans That Is Toxic, Antifeedant, and Repellent to the Yellow Fever Mosquito Aedes aegypti. PLoS Negl. Trop. Dis. 2018, 12, e0006265. [Google Scholar] [CrossRef] [PubMed]
- Martínez Rodríguez, E.J.; Evans, P.; Kalsi, M.; Rosenblatt, N.; Stanley, M.; Piermarini, P.M. Larvicidal Activity of Carbon Black against the Yellow Fever Mosquito Aedes aegypti. Insects 2022, 13, 307. [Google Scholar] [CrossRef] [PubMed]
- Pridgeon, J.W.; Becnel, J.J.; Clark, G.G.; Linthicum, K.J. A High-Throughput Screening Method to Identify Potential Pesticides for Mosquito Control. J. Med. Entomol. 2009, 46, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Abbott, W.S. A Method of Computing the Effectiveness of an Insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Cerceau, C.I.; Barbosa, L.C.A.; Alvarenga, E.S.; Ferreira, A.G.; Thomasi, S.S. A Validated 1H NMR Method for Quantitative Analysis of α-Bisabolol in Essential Oils of Eremanthus erythropappus. Talanta 2016, 161, 71–79. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Petrelli, R.; Cappellacci, L.; Santini, G.; Fiorini, D.; Sut, S.; Dall’Acqua, S.; Canale, A.; Maggi, F. The Essential Oil from Industrial Hemp (Cannabis sativa L.) by-Products as an Effective Tool for Insect Pest Management in Organic Crops. Ind. Crops Prod. 2018, 122, 308–315. [Google Scholar] [CrossRef]
- Şengül Demirak, M.Ş.; Canpolat, E. Plant-Based Bioinsecticides for Mosquito Control: Impact on Insecticide Resistance and Disease Transmission. Insects 2022, 13, 162. [Google Scholar] [CrossRef] [PubMed]
- Reid, W.R.; Thornton, A.; Pridgeon, J.W.; Becnel, J.J.; Tang, F.; Estep, A.; Clark, G.G.; Allan, S.; Liu, N. Transcriptional Analysis of Four Family 4 P450s in a Puerto Rico Strain of Aedes aegypti (Diptera: Culicidae) Compared With an Orlando Strain and Their Possible Functional Roles in Permethrin Resistance. J. Med. Entomol. 2014, 51, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R.; Benelli, G. Essential Oils as Ecofriendly Biopesticides? Challenges and Constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Acheuk, F.; Basiouni, S.; Shehata, A.A.; Dick, K.; Hajri, H.; Lasram, S.; Yilmaz, M.; Emekci, M.; Tsiamis, G.; Spona-Friedl, M.; et al. Status and Prospects of Botanical Biopesticides in Europe and Mediterranean Countries. Biomolecules 2022, 12, 311. [Google Scholar] [CrossRef] [PubMed]
- Kleinhenz, M.D.; Magnin, G.; Ensley, S.M.; Griffin, J.J.; Goeser, J.; Lynch, E.; Coetzee, J.F. Nutrient Concentrations, Digestibility, and Cannabinoid Concentrations of Industrial Hemp Plant Components. Appl. Anim. Sci. 2020, 36, 489–494. [Google Scholar] [CrossRef]
- Hanuš, L.O.; Hod, Y. Terpenes/Terpenoids in Cannabis: Are They Important? Med. Cannabis Cannabinoids 2020, 3, 25–60. [Google Scholar] [CrossRef] [PubMed]
- Namdar, D.; Mazuz, M.; Ion, A.; Koltai, H. Variation in the Compositions of Cannabinoid and Terpenoids in Cannabis Sativa Derived from Inflorescence Position along the Stem and Extraction Methods. Ind. Crops Prod. 2018, 113, 376–382. [Google Scholar] [CrossRef]
- Jackson, B.; Gilbert, L.; Tolosa, T.; Henry, S.; Volkis, V.; Zebelo, S. The Impact of Insect Herbivory in the Level of Cannabinoids in CBD Hemp Varieties. 2021; in review. [Google Scholar] [CrossRef]
- Abendroth, J.A.; Gondhalekar, A.D.; Scharf, M.E.; Couture, J.J. Cannabidiol Reduces Fall Armyworm (Spodoptera frugiperda) Growth by Reducing Consumption and Altering Detoxification and Nutritional Enzyme Activity in a Dose-Dependent Manner. Arthropod-Plant Interact. 2023, 17, 195–204. [Google Scholar] [CrossRef]
- Fine, P.G.; Rosenfeld, M.J. The Endocannabinoid System, Cannabinoids, and Pain. Rambam. Maimonides Med. J. 2013, 4, e0022. [Google Scholar] [CrossRef]
- McPartland, J.; Di Marzo, V.; De Petrocellis, L.; Mercer, A.; Glass, M. Cannabinoid Receptors Are Absent in Insects. J. Comp. Neurol. 2001, 436, 423–429. [Google Scholar] [CrossRef]
- Berman, P.; de Haro, L.A.; Jozwiak, A.; Panda, S.; Pinkas, Z.; Dong, Y.; Cveticanin, J.; Barbole, R.; Livne, R.; Scherf, T.; et al. Parallel Evolution of Cannabinoid Biosynthesis. Nat. Plants 2023, 9, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Norris, E.J.; Gross, A.D.; Bartholomay, L.C.; Coats, J.R. Plant Essential Oils Synergize Various Pyrethroid Insecticides and Antagonize Malathion in Aedes aegypti. Med. Vet Entomol. 2019, 33, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Ghovanloo, M.-R.; Ruben, P.C. Cannabidiol and Sodium Channel Pharmacology: General Overview, Mechanism, and Clinical Implications. Neuroscientist 2022, 28, 318–334. [Google Scholar] [CrossRef] [PubMed]
- Puopolo, T.; Liu, C.; Ma, H.; Seeram, N.P. Inhibitory Effects of Cannabinoids on Acetylcholinesterase and Butyrylcholinesterase Enzyme Activities. Med. Cannabis Cannabinoids 2022, 5, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Ghovanloo, M.-R.; Shuart, N.G.; Mezeyova, J.; Dean, R.A.; Ruben, P.C.; Goodchild, S.J. Inhibitory Effects of Cannabidiol on Voltage-Dependent Sodium Currents. J. Biol. Chem. 2018, 293, 16546–16558. [Google Scholar] [CrossRef] [PubMed]
- Fiorini, D.; Molle, A.; Nabissi, M.; Santini, G.; Benelli, G.; Maggi, F. Valorizing Industrial Hemp (Cannabis Sativa L.) by-Products: Cannabidiol Enrichment in the Inflorescence Essential Oil Optimizing Sample Pre-Treatment Prior to Distillation. Ind. Crops Prod. 2019, 128, 581–589. [Google Scholar] [CrossRef]
- Adesina, I.; Bhowmik, A.; Sharma, H.; Shahbazi, A. A Review on the Current State of Knowledge of Growing Conditions, Agronomic Soil Health Practices and Utilities of Hemp in the United States. Agriculture 2020, 10, 129. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, J.; Cheng, C.; Lv, J.; Lambo, M.T.; Zhang, G.; Li, Y.; Zhang, Y. Nutritional Values of Industrial Hemp Byproducts for Dairy Cattle. Animals 2022, 12, 3488. [Google Scholar] [CrossRef] [PubMed]
- Ranalli, P.; Venturi, G. Hemp as a Raw Material for Industrial Applications. Euphytica 2004, 140, 1–6. [Google Scholar] [CrossRef]
- Schluttenhofer, C.; Yuan, L. Challenges towards Revitalizing Hemp: A Multifaceted Crop. Trends Plant Sci. 2017, 22, 917–929. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Izzo, L.; Castaldo, L.; Narváez, A.; Graziani, G.; Gaspari, A.; Rodríguez-Carrasco, Y.; Ritieni, A. Analysis of Phenolic Compounds in Commercial Cannabis sativa L. Inflorescences Using UHPLC-Q-Orbitrap HRMS. Molecules 2020, 25, 631. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez Rodríguez, E.J.; Phelan, P.L.; Canas, L.; Acosta, N.; Rakotondraibe, H.L.; Piermarini, P.M. Larvicidal Activity of Hemp Extracts and Cannabidiol against the Yellow Fever Mosquito Aedes aegypti. Insects 2024, 15, 517. https://doi.org/10.3390/insects15070517
Martínez Rodríguez EJ, Phelan PL, Canas L, Acosta N, Rakotondraibe HL, Piermarini PM. Larvicidal Activity of Hemp Extracts and Cannabidiol against the Yellow Fever Mosquito Aedes aegypti. Insects. 2024; 15(7):517. https://doi.org/10.3390/insects15070517
Chicago/Turabian StyleMartínez Rodríguez, Erick J., P. Larry Phelan, Luis Canas, Nuris Acosta, Harinantenaina L. Rakotondraibe, and Peter M. Piermarini. 2024. "Larvicidal Activity of Hemp Extracts and Cannabidiol against the Yellow Fever Mosquito Aedes aegypti" Insects 15, no. 7: 517. https://doi.org/10.3390/insects15070517
APA StyleMartínez Rodríguez, E. J., Phelan, P. L., Canas, L., Acosta, N., Rakotondraibe, H. L., & Piermarini, P. M. (2024). Larvicidal Activity of Hemp Extracts and Cannabidiol against the Yellow Fever Mosquito Aedes aegypti. Insects, 15(7), 517. https://doi.org/10.3390/insects15070517







