Molecular Identification of the Glutaredoxin 5 Gene That Plays Important Roles in Antioxidant Defense in Arma chinensis (Fallou)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing and Sample Preparation
2.2. Gene Cloning and Sequence Analysis
2.3. mRNA Expression by RT-PCR Analysis
2.4. Tissue-Specific Expression of AcGrx5 mRNA
2.5. Low Temperature Treatment
2.6. Gene Expression Was Compared between Diapause Induction and Normal Developmental Stages
2.7. RNA Interference (RNAi)
2.8. Quantification of Antioxidant Genes after AcGrx5 Silencing
2.9. Comparison of Each Oxide Content after Interference
2.10. Statistical Analysis
3. Results
3.1. The Identification of AcGrx5 Genes and Phylogenetic Analysis of AcGrx5 Proteins
3.2. Expression Profiles of AcGrx5 at Different Stages
3.3. Expression Profiles of AcGrx5 at Different Tissues
3.4. Expression Profiles of AcGrx5 at Different Temperatures and Different Times
3.5. Expression Profiles of Other Antioxidant Genes after AcGrx5 Silencing
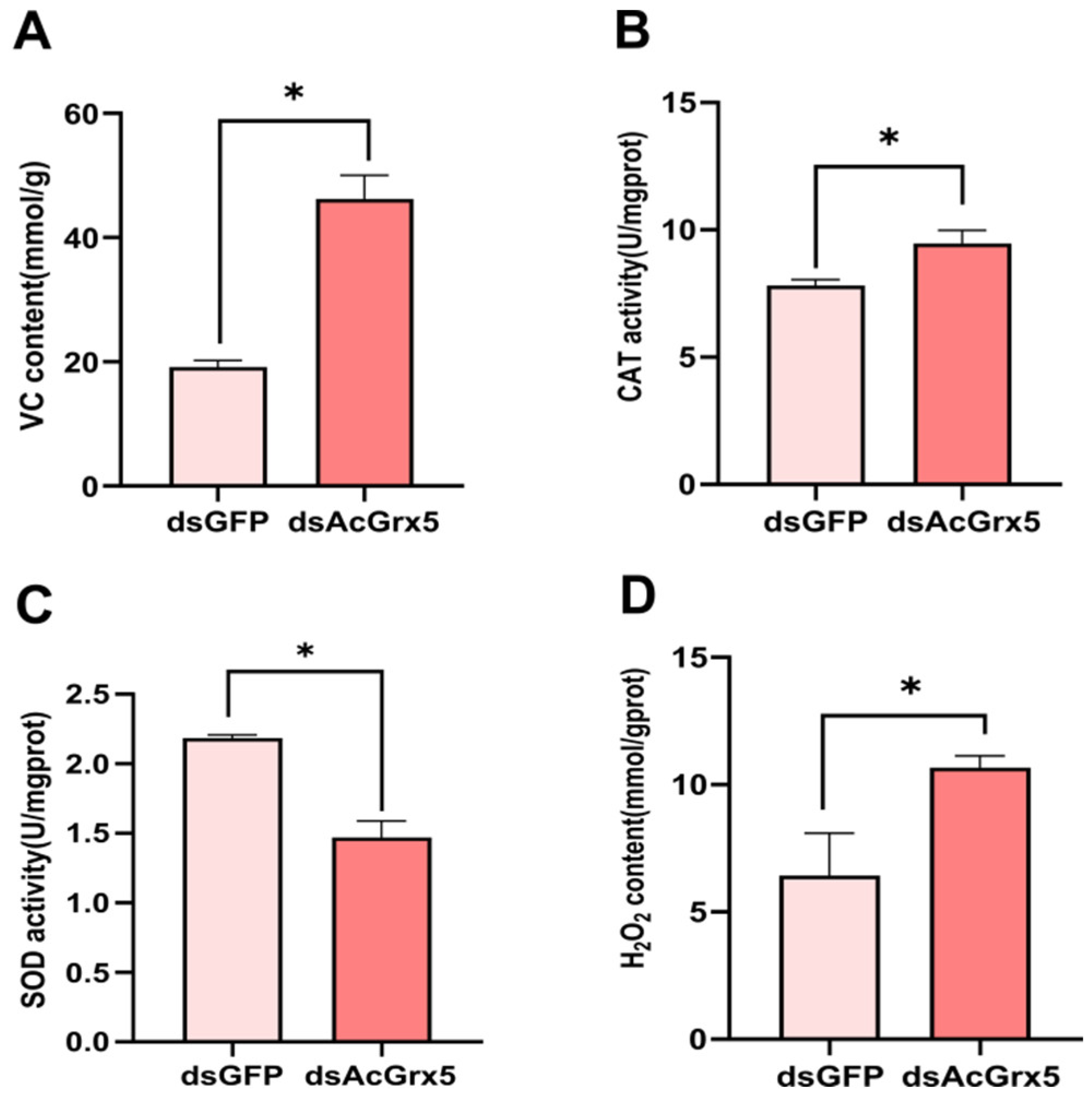
3.6. Assay of Antioxidant Enzyme Activities and Metabolite Contents after AcGrx5 Silencing
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hahn, D.A.; Denlinger, D.L. Meeting the energetic demands of insect diapause: Nutrient storage and utilization. J. Insect Physiol. 2007, 53, 760–773. [Google Scholar] [CrossRef]
- Zou, D.; Wang, M.; Zhang, L.; Zhang, Y.; Zhang, X.J.; Chen, H. Taxonomic and bionomic notes on Arma chinensis (Fallou) (Hemiptera: Pentatomidae: Asopinae). Zootaxa 2012, 3382, 41–52. Available online: https://www.x-mol.com/paperRedirect/1368303225021292544 (accessed on 23 April 2024). [CrossRef]
- Mhamdi, A.; Van-Breusegem, F. Reactive oxygen species in plant development. Development 2018, 145, dev164376. [Google Scholar] [CrossRef] [PubMed]
- Cross, F.W.; Al-Dhahir, R.K.; Dyer, P.E.; MacRobert, A.J. Time-resolved photoacoustic studies of vascular tissue ablation at three laser wavelengths. Appl. Phys. Lett. 1987, 50, 1019–1021. [Google Scholar] [CrossRef]
- Chiu, P.W.; Ng, E.K.; Cheung, F.K.; Chan, F.K.; Leung, W.K.; Wu, J.C.; Wong, V.W.; Yung, M.Y.; Tsoi, K.; Lau, J.Y.; et al. Predicting mortality in patients with bleeding peptic ulcers after therapeutic endoscopy. Clin. Gastroenterol. Hepatol. 2009, 7, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, C.M.; Kondo, T.; Sa-jan, M.; Luo, J.; Bronson, R.; Asano, T.; Farese, R.; Cantley, L.C.; Kahn, C.R. Divergent regulation of hepatic glucose and lipid metabolism by phosphoinositide 3-kinase via Akt and PKClambda/zeta. Cell Metab. 2006, 3, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Dutilleul, C.; Garmier, M.; Noctor, G.; Mathieu, C.; Chétrit, P.; Foyer, C.H.; de-Paepe, R. Leaf mitochondria modulate whole cell redox homeostasis, set antioxidant capacity, and determine stress resistance through altered signaling and diurnal regulation. Plant Cell 2003, 15, 1212–1226. [Google Scholar] [CrossRef] [PubMed]
- Daniel, T.; Faruq, H.M.; Laura-Magdalena, J.; Manuela, G.; Christopher-Horst, L. Role of GSH and Iron-Sulfur Glutaredoxins in Iron Metabolism—Review. Molecules 2020, 25, 3860. [Google Scholar] [CrossRef]
- Holmgren, A. Hydrogen donor system for Escherichia coli ribonucleoside-diphosphate reductase dependent upon glutathione. Proc. Natl. Acad. Sci. USA 1976, 73, 2275–2279. [Google Scholar] [CrossRef]
- Colville, L.; Kranner, I. Desiccation tolerant plants as model systems to study redox regulation of protein thiols. Plant Growth Regul. 2010, 62, 241–255. [Google Scholar] [CrossRef]
- Popović, Ž.D.; Subotić, A.; Nikolić, T.V.; Radojičić, R.; Blagojević, D.P.; Grubor-Lajšić, G.; Koštál, V. Expression of stress-related genes in diapause of European corn borer (Ostrinia nubilalis Hbn.). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2015, 186, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mercer, S.W.; Burke, R. Evidence for a role for the putative Drosophila hGRX1 orthologue in copper homeostasis. Biometals 2016, 29, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.D.; Shen, Z.J.; Liu, X.M.; Li, Z.; Zhang, Q.W.; Liu, X.X. Molecular identification of three novel glutaredoxin genes that play important roles in antioxidant defense in Helicoverpa armigera. Insect Biochem. Mol. Biol. 2016, 75, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Storey, K.B.; Storey, J.M. Tribute to P. L. Lutz: Putting life on ‘pause’–molecular regulation of hypometabolism. J. Exp. Biol. 2007, 210, 1700–1714. [Google Scholar] [CrossRef]
- MacRae, T.H. Gene expression, metabolic regulation and stress tolerance during diapause. Cell Mol. Life Sci. 2010, 67, 2405–2424. [Google Scholar] [CrossRef] [PubMed]
- Camaschella, C.; Campanella, A.; De-Falco, L.; Boschetto, L.; Merlini, R.; Silvestri, L.; Levi, S.; Iolascon, A. The human counterpart of zebrafish shiraz shows sideroblastic-like microcytic anemia and iron overload. Blood 2007, 110, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Shakamuri, P.; Zhang, B.; Johnson, M.K. Monothiol Glutaredoxins Function in Storingand Transporting [Fe2S2] Clusters Assembled on IscU Scaffold Proteins. J. Am. Chem. Soc. 2012, 134, 15213–15216. [Google Scholar] [CrossRef]
- Mapolelo, D.T.; Zhang, B.; Randeniya, S.; Albetel, A.N.; Li, H.; Couturier, J.; Outten, C.E.; Rouhier, N.; Johnson, M.K. Monothiol glutaredoxins and A-type proteins: Partners in Fe–S cluster trafficking. Dalton Trans. 2013, 42, 3107–3115. [Google Scholar] [CrossRef]
- An, S.; Zhang, Y.; Wang, T.; Luo, M.; Li, C. Molecular characterization of glutaredoxin 2 from Ostrinia furnacalis. Integr. Zool. 2013, 8, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.J.; Liu, Y.J.; Gao, X.H.; Liu, X.M.; Zhang, S.D.; Li, Z.; Zhang, Q.W.; Liu, X.X. Molecular identification of two thioredoxin genes from Grapholita molesta and their function in resistance to emamectin benzoate. Front. Physiol. 2018, 9, 1421. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.; Lu, W.; Meng, F.; Wang, X.; Xu, B.; Guo, X. Molecular cloning, expression and oxidative stress response of a mitochondrial thioredoxin peroxidase gene (AccTpx-3) from Apis cerana cerana. J. Insect Physiol. 2013, 59, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.; Gama, F.; Molina-Navarro, M.M.; Gualberto, J.M.; Claxton, R.; Naik, S.G.; Huynh, B.H.; Herrero, E.; Jacquot, J.P.; Johnson, M.K.; et al. Chloroplast monothiol glutaredoxins as scaffold proteins for the assembly and delivery of [2Fe–2S] clusters. EMBO J. 2008, 27, 1122–1133. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.H.; Zhang, W.; Chen, W.Q.; Jin, J.; Cui, X.; Butte, N.F.; Chan, L.; Hirschi, K.D. A mammalian monothiol glutaredoxin, Grx3, is critical for cell cycle progressionduring embryogenesis. FEBS J. 2011, 278, 2525–2539. [Google Scholar] [CrossRef] [PubMed]
- Kozak, G.M.; Wadsworth, C.B.; Kahne, S.C.; Bogdanowicz, S.M.; Harrison, R.G.; Coates, B.S.; Dopman, E.B. Genomic basis of circannual rhythm in the European corn borer moth. Curr. Biol. 2019, 29, 3501–3509. [Google Scholar] [CrossRef] [PubMed]
- Reznik, S.; Voinovich, N. Photoperiod-independent diapause-inducing thermal responseof Trichogramma larvae: Pattern and mechanisms. J. Appl. Entomol. 2022, 146, 586–595. [Google Scholar] [CrossRef]
- Shintani, Y.; Masuzawa, Y.; Hirose, Y.; Miyahara, R.; Watanabe, F.; Tajima, J.Y. Seasonal occurrence and diapause induction of a predatory bug Andrallus spinidens (F.) (Heteroptera: Pentatomidae). Entomol. Sci. 2010, 13, 273–279. [Google Scholar] [CrossRef]
- Jahns, H.; Degaonkar, R.; Podbevsek, P.; Gupta, S.; Bisbe, A.; Aluri, K.; Szeto, J.; Kumar, P.; LeBlanc, S.; Racie, T.; et al. Small circular interfering RNAs (sciRNAs) as a potent therapeutic platform for gene-silencing. Nucleic Acids Res. 2021, 49, 10250–10264. [Google Scholar] [CrossRef]
- Cooper, A.M.; Silver, K.; Zhang, J.; Park, Y.; Zhu, K.Y. Molecular mechanisms influencing efficiency of RNA interference in insects. Pest Manag. Sci. 2019, 75, 18–28. [Google Scholar] [CrossRef]
- Lu, W.; Kang, M.; Liu, X.; Zhao, X.; Guo, X.; Xu, B. Identification and antioxidant characterisation of thioredoxin-like1 from Apis cerana cerana. Apidologie 2012, 43, 737–752. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, Y.; Han, T.; Cui, X.; Lu, X. Chronic intermittent hypoxia aggravates skeletal muscle aging by down-regulating Klc1/grx1 expression via Wnt/β-catenin pathway. Arch Gerontol. Geriatr. 2021, 96, 104460. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liao, Z.; Xiao, Q. Metformin ameliorates skeletal muscle atrophy in Grx1 KO mice by regulating intramuscular lipid accumulation and glucose utilization. Biochem. Biophys. Res. Commun. 2020, 17, 1226–1232. [Google Scholar] [CrossRef] [PubMed]






| Primers | Sequences | Tm/°C | Product Size/bp |
|---|---|---|---|
| AcGrx5-F | TAGCTGCCCAACGTTGATAAC | 58 | 779 |
| AcGrx5-R | TGGTGTACTCTTCAGTAAACA | 58 | 779 |
| qAcGrx5-F | ATGAATTATTTGATTAGGTC | 53 | 82 |
| qAcGrx5-R | CCGCAGCACTTGAAAGTAGTCT | 53 | 82 |
| dsAcGrx5-F | T7-TATGAAAGGTGTCCCAGATGA | 60 | 301 |
| dsAcGrx5-R | T7-CTATTTCTTCTCCTTTTCT | 60 | 301 |
| qAcTrx2-F | ATGGTTTCTTTTCATTTTC | 53 | 120 |
| qAcTrx2-R | AATTTTGCGGCAAGGACC | 53 | 120 |
| qAcTrx-like-F | ATGGCACTTTCAGTTTTAATCG | 53 | 84 |
| qAcTrx-like-R | GCATATGAGCCGGTTGCAT | 53 | 84 |
| qAcPDI-F | TCAAGGTGACAGGACAAAGG | 53 | 86 |
| qAcPDI-R | TTTCTTGGCGTGTTATTTGC | 53 | 86 |
| RPL27-F | CCACCTTAGACGTAACCAGAA | 53 | 116 |
| RPL27-R | ACAGTCGATAACTGGTGCCT | 53 | 116 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Q.; Shen, Z.; Kanjana, N.; Guo, X.; Wu, H.; Zhang, L. Molecular Identification of the Glutaredoxin 5 Gene That Plays Important Roles in Antioxidant Defense in Arma chinensis (Fallou). Insects 2024, 15, 537. https://doi.org/10.3390/insects15070537
Luo Q, Shen Z, Kanjana N, Guo X, Wu H, Zhang L. Molecular Identification of the Glutaredoxin 5 Gene That Plays Important Roles in Antioxidant Defense in Arma chinensis (Fallou). Insects. 2024; 15(7):537. https://doi.org/10.3390/insects15070537
Chicago/Turabian StyleLuo, Qiaozhi, Zhongjian Shen, Nipapan Kanjana, Xingkai Guo, Huihui Wu, and Lisheng Zhang. 2024. "Molecular Identification of the Glutaredoxin 5 Gene That Plays Important Roles in Antioxidant Defense in Arma chinensis (Fallou)" Insects 15, no. 7: 537. https://doi.org/10.3390/insects15070537




