Exploring Biodiversity through the Lens of Knautia arvensis Pollinators: Knautia Pollinator Walks as a Monitoring Method
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
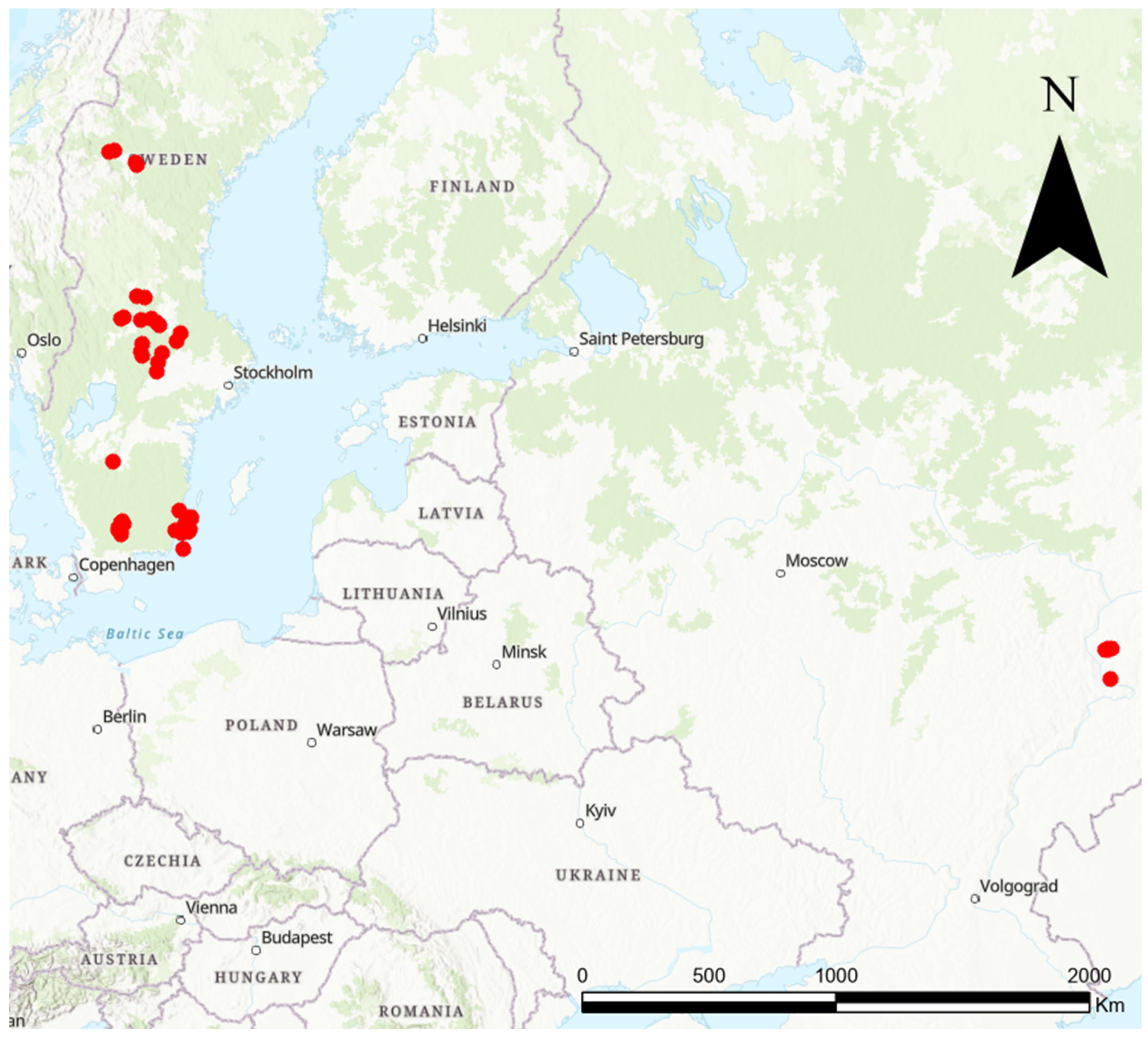
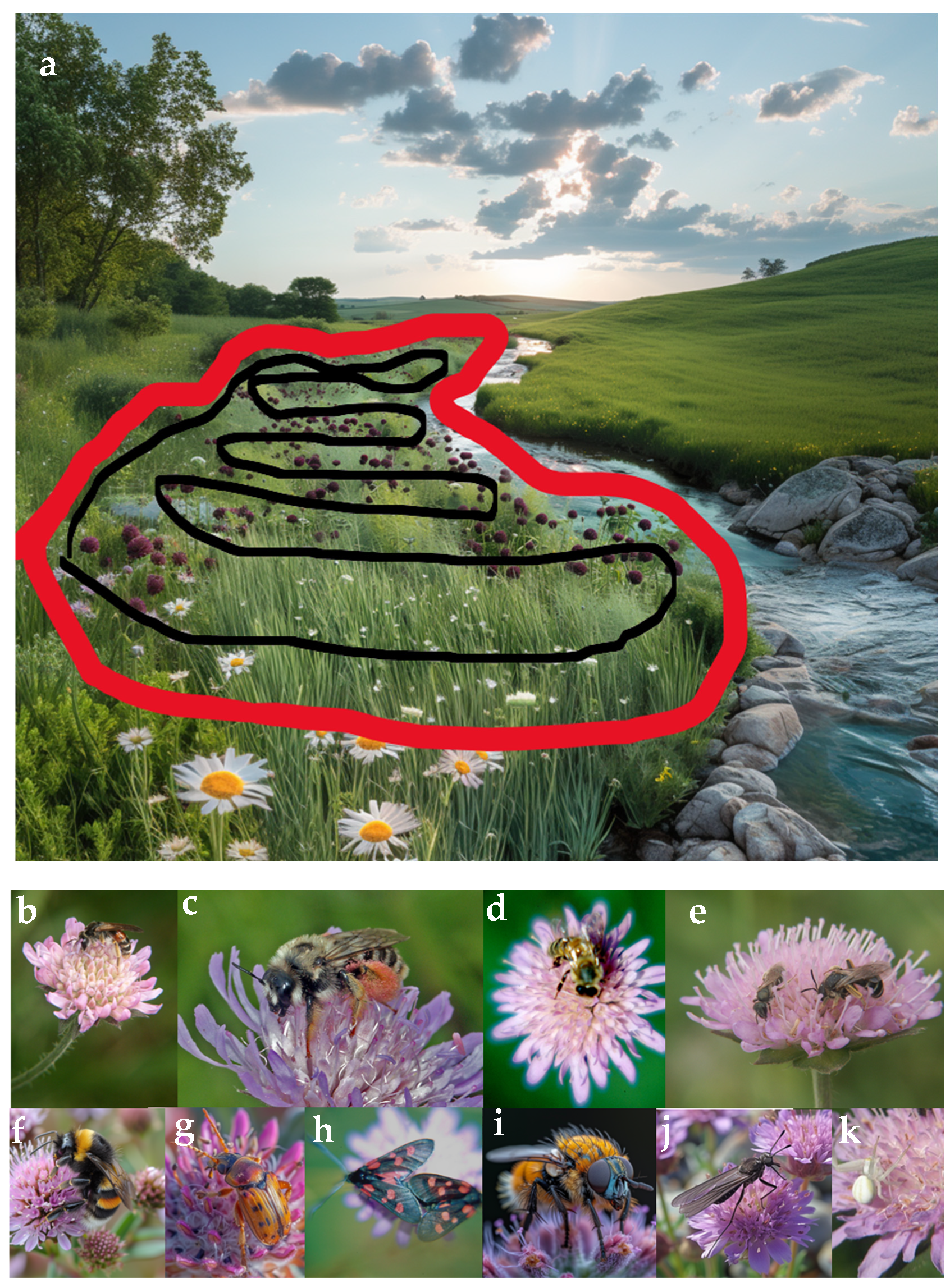
2.1. Study Area and the ‘Knautia Pollinator Walk’
2.2. The Studied Plant Species K. arvensis
2.3. Studied Pollinator Groups
2.4. Datasets
2.5. Land Cover Data Acquisition
2.6. Statistical Analyses
3. Results
3.1. Pollinator Diversity and Prevalence across Sites
3.2. Flower Visitor Species, Taxonomic Group Richness, and Density of Land Cover
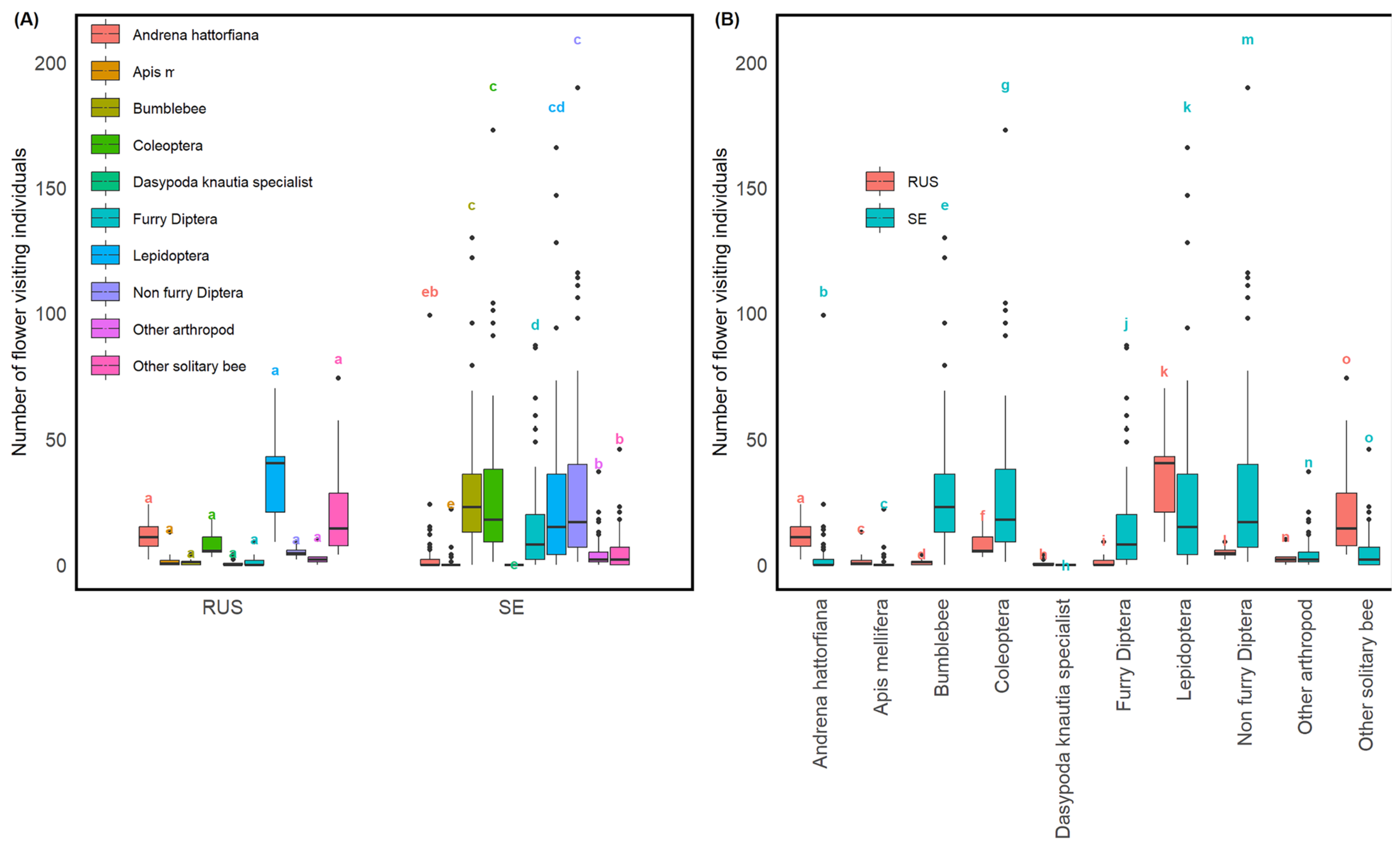
3.3. Contrasting Pollinator Frequencies between Sweden and Russia
4. Discussion
4.1. The Importance of an Easy Method to Monitor Groups, Differences, and Changes
4.2. Habitat Transformations and Biodiversity Indicators
4.3. The Relationship between Land Use and Pollinator Communities Is Complex and Multifaceted
4.4. The Imperative of Longitudinal Monitoring of Flower-Visiting Insect Populations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ceballos, G.; Ehrlich, P.R.; Raven, P.H. Vertebrates on the brink as indicators of biological annihilation and the sixth mass extinction. Proc. Natl. Acad. Sci. USA 2020, 117, 13596–13602. [Google Scholar] [CrossRef] [PubMed]
- Parmesan, C.; Burrows, M.T.; Duarte, C.M.; Poloczanska, E.S.; Richardson, A.J.; Schoeman, D.S.; Singer, M.C. Beyond climate change attribution in conservation and ecological research. Ecol. Lett. 2013, 16, 58–71. [Google Scholar] [CrossRef]
- Dirzo, R.; Young, H.S.; Galetti, M.; Ceballos, G.; Isaac, N.J.B.; Collen, B. Defaunation in the Anthropocene. Science 2014, 345, 401. [Google Scholar] [CrossRef]
- Franzén, M.; Nilsson, S.G. High population variability and source-sink dynamics in a solitary bee species. Ecology 2013, 94, 1400–1408. [Google Scholar] [CrossRef]
- Seibold, S.; Gossner, M.M.; Simons, N.K.; Blüthgen, N.; Müller, J.; Ambarlı, D.; Ammer, C.; Bauhus, J.; Fischer, M.; Habel, J.C. Arthropod decline in grasslands and forests is associated with landscape-level drivers. Nature 2019, 574, 671–674. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; et al. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef]
- Betzholtz, P.-E.; Forsman, A.; Franzén, M. Increased Abundance Coincides with Range Expansions and Phenology Shifts: A Long-Term Case Study of Two Noctuid Moths in Sweden. Diversity 2023, 15, 1177. [Google Scholar] [CrossRef]
- Hopper, K.R. Risk-spreading and bet-hedging in insect population biology. Annu. Rev. Entomol. 1999, 44, 535–560. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Hothorn, T.; Yuan, Y.; Seibold, S.; Mitesser, O.; Rothacher, J.; Freund, J.; Wild, C.; Wolz, M.; Menzel, A. Weather explains the decline and rise of insect biomass over 34 years. Nature 2023, 628, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Franzén, M.; Francioli, Y.; Sjöberg, G.; Forsman, A. Positive shifts in species richness and abundance of moths over five decades coincide with community-wide phenotypic trait homogenisation. J. Insect Conserv. 2023, 27, 323–333. [Google Scholar] [CrossRef]
- Franzén, M.; Forsman, A.; Karimi, B. Anthropogenic Influence on Moth Populations: A Comparative Study in Southern Sweden. Insects 2023, 14, 702. [Google Scholar] [CrossRef] [PubMed]
- Sunde, J.; Franzén, M.; Betzholtz, P.-E.; Francioli, Y.; Pettersson, L.B.; Pöyry, J.; Ryrholm, N.; Forsman, A. Century-long butterfly range expansions in northern Europe depend on climate, land use and species traits. Commun. Biol. 2023, 6, 601. [Google Scholar] [CrossRef] [PubMed]
- Pöyry, J.; Carvalheiro, L.G.; Heikkinen, R.K.; Kühn, I.; Kuussaari, M.; Schweiger, O.; Valtonen, A.; van Bodegom, P.M.; Franzén, M. The effects of soil eutrophication propagate to higher trophic levels. Glob. Ecol. Biogeogr. 2016, 26, 18–30. [Google Scholar] [CrossRef]
- Valtonen, A.; Hirka, A.; Szőcs, L.; Ayres, M.P.; Roininen, H.; Csóka, G. Long-term species loss and homogenization of moth communities in Central Europe. J. Anim. Ecol. 2017, 86, 730–738. [Google Scholar] [CrossRef]
- Ollerton, J.; Winfree, R.; Tarrant, S. How many flowering plants are pollinated by animals? Oikos 2011, 120, 321–326. [Google Scholar] [CrossRef]
- Klein, A.-M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B Biol. Sci. 2007, 274, 303. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Garibaldi, L.A.; Steffan-Dewenter, I.; Winfree, R.; Aizen, M.A.; Bommarco, R.; Cunningham, S.A.; Kremen, C.; Carvalheiro, L.G.; Harder, L.D.; Afik, O.; et al. Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science 2013, 339, 1608–1611. [Google Scholar] [CrossRef]
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Aizen, M.A.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J.; et al. Safeguarding pollinators and their values to human well-being. Nature 2016, 540, 220–229. [Google Scholar] [CrossRef]
- Prishchepov, A.V.; Radeloff, V.C.; Baumann, M.; Kuemmerle, T.; Müller, D. Effects of institutional changes on land use: Agricultural land abandonment during the transition from state-command to market-driven economies in post-Soviet Eastern Europe. Environ. Res. Lett. 2012, 7, 024021. [Google Scholar] [CrossRef]
- Parreño, M.A.; Alaux, C.; Brunet, J.-L.; Buydens, L.; Filipiak, M.; Henry, M.; Keller, A.; Klein, A.-M.; Kuhlmann, M.; Leroy, C. Critical links between biodiversity and health in wild bee conservation. Trends Ecol. Evol. 2022, 37, 309–321. [Google Scholar] [CrossRef]
- Levenson, H.K.; Metz, B.N.; Tarpy, D.R. Effects of study design parameters on estimates of bee abundance and richness in agroecosystems: A meta-analysis. Ann. Entomol. Soc. Am. 2024, 117, 92–106. [Google Scholar] [CrossRef]
- Klaus, F.; Ayasse, M.; Classen, A.; Dauber, J.; Diekötter, T.; Everaars, J.; Fornoff, F.; Greil, H.; Hendriksma, H.P.; Jütte, T. Improving wild bee monitoring, sampling methods, and conservation. Basic Appl. Ecol. 2024, 75, 2–11. [Google Scholar] [CrossRef]
- Roy, H.E.; Baxter, E.; Saunders, A.; Pocock, M.J. Focal plant observations as a standardised method for pollinator monitoring: Opportunities and limitations for mass participation citizen science. PLoS ONE 2016, 11, e0150794. [Google Scholar]
- Larsson, M. Higher pollinator effectiveness by specialist than generalist flower-visitors of unspecialized Knautia arvensis (Dipsacaceae). Oecologia 2005, 146, 394–403. [Google Scholar] [CrossRef]
- Knuth, P. Handbuch der Blütenbiologie; Engelmann: Leipzig, Germany, 1899. [Google Scholar]
- Larsson, M.; Franzen, M. Critical resource levels of pollen for the declining bee Andrena hattorfiana (Hymenoptera, Andrenidae). Biol. Conserv. 2007, 134, 405–414. [Google Scholar] [CrossRef]
- Nieto, A.; Roberts, S.P.M.; Kemp, J.; Rasmont, P.; Kuhlmann, M.; García Criado, M.; Biesmeijer, J.C.; Bogusch, P.; Dathe, H.H.; De la Rúa, P.; et al. European Red List of Bees; Publication Office of the European Union: Luxembourg, 2014. [Google Scholar]
- Eide, W.; Ahrné, K.; Bjelke, U.; Nordström, S.; Ottosson, E.; Sandström, J.; Sundberg, S. Tillstånd och Trender för arter och Deras Livsmiljöer–Rödlistade Arter i Sverige 2020; SLU Artdatabanken: Uppsala, Sweden, 2020. [Google Scholar]
- Gomez, J.M.; Abdelaziz, M.; Lorite, J.; Jesus Munoz-Pajares, A.; Perfectti, F. Changes in pollinator fauna cause spatial variation in pollen limitation. J. Ecol. 2010, 98, 1243–1252. [Google Scholar] [CrossRef]
- Ashman, T.L.; Knight, T.M.; Streets, J.A.; Amarasekare, P.; Burd, M.; Campbell, D.R.; Dudash, M.R.; Johnston, M.O.; Mazer, S.J.; Mitchell, R.J.; et al. Pollen limitation of plant reproduction: Ecological andevolutionary causes and consequences. Ecology 2004, 85, 2408–2421. [Google Scholar] [CrossRef]
- Franzén, M.; Larsson, M. Seed set differs in relation to pollen and nectar foraging flower visitors in an insect-pollinated herb. Nord. J. Bot. 2009, 27, 274–283. [Google Scholar] [CrossRef]
- Clausen, H.D.; Holbeck, H.B.; Reddersen, J. Factors influencing abundance of butterflies and burnet moths in the uncultivated habitats of an organic farm in Denmark. Biol. Conserv. 2001, 98, 167–178. [Google Scholar] [CrossRef]
- Jennersten, O.; Nilsson, S.G. Insect flower visitation frequency and seed production in relation to patch size of Viscaria vulgaris (Caryophyllaceae). Oikos 1993, 68, 283–292. [Google Scholar] [CrossRef]
- Westrich, P. Die Wildbienen Baden-Württembergs; Eugen Ulmer: Hohenheim, Germany, 1990. [Google Scholar]
- Grime, J.P.; Hodgson, J.G.; Hunt, R. Comparative Plant Ecology; Springer: Amsterdam, The Netherlands, 1988. [Google Scholar]
- Powney, G.D.; Carvell, C.; Edwards, M.; Morris, R.K.A.; Roy, H.E.; Woodcock, B.A.; Isaac, N.J.B. Widespread losses of pollinating insects in Britain. Nat. Commun. 2019, 10, 1018. [Google Scholar] [CrossRef] [PubMed]
- Steele, D.J.; Baldock, K.; Breeze, T.D.; Brown, M.J.; Carvell, C.; Dicks, L.V.; Garratt, M.P.; Norman, H.; Potts, S.G.; Senapathi, D. Management and Drivers of Change of Pollinating Insects and Pollination Services. National Pollinator Strategy: For Bees and Other Pollinators in England, Evidence Statements and Summary of Evidence; Northumbria University: Tyne, UK, 2019. [Google Scholar]
- Mahon, N.; Hodge, S. Evaluating the effects of observation period, floral density, and weather conditions on the consistency and accuracy of timed pollinator counts. J. Pollinat. Ecol. 2022, 32, 124–138. [Google Scholar] [CrossRef]
- Kearns, C.A.; Inouye, D.W.; Waser, N.M. Endangered mutualisms: The conservation of plant-pollinator interactions. Annu. Rev. Ecol. Syst. 1998, 29, 83–112. [Google Scholar] [CrossRef]
- Vange, V. Breeding system and inbreeding depression in the clonal plant species Knautia arvensis (Dipsacaceae): Implications for survival in abandoned grassland. Biol. Conserv. 2002, 108, 59–67. [Google Scholar] [CrossRef]
- Goulson, D. Causes of rarity in bumblebees. Biol. Conserv. 2005, 122, 1–8. [Google Scholar] [CrossRef]
- Franzén, M.; Nilsson, S.G. How can we preserve and restore species richness of pollinating insects on agricultural land? Ecography 2008, 31, 698–708. [Google Scholar] [CrossRef]
- Franzen, M.; Larsson, M.; Nilsson, S.G. Small local population sizes and high habitat patch fidelity in a specialised solitary bee. J. Insect Conserv. 2009, 13, 89–95. [Google Scholar] [CrossRef]
- Larsson, M.; Franzén, M. Estimating the population size of specialized solitary bees. Ecol. Entomol. 2007, 33, 232–238. [Google Scholar] [CrossRef]
- Celary, W. The ground-nesting solitary bee, Dasypoda thoracica Baer, 1853 (Hymenoptera: Apoidea: Melittidae) and its life history. Folia Biol. 2002, 50, 191–198. [Google Scholar]
- Anonymous. Nationella Marktäckedata 2018 Basskikt: Produktbeskrivning [National Land-Cover Data 2018 Basic Layer: Product Description]. Version 2.2; 7 July 2020; Swedish Environmental Protection Agency: Stockholm, Sweden, 2020. Available online: https://www.naturvardsverket.se/4a43ca/contentassets/37e8b38528774982b5840554f02a1f81/produktbeskrivning-nmd-2018-basskikt-v2-2.pdf (accessed on 20 October 2020). (In Swedish)
- Akaike, H. A new look at the statistical model identification. Autom. Control IEEE Trans. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical. R Version 4.3.0; R Core Team: Vienna, Austria, 2023. [Google Scholar]
- Neil, K.; Wu, J.; Bang, C.; Faeth, S. Urbanization affects plant flowering phenology and pollinator community: Effects of water availability and land cover. Ecol. Process. 2014, 3, 17. [Google Scholar] [CrossRef]
- de Souza, G.F.; Ferreira, M.C.; Munhoz, C.B.R. Decrease in species richness and diversity, and shrub encroachment in Cerrado grasslands: A 20 years study. Appl. Veg. Sci. 2022, 25, e12668. [Google Scholar] [CrossRef]
- Ekroos, J.; Kleijn, D.; Batáry, P.; Albrecht, M.; Báldi, A.; Blüthgen, N.; Knop, E.; Kovács-Hostyánszki, A.; Smith, H.G. High land-use intensity in grasslands constrains wild bee species richness in Europe. Biol. Conserv. 2020, 241, 108255. [Google Scholar] [CrossRef]
- Li, P.; Kleijn, D.; Badenhausser, I.; Zaragoza-Trello, C.; Gross, N.; Raemakers, I.; Scheper, J. The relative importance of green infrastructure as refuge habitat for pollinators increases with local land-use intensity. J. Appl. Ecol. 2020, 57, 1494–1503. [Google Scholar] [CrossRef]
- Clough, Y.; Ekroos, J.; Báldi, A.; Batáry, P.; Bommarco, R.; Gross, N.; Holzschuh, A.; Hopfenmüller, S.; Knop, E.; Kuussaari, M. Density of insect-pollinated grassland plants decreases with increasing surrounding land-use intensity. Ecol. Lett. 2014, 17, 1168–1177. [Google Scholar] [CrossRef] [PubMed]
- Herrera, C.M. Variation in mutualisms: The spatiotemporal mosaic of a pollinator assemblage. Biol. J. Linn. Soc. 1988, 35, 95–125. [Google Scholar] [CrossRef]
- Carvalheiro, L.G.; Kunin, W.E.; Keil, P.; Aguirre-Gutiérrez, J.; Ellis, W.N.; Fox, R.; Groom, Q.; Hennekens, S.; Van Landuyt, W.; Maes, D.; et al. Species richness declines and biotic homogenisation have slowed down for NW-European pollinators and plants. Ecol. Lett. 2013, 16, 870–878. [Google Scholar] [CrossRef]
- Westphal, C.; Bommarco, R.; Carre, G.; Lamborn, E.; Morison, N.; Petanidou, T.; Potts, S.G.; Roberts, S.P.; Szentgyoergyi, H.; Tscheulin, T.; et al. Measuring bee diversity in different european habitats and biogeographical regions. Ecol. Monogr. 2008, 78, 653–671. [Google Scholar] [CrossRef]
- Wilson, J.S.; Griswold, T.; Messinger, O.J. Sampling bee communities (Hymenoptera: Apiformes) in a desert landscape: Are pan traps sufficient? J. Kans. Entomol. Soc. 2008, 81, 288–300. [Google Scholar] [CrossRef]
- Dally, T.M. Pollinator Monitoring: Comparing Standardised and Novel Survey Methods; University of Leeds: Leeds, UK, 2019. [Google Scholar]
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef]
- Millard, J.; Outhwaite, C.L.; Kinnersley, R.; Freeman, R.; Gregory, R.D.; Adedoja, O.; Gavini, S.; Kioko, E.; Kuhlmann, M.; Ollerton, J. Global effects of land-use intensity on local pollinator biodiversity. Nat. Commun. 2021, 12, 2902. [Google Scholar] [CrossRef]
- Ferreira, P.A.; Boscolo, D.; Viana, B.F. What do we know about the effects of landscape changes on plant–pollinator interaction networks? Ecol. Indic. 2013, 31, 35–40. [Google Scholar] [CrossRef]
- Kleijn, D.; Winfree, R.; Bartomeus, I.; Carvalheiro, L.G.; Henry, M.; Isaacs, R.; Klein, A.-M.; Kremen, C.; M’gonigle, L.K.; Rader, R. Delivery of crop pollination services is an insufficient argument for wild pollinator conservation. Nat. Commun. 2015, 6, 7414. [Google Scholar] [CrossRef]
- Gill, R.J.; Baldock, K.C.; Brown, M.J.; Cresswell, J.E.; Dicks, L.V.; Fountain, M.T.; Garratt, M.P.; Gough, L.A.; Heard, M.S.; Holland, J.M. Protecting an ecosystem service: Approaches to understanding and mitigating threats to wild insect pollinators. In Advances in Ecological Research; Elsevier: Amsterdam, The Netherlands, 2016; Volume 54, pp. 135–206. [Google Scholar]
- LeBuhn, G.; Luna, J.V. Pollinator decline: What do we know about the drivers of solitary bee declines? Curr. Opin. Insect Sci. 2021, 46, 106–111. [Google Scholar] [CrossRef]
- Franzén, M.; Ranius, T. Habitat associations and occupancy patterns of burnet moths (Zygaenidae) in semi-natural pastures in Sweden. Entomol. Fenn. 2004, 15, 91–101. [Google Scholar] [CrossRef]
- Jennersten, O.; Kwak, M.M. Competition for bumblebee visitation between Melampyrum pratense and Viscaria vulgaris with healthy and Ustilago infected flowers. Oecologia 1991, 86, 88–98. [Google Scholar] [CrossRef]
- Goulson, D.; Lye, G.C.; Darvill, B. Decline and conservation of bumble bees. Annu. Rev. Entomol. 2008, 53, 191–208. [Google Scholar] [CrossRef]
- Winfree, R. The conservation and restoration of wild bees. Ann. N. Y. Acad. Sci. 2010, 1195, 169–197. [Google Scholar] [CrossRef]
- Wratten, S.D.; Gillespie, M.; Decourtye, A.; Mader, E.; Desneux, N. Pollinator habitat enhancement: Benefits to other ecosystem services. Agric. Ecosyst. Environ. 2012, 159, 112–122. [Google Scholar] [CrossRef]
- Batáry, P.; Báldi, A.; Szél, G.; Podlussány, A.; Rozner, I.; Erdős, S. Responses of grassland specialist and generalist beetles to management and landscape complexity. Divers. Distrib. 2007, 13, 196–202. [Google Scholar] [CrossRef]
- Johansson, V.; Kindvall, O.; Askling, J.; Franzén, M. Extreme weather affects colonization–extinction dynamics and the persistence of a threatened butterfly. J. Appl. Ecol. 2020, 57, 1068–1077. [Google Scholar] [CrossRef]
- Johansson, V.; Kindvall, O.; Askling, J.; Säwenfalk, D.S.; Norman, H.; Franzén, M. Quick recovery of a threatened butterfly in well-connected patches following an extreme drought. Insect Conserv. Divers. 2022, 15, 572–582. [Google Scholar] [CrossRef]
- Ståhl, G.; Allard, A.; Esseen, P.-A.; Glimskär, A.; Ringvall, A.; Svensson, J.; Sundquist, S.; Christensen, P.; Torell, Å.G.; Högström, M. National Inventory of Landscapes in Sweden (NILS)—Scope, design, and experiences from establishing a multiscale biodiversity monitoring system. Environ. Monit. Assess. 2011, 173, 579–595. [Google Scholar] [CrossRef]
- Brown, A.; Hedenås, H.; Holm, E.; Lind, T.; Richards, A.E.; Prober, S.M.; Schmidt, B. Designing and adapting biodiversity monitoring schemes. In Monitoring Biodiversity; Routledge: London, UK, 2023; pp. 275–304. [Google Scholar]
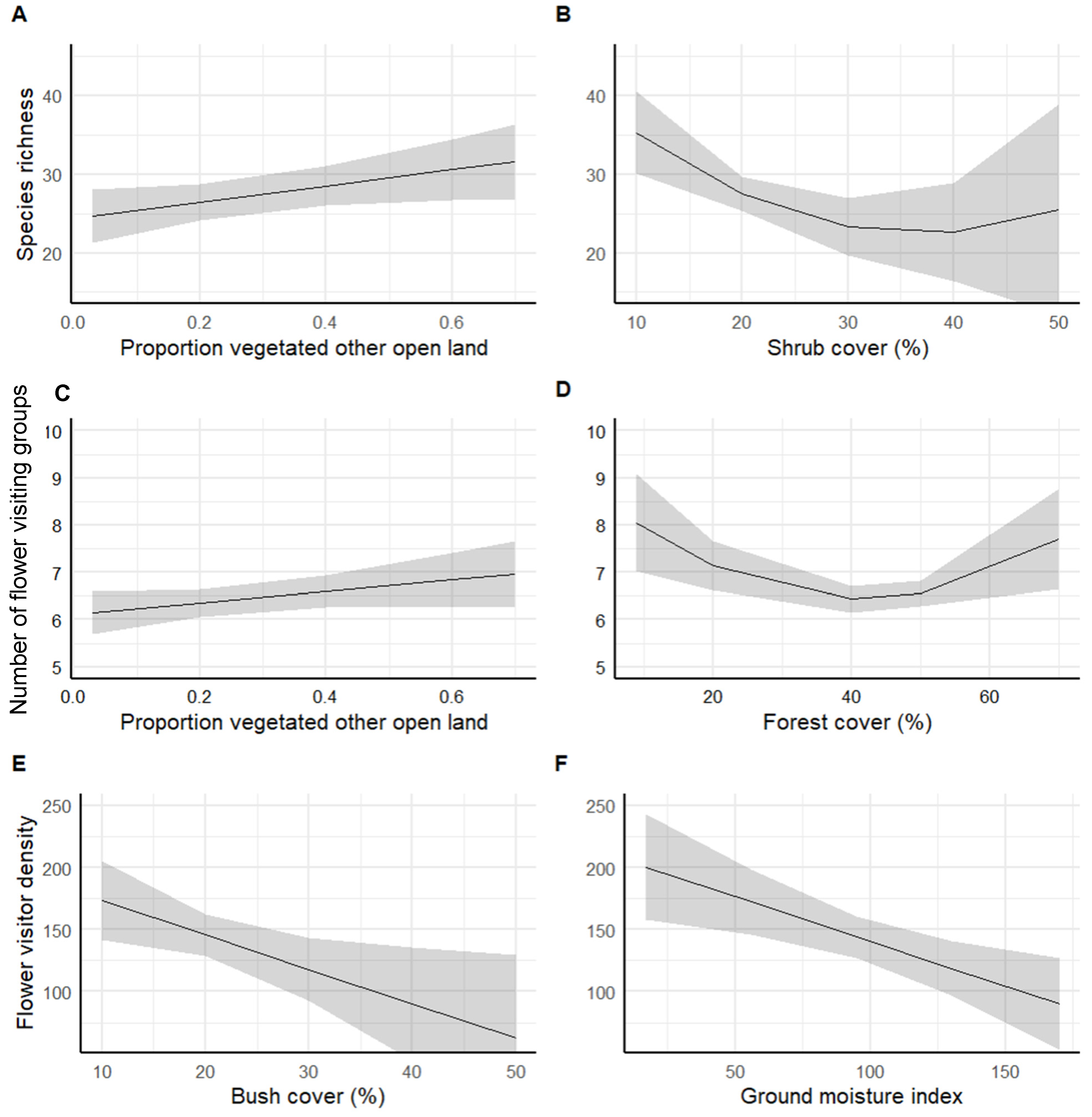
| Model | Predictor | Estimate | Std. Error | t Value | p-Value |
|---|---|---|---|---|---|
| Species richness | (Intercept) | 25.231 | 1.721 | 14.658 | <0.001 |
| Vegetated other open land | 10.382 | 5.034 | 2.062 | 0.045 | |
| Shrub cover | −18.076 | 6.567 | −2.753 | 0.008 | |
| Shrub cover2 | 10.633 | 6.51 | 1.633 | 0.10972 | |
| Morphogroup richness | (Intercept) | 6.3602 | 0.2276 | 27.939 | <0.001 |
| Vegetated other open land | 1.1971 | 0.7567 | 1.582 | 0.117 | |
| Forest cover | −0.8701 | 1.0885 | −0.799 | 0.426 | |
| Forest cover2 | 2.7907 | 0.9677 | 2.884 | 0.005 | |
| Flower visitor density | (Intercept) | 272.9613 | 32.2213 | 8.471 | <0.001 |
| Shrub cover | −2.7732 | 1.1654 | −2.38 | 0.019 | |
| Ground moisture index | −0.7205 | 0.2368 | −3.042 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franzén, M.; Stenmark, M. Exploring Biodiversity through the Lens of Knautia arvensis Pollinators: Knautia Pollinator Walks as a Monitoring Method. Insects 2024, 15, 563. https://doi.org/10.3390/insects15080563
Franzén M, Stenmark M. Exploring Biodiversity through the Lens of Knautia arvensis Pollinators: Knautia Pollinator Walks as a Monitoring Method. Insects. 2024; 15(8):563. https://doi.org/10.3390/insects15080563
Chicago/Turabian StyleFranzén, Markus, and Magnus Stenmark. 2024. "Exploring Biodiversity through the Lens of Knautia arvensis Pollinators: Knautia Pollinator Walks as a Monitoring Method" Insects 15, no. 8: 563. https://doi.org/10.3390/insects15080563
APA StyleFranzén, M., & Stenmark, M. (2024). Exploring Biodiversity through the Lens of Knautia arvensis Pollinators: Knautia Pollinator Walks as a Monitoring Method. Insects, 15(8), 563. https://doi.org/10.3390/insects15080563





