Intergenerational Sublethal Effects of Flonicamid on Cotton Aphid, Aphis gossypii: An Age-Stage, Two-Sex Life Table Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Insect and Insecticide
2.2. Bioassays
2.3. Sublethal Effects of Flonicamid on Life-History Traits of the F0 Generation
2.4. Intergenerational Effects of Flonicamid on the Biological Traits of F1 A. gossypii
2.5. Statistical and Life Table Analysis
2.6. Population Projection
3. Results
3.1. Toxicity of Flonicamid on Adult Aphis gossypii
3.2. Sublethal Effects of Flonicamid on Parental Aphis gossypii (F0)
3.3. Duration of Development and Adult Longevity of F1 Aphis gossypii
3.4. Reproduction and Life Table Parameters of F1 Aphis gossypii
3.5. Population Projection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hullé, M.; Chaubet, B.; Turpeau, E.; Simon, J. Encyclop’Aphid: A website on aphids and their natural enemies. Entomol. Gen. 2020, 40, 97–101. [Google Scholar] [CrossRef]
- Verheggen, F.; Barrès, B.; Bonafos, R.; Desneux, N.; Escobar-Gutiérrez, A.J.; Gachet, E.; Laville, J.; Siegwart, M.; Thiéry, D.; Jactel, H. Producing sugar beets without neonicotinoids: An evaluation of alternatives for the management of viruses-transmitting aphids. Entomol. Gen. 2022, 42, 491–498. [Google Scholar] [CrossRef]
- Zang, L.-S.; Wang, S.; Zhang, F.; Desneux, N. Biological control with Trichogramma in China: History, present status and perspectives. Annu. Rev. Entomol. 2021, 66, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Monticelli, L.S.; Desneux, N.; Biondi, A.; Mohl, E.; Heimpel, G.E. Post-introduction changes of host specificity traits in the aphid parasitoid Lysiphlebus testaceipes. Entomol. Gen. 2022, 42, 559–569. [Google Scholar] [CrossRef]
- Gao, X.; Xue, H.; Zhu, X.; Wang, L.; Zhang, K.; Li, D.; Ji, J.; Liang, J.; Luo, J.; Cui, J. Parasitism by Lysiphlebia japonica alters the microbiome of Aphis gossypii offspring. Entomol. Gen. 2023, 43, 1071–1075. [Google Scholar] [CrossRef]
- Abbas, A.; Zhao, C.R.; Arshad, M.; Han, X.; Iftikhar, A.; Hafeez, F.; Aslam, A.; Ullah, F. Sublethal effects of spinetoram and emamectin benzoate on key demographic parameters of fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) under laboratory conditions. Environ. Sci. Pollut. Res. 2023, 30, 82990–83003. [Google Scholar] [CrossRef]
- Dai, H.; Tian, L.; Sun, X.; Wang, X.; Zhu, X.; Monticelli, L.S.; Ullah, F.; Zhao, J.; Desneux, N.; Wang, S. Spirotetramat-and thiamethoxam-induced sublethal effects increase spread of tomato chlorosis virus by its vector Bemisia tabaci. Entomol. Gen. 2023, 43, 1051–1059. [Google Scholar] [CrossRef]
- Shi, D.; Luo, C.; Lv, H.; Zhang, L.; Desneux, N.; You, H.; Li, J.; Ullah, F.; Ma, K. Impact of sublethal and low lethal concentrations of flonicamid on key biological traits and population growth associated genes in melon aphid, Aphis gossypii Glover. Crop Prot. 2022, 152, 105863. [Google Scholar] [CrossRef]
- Roditakis, E.; Fytrou, N.; Staurakaki, M.; Vontas, J.; Tsagkarakou, A. Activity of flonicamid on the sweet potato whitely Bemisia tabaci (Homoptera: Aleyrodidae) and its natural enemies. Pest Manag. Sci. 2014, 70, 1460–1467. [Google Scholar] [CrossRef]
- Ren, M.; Niu, J.; Hu, B.; Wei, Q.; Zheng, C.; Tian, X.; Gao, C.; He, B.; Dong, K.; Su, J. Block of Kir channels by flonicamid disrupts salivary and renal excretion of insect pests. Insect Biochem. Mol. Biol. 2018, 99, 17–26. [Google Scholar] [CrossRef]
- Kodandaram, M.; Rai, A.; Halder, J. Novel insecticides for management of insect pests in vegetable crops: A review. Veg. Sci 2010, 37, 109–123. [Google Scholar]
- Gul, H.; ul Haq, I.; Ullah, F.; Khan, S.; Yaseen, A.; Shah, S.H.; Tariq, K.; Güncan, A.; Desneux, N.; Liu, X. Impact of sublethal concentrations of flonicamid on key demographic parameters and feeding behavior of Schizaphis graminum. Ecotoxicology 2023, 32, 756–767. [Google Scholar] [CrossRef]
- Cho, S.-R.; Koo, H.-N.; Yoon, C.; Kim, G.-H. Sublethal effects of flonicamid and thiamethoxam on green peach aphid, Myzus persicae and feeding behavior analysis. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 889–898. [Google Scholar] [CrossRef]
- Desneux, N.; Fauvergue, X.; Dechaume-Moncharmont, F.-X.; Kerhoas, L.; Ballanger, Y.; Kaiser, L. Diaeretiella rapae limits Myzus persicae populations after applications of deltamethrin in oilseed rape. J. Econ. Entomol. 2005, 98, 9–17. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.-M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- Ullah, F.; Gul, H.; Desneux, N.; Tariq, K.; Ali, A.; Gao, X.; Song, D. Clothianidin-induced sublethal effects and expression changes of vitellogenin and ecdysone receptors genes in the melon aphid, Aphis gossypii. Entomol. Gen. 2019, 39, 137–149. [Google Scholar] [CrossRef]
- Gul, H.; Ullah, F.; Hafeez, M.; Tariq, K.; Desneux, N.; Gao, X.; Song, D. Sublethal concentrations of clothianidin affect fecundity and key demographic parameters of the chive maggot, Bradysia odoriphaga. Ecotoxicology 2021, 30, 1150–1160. [Google Scholar] [CrossRef]
- Ullah, F.; Gul, H.; Desneux, N.; Gao, X.; Song, D. Imidacloprid-induced hormesis effects on demographic traits of the melon aphid, Aphis gossypii. Entomol. Gen. 2019, 39, 325–337. [Google Scholar] [CrossRef]
- Ullah, F.; Gul, H.; Tariq, K.; Desneux, N.; Gao, X.; Song, D. Thiamethoxam induces transgenerational hormesis effects and alteration of genes expression in Aphis gossypii. Pestic. Biochem. Physiol. 2020, 165, 104557. [Google Scholar] [CrossRef]
- Jia, Z.Q.; Zhan, E.L.; Zhang, S.G.; Wang, Y.; Song, P.P.; Jones, A.K.; Han, Z.J.; Zhao, C.Q. Broflanilide prolongs the development of fall armyworm Spodoptera frugiperda by regulating biosynthesis of juvenile hormone. Entomol. Gen. 2022, 42, 61. [Google Scholar] [CrossRef]
- Cutler, G.C. Insects, insecticides and hormesis: Evidence and considerations for study. Dose-Response 2013, 11. [Google Scholar] [CrossRef]
- Decourtye, A.; Henry, M.; Desneux, N. Overhaul pesticide testing on bees. Nature 2013, 497, 188. [Google Scholar] [CrossRef] [PubMed]
- Rix, R.R.; Cutler, G.C. Review of molecular and biochemical responses during stress induced stimulation and hormesis in insects. Sci. Total Environ. 2022, 827, 154085. [Google Scholar] [CrossRef] [PubMed]
- Cutler, G.C.; Amichot, M.; Benelli, G.; Guedes, R.N.C.; Qu, Y.; Rix, R.R.; Ullah, F.; Desneux, N. Hormesis and insects: Effects and interactions in agroecosystems. Sci. Total Environ. 2022, 825, 153899. [Google Scholar] [CrossRef]
- Guedes, R.; Smagghe, G.; Stark, J.; Desneux, N. Pesticide-induced stress in arthropod pests for optimized integrated pest management programs. Annu. Rev. Entomol. 2016, 61, 43–62. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Gul, H.; Desneux, N.; Qu, Y.; Xiao, X.; Khattak, A.M.; Gao, X.; Song, D. Acetamiprid-induced hormetic effects and vitellogenin gene (Vg) expression in the melon aphid, Aphis gossypii. Entomol. Gen. 2019, 39, 259–270. [Google Scholar] [CrossRef]
- Aeinehchi, P.; Naseri, B.; Rafiee Dastjerdi, H.; Nouri-Ganbalani, G.; Golizadeh, A. Lethal and sublethal effects of thiacloprid on Schizaphis graminum (Rondani) (Hemiptera: Aphididae) and its predator Hippodamia variegata (Goeze) (Coleoptera: Coccinellidae). Toxin Rev. 2021, 40, 1261–1271. [Google Scholar] [CrossRef]
- Hafeez, M.; Ullah, F.; Khan, M.M.; Wang, Z.; Gul, H.; Li, X.; Huang, J.; Siddiqui, J.A.; Qasim, M.; Wang, R.-L. Comparative low lethal effects of three insecticides on demographical traits and enzyme activity of the Spodoptera exigua (Hübner). Environ. Sci. Pollut. Res. 2022, 29, 60198–60211. [Google Scholar] [CrossRef]
- Ullah, F.; Güncan, A.; Gul, H.; Hafeez, M.; Zhou, S.; Wang, Y.; Zhang, Z.; Huang, J.; Ghramh, H.A.; Guo, W.; et al. Spinosad-induced intergenerational sublethal effects on Tuta absoluta: Biological traits and related genes expressions. Entomol. Gen. 2024, 44, 395–404. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, K.; Jiang, Y.; Guo, Y.; Desneux, N. Widespread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nature 2012, 487, 362. [Google Scholar] [CrossRef]
- Abd Allah, A.; Desneux, N.; Monticelli, L.S.; Fan, Y.; Shi, X.; Guedes, R.N.; Gao, X. Potential for insecticide-mediated shift in ecological dominance between two competing aphid species. Chemosphere 2019, 226, 651–658. [Google Scholar]
- Chi, H.; You, M.; Atlıhan, R.; Smith, C.L.; Kavousi, A.; Özgökçe, M.S.; Güncan, A.; Tuan, S.-J.; Fu, J.-W.; Xu, Y.-Y. Age-Stage, two-sex life table: An introduction to theory, data analysis, and application. Entomol. Gen. 2020, 40, 102–123. [Google Scholar] [CrossRef]
- Chi, H.; Güncan, A.; Kavousi, A.; Gharakhani, G.; Atlihan, R.; Özgökçe, M.S.; Shirazi, J.; Amir-Maafi, M.; Maroufpoor, M.; Taghizadeh, R. TWOSEX-MSChart: The key tool for life table research and education. Entomol. Gen. 2022, 42, 845–849. [Google Scholar] [CrossRef]
- Chi, H.; Kavousi, A.; Gharekhani, G.; Atlihan, R.; Özgökçe, M.S.; Güncan, A.; Gökçe, A.; Smith, C.L.; Benelli, G.; Guedes, R.N.C. Advances in theory, data analysis, and application of the age-stage, two-sex life table for demographic research, biological control, and pest management. Entomol. Gen. 2023, 43, 705–735. [Google Scholar] [CrossRef]
- Chi, H.; Kara, H.; Özgökçe, M.S.; Atlihan, R.; Güncan, A.; Rişvanlı, M.R. Innovative application of set theory, Cartesian product, and multinomial theorem in demographic research. Entomol. Gen. 2022, 42, 863–874. [Google Scholar] [CrossRef]
- Liu, X.; Fu, Z.; Zhu, Y.; Gao, X.; Liu, T.-X.; Liang, P. Sublethal and transgenerational effects of afidopyropen on biological traits of the green peach aphid Myzus persicae (Sluzer). Pestic. Biochem. Physiol. 2022, 180, 104981. [Google Scholar] [CrossRef]
- Ju, D.; Liu, Y.-X.; Liu, X.; Dewer, Y.; Mota-Sanchez, D.; Yang, X.-Q. Exposure to lambda-cyhalothrin and abamectin drives sublethal and transgenerational effects on the development and reproduction of Cydia pomonella. Ecotoxicol. Environ. Saf. 2023, 252, 114581. [Google Scholar] [CrossRef]
- Moores, G.D.; Gao, X.; Denholm, I.; Devonshire, A.L. Characterisation of insensitive acetylcholinesterase in insecticide-resistant cotton aphids, Aphis gossypii glover (homoptera: Aphididae). Pestic. Biochem. Physiol. 1996, 56, 102–110. [Google Scholar] [CrossRef]
- Gul, H.; Güncan, A.; Ullah, F.; Ning, X.; Desneux, N.; Liu, X. Sublethal concentrations of thiamethoxam induce transgenerational hormesis in cotton aphid, Aphis gossypii Glover. CABI Agric. Biosci. 2023, 4, 50. [Google Scholar] [CrossRef]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX-MS Chart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis. 2023. Available online: http://140.120.197.173/ecology/prod02.htm (accessed on 22 November 2023).
- Akca, I.; Ayvaz, T.; Yazici, E.; Smith, C.L.; Chi, H. Demography and population projection of Aphis fabae (Hemiptera: Aphididae): With additional comments on life table research criteria. J. Econ. Entomol. 2015, 108, 1466–1478. [Google Scholar] [CrossRef]
- Efron, B.; Tibshirani, R. An Introduction to the Bootstrap; Chapman and Hall Inc.: New York, NY, USA, 1993; Volune 914. [Google Scholar]
- Huang, Y.B.; Chi, H. Age-stage, two-sex life tables of Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae) with a discussion on the problem of applying female age-specific life tables to insect populations. Insect Sci. 2012, 19, 263–273. [Google Scholar] [CrossRef]
- Akköprü, E.P.; Atlıhan, R.; Okut, H.; Chi, H. Demographic assessment of plant cultivar resistance to insect pests: A case study of the dusky-veined walnut aphid (Hemiptera: Callaphididae) on five walnut cultivars. J. Econ. Entomol. 2015, 108, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Chi, H.; Guo, Y.; Li, X.; Zhao, L.; Ma, R. Demography of Cacopsylla chinensis (Hemiptera: Psyllidae) reared on four cultivars of Pyrus bretschneideri (Rosales: Rosaceae) and P. communis pears with estimations of confidence intervals of specific life table statistics. J. Econ. Entomol. 2020, 113, 2343–2353. [Google Scholar] [CrossRef]
- Chi, H. TIMING-MSChart: A Computer Program for the Population Projection Based on Age-Stage, Two-Sex Life Table; National Chung Hsing University: Taichung, Taiwan, 2023; Available online: http://140.120.197.173/ecology/prod02.htm (accessed on 28 November 2023).
- Chi, H. Timing of control based on the stage structure of pest populations: A simulation approach. J. Econ. Entomol. 1990, 83, 1143–1150. [Google Scholar] [CrossRef]
- Huang, H.-W.; Chi, H.; Smith, C.L. Linking demography and consumption of Henosepilachna vigintioctopunctata (Coleoptera: Coccinellidae) fed on Solanum photeinocarpum (Solanales: Solanaceae): With a new method to project the uncertainty of population growth and consumption. J. Econ. Entomol. 2018, 111, 1–9. [Google Scholar] [CrossRef]
- Gul, H.; Haq, I.U.; Güncan, A.; Abbas, A.; Khan, S.; Yaseen, A.; Ullah, F.; Desneux, N.; Liu, X. Thiamethoxam-Induced Intergenerational Sublethal Effects on the Life History and Feeding Behavior of Rhopalosiphum padi. Plants 2024, 13, 865. [Google Scholar] [CrossRef]
- Ma, K.-S.; Tang, Q.-L.; Liang, P.-Z.; Li, J.-H.; Gao, X.-W. A sublethal concentration of afidopyropen suppresses the population growth of the cotton aphid, Aphis gossypii Glover (Hemiptera: Aphididae). J. Integr. Agric. 2022, 21, 2055–2064. [Google Scholar] [CrossRef]
- Tang, Q.; Ma, K.; Chi, H.; Hou, Y.; Gao, X. Transgenerational hormetic effects of sublethal dose of flupyradifurone on the green peach aphid, Myzus persicae (Sulzer) (Hemiptera: Aphididae). PLoS ONE 2019, 14, e0208058. [Google Scholar] [CrossRef]
- Vakhide, N.; Safavi, S.A. Lethal and sublethal effects of direct exposure to acetamiprid on reproduction and survival of the greenbug, Schizaphis graminum (Hemiptera: Aphididae). Arch. Phytopathol. Plant Prot. 2014, 47, 339–348. [Google Scholar] [CrossRef]
- Cui, L.; Yuan, H.; Wang, Q.; Wang, Q.; Rui, C. Sublethal effects of the novel cis-nitromethylene neonicotinoid cycloxaprid on the cotton aphid Aphis gossypii Glover (Hemiptera: Aphididae). Sci. Rep. 2018, 8, 8915. [Google Scholar] [CrossRef] [PubMed]
- Mostafiz, M.M.; Alam, M.B.; Chi, H.; Hassan, E.; Shim, J.-K.; Lee, K.-Y. Effects of sublethal doses of methyl benzoate on the life history traits and acetylcholinesterase (AChE) activity of Aphis gossypii. Agronomy 2020, 10, 1313. [Google Scholar] [CrossRef]
- Yuan, H.B.; Li, J.H.; Liu, Y.Q.; Cui, L.; Lu, Y.H.; Xu, X.Y.; Li, Z.; Wu, K.M.; Desneux, N. Lethal, sublethal and transgenerational effects of the novel chiral neonicotinoid pesticide cycloxaprid on demographic and behavioral traits of Aphis gossypii (Hemiptera: Aphididae). Insect Sci. 2017, 24, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Gul, H.; Yousaf, H.K.; Xiu, W.; Qian, D.; Gao, X.; Tariq, K.; Han, P.; Desneux, N.; Song, D. Impact of low lethal concentrations of buprofezin on biological traits and expression profile of chitin synthase 1 gene (CHS1) in melon aphid, Aphis gossypii. Sci. Rep. 2019, 9, 12291. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.-Z.; Ma, K.-S.; Chen, X.-W.; Tang, C.-Y.; Xia, J.; Chi, H.; Gao, X.-W. Toxicity and Sublethal Effects of Flupyradifurone, a Novel Butenolide Insecticide, on the Development and Fecundity of Aphis gossypii (Hemiptera: Aphididae). J. Econ. Entomol. 2018, 112, 852–858. [Google Scholar] [CrossRef]
- Ali, E.; Liao, X.; Yang, P.; Mao, K.; Zhang, X.; Shakeel, M.; Salim, A.M.; Wan, H.; Li, J. Sublethal effects of buprofezin on development and reproduction in the white-backed planthopper, Sogatella furcifera (Hemiptera: Delphacidae). Sci. Rep. 2017, 7, 16913. [Google Scholar] [CrossRef] [PubMed]
- Pakyari, H.; Enkegaard, A. Sublethal and transgenerational effects of abamectin on the biological performance of the predatory thrips Scolothrips longicornis (Thysanoptera: Thripidae). J. Econ. Entomol. 2015, 108, 559–565. [Google Scholar] [CrossRef]
- Jager, T.; Barsi, A.; Ducrot, V. Hormesis on life-history traits: Is there such thing as a free lunch? Ecotoxicology 2013, 22, 263–270. [Google Scholar] [CrossRef]
- Guo, R.; Ren, X.; Ren, H. Effects of dimethoate on rotifer Brachionus calyciflorus using multigeneration toxicity tests. J. Environ. Sci. Health Part B 2012, 47, 883–890. [Google Scholar] [CrossRef]
- Hannig, G.T.; Ziegler, M.; Marçon, P.G. Feeding cessation effects of chlorantraniliprole, a new anthranilic diamide insecticide, in comparison with several insecticides in distinct chemical classes and mode-of-action groups. Pest Manag. Sci. Former. Pestic. Sci. 2009, 65, 969–974. [Google Scholar] [CrossRef]
- Mallqui, K.V.; Vieira, J.; Guedes, R.; Gontijo, L. Azadirachtin-induced hormesis mediating shift in fecundity-longevity trade-off in the Mexican bean weevil (Chrysomelidae: Bruchinae). J. Econ. Entomol. 2014, 107, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Atta, B.; Rizwan, M.; Sabir, A.M.; Gogi, M.D.; Farooq, M.A.; Jamal, A. Lethal and sublethal effects of clothianidin, imidacloprid and sulfoxaflor on the wheat aphid, Schizaphis graminum (Hemiptera: Aphididae) and its coccinellid predator, Coccinella septempunctata. Int. J. Trop. Insect Sci. 2021, 41, 345–358. [Google Scholar] [CrossRef]
- Koo, H.N.; Lee, S.W.; Yun, S.H.; Kim, H.K.; Kim, G.H. Feeding response of the cotton aphid, Aphis gossypii, to sublethal rates of flonicamid and imidacloprid. Entomol. Exp. Appl. 2015, 154, 110–119. [Google Scholar] [CrossRef]
- Chen, X.; Ma, K.; Li, F.; Liang, P.; Liu, Y.; Guo, T.; Song, D.; Desneux, N.; Gao, X. Sublethal and transgenerational effects of sulfoxaflor on the biological traits of the cotton aphid, Aphis gossypii Glover (Hemiptera: Aphididae). Ecotoxicology 2016, 25, 1841–1848. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Shi, X.; Desneux, N.; Gao, X. Effects of spirotetramat treatments on fecundity and carboxylesterase expression of Aphis gossypii Glover. Ecotoxicology 2016, 25, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Stark, J.D.; Banks, J.E. Population-level effects of pesticides and other toxicants on arthropods. Annu. Rev. Entomol. 2003, 48, 505–519. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-M.; Chi, H.; Wang, R.-C.; Wang, Y.-P.; Xu, Y.-Y.; Li, X.-D.; Yin, P.; Zheng, F.-Q. Demography and uncertainty of population growth of Conogethes punctiferalis (Lepidoptera: Crambidae) reared on five host plants with discussion on some life history statistics. J. Econ. Entomol. 2018, 111, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Goodman, D. Optimal life histories, optimal notation, and the value of reproductive value. Am. Nat. 1982, 119, 803–823. [Google Scholar] [CrossRef]
- Chi, H.; Su, H.-Y. Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ. Entomol 2006, 35, 10–21. [Google Scholar] [CrossRef]
- Tuan, S.J.; Lee, C.C.; Chi, H. Population and damage projection of Spodoptera litura (F.) on peanuts (Arachis hypogaea L.) under different conditions using the age-stage, two-sex life table. Pest Manag. Sci. 2014, 70, 805–813. [Google Scholar] [CrossRef]
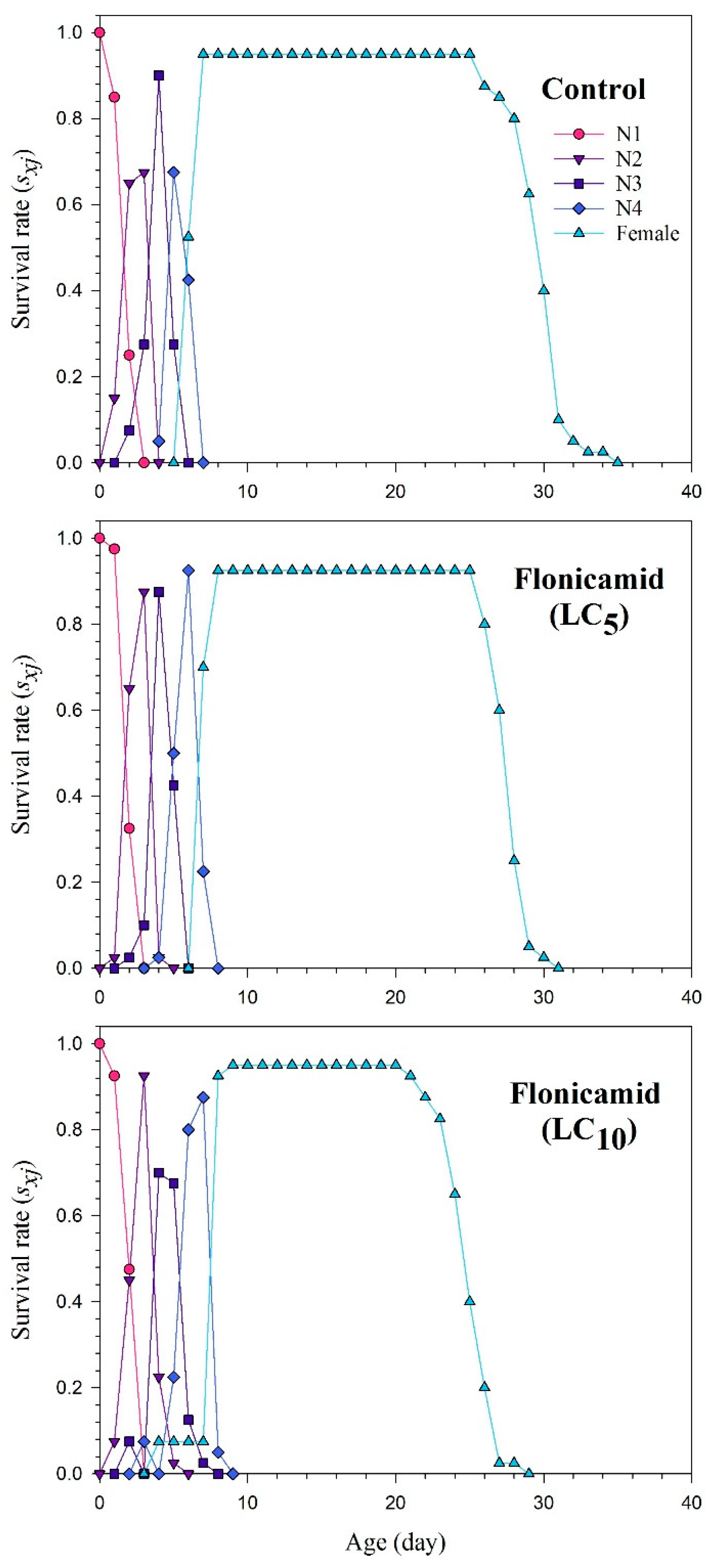
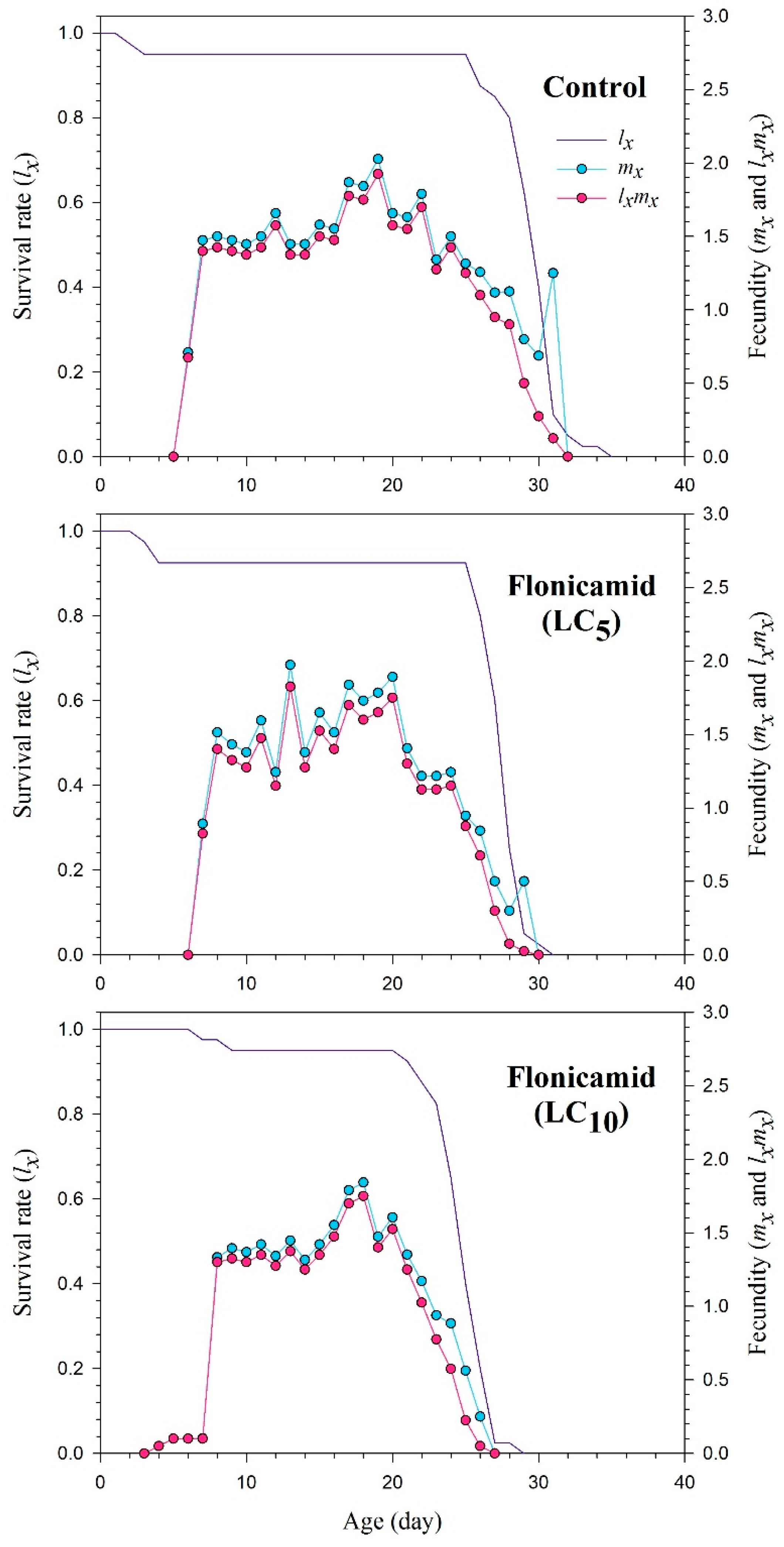

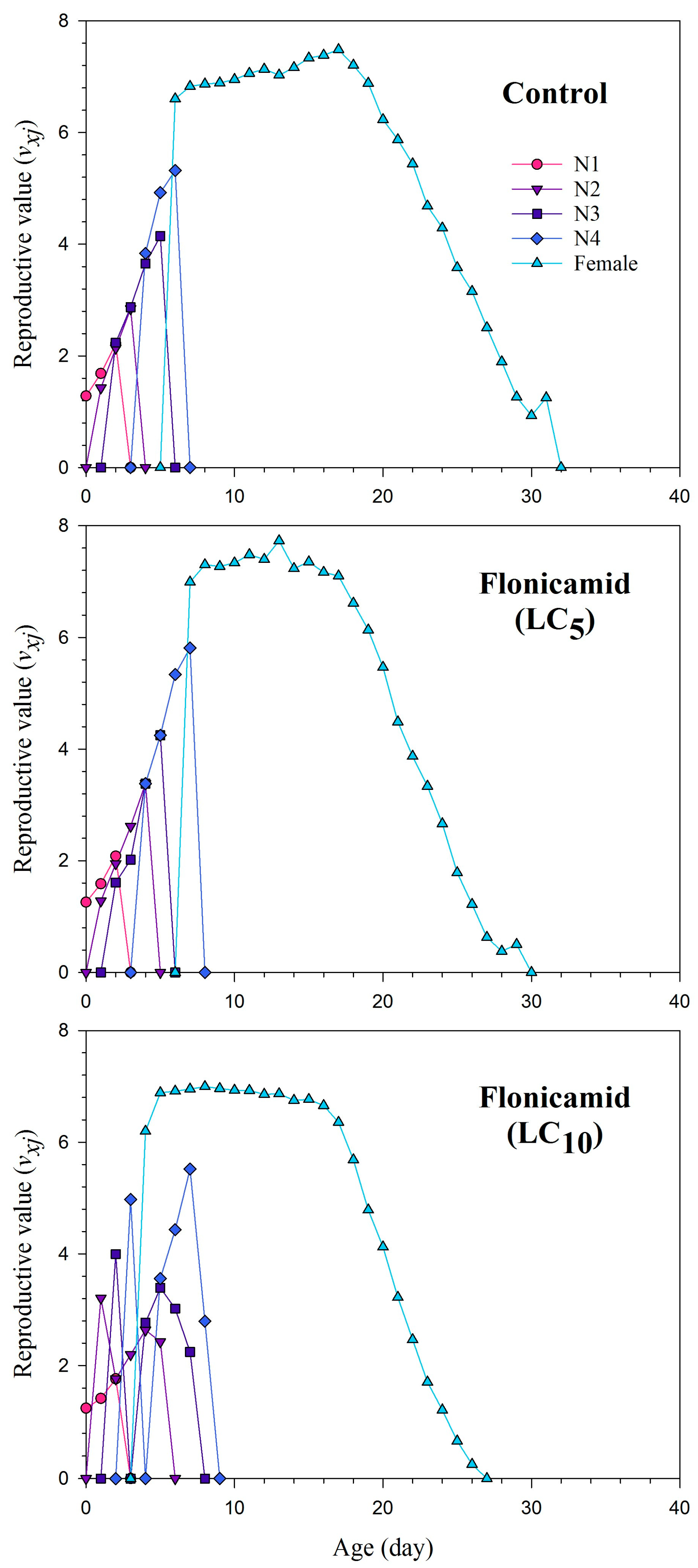
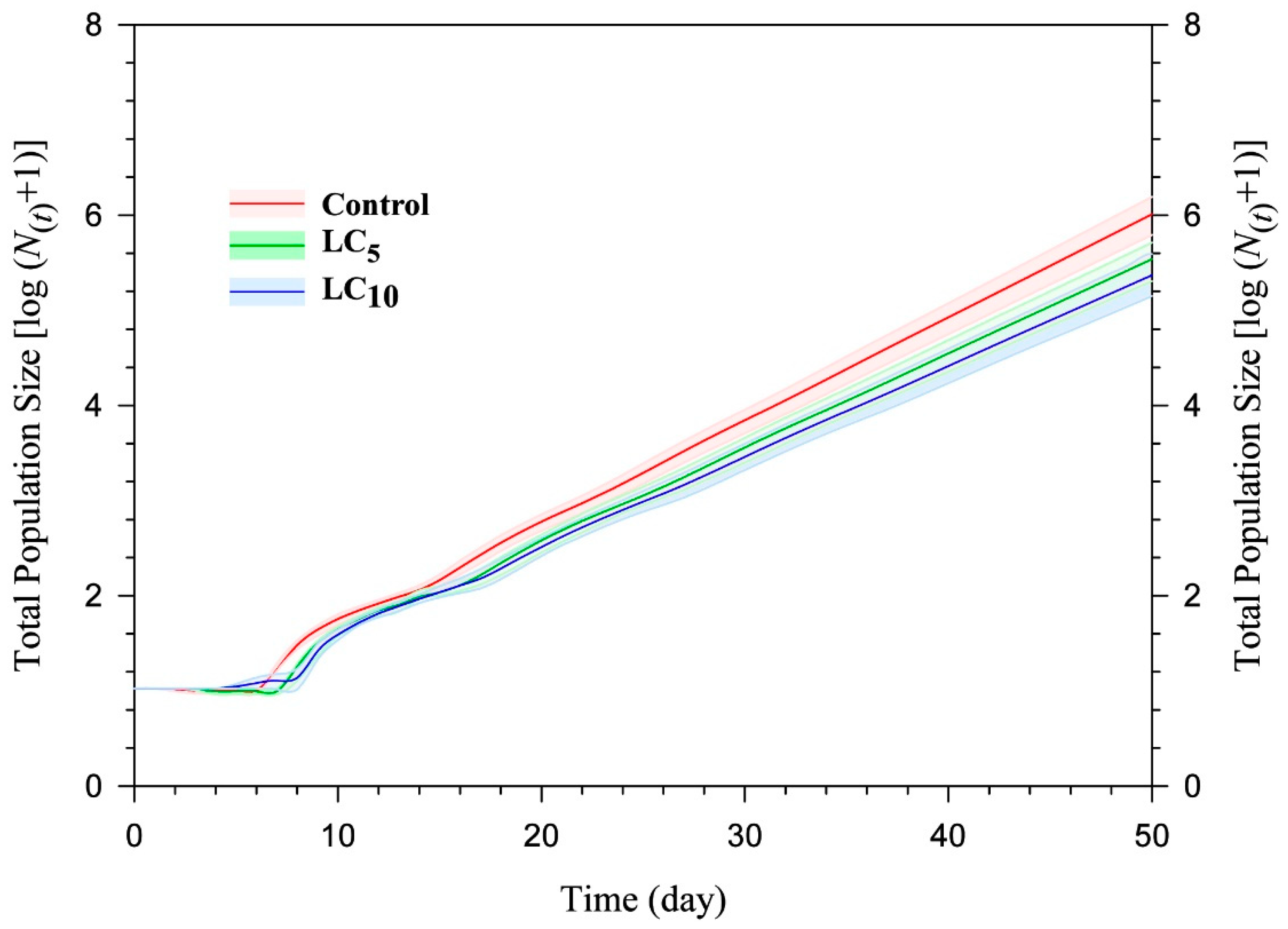
| Parameters | Control | Flonicamid (LC5) | Flonicamid (LC10) |
|---|---|---|---|
| Mean ± SE | Mean ± SE | Mean ± SE | |
| Adult longevity (days) | 22.80 ± 0.22 a | 19.30 ± 0.20 b | 15.80 ± 0.20 c |
| Fecundity (nymphs/female) | 35.10 ± 0.60 a | 26.40 ± 0.34 b | 21.70 ± 0.38 c |
| Reproductive days (days) | 21.50 ± 0.27 a | 17.03 ± 0.23 b | 14.05 ± 0.20 c |
| Stage | Control | Flonicamid (LC5) | Flonicamid (LC10) | |||
|---|---|---|---|---|---|---|
| n | Mean ± SE | n | Mean ± SE | n | Mean ± SE | |
| First-instar nymph | 40 | 2.10 ± 0.10 b | 40 | 2.30 ± 0.08 ab | 40 | 2.40 ± 0.10 a |
| Second-instar nymph | 38 | 1.50 ± 0.09 a | 38 | 1.61 ± 0.08 a | 40 | 1.70 ± 0.09 a |
| Third-instar nymph | 38 | 1.61 ± 0.10 a | 37 | 1.51 ± 0.09 a | 39 | 1.62 ± 0.09 a |
| Fourth-instar nymph | 38 | 1.21 ± 0.07 c | 37 | 1.81 ± 0.10 b | 38 | 2.11 ± 0.10 a |
| Pre-adult | 38 | 6.45 ± 0.08 c | 37 | 7.24 ± 0.07 b | 38 | 7.71 ± 0.18 a |
| Adult (Female) | 38 | 23.50 ± 0.27 a | 37 | 20.62 ± 0.19 b | 38 | 17.42 ± 0.20 c |
| Total longevity (Female) | 38 | 29.95 ± 0.30 a | 37 | 27.86 ± 0.19 b | 38 | 25.13 ± 0.26 c |
| Parameters a | Control | Flonicamid (LC5) | Flonicamid (LC10) |
|---|---|---|---|
| Mean ± SE | Mean ± SE | Mean ± SE | |
| R0 (offspring/individual) | 33.08 ± 1.32 a | 26.83 ± 1.33 b | 22.63 ± 0.91 c |
| r (day−1) | 0.2494 ± 0.0044 a | 0.2281 ± 0.0045 b | 0.2194 ± 0.0050 b |
| λ (day−1) | 1.2832 ± 0.0057 a | 1.2562 ± 0.0057 b | 1.2453 ± 0.0062 b |
| T (days) | 14.03 ± 0.16 a | 14.42 ± 0.14 a | 14.22 ± 0.25 a |
| F (nymphs/female) | 34.82 ± 0.59 a | 29.00 ± 0.61 b | 23.82 ± 0.41 c |
| RPd (days) | 22.05 ± 0.30 a | 18.65 ± 0.21 b | 16.00 ± 0.25 c |
| APRP (days) | 0.13 ± 0.05 a | 0.19 ± 0.07 a | 0.11 ± 0.05 a |
| TPRP (days) | 6.58 ± 0.09 b | 7.43 ± 0.10 a | 7.82 ± 0.17 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gul, H.; Güncan, A.; Ullah, F.; Desneux, N.; Liu, X. Intergenerational Sublethal Effects of Flonicamid on Cotton Aphid, Aphis gossypii: An Age-Stage, Two-Sex Life Table Study. Insects 2024, 15, 529. https://doi.org/10.3390/insects15070529
Gul H, Güncan A, Ullah F, Desneux N, Liu X. Intergenerational Sublethal Effects of Flonicamid on Cotton Aphid, Aphis gossypii: An Age-Stage, Two-Sex Life Table Study. Insects. 2024; 15(7):529. https://doi.org/10.3390/insects15070529
Chicago/Turabian StyleGul, Hina, Ali Güncan, Farman Ullah, Nicolas Desneux, and Xiaoxia Liu. 2024. "Intergenerational Sublethal Effects of Flonicamid on Cotton Aphid, Aphis gossypii: An Age-Stage, Two-Sex Life Table Study" Insects 15, no. 7: 529. https://doi.org/10.3390/insects15070529
APA StyleGul, H., Güncan, A., Ullah, F., Desneux, N., & Liu, X. (2024). Intergenerational Sublethal Effects of Flonicamid on Cotton Aphid, Aphis gossypii: An Age-Stage, Two-Sex Life Table Study. Insects, 15(7), 529. https://doi.org/10.3390/insects15070529








