Acoustic Communication in Dendroctonus adjunctus Blandford (Curculionidae Scolytinae): Description of Calls and Sound Production Mechanism
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Optical and Scanning Electron Microscopy of Stridulatory Structures
2.2. Sound Recording
2.3. Analyses
Spectro–Temporal Features
3. Results
3.1. Stridulatory Apparatus of D. adjunctus
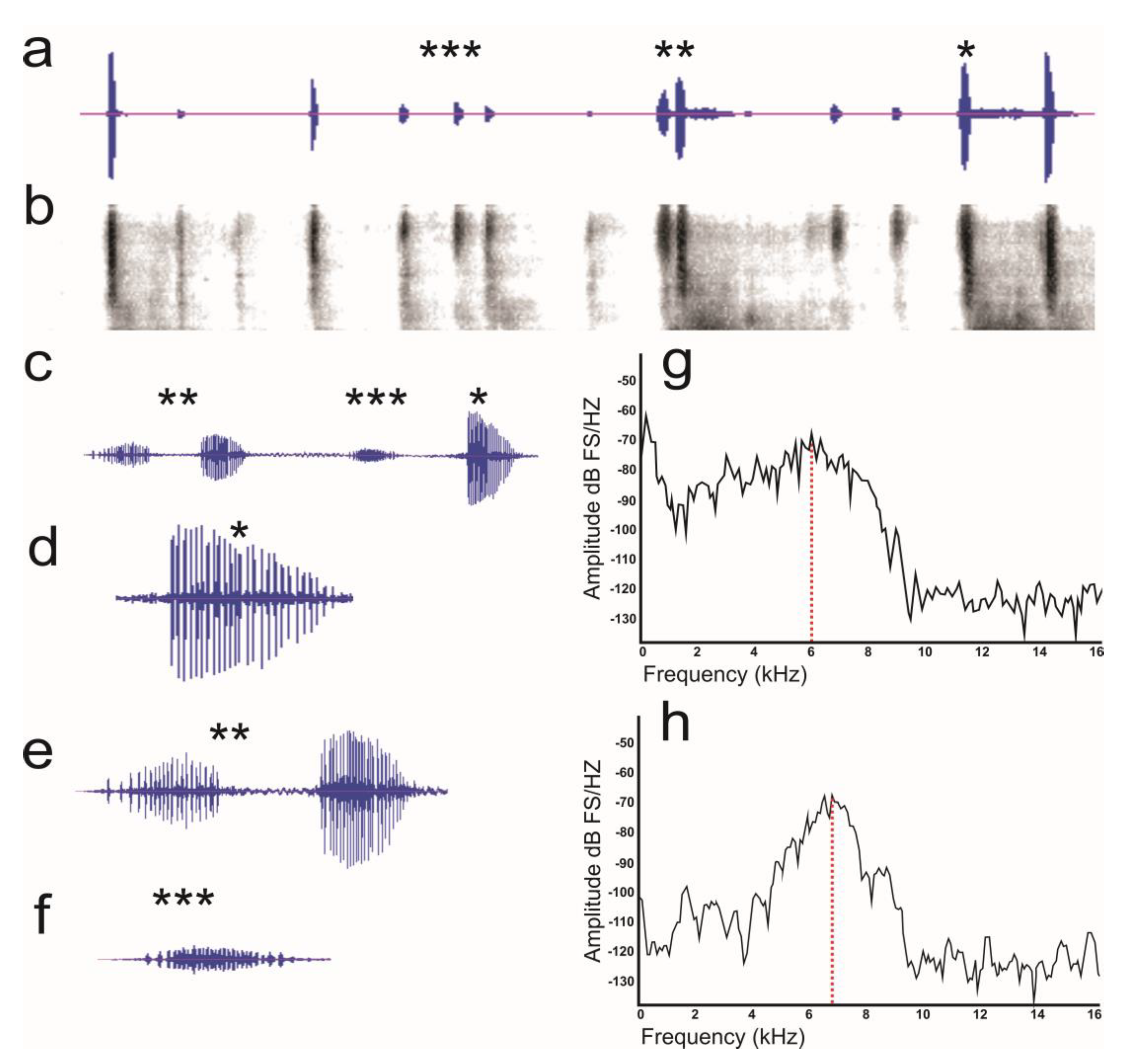
3.2. Sound Recording
3.2.1. Stress Calls
3.2.2. Female–Male Interaction
3.2.3. Male–Male Interaction
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greenfield, M.D. Evolution of Acoustic Communication in Insects. In Insect Hearing; Pollack, G.S., Mason, A.C., Popper, A.N., Fay, R.R., Eds.; Springer International Publishing: Cham, Switzeland, 2016; Volume 55, pp. 17–48. [Google Scholar]
- Neil, T.R.; Holdereid, M.W. Sound production and hearing in insects. In Advances in Insect Physiology; Jurenka, R., Ed.; Academic Press: Amsterdam, The Netherlands, 2021; Volume 61, pp. 101–139. [Google Scholar] [CrossRef]
- Cocroft, R.B.; Rodríguez, R.L. The Behavioral Ecology of Insect Vibrational Communication. Bioscience 2005, 61, 323–334. [Google Scholar] [CrossRef]
- Sanborn, A. Acoustic Communication in Insects. In Encyclopedia of Entomology; Springer: Dordrecht, The Netherlands, 2008; pp. 33–38. [Google Scholar]
- Virant-Doberlet, M.; Stritih-Peljhan, N.; Žunic-Kosi, A.; Polajnar, J. Functional Diversity of Vibrational Signaling Systems in Insects. Annu. Rev. Entomol. 2023, 68, 191–210. [Google Scholar] [CrossRef]
- Henry, C.S.; Brooks, S.J.; Dueli, P.; Johnson, J.B.; Wells, M.M.; Mochizuki, A. Obligatory duetting behaviour in the Chrysoperla carnea–group of cryptic species (Neuroptera: Chrysopidae): Its role in shaping evolutionary history. Biol. Rev. 2013, 88, 787–808. [Google Scholar] [CrossRef]
- Simmons, L.W.; Thomas, M.L.; Simmons, F.W.; Zuk, M. Female preferences for acoustic and olfactory signals during courtship: Male crickets send multiple messages. Behav. Ecol. 2013, 24, 1099–1107. [Google Scholar] [CrossRef]
- Padimi, V.; Manisha, B.L.; Singh, S.K.; Mishra, V.K. Communication in Insects: A Review. J. Exp. Zool. India 2023, 26, 1317–1327. [Google Scholar] [CrossRef]
- Seeley, T.D. Thoughts on information and integration in honey bee colonies. Apidologie 1998, 29, 67–80. [Google Scholar] [CrossRef]
- Grüter, C.; Czaczkes, T.J. Communication in social insects and how it is shaped by individual experience. Anim. Behav. 2019, 151, 207–215. [Google Scholar] [CrossRef]
- Montealegre, Z.F.; Soulsbury, C.D.; Elias, D.O. Editorial: Evolutionary Biomechanics of Sound Production and Reception. Front. Ecol. Evol. 2023, 19, 1–3. [Google Scholar] [CrossRef]
- Stumpner, A.; Von Helversen, D. Evolution and function of auditory systems in insects. Naturwissenschaften 2001, 88, 159–170. [Google Scholar] [CrossRef]
- Eskov, E.K. The Diversity of Ethological and Physiological Mechanisms of Acoustic Communication in Insects. Int. Max Planck Res. Sch. 2017, 62, 466–478. [Google Scholar] [CrossRef]
- Yack, J. Vibrational signaling. In Insect Hearing; Pollack, G.S., Mason, A.C., Popper, A.N., Fay, R.R., Eds.; Springer International Publishing: Cham, Switzeland, 2016; Volume 55, pp. 99–124. [Google Scholar]
- Ronacher, B.; Römer, H. Insect hearing: From physics to ecology. J. Comp. Physiol. 2015, 201, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Bedoya, C.L.; Hofstetter, R.W.; Nelson, X.J.; Hayes, M.; Miller, D.R.; Brockerhoff, E.G. Sound production in bark and ambrosia beetles. Bioacoustics 2019, 30, 58–73. [Google Scholar] [CrossRef]
- Wessel, A. Stridulation in the Coleoptera—An overview. In Insect Sounds and Communication. Physiology, Behaviour, Ecology and Evolution; Drosopoulos, S., Claridge, M.F., Eds.; Taylor & Francis: Boca Raton, FL, USA, 2006; pp. 397–403. [Google Scholar]
- Safrayik, L.; Carroll, A. The biology and epidemiology of mountain pine beetle in Lodgepole pine forest. In The Mountain Pine Beetle: A Synthesis of Biology, Management, and Impacts on Lodgepole Pine; Safrayik, L., Wilson, B., Eds.; Natural Resources Canada, Canadian Forest Service, Pacific Forestry Centre: Victoria, BC, Canada, 2006; pp. 3–36. [Google Scholar]
- Six, D.L.; Bracewell, R. Chapter 8 Dendroctonus. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Vega, F.E., Hofstetter, R.W., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 305–350. [Google Scholar]
- Wegensteiner, R.; Wermelinger, B.; Herrmann, M. Chapter 7 Natural enemies of bark beetles: Predators, parasitoids, pathogens, and nematodes. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Vega, F.E., Hofstetter, R.W., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 247–304. [Google Scholar]
- Six, D.L. The bark beetle holobiont: Why microbes matter. J. Chem. Ecol 2013, 39, 989–1002. [Google Scholar] [CrossRef]
- Simon, J.C.; Marchesi, J.R.; Moguel, C.; Selosse, M.A. Host–microbiota interactions; from holobiont theory to analysis. Microbiome 2019, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Moreno, Y.; Ager, A.; Vargas, C.F.; Hayes, J.L.; Zúñiga, G. Determing the vulnerability of Mexican pine forest to bark beetles of the genus Dendroctonus Erichson (Coleoptera: Curculionidae: Scolytinae). For. Ecol. Manag. 2010, 260, 52–61. [Google Scholar] [CrossRef]
- Raffa, K.F.; Gregoire, J.C.; Lindgren, B.S. Chapter 1 Natural history and ecology of bark beetles. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Vega, F.E., Hofstetter, R.W., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 1–40. [Google Scholar]
- Wood, D.L. The role of pheromones, kairomones, and allomones in the host selection and colonization behavior of bark beetles. Annu. Rev. Entomol. 1982, 27, 411–446. [Google Scholar] [CrossRef]
- Gitau, C.W.; Bashford, R.; Carneige, A.J.; Gurr, G.M. A review of semiochemicals associated with bark beetle (Coleoptera: Curculionidae: Scolytinae) pest of coniferous trees: A focus on beetle interactions with other pest and associates. For. Ecol. Manag. 2013, 297, 1–14. [Google Scholar] [CrossRef]
- Čokl, A.; Virant–Doberlet, M. Communication with substrate–borne signals in small plant–dwelling insects. Annu. Rev. Entomol. 2003, 48, 29–50. [Google Scholar] [CrossRef]
- Lyal, C.H.C.; King, T. Elytro–tergal stridulation in weevils (Insecta: Coleoptera: Curculionoidea). J. Nat. Hist. 1996, 30, 703–773. [Google Scholar] [CrossRef]
- Fleming, A.J.; Lindeman, A.A.; Carrol, A.L.; Yack, J.E. Acoustics of the mountain pine beetle (Dendroctonus ponderosae) (Curculionidae, Scolytinae): Sonic, ultrasonic, and vibration characteristics. Can. J. Zool. 2013, 91, 235–244. [Google Scholar] [CrossRef]
- Yturralde, K.M.; Hofstetter, R.W. Characterization of stridulatory structures and sounds of the larger Mexican pine beetle, Dendroctonus approximatus (Coleoptera: Curculionidae: Scolytinaae). Fla. Entomol. 2015, 98, 515–527. [Google Scholar] [CrossRef]
- Bedoya, C.L.; Brockerhoff, E.G.; Haye, M.; Pawson, S.M.; Najar-Rodríguez, A.; Nelson, X.J. Acoustic communication of the red–haired bark beetle Hylurgus ligniperda. Physiol. Entomol. 2019, 44, 252–265. [Google Scholar] [CrossRef]
- Lukic, I.; Bedoya, C.L.; Hofstetter, E.M.; Hofstetter, R.W. Pinyon engraver beetle acoustics: Stridulation apparatus, sound production and behavioral response to vibroacoustic treatments in logs. Insects 2021, 12, 496. [Google Scholar] [CrossRef]
- Arjomandi, E.; Turchen, L.M.; Conolly, A.A.; Léveillée, M.B.; Yack, J.E. Acoustic communication in bark beetles (Scolytinae): 150 years of research. Physiol. Entomol. 2024, 24, 1–20. [Google Scholar] [CrossRef]
- Rudinsky, J.A.; Michael, R.R. Sound production in Scolytidae: “Rivalry” Behaviour of male Dendroctonus beetles. J. Insect Physiol. 1974, 20, 1219–1230. [Google Scholar] [CrossRef]
- Ryker, L.C. Acoustics studies of Dendroctonus bark beetles. Fla. Entomol. 1988, 77, 447–461. [Google Scholar] [CrossRef]
- Lindeman, A.A.; Yack, J.E. What is the password? Female bark beetles (Scolytinae) grant males access to their galleries based on courtship song. Behav. Process 2015, 115, 123–131. [Google Scholar] [CrossRef]
- Wood, S.L. The bark and ambrosia beetles of North and Central America (Coleoptera: Scolytidae), a taxonomic monograph. Great Basin Nat. 1982, 6, 1–1359. [Google Scholar]
- Salinas-Moreno, Y.; Vargas, C.F.; Zúñiga, G.; Víctor, J.; Ager, A.; Hayes, J.L. Atlas de Distribución Geográfica de Los Descortezadores del Género Dendroctonus (Curculionidae: Scolytinae) en México/Atlas of the Geographic Distribution of Bark Beetles of the Genus Dendroctonus (Curculionidae: Scolytinae) in Mexico; Instituto Politécnico Nacional, Comisión Nacional Forestal Distrito Federal: Mexico City, México, 2010. [Google Scholar]
- Lyon, R.L. A useful secondary sex character in Dendroctonus bark beetles. Can. Entomol. 1958, 90, 582–584. [Google Scholar] [CrossRef]
- Barr, B.A. Sound production in Scolytidae with emphasis on the genus Ips. Can. Entomol. 1969, 101, 636–672. [Google Scholar] [CrossRef]
- Rudinsky, J.A.; Ryker, L.C. Sound production in Scolytidae: Rivalry and premating stridulation of male Douglas–fir beetle. J. Insect Physiol. 1976, 22, 997–1003. [Google Scholar] [CrossRef]
- Michael, R.R.; Rudinsky, J.A. Sound production in Scolytidae: Specificity in male Dendroctonus beetles. J. Insect Physiol. 1972, 18, 2189–2201. [Google Scholar] [CrossRef]
- Pajares, J.A.; Lanier, G.L. Biosystematics of the turpentine beetle Dendroctons terebrans and D. valens (Coleoptera: Scolytidae). Ann. Entomol. Soc. Am. 1990, 83, 171–188. [Google Scholar] [CrossRef]
- Cerrillo-Mancilla, L.L.; (Escuela Nacional de Ciencias Biológicas, Ciudad de México, México). Personal communication, 2024.
- Rudinsky, J.A.; Michael, R.R. Sound production in Scolytidae: Stridulation by female Dendroctonus beetles. J. Insect Physiol. 1973, 19, 689–705. [Google Scholar] [CrossRef]
- Munro, H.L.; Sullivan, B.T.; Villari, C.; Gandhi, K.J. A review of the ecology and management of black turpentine beetle (Coleoptera: Curculionidae). Environ. Entomol. 2019, 48, 765–783. [Google Scholar] [CrossRef]
- Liu, Z.D.; Wickham, J.D.; Sun, J.H. Fighting and aggressive sound determines larger male to win male-male competition in a bark beetle. Insect Sci. 2021, 28, 203–214. [Google Scholar] [CrossRef]
- Fonseca, P.J. Cicada acoustic communication. In Insect Hearing and Acoustic Communication; Hedwig, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 101–121. [Google Scholar]
- Hall, M.; Robinson, D. Acoustic signalling in Orthoptera. In Advances in Insect Physiology; Academic Press: Cambridge, MA, USA, 2021; Volume 61, pp. 1–99. [Google Scholar]
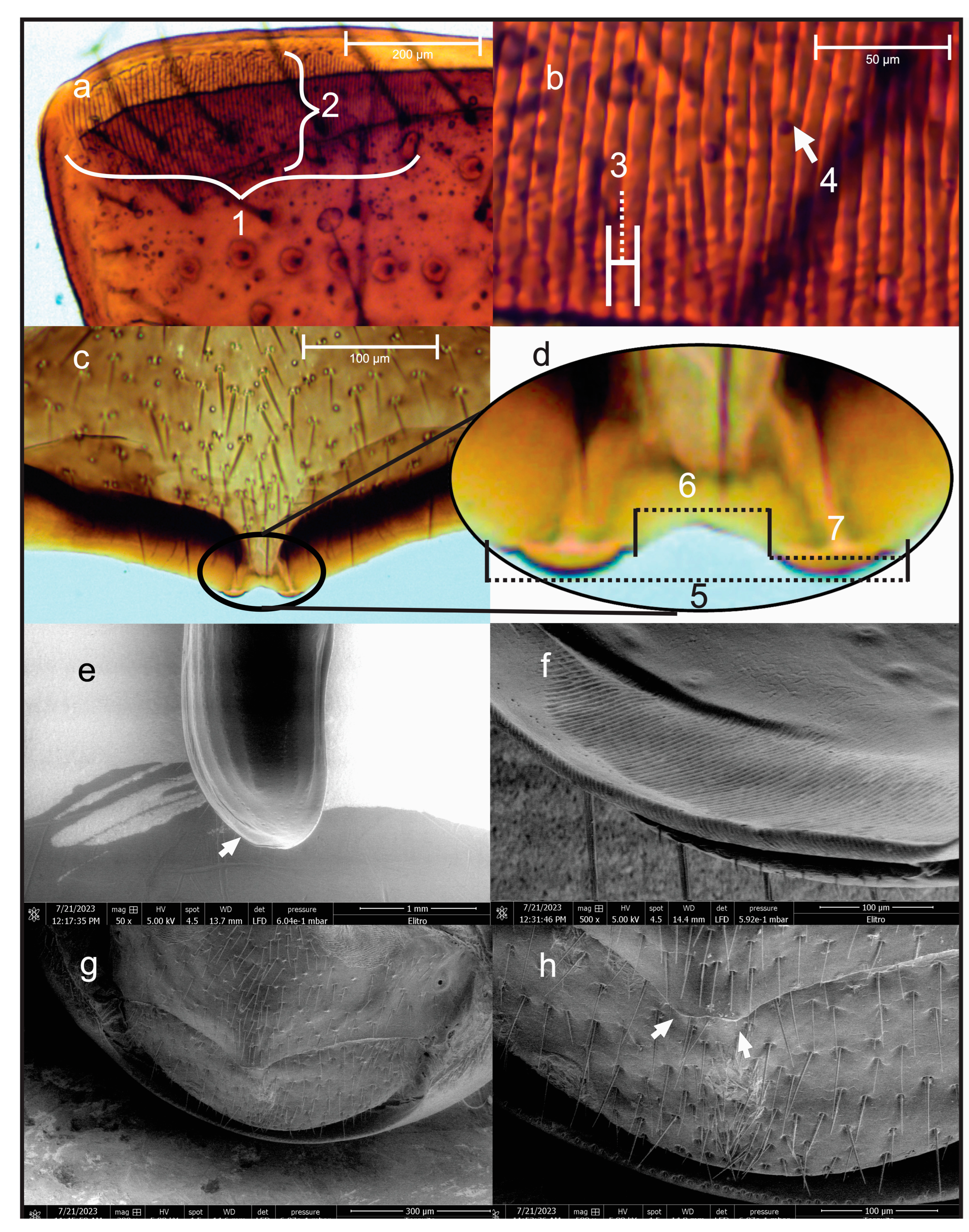

| * Pars Stridens | Width (µm) | Length (µm) | Ridge Width (µm) |
| Range | 170–260 | 550–690 | 6–8 |
| Media | 219.71 | 610.85 | 6.71 |
| Standard error | 25.26 | 42.31 | 0.78 |
| * Plectrum | Internal width (µm) | External width (µm) | Lobe width (µm) |
| Range | 26–31 | 57–71 | 15–21 |
| Media | 26.54 | 65.12 | 19.56 |
| Standard error | 3.74 | 4.73 | 2.64 |
| Call Context | Call Type | n | Proportion (%) | Temporal | Spectral | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tooth Strike (No./Call) | Tooth Strike Rate (No./s) | Intertooth Strike Interval (ms) | Call Duration (ms) | Minimum Frequency (kHz) | Maximum Frequency (kHz) | Dominant Frequency (kHz) | ||||||
| Stress | Single | 30 | 0.9 | 41.4 ± 0.7 | 1034.3 ± 26.5 | 0.9 ± 0.03 | 40 ± 1 | 2.1 ± 0.14 | a | 8.6 ± 0.03 | a | 6.5 ± 0.06 |
| Multiple | 13 | 0.1 | 27.6 ± 6.5 | 585.7 ± 75.2 | 1.6 ± 0.23 | 47.2 ± 5.5 | 4.3 ± 0.04 | 8.7 ± 0.08 | 6.7 ± 0.3 | |||
| Female–male | Single | 24 | 0.74 | 23.8 ± 1.8 | 675 ± 20.7 | 1.4 ± 0.04 | 35.3 ± 3.7 | 5.6 ± 0.07 | b | 7.8 ± 0.08 | b | 6.7 ± 0.07 |
| Multiple | 12 | 0.26 | 36.9 ± 1.9 | 532 ± 27.4 | 1.8 ± 0.09 | 69.5 ± 1.3 | 4.3 ± 0.1 | 7.1 ± 0.07 | 6 ± 0.06 | |||
| Male–male remains | Single | 15 | 0.857 | 16.4 ± 1 | 611.7 ± 15.5 | 1.6 ± 0.04 | 26.8 ± 2 | 2.7 ± 0.19 | a | 8.4 ± 0.07 | c | 6.6 ± 0.06 |
| Multiple | 7 | 0.143 | 21.9 ± 0.3 | 549.6 ± 24 | 1.8 ± 0.07 | 40 ± 1.5 | 2.5 ± 0.41 | 8.5 ± 0.26 | 6.2 ± 0.11 | |||
| Male–male withdrawal | Single | 10 | 1 | 24 ± 1.9 | 616.3 ± 24 | 1.6 ± 0.05 | 39 ± 2.8 | 5 ± 0.21 | b | 7.3 ± 0.11 | d | 6.3 ± 0.11 |
| Significance level Paired t–test | ||||||||||||
| Comparison between call type | Stress | t= | 3.32 (p < 0.05) | 4.68 (p < 0.05) | 5.42 (p < 0.05) | 1.84 (p = 0.06) | 3.75 (p < 0.05) | 1.05 (p = 0.2) | 1.04 (p = 0.3) | |||
| Female–male | t= | 3.68 (p < 0.05) | 11.44 (p < 0.05) | 17.63 (p < 0.05) | 15.54 (p < 0.05) | 8.41 (p < 0.05) | 4.83 (p < 0.05) | 5.88 (p = 0.3) | ||||
| Male–male remains | t= | 1.68 (p < 0.05) | 1.66 (p = 0.1) | 1.58 (p < 0.1) | 2.11 (p < 0.05) | 0.44 (p = 0.6) | 0.66 (p = 0.5) | 2.65 (p < 0.05) | ||||
| Significance level one way ANOVA | ||||||||||||
| Comparison between context | Single | F= | 153 (p < 0.05) | 76.4 (p < 0.05) | 78.58 (p < 0.05) | 12.29 (p < 0.05) | 68.26 (p < 0.05) | 37.59 (p < 0.05) | 2.7 (p = 0.06) | |||
| Multiple | F= | 3.4 (p < 0.05) | 17.76 (p < 0.05) | 42.69 (p < 0.05) | 238.3 (p < 0.05) | 19.58 (p < 0.05) | 42.71 (p < 0.05) | 4.1 (p = 0.08) | ||||
| Species | Temporal | Spectral | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tooth Strike (No./Call) | Tooth Strike Rate (No./s) | Intertooth Strike Interval (ms) | Call Duration (ms) | Minimum Frequency (kHz) | Maximum Frequency (kHz) | Dominant Frequency (kHz) | ||||
| Dendroctonus adjunctus | Stress | Single–noted | 41.4 ± 0.7 | 1034.3 ± 26.5 | 0.9 ± 0.03 | 40 ± 1 | 2.1 ± 0.14 | 8.6 ± 0.03 | 6.5 ± 0.06 | Our data |
| Multiple–noted | 27.6 ± 6.5 | 585.7 ± 75.2 | 1.6 ± 0.23 | 47.2 ± 5.5 | 4.3 ± 0.04 | 8.7 ± 0.08 | 6.7 ± 0.3 | |||
| Female–male | Single–noted | 23.8 ± 1.8 | 675 ± 20.7 | 1.4 ± 0.04 | 35.3 ± 3.7 | 5.6 ± 0.07 | 7.8 ± 0.08 | 6.7 ± 0.07 | ||
| Multiple–noted | 36.9 ± 1.9 | 532 ± 27.4 | 1.8 ± 0.09 | 69.5 ± 1.3 | 4.3 ± 0.1 | 7.1 ± 0.07 | 6 ± 0.06 | |||
| Male–male | Single–noted | 16.4 ± 1 | 611.7 ± 15.5 | 1.6 ± 0.04 | 26.8 ± 2 | 2.7 ± 0.19 | 8.4 ± 0.07 | 6.6 ± 0.06 | ||
| Multiple–noted | 21.9 ± 0.3 | 549.6 ± 24 | 1.8 ± 0.07 | 40 ± 1.5 | 2.5 ± 0.41 | 8.5 ± 0.26 | 6.2 ± 0.11 | |||
| Dendroctonus ponderosae | Stress | Single–noted | 17.4 ± 1.8 | 828.5 ± 59.8 | 1.4 ± 0.1 | 21.8 ± 1.7 | ND | ND | 15.6 ± 2.8 | [29] |
| Multiple–noted | 27.9 ± 4.1 | 593.2 ± 89.5 | 2.2 ± 2.8 | 56.3 ± 8.0 | ND | ND | 18.3 ± 5.8 | |||
| Female–male | Single–noted | 21.3 ± 3.5 | 786.0 ± 63.7 | 1.4 ± 0.1 | 30.0 ± 6.8 | ND | ND | 26.0 ± 4.6 | ||
| Multiple–noted | 35.0 ± 3.5 | 433.1 ± 18.8 | 2.6 ± 0.1 | 90.0 ± 6.5 | ND | ND | 21.9 ± 5.9 | |||
| Male–male | Single–noted | 16.9 ± 2.2 | 709.5 ± 40.2 | 2.6 ± 1.0 | 28.8 ± 6.3 | ND | ND | 17.4 ± 1.6 | ||
| Multiple–noted | 22.2 ± 2.2 | 464.0 ± 44.7 | 2.6 ± 0.2 | 56.1 ± 8.5 | ND | ND | 26.4 ± 3.4 | |||
| Dendroctonus approximatus | Stress | Single–noted | 55.1 ± 0.6 | 517.6 ± 23.0 | ND | 108.6 ± 1.2 | ND | ND | 5.2 ± 0.1 | [30] |
| Female–male | Single–noted | 61.7 ± 1.2 | 505.7 ± 12.0 | ND | 124.5 ± 2.7 | ND | ND | 5.6 ± 0.12 | ||
| Dendroctonus adjunctus | Stress | Single–noted | ND | ND | ND | 39.0 ± 12.2 | 2.99 ± 0.13 | 10.3 ± 1.68 | 5.94 ± 1.94 | [16] |
| Dendroctonus brevicomis | Stress | Single–noted | ND | ND | ND | 68.1 ± 27.4 | 3.86 ± 0.74 | 14.3 ± 4.21 | 6.03 ± 1.50 | |
| Dendroctonus frontalis | Stress | Single–noted | ND | ND | ND | 59.9 ± 19.2 | 3.85 ± 0.73 | 18.2 ± 3.84 | 7.99 ± 4.36 | |
| Dendroctonus pseudotsugae | Stress | Single–noted | ND | ND | ND | 36.4 ± 8.8 | 2.86 ± 0.42 | 8.16 ± 2.03 | 4.92 ± 1.25 | |
| Dendroctonus terebrans | Stress | Single–noted | ND | ND | ND | 99.3 ± 21.9 | 6.08 ± 2.23 | 39.2 ± 3.57 | 22.6 ± 6.05 | |
| Dendroctonus terebrans | Stress | Single–noted | 11.3 ± 0.6 | 375 ± 79 | ND | 31 ± 6 | ND | ND | ND | [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerrillo-Mancilla, L.L.; Cano-Ramírez, C.; Zúñiga, G. Acoustic Communication in Dendroctonus adjunctus Blandford (Curculionidae Scolytinae): Description of Calls and Sound Production Mechanism. Insects 2024, 15, 542. https://doi.org/10.3390/insects15070542
Cerrillo-Mancilla LL, Cano-Ramírez C, Zúñiga G. Acoustic Communication in Dendroctonus adjunctus Blandford (Curculionidae Scolytinae): Description of Calls and Sound Production Mechanism. Insects. 2024; 15(7):542. https://doi.org/10.3390/insects15070542
Chicago/Turabian StyleCerrillo-Mancilla, León L., Claudia Cano-Ramírez, and Gerardo Zúñiga. 2024. "Acoustic Communication in Dendroctonus adjunctus Blandford (Curculionidae Scolytinae): Description of Calls and Sound Production Mechanism" Insects 15, no. 7: 542. https://doi.org/10.3390/insects15070542
APA StyleCerrillo-Mancilla, L. L., Cano-Ramírez, C., & Zúñiga, G. (2024). Acoustic Communication in Dendroctonus adjunctus Blandford (Curculionidae Scolytinae): Description of Calls and Sound Production Mechanism. Insects, 15(7), 542. https://doi.org/10.3390/insects15070542






