Influence of Parental Age on Reproductive Potential and Embryogenesis in the Pepper Weevil, Anthonomus eugenii (Cano) (Col.: Curculionidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Location
2.2. Collection of A. eugenii Adults from Infested Fruit
2.3. Effect of Age on Egg Production
2.4. Effect of Age on Embryonic Development, Hatching Rate, and Incubation Period
2.5. Statistical Analyses
3. Results
3.1. Effect of Age on the Egg Production of the Pepper Weevil
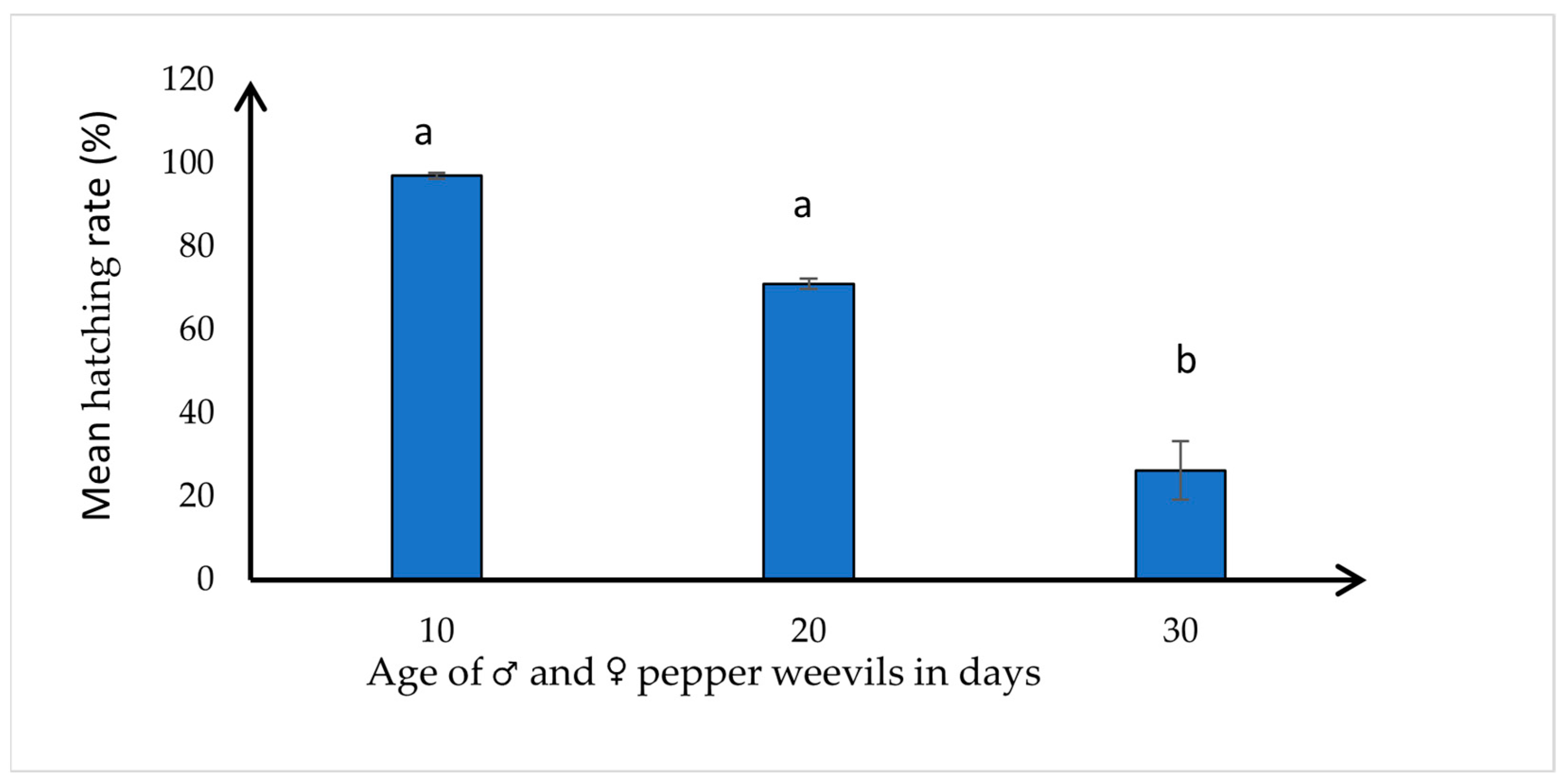
3.2. Effect of Age on the Hatching Rate
3.3. Effect of Age on the Incubation Period (Development Time between Egg to First Instar Larvae)
3.4. Effect of Parental Age on Embryonic Development
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dagnoko, S.; Yaro-Diarisso, N.; Sanago, P.N.; Adetula, O.; Dolo-Nantoume, A.; Gamby-Toure, K.; Traore-Thera, A.; Katile, S.; Diallo-Ba, D. Overview of pepper (Capsicum spp.) breeding in West Africa. Afr. J. Agric. Res. 2013, 8, 1108–1114. [Google Scholar]
- Elmore, J.C.; Davis, A.C.; Campbell, R.E. The pepper weevil. USDA Agric. Tech. Bull. 1934, 447, 1–27. [Google Scholar]
- Riley, D.; Sparks, A.N. The Pepper Weevil and Its Management; AgriLife Extension, L-5069; Texas A&M University: College Station, TX, USA, 1995; Available online: https://hdl.handle.net/1969.1/87688 (accessed on 22 November 2023).
- Toapanta, M. Population Ecology, Life History and Biological Control of the Pepper Weevil, Anthonomus eugenii Cano (Coleoptera: Curculionidae). Doctoral Dissertation, University of Florida, Gainesville, FL, USA, 2001. Available online: https://www.proquest.com/dissertations-theses/population-ecology-life-history-biological/docview/304694207/se-2 (accessed on 22 November 2023).
- Capinera, J.L. Pepper weevil, Anthonomus eugenii Cano (Coleoptera: Curculionidae). Featured Creatures. University of Florida, Department of Entomology and Nematology. 2002. Available online: http://entnemdept.ufl.edu/creatures/veg/pepper_weevil.htm (accessed on 22 November 2023).
- Toapanta, M.A.; Schuster, D.J.; Stansly, P.A. Development and Life History of Anthonomus eugenii (Coleoptera: Curculionidae) at Constant Temperatures. Environ. Entomol. 2005, 34, 999–1008. [Google Scholar] [CrossRef]
- Rodríguez-Leyva, E. Life History of Triaspis eugenii Wharton and Lopez-Martinez (Hymenoptera: Braconidae) and Evaluation of Its Potential for Biological Control of Pepper Weevil Anthonomus eugenii Cano (Coleoptera: Curculionidae). Doctoral Dissertation, University of Florida, Gainesville, FL, USA, 2006. Available online: https://www.proquest.com/dissertations-theses/life-history-i-triaspis-eugenii-wharton-lopez/docview/305329657/se-2 (accessed on 23 December 2023).
- U. S. Department of Agriculture (USDA). Pepper weevils: New trap aids IPM. In Ag Research Magazine; USDA: Washington, DC, USA, 1995. [Google Scholar]
- Carey, J.R.; Roach, D.A. Biodemography: An Introduction to Concepts and Methods; Princeton University Press: Princeton, NJ, USA, 2020. [Google Scholar]
- Leather, S.R. Factors Affecting Fecundity, Fertility, Oviposition, and Larviposition in Insects. In Insect Reproduction; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Shelly, T.E. Fecundity of female oriental fruit flies (Diptera: Tephritidae): Effects of methyl eugenol-fed and multiple mates. Ann. Entomol. Soc. Am. 2000, 93, 559–564. [Google Scholar] [CrossRef]
- Charlesworth, B. Evolution in Age-Structured Populations; Cambridge University Press: New York, NY, USA, 1994. [Google Scholar]
- Cole, L.C. Sketches of general and comparative demography. In Cold Spring Harbor Symposia on Quantitative Biology; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1957; pp. 1–15. [Google Scholar]
- Iliadi, K.G.; Boulianne, G.L. Age-related behavioral changes in Drosophila. Ann. N. Y. Acad. Sci. 2010, 1197, 9–18. [Google Scholar] [CrossRef]
- Johnson, S.L.; Gemmell, N.J. Are old males still good males and can females tell the difference? Do female guppies distinguish between old and young males? Ethology 2012, 34, 609–619. [Google Scholar] [CrossRef]
- Leather, S.R. Insect Reproduction; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Túler, A.C.; Silva-Torres, C.; Torres, J.; Moraes, R.; Rodrigues, A. Mating system, age, and reproductive performance in Tenuisvalvae notata, a long-lived ladybird beetle. Bull. Entomol. Res. 2018, 108, 616–624. [Google Scholar] [CrossRef]
- Parsons, P.A. Parental age and the offspring. Q. Rev. Biol. 1964, 39, 258–275. [Google Scholar] [CrossRef]
- Mousseau, T.A.; Dingle, H. Maternal effects in insect life histories. Annu. Rev. Entomol. 1991, 36, 511–534. [Google Scholar] [CrossRef]
- Opit, G.P.; Throne, J.E. Influence of Maternal Age on the Fitness of Progeny in the Rice Weevil, Sitophilus oryzae (Coleoptera: Curculionidae). Environ. Entomol. 2007, 36, 83–89. [Google Scholar] [CrossRef]
- David, J.; Cohet, Y.; Fouillet, P. The variability between individuals as a measure of senescence: A study of the number of eggs laid and the percentage of hatched eggs in the case of Drosophila melanogaster. Exp. Gerontol. 1975, 10, 17–25. [Google Scholar] [CrossRef]
- Hercus, M.J.; Hoffmann, A.A. Maternal and grandmaternal age influence offspring fitness in Drosophila. Proc. R. Soc. Lond. B Biol. Sci. 2000, 267, 2105–2110. [Google Scholar] [CrossRef]
- Kern, A.D.; Jones, C.D.; Begun, D.J. Decline in offspring viability as a manifestation of aging in Drosophila melanogaster. Evolution 2001, 55, 1822–1831. [Google Scholar] [CrossRef]
- Priest, N.K.; Mackowiak, B.; Promislow, D.E. The role of parental age effects on the evolution of aging. Evolution 2002, 56, 927–935. [Google Scholar] [CrossRef]
- Lansing, A.I. A transmissible, cumulative, and reversible factor in aging. J. Gerontol. 1947, 2, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.; Fiore, C. Further studies on the relationship between parental age and the life cycle of the mealworm, Tenebrio molitor. Ann. Entomol. Soc. Am. 1960, 53, 595–600. [Google Scholar] [CrossRef]
- Raychaudhuri, A.; Butz, A. Aging I. Effects of parental age on the life cycle of Tribolium confusum (Coleoptera, Tenehrionidae). Ann. Entomol. Soc. Am. 1965, 58, 535–540. [Google Scholar] [CrossRef]
- Fox, C.M.; Bush, M.L.; Wallis, W.G. Maternal age affects offspring lifespan in the seed beetle Callosobruchus maculates. Funct. Ecol. 2003, 17, 811–820. [Google Scholar] [CrossRef]
- Hale, J.M.; Elgar, M.A.; Jones, T.M. Sperm quantity explains age-related variation in fertilization success in the hide beetle. Ethology 2008, 114, 797–807. [Google Scholar] [CrossRef]
- Ponlawat, A.; Harrington, L.C. Age and body size influence male sperm capacity of the dengue vector Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2007, 44, 422–426. [Google Scholar] [CrossRef]
- Seal, D.R.; Martin, C.G. Laboratory rearing of pepper weevils (Coleoptera: Curculionidae) using artificial leaf balls and a boll weevil diet. J. Entomol. Sci. 2017, 52, 395–410. [Google Scholar] [CrossRef]
- SAS Institute. SAS/STAT 9.4 User’s Guide; SAS Institute: Cary, NC, USA, 2013. [Google Scholar]
- Tasnin, M.S.; Kay, B.J.; Peek, T.; Merkel, K.; Clarke, A.R. Age-related changes in the reproductive potential of the Queensland fruit fly. J. Insect Physiol. 2022, 131, 104245. [Google Scholar] [CrossRef] [PubMed]
- Mishra, G.; Omkar. Influence of parental age on reproductive performance of an aphidophagous ladybird, Propylea dissecta (Mulsant). J. Appl. Entomol. 2004, 128, 605–609. [Google Scholar] [CrossRef]
- Rahman, M.A.; Zohora, F.T.; Mohanta, M.K.; Saha, A.K.; Rahman, M.H. Maternal age induced changes in the reproductive traits of the bruchid beetle, Callosobruchus chinensis (L.) (Coleoptera: Bruchidae). Int. J. Entomol. Res. 2021, 6, 189–195. [Google Scholar]
- Yanagi, S.; Miyatake, T. Effects of maternal age on reproductive traits and fitness components of the offspring in the bruchid beetle, Callosobruchus chinensis (Coleoptera: Bruchidae). Physiol. Entomol. 2002, 27, 261–266. [Google Scholar] [CrossRef]
- Mohaghegh, D.; Clercq, P.D.; Tirry. Functional response of the predators Podisus maculiventris (Say) and Podisus nigrispinus (Dallas) (Het., Pentatomidae) to the beet armyworm, Spodoptera exigua (Hübner) (Lep., Noctuidae): Effect of temperature. J. Appl. Entomol. 2001, 125, 31–134. [Google Scholar] [CrossRef]
- Giron, D.; Casas, J. Mothers reduce egg provisioning with age. Ecol. Lett. 2003, 6, 273–277. [Google Scholar] [CrossRef]
- McIntyre, N.E.; Gooding, R.H. Effects of maternal age and egg-density on offspring quality in Musca domestica (Diptera: Muscidae). J. Med. Entomol. 2000, 37, 242–249. [Google Scholar]
- Carlson, K.A.; Harshman, L.G. Extended Longevity Lines of Drosophila melanogaster: Characterization of Oocyte Stages and Ovariole Numbers as a Function of Age and Diet. J. Gerontol. Ser. A 1999, 54, B432–B440. [Google Scholar] [CrossRef]

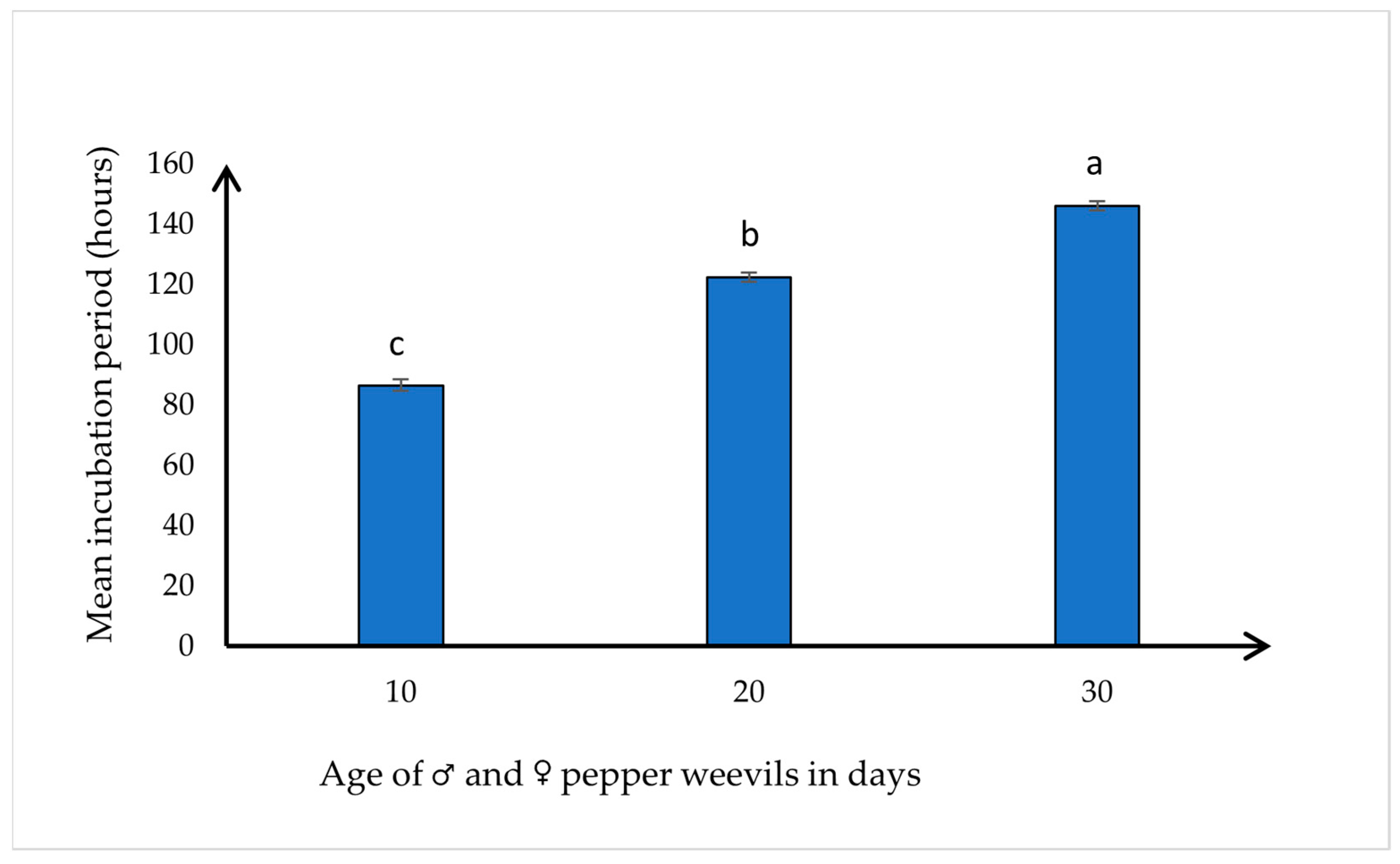
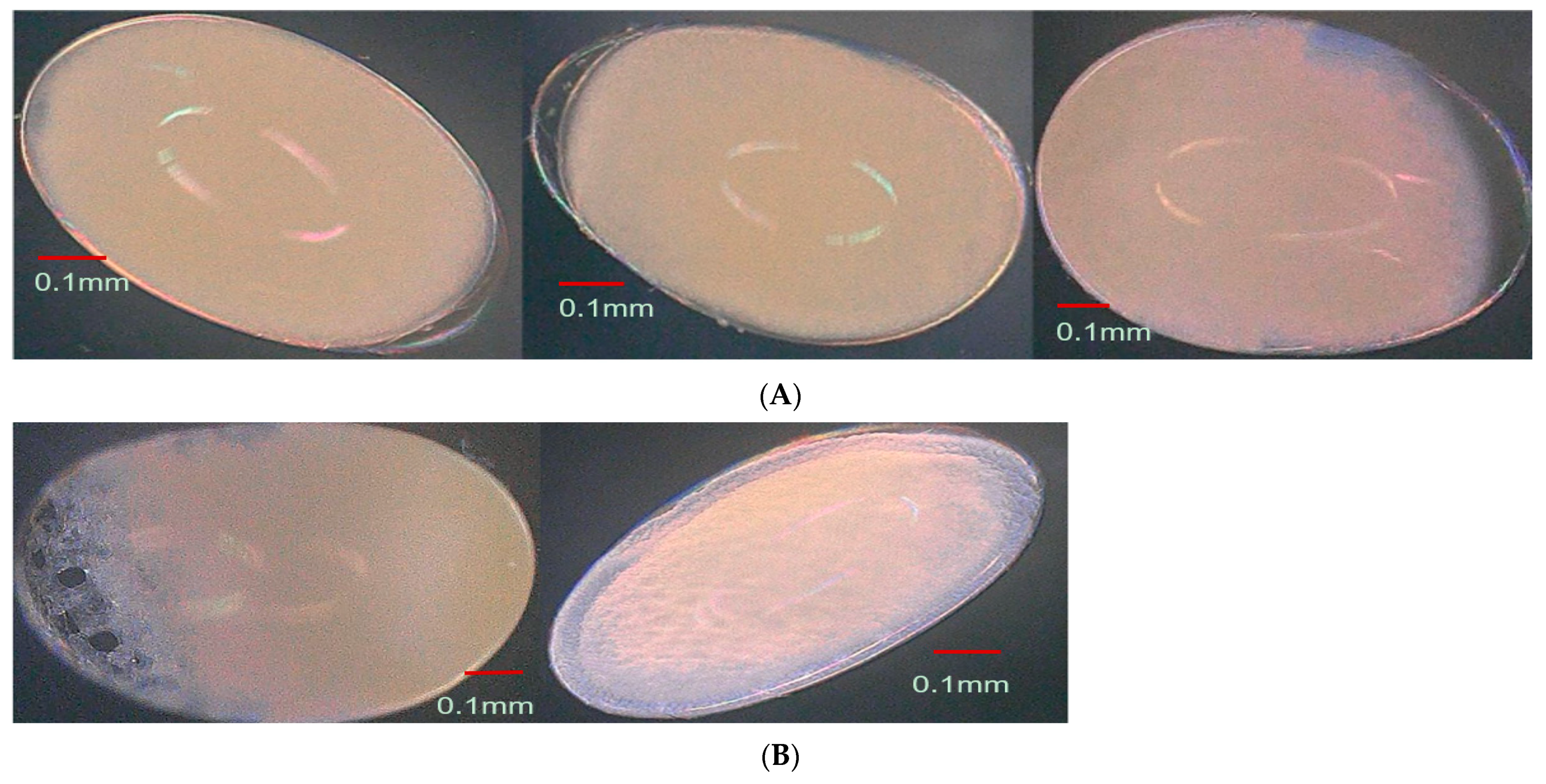
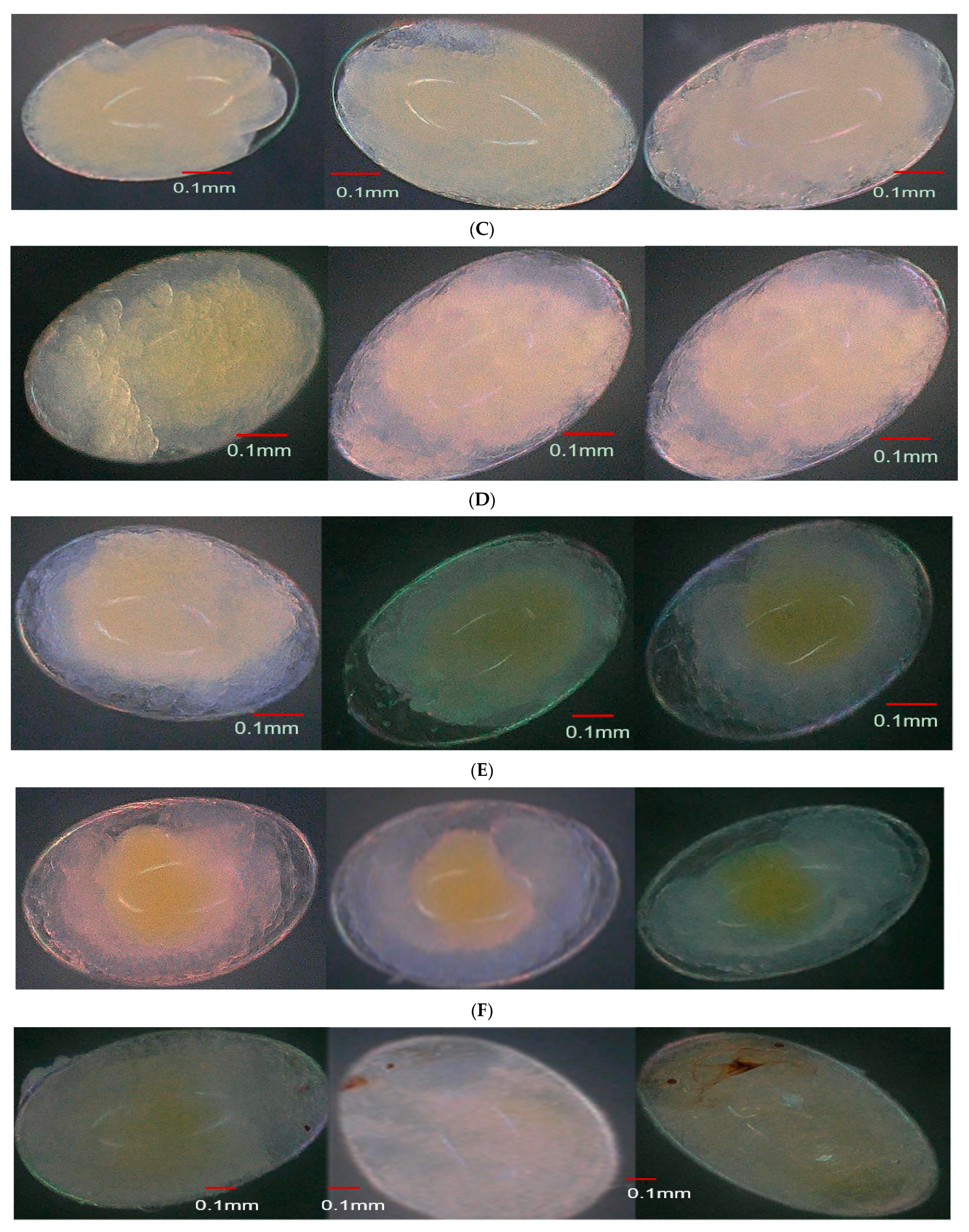
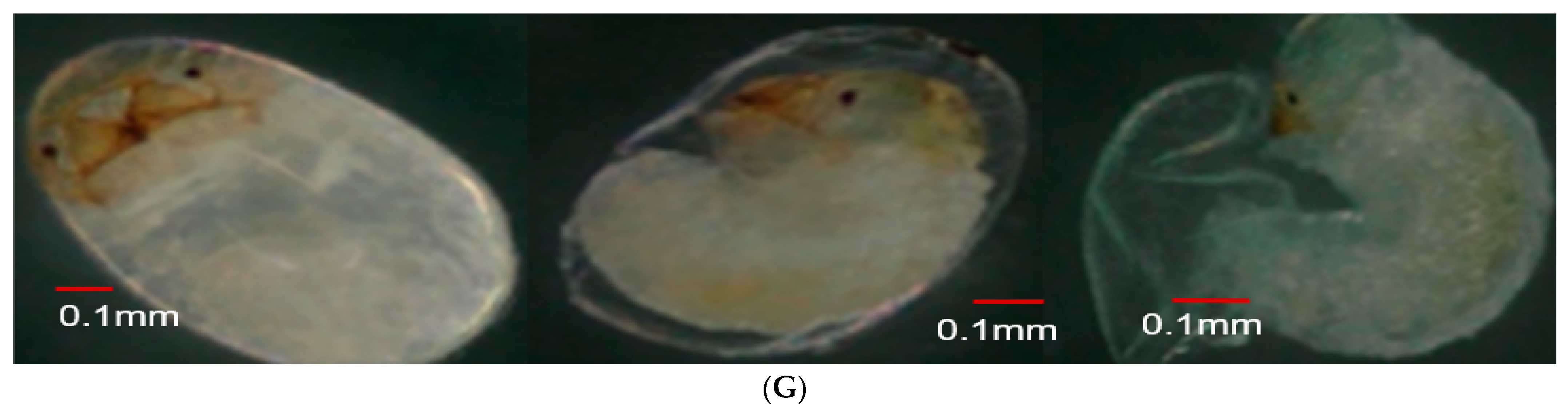

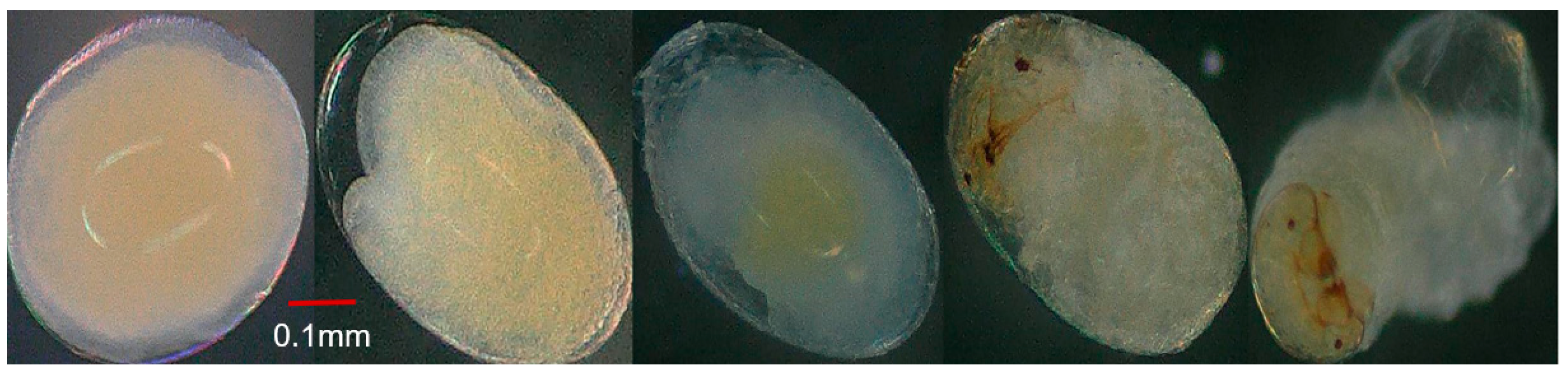
| Stage | Pepper Weevil Age | Statistics (df = 2126) F; p | ||
|---|---|---|---|---|
| 10 Days | 20 Days | 30 Days | ||
| Blastoderm | 9.25 ± 0.76 c | 13.53 ± 1.00 b | 17.91 ± 1.29 a | 126.01; < 0.0001 |
| Germband formation | 4.13 ± 1.14 b | 5.63 ± 1.00 ab | 8.73 ± 0.52 a | 248.81; < 0.0001 |
| Early Gastrulation | 0.93 ± 0.34 c | 2.92 ± 0.33 b | 4.73 ± 0.51 a | 135.04; < 0.0001 |
| Segmentation | 42.00 ± 1.22 c | 50.87 ± 2.08 b | 60.21 ± 1.35 a | 145.17; < 0.0001 |
| Appendage formation | 30.18 ± 1.33 c | 43.50 ± 0.96 b | 47.85 ± 1.00 a | 197.88; < 0.0001 |
| First instar | 2.34 ± 0.62 b | 4.52 ± 0.79 ab | 7.86 ± 0.99 a | 139.38; < 0.0001 |
| Total | 88.3 ± 5.41 c | 120.97 ± 6.163 b | 147.29 ± 5.66 a | 298.50; < 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanchupati, N.M.; Seal, D.R.; Jangra, S.; Schaffer, B.; Liburd, O.E.; Beuzelin, J. Influence of Parental Age on Reproductive Potential and Embryogenesis in the Pepper Weevil, Anthonomus eugenii (Cano) (Col.: Curculionidae). Insects 2024, 15, 562. https://doi.org/10.3390/insects15080562
Kanchupati NM, Seal DR, Jangra S, Schaffer B, Liburd OE, Beuzelin J. Influence of Parental Age on Reproductive Potential and Embryogenesis in the Pepper Weevil, Anthonomus eugenii (Cano) (Col.: Curculionidae). Insects. 2024; 15(8):562. https://doi.org/10.3390/insects15080562
Chicago/Turabian StyleKanchupati, Naga Mani, Dakshina R. Seal, Sumit Jangra, Bruce Schaffer, Oscar E. Liburd, and Julien Beuzelin. 2024. "Influence of Parental Age on Reproductive Potential and Embryogenesis in the Pepper Weevil, Anthonomus eugenii (Cano) (Col.: Curculionidae)" Insects 15, no. 8: 562. https://doi.org/10.3390/insects15080562









