Predicting the Current and Future Distribution of Monolepta signata (Coleoptera: Chrysomelidae) Based on the Maximum Entropy Model
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
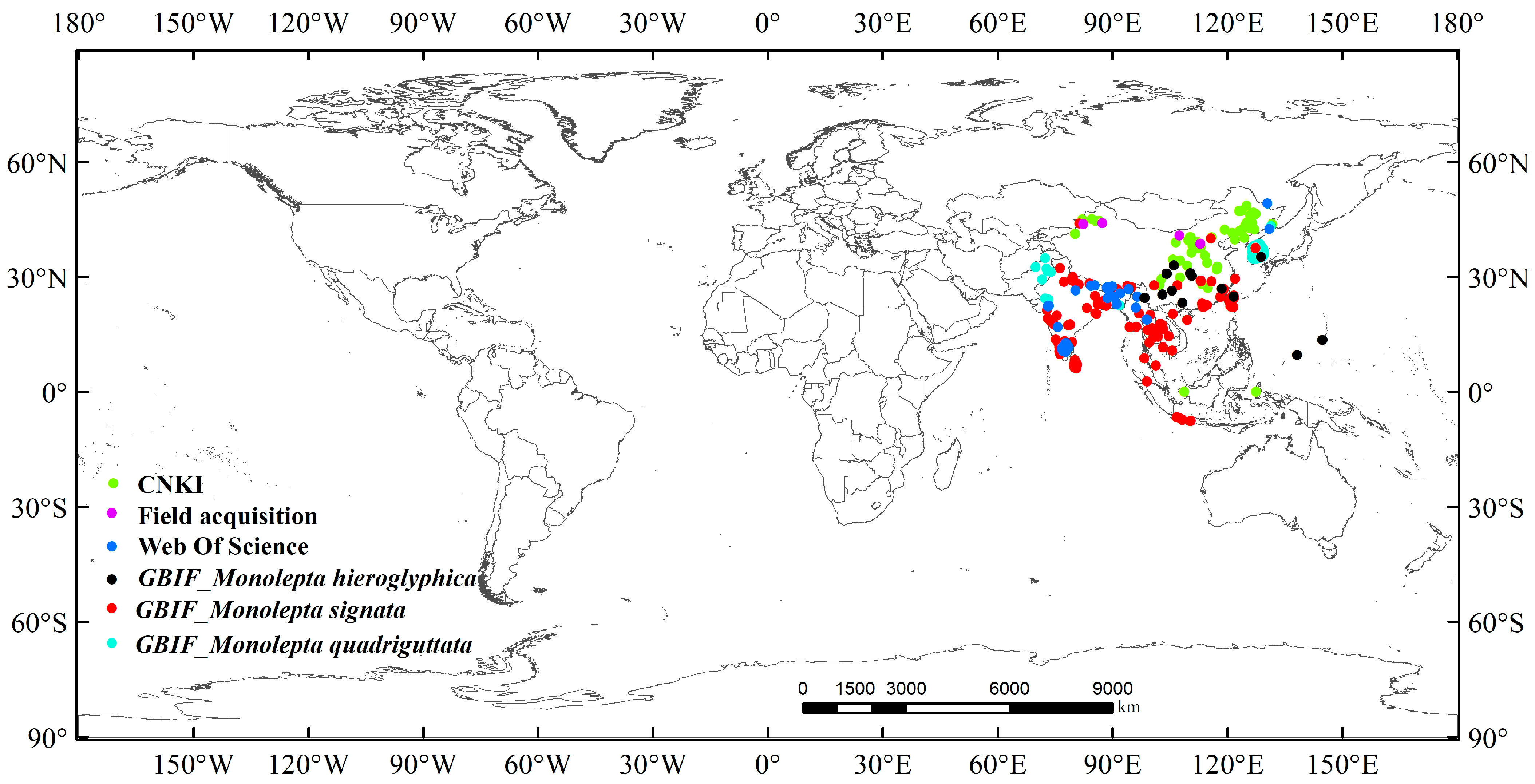
2.1. Collection and Screening of Sample Data
2.2. Environmental Variables
2.3. Model Establishment and Optimization
2.4. Suitable Area Prediction
2.5. Accuracy of the Prediction Results of the MaxEnt Model
3. Results
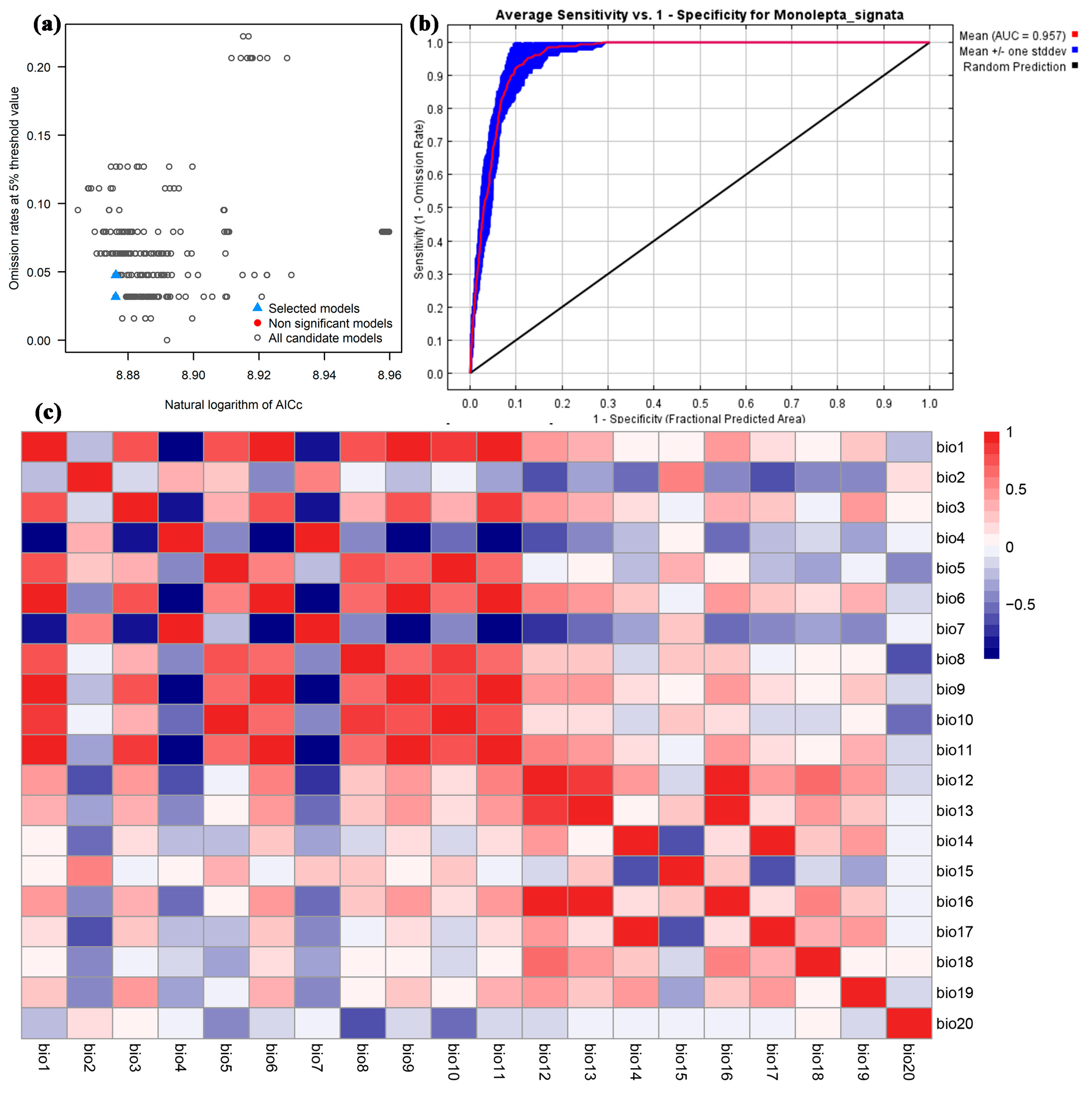
3.1. Modeling Performance
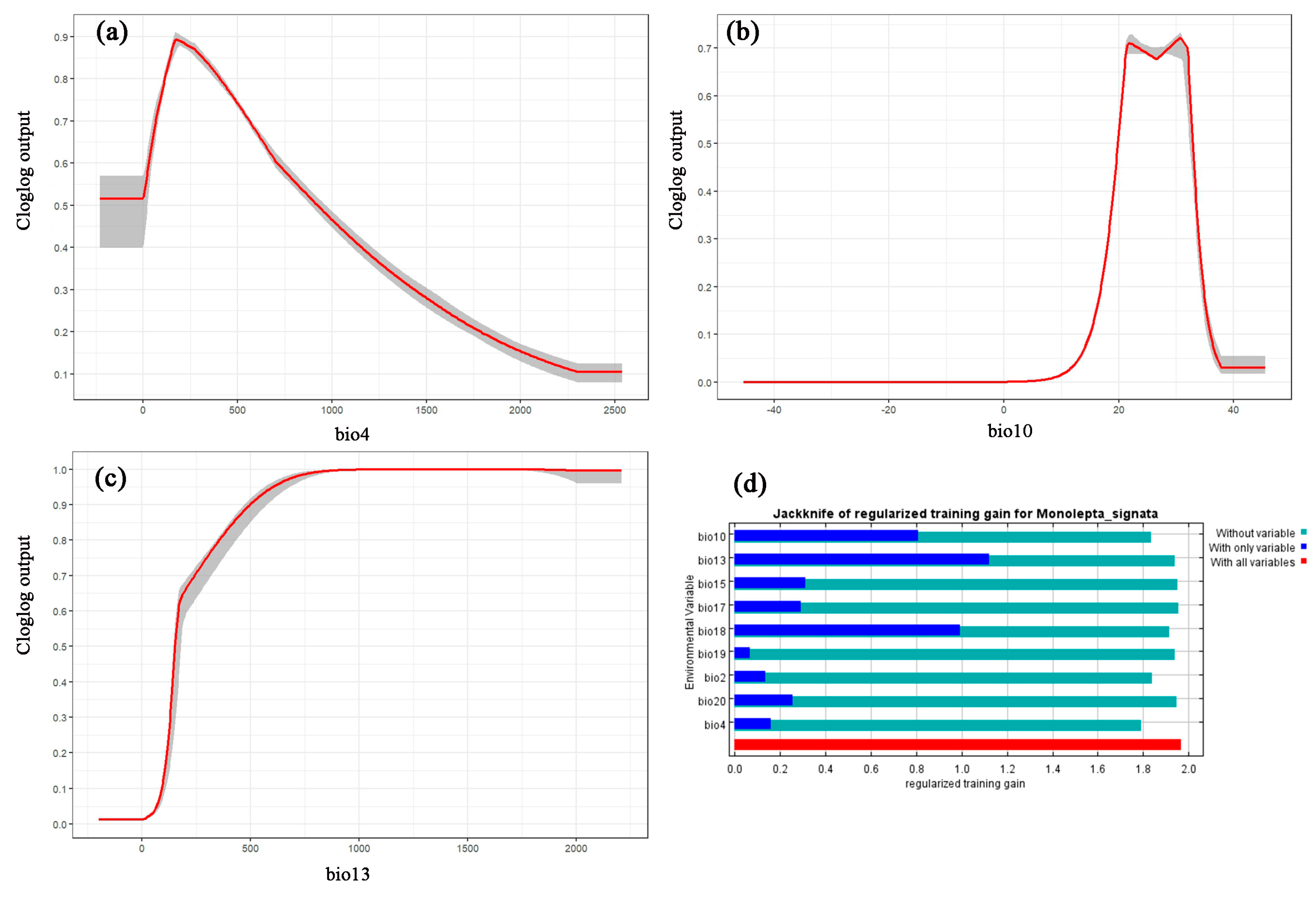
3.2. Contribution Analysis of Environmental Variables
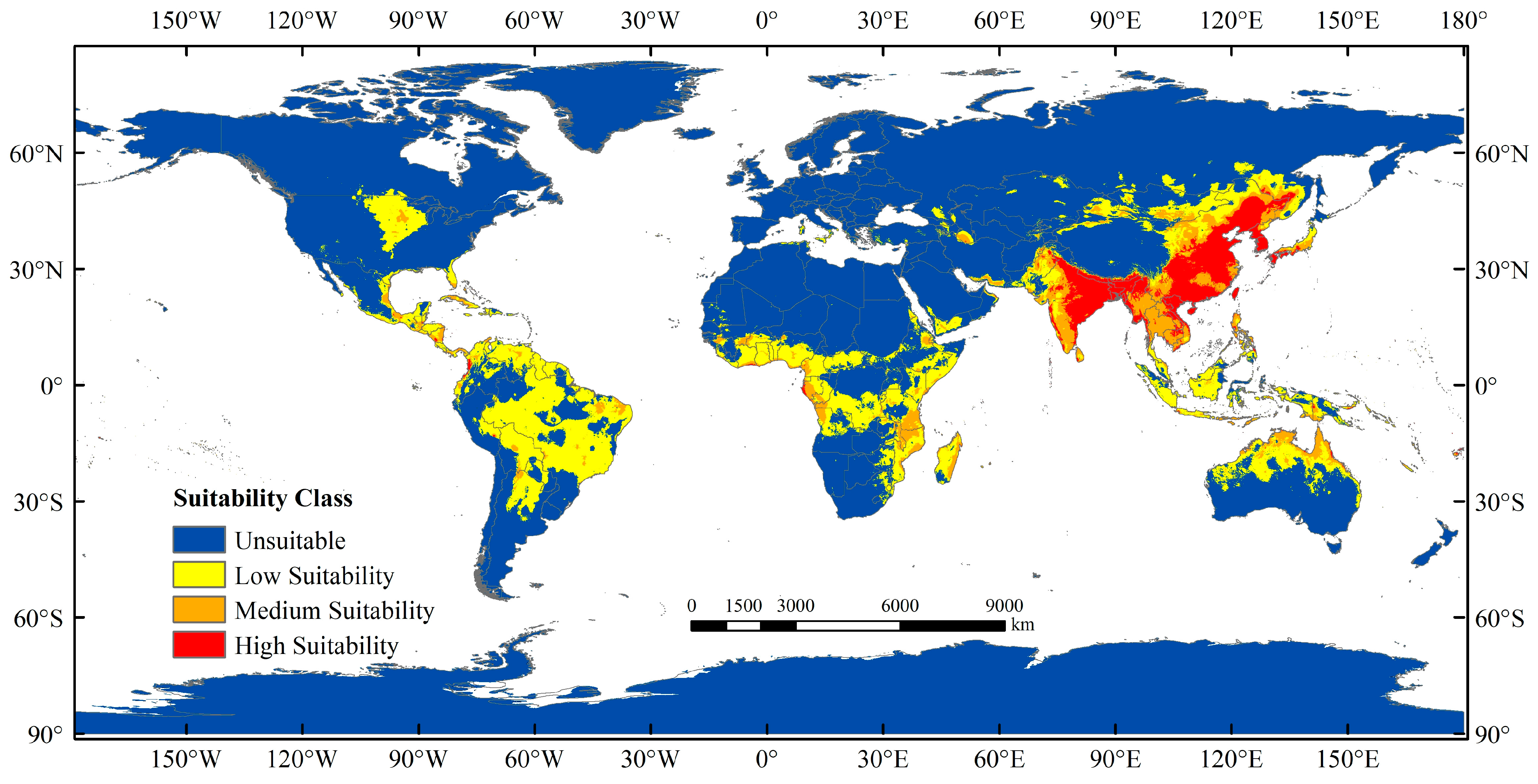
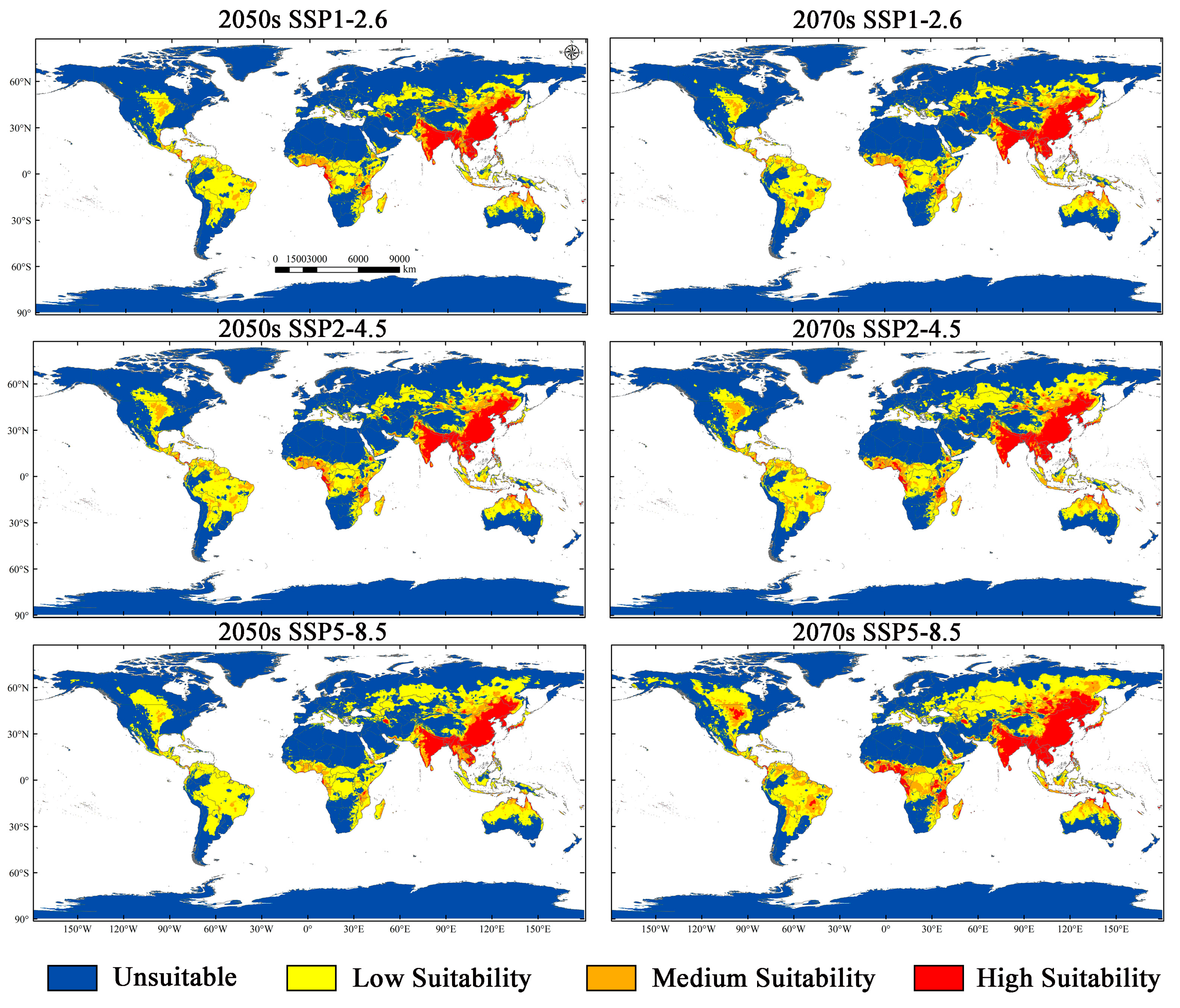
3.3. Current Potentially Suitable Region
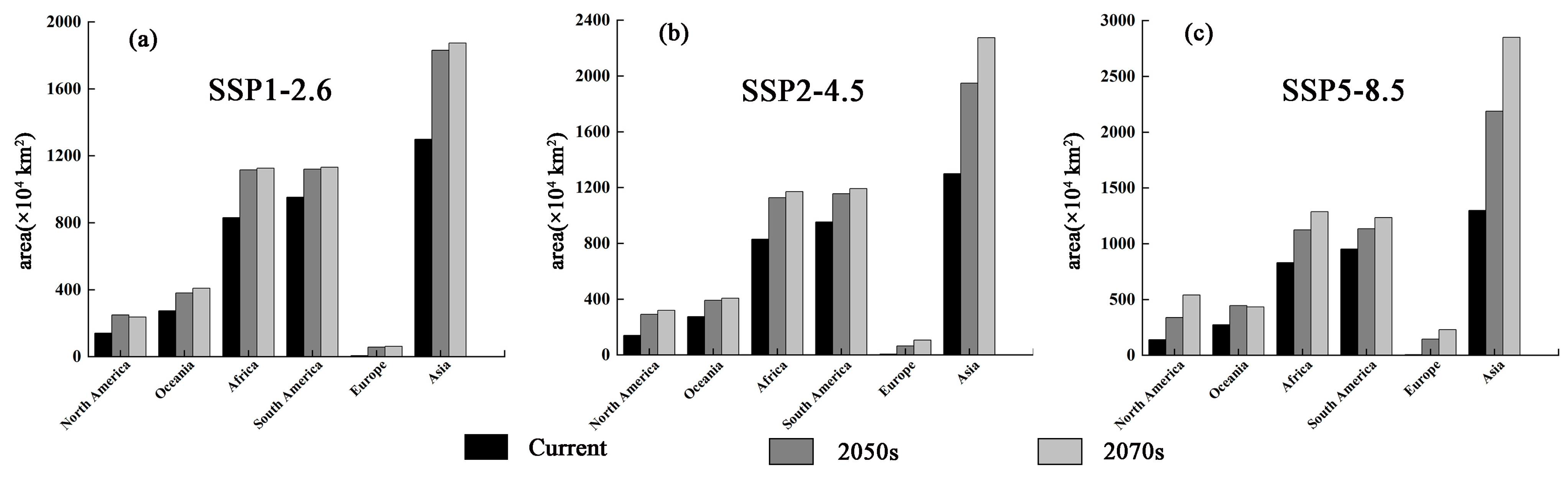
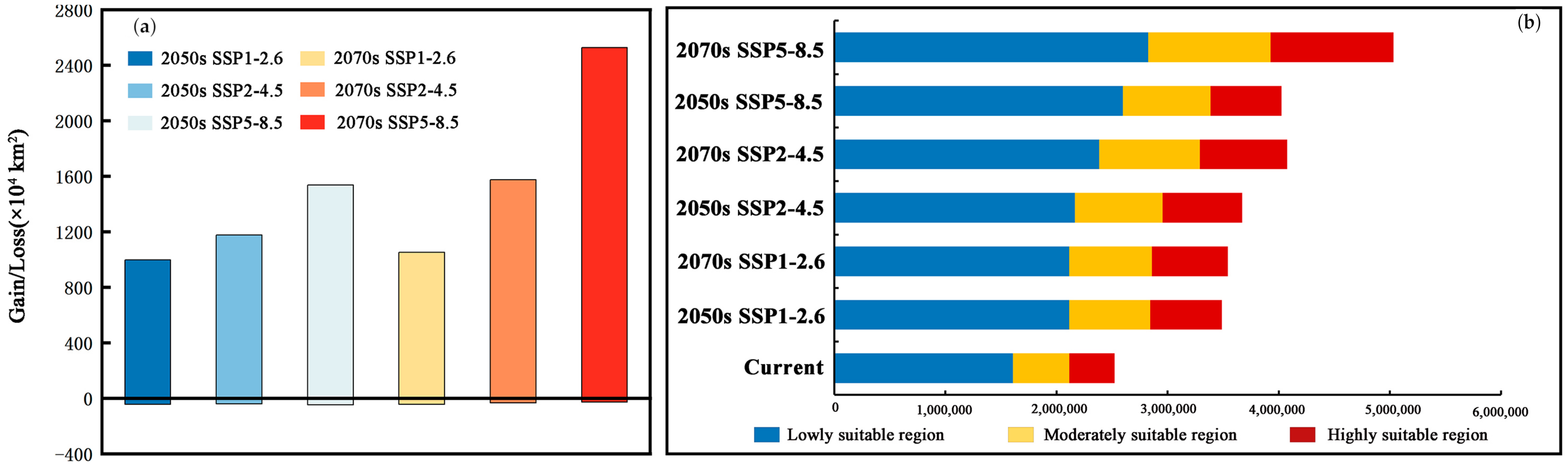
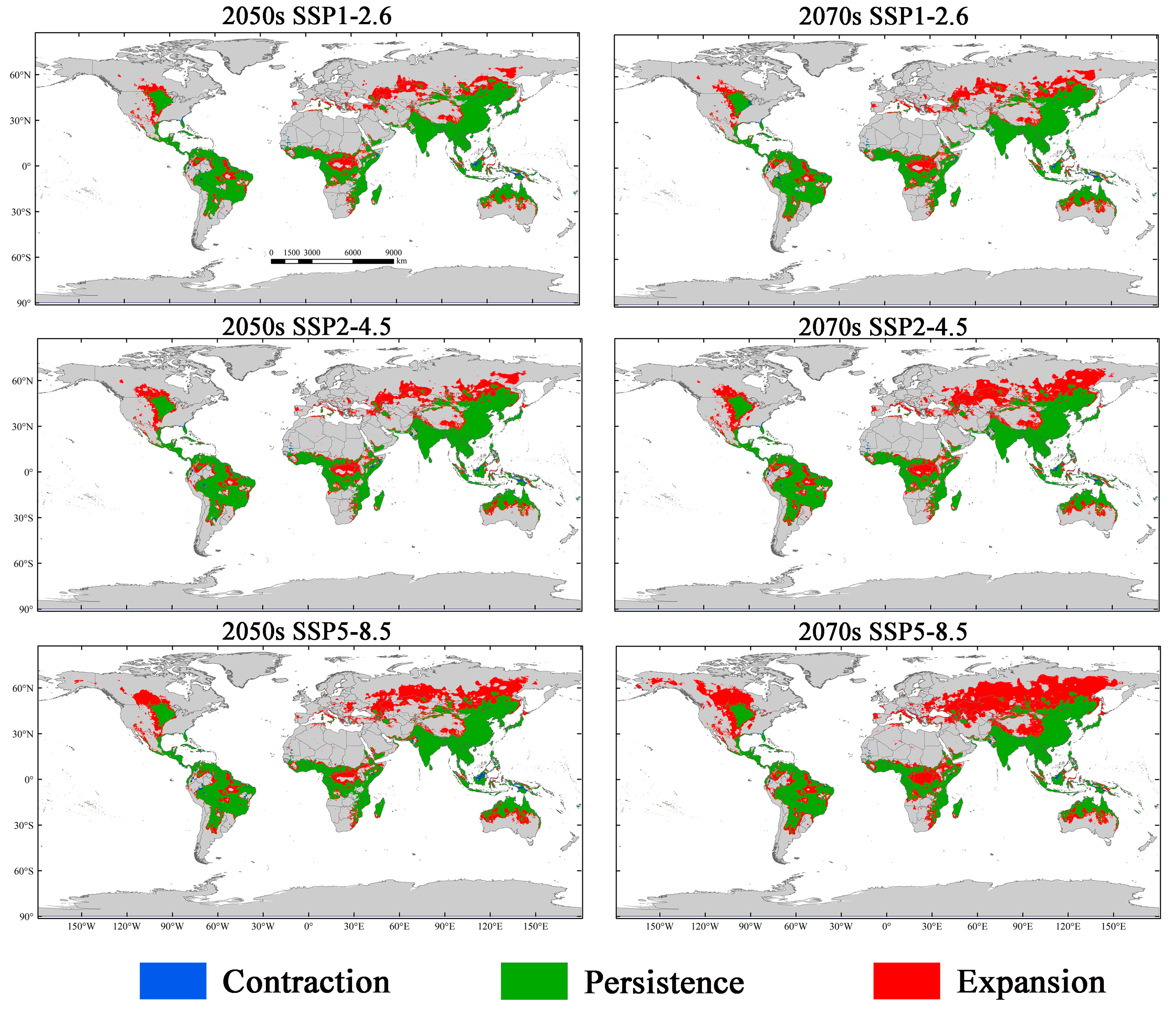
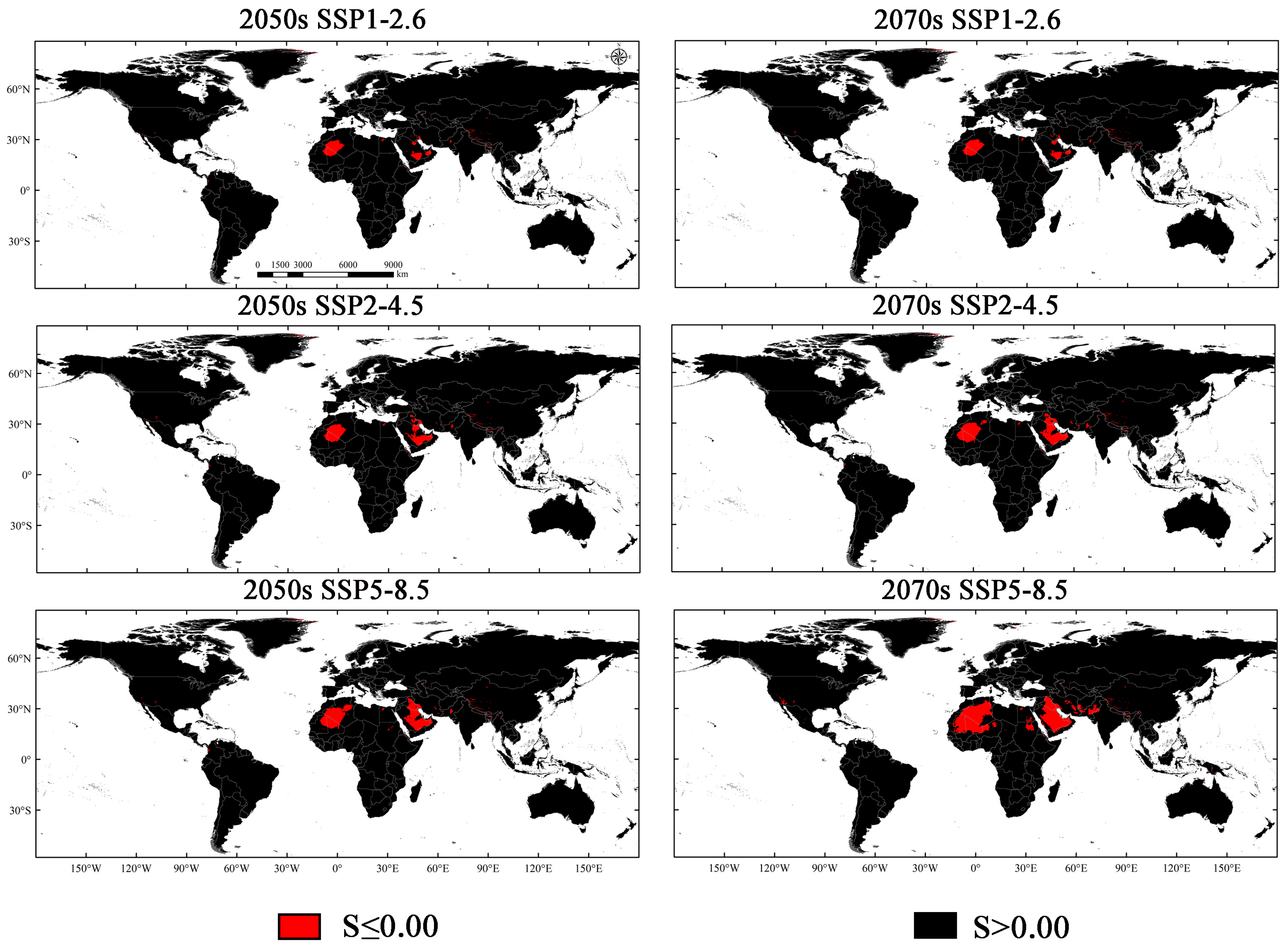
3.4. Potential Suitability Regions Change for M. Signata under Future Climate Scenarios
3.5. Potential Suitability Regions Change of M. Signata under Future Climate Scenarios
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Zhang, R.; Li, L.; Yang, Y.; Ding, Y.; Guan, H.; Wang, X.; Zhang, A.; Wen, H. Negligible transcriptome and metabolome alterations in RNAi insecticidal maize against Monolepta hieroglyphica. Plant Cell Rep. 2020, 39, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zheng, X.; Guo, J.; Wang, L.; Luo, B.; Wang, L.; Li, Q.; Liu, Y.; Han, Y.; Wang, Z. Influences of damaged silks by Monolepta hieroglyphica (Motschulsky) on corn yield. Plant Prot. 2021, 47, 109–114. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, J.; Tang, S.; Zhang, J.; Li, L. Olfactory Behavioral Response of the Monolepta hieroglyphica (Motschulsky) to volatiles of cotton and corn such as Dragosantol. Xinjiang Agric. Sci. 2018, 55, 1117. [Google Scholar] [CrossRef]
- Zhang, C. Study on the Occurrence and Biological Characteristics of Monolepta hieroglyphica in Corn Field; Chinese Academy of Agricultural Sciences: Beijing, China, 2012. [Google Scholar]
- Liu, H. A Research on Laws of Host Selection Migration and Optimum Period of Chemical to Monolepta hieroglyphica (Motschulsky); Jilin Agricultural University: Changchun, China, 2018. [Google Scholar]
- Paing, T.; Mying, H.; Lwin, H.H.; Nyo, K.M. Occurrence and Composition of Beetles in Yadanabon University Campus and Surrounding Area, Amarapura Township, Mandalay Region. MERAL Portal. 2020. Available online: http://hdl.handle.net/20.500.12678/0000007446 (accessed on 2 October 2023).
- Patra, S.; Rahman, Z.; Bhumita, P.; Saikia, K.; Thakur, N.A. Study on pest complex and crop damage in maize in medium altitude hill of Meghalaya. Bioscan 2013, 8, 825–828. [Google Scholar]
- Umemura, S.; Tayutivutukul, J.; Nakamura, H. Leaf Beetles (Coleoptera; Chrysomelidae) in the Campus and Agricultural Research Stations of Chiang Mai University, Thailand. J. Fac. Agric. Shinshu Univ. 2005, 41, 2. [Google Scholar]
- Zheng, F.; Jiang, H.; Jia, J.; Wang, R.; Zhang, Z.; Xu, H. Effect of dimethoate in controlling Monolepta hieroglyphica (Motschulsky) and its distribution in maize by drip irrigation. Pest Manag. Sci. 2020, 76, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Zheng, Q.; Yang, L.; Zhu, S.; Zhang, Z.; Xu, H. Efficacy of drip irrigation with thiamethoxam on control of Monolepta hieroglyphica, and uptake, translocation and dietary risk of thiamethoxam in maize. Pest Manag. Sci. 2023, 79, 4931–4941. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, U.; Sappington, T.; Wang, Z. Special issue highlighting research presented at the 25th IWGO Conference, Chicago 2014. J. Appl. Entomol. 2015, 139, 401–402. [Google Scholar] [CrossRef]
- Wagner, T.; Bieneck, S. Galerucine type material described by Victor Motschulsky in 1858 and 1866 from the Zoological Museum Moscow (Coleoptera: Chrysomelidae, Galerucinae). Entomol. Z. Mit Insekten-Börse 2012, 122, 205–216. [Google Scholar]
- Weise, J. Über Chrysomeliden und Coccinelliden der Philippinen: II Teil. Philipp. J. Sci. 1913, 8, 215–242. [Google Scholar]
- Ge, Y.; Shi, C.; Bai, M.; Cao, Z.; Cao, L.; Wang, Z.; Dong, J.; Wang, Y. Molecular data confirm Monolepta hieroglyphica (Motschulsky, 1858) and M. quadriguttata (Motschulsky, 1860) being synonyms of M. signata (Oliver, 1808). Insect Syst. Evol. 2023, 54, 402–412. [Google Scholar] [CrossRef]
- Van Kleunen, M.; Dawson, W.; Essl, F.; Pergl, J.; Winter, M.; Weber, E.; Kreft, H.; Weigelt, P.; Kartesz, J.; Nishino, M. Global exchange and accumulation of non-native plants. Nature 2015, 525, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, C.A.; Tewksbury, J.J.; Tigchelaar, M.; Battisti, D.S.; Merrill, S.C.; Huey, R.B.; Naylor, R.L. Increase in crop losses to insect pests in a warming climate. Science 2018, 361, 916–919. [Google Scholar] [CrossRef]
- Sala, O.E.; Stuart Chapin, F.; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, P.; Sharma, A.K.; Das, S. First record of the white-spotted leaf beetle, Monolepta signata (Olivier) (Coleoptera: Chrysomelidae) on Ashwagandha, Withania somnifera (L.) Dunal. International Journal of Advanced Biochemistry Research 2024, 8, 310–312. [Google Scholar] [CrossRef]
- Tiwari, S.; Sharma, S.; Wratten, S.D. Flowering alyssum (Lobularia maritima) promote arthropod diversity and biological control of Myzus persicae. J. Asia-Pac. Entomol. 2020, 23, 634–640. [Google Scholar] [CrossRef]
- He, Q.; Song, X.; Ma, H.; Yin, Y. The complete mitochondrial genome of Monolepta hieroglyphica (Motschulsky) (Coleoptera: Chrysomelidae). Mitochondrial DNA Part B 2021, 6, 2019–2021. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.N.; Seo, C.; Thorne, J.; Nelson, J.K.; Erwin, S.; O’Brien, J.M.; Schwartz, M.W. Using species distribution models to predict new occurrences for rare plants. Divers. Distrib. 2009, 15, 565–576. [Google Scholar] [CrossRef]
- Lin, C.-H.; Wen, T.-H. Using geographically weighted regression (GWR) to explore spatial varying relationships of immature mosquitoes and human densities with the incidence of dengue. Int. J. Environ. Res. Public Health 2011, 8, 2798–2815. [Google Scholar] [CrossRef]
- Stefánsson, G. Analysis of groundfish survey abundance data: Combining the GLM and delta approaches. ICES J. Mar. Sci. 1996, 53, 577–588. [Google Scholar] [CrossRef]
- Zhao, J.; Zou, X.; Yuan, F.; Luo, Y.; Shi, J. Predicting the current and future distribution of Monochamus carolinensis (Coleoptera: Cerambycidae) based on the maximum entropy model. Pest Manag. Sci. 2023, 79, 5393–5404. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A., Jr. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Li, C.; Liu, Z. Optimized maxent model predictions of climate change impacts on the suitable distribution of cunninghamia lanceolata in China. Forests 2020, 11, 302. [Google Scholar] [CrossRef]
- Thibaud, E.; Petitpierre, B.; Broennimann, O.; Davison, A.; Guisan, A. Measuring the relative effect of factors affecting species distribution model predictions. Methods Ecol. Evol. 2014, 5, 947–955. [Google Scholar] [CrossRef]
- Yackulic, C.B.; Chandler, R.; Zipkin, E.F.; Royle, J.A.; Nichols, J.D.; Campbell Grant, E.H.; Veran, S. Presence–only modelling using MAXENT: When can we trust the inferences? Methods Ecol. Evol. 2013, 4, 236–243. [Google Scholar] [CrossRef]
- Hernandez, P.A.; Graham, C.H.; Master, L.L.; Albert, D.L. The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography 2006, 29, 773–785. [Google Scholar] [CrossRef]
- Aidoo, O.F.; Souza, P.G.C.; da Silva, R.S.; Santana, P.A., Jr.; Picanço, M.C.; Kyerematen, R.; Sètamou, M.; Ekesi, S.; Borgemeister, C. Climate–induced range shifts of invasive species (Diaphorina citri Kuwayama). Pest Manag. Sci. 2022, 78, 2534–2549. [Google Scholar] [CrossRef]
- Tortorici, F.; Bombi, P.; Loru, L.; Mele, A.; Moraglio, S.T.; Scaccini, D.; Pozzebon, A.; Pantaleoni, R.A.; Tavella, L. Halyomorpha halys and its egg parasitoids Trissolcus japonicus and T. mitsukurii: The geographic dimension of the interaction. NeoBiota 2023, 85, 197–221. [Google Scholar] [CrossRef]
- Wu, T.; Yu, R.; Lu, Y.; Jie, W.; Fang, Y.; Zhang, J.; Zhang, L.; Xin, X.; Li, L.; Wang, Z. BCC-CSM2-HR: A high-resolution version of the Beijing Climate Center Climate System Model. Geosci. Model Dev. 2021, 14, 2977–3006. [Google Scholar] [CrossRef]
- Rank, A.; Ramos, R.S.; da Silva, R.S.; Soares, J.R.S.; Picanço, M.C.; Fidelis, E.G. Risk of the introduction of Lobesia botrana in suitable areas for Vitis vinifera. J. Pest Sci. 2020, 93, 1167–1179. [Google Scholar] [CrossRef]
- O’Neill, B.C.; Tebaldi, C.; Van Vuuren, D.P.; Eyring, V.; Friedlingstein, P.; Hurtt, G.; Knutti, R.; Kriegler, E.; Lamarque, J.-F.; Lowe, J. The scenario model intercomparison project (ScenarioMIP) for CMIP6. Geosci. Model Dev. 2016, 9, 3461–3482. [Google Scholar] [CrossRef]
- Gidden, M.J.; Riahi, K.; Smith, S.J.; Fujimori, S.; Luderer, G.; Kriegler, E.; Van Vuuren, D.P.; Van Den Berg, M.; Feng, L.; Klein, D. Global emissions pathways under different socioeconomic scenarios for use in CMIP6: A dataset of harmonized emissions trajectories through the end of the century. Geosci. Model Dev. 2019, 12, 1443–1475. [Google Scholar] [CrossRef]
- Liu, X.; Wu, T.; Yang, S.; Jie, W.; Nie, S.; Li, Q.; Cheng, Y.; Liang, X. Performance of the seasonal forecasting of the Asian summer monsoon by BCC_CSM1. 1 (m). Adv. Atmos. Sci. 2015, 32, 1156–1172. [Google Scholar] [CrossRef]
- Riahi, K.; Van Vuuren, D.P.; Kriegler, E.; Edmonds, J.; O’neill, B.C.; Fujimori, S.; Bauer, N.; Calvin, K.; Dellink, R.; Fricko, O. The Shared Socioeconomic Pathways and their energy, land use, and greenhouse gas emissions implications: An overview. Glob. Environ. Chang. 2017, 42, 153–168. [Google Scholar] [CrossRef]
- Chan, J.Y.-L.; Leow, S.M.H.; Bea, K.T.; Cheng, W.K.; Phoong, S.W.; Hong, Z.-W.; Chen, Y.-L. Mitigating the multicollinearity problem and its machine learning approach: A review. Mathematics 2022, 10, 1283. [Google Scholar] [CrossRef]
- Cobos, M.E.; Peterson, A.T.; Barve, N.; Osorio-Olvera, L. kuenm: An R package for detailed development of ecological niche models using Maxent. PeerJ 2019, 7, e6281. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open–source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Chen, J.; Yang, S.; Li, H.; Zhang, B.; Lv, J. Research on geographical environment unit division based on the method of natural breaks (Jenks). Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2013, 40, 47–50. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, H.; Chen, C.; Fan, S.; Wei, J.; Cai, B.; Zhang, H. Potential global distribution of Paracoccus marginatus, under climate change conditions, using MaxEnt. Insects 2024, 15, 98. [Google Scholar] [CrossRef] [PubMed]
- Elith, J.; Kearney, M.; Phillips, S. The art of modelling range–shifting species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Lobo, J.M.; Jiménez–Valverde, A.; Real, R. AUC: A misleading measure of the performance of predictive distribution models. Glob. Ecol. Biogeogr. 2008, 17, 145–151. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Borkakati, R.; Venkatesh, M.; Saikia, D. Insect pests of Brinjal and their natural enemies. J. Entomol. Zool. Stud. 2019, 7, 932–937. [Google Scholar] [CrossRef]
- Sergeev, M. The leaf beetles (Coleoptera: Chrysomelidae) of the Far Eastern State Marine Reserve, Primorskii krai. Far East. Entomol. 2019, 375, 11–19. [Google Scholar] [CrossRef]
- Zhao, X.; Lei, M.; Wei, C.; Guo, X. Assessing the suitable regions and the key factors for three Cd-accumulating plants (Sedum alfredii, Phytolacca americana, and Hylotelephium spectabile) in China using MaxEnt model. Sci. Total Environ. 2022, 852, 158202. [Google Scholar] [CrossRef]
- Jarnevich, C.S.; Stohlgren, T.J.; Kumar, S.; Morisette, J.T.; Holcombe, T.R. Caveats for correlative species distribution modeling. Ecol. Inform. 2015, 29, 6–15. [Google Scholar] [CrossRef]
- Brown, J.L. SDM toolbox: A python–based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. Methods Ecol. Evol. 2014, 5, 694–700. [Google Scholar] [CrossRef]
- Wu, T.; Lu, Y.; Fang, Y.; Xin, X.; Li, L.; Li, W.; Jie, W.; Zhang, J.; Liu, Y.; Zhang, L. The Beijing Climate Center climate system model (BCC-CSM): The main progress from CMIP5 to CMIP6. Geosci. Model Dev. 2019, 12, 1573–1600. [Google Scholar] [CrossRef]
- Yazdandoost, F.; Moradian, S.; Izadi, A.; Aghakouchak, A. Evaluation of CMIP6 precipitation simulations across different climatic zones: Uncertainty and model intercomparison. Atmos. Res. 2021, 250, 105369. [Google Scholar] [CrossRef]
- Tanabe, S.; Shibasaki, R.; Kato, H. Impact assessment model of international transportation infrastructure development: Focusing on trade and freight traffic in Central Asia. Asian Transp. Stud. 2016, 4, 159–177. [Google Scholar]
- Zhao, X.; Zheng, X.; Guo, J.; Liu, Y.; Luo, B.; Wang, L.; Wang, L.; Liu, Y.; Li, Q.; Wang, Z. Occurrence of Monolepta hieroglyphica adults in cornfields in Qiqihar. Chin. J. Appl. Entomol. 2021, 58, 979–984. [Google Scholar] [CrossRef]
- Zhang, C.; Yuan, Z.; Wang, Z.; He, K.; Bai, S. Population dynamics of Monolepta hieroglyphica (Motschulsky) in cornfields. Chin. J. Appl. Entomol. 2014, 51, 668–675. [Google Scholar]
- Boopathi, T.; Singh, S.; Manju, T.; Dutta, S.; Saha, S.; Singh, A.; Ramakrishna, Y.; Chowdhury, S.; Dayal, V.; Ngachan, S. Effect of weather factors on the population of Chaetocnema spp., Monolepta signata Oliver and Aulacophora foveicollis (Lucas) on paddy under rainfed lowland conditions in Mizoram, India. J. Agrometeorol. 2017, 19, 145–148. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, X.; Zhang, Q.; Lin, Y.; Hao, M.; Luo, Y.; Zhao, Z.; Yao, Y.; Chen, X.; Wang, L. Recently amplified arctic warming has contributed to a continual global warming trend. Nat. Clim. Chang. 2017, 7, 875–879. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Z.; Chen, J.; Han, X. Effects of high temperature stress on total protein and two protective enzymes in adult Monolepta hieroglyphica (Motschulsky). J. Environ. Entomol. 2018, 40, 440–445. (In Chinese) [Google Scholar] [CrossRef]
- Petri, P.A. Is East Asia becoming more interdependent? J. Asian Econ. 2006, 17, 381–394. [Google Scholar] [CrossRef]
- Lyu, Z.; Li, J.; Wang, Z.; Liu, G. Preli mi nary studies on two spotted leaf beetle, Monolepta hieroglyphica on cotton. Plant Prot. 2007, 33, 97–99. (In Chinese) [Google Scholar]
- Wang, Q.; Zhai, P.-M.; Qin, D.-H. New perspectives on ‘warming–wetting’trend in Xinjiang, China. Adv. Clim. Chang. Res. 2020, 11, 252–260. [Google Scholar] [CrossRef]
- Dong, T.; Liu, J.; Liu, D.; He, P.; Li, Z.; Shi, M.; Xu, J. Spatiotemporal variability characteristics of extreme climate events in Xinjiang during 1960–2019. Environ. Sci. Pollut. Res. 2023, 30, 57316–57330. [Google Scholar] [CrossRef] [PubMed]
- Krauss, J.; Steffan-Dewenter, I.; Tscharntke, T. Landscape occupancy and local population size depends on host plant distribution in the butterfly Cupido minimus. Biol. Conserv. 2004, 120, 355–361. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Marotzke, J.; Bala, G.; Cao, L.; Corti, S.; Dunne, J.P.; Engelbrecht, F.; Fischer, E.; Fyfe, J.C.; Jones, C. Future global climate: Scenario-based projections and near-term information. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021; pp. 553–672. [Google Scholar]
- Mai, J.; Liu, G. Modeling and predicting the effects of climate change on cotton-suitable habitats in the Central Asian arid zone. Ind. Crops Prod. 2023, 191, 115838. [Google Scholar] [CrossRef]
- Santana, P.A., Jr.; Kumar, L.; Da Silva, R.S.; Pereira, J.L.; Picanço, M.C. Assessing the impact of climate change on the worldwide distribution of Dalbulus maidis (DeLong) using MaxEnt. Pest Manag. Sci. 2019, 75, 2706–2715. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, C.; Zhao, J.; Wang, K.; Chen, F.; Chu, Q. Increasing inconsistency between climate suitability and production of cotton (Gossypium hirsutum L.) in China. Ind. Crops Prod. 2021, 171, 113959. [Google Scholar] [CrossRef]
- Ranum, P.; Peña–Rosas, J.P.; Garcia–Casal, M.N. Global maize production, utilization, and consumption. Ann. N. Y. Acad. Sci. 2014, 1312, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Poland, T.M.; Rassati, D. Improved biosecurity surveillance of non-native forest insects: A review of current methods. J. Pest Sci. 2019, 92, 37–49. [Google Scholar] [CrossRef]
- Guo, D.; Zhang, Z.; Chen, J.; Wang, S. Electrophysiological and behavioral responses of Monolepta hieroglyphica (Motschulsky) to 7 cotton and corn volatiles. Chin. J. Appl. Entomol. 2018, 55, 79–86. [Google Scholar]
- Ji, W.; Han, K.; Lu, Y.; Wei, J. Predicting the potential distribution of the vine mealybug, Planococcus ficus under climate change by MaxEnt. Crop Prot. 2020, 137, 105268. [Google Scholar] [CrossRef]
- Pearson, R.G.; Dawson, T.P. Predicting the impacts of climate change on the distribution of species: Are bioclimate envelope models useful? Glob. Ecol. Biogeogr. 2003, 12, 361–371. [Google Scholar] [CrossRef]

| Variable | Percentage Contribution (%) | Permutation Importance |
|---|---|---|
| Annual Mean Temperature (bio1, °C) | - | - |
| Mean Diurnal Range (bio2,°C) | 4.2 | 8.3 |
| Isothermality (bio3) | - | - |
| Temperature Seasonality (standard deviation ×100) (bio4) | 9.9 | 6.9 |
| Max Temperature of Warmest Month (bio5, °C) | - | - |
| Min Temperature of Coldest Month (bio6, °C) | - | - |
| Temperature Annual Range (bio7, mm) | - | - |
| Mean Temperature of Wettest Quarter (bio8, °C) | - | - |
| Mean Temperature of Driest Quarter (bio9, °C) | - | - |
| Mean Temperature of Warmest Quarter (bio10,°C) | 12.2 | 56.7 |
| Mean Temperature of Coldest Quarter (bio11, °C) | - | - |
| Annual Precipitation (bio12, mm) | - | - |
| Precipitation of Wettest Month (bio13, mm) | 55.3 | 1.7 |
| Precipitation of Driest Month (bio14, mm) | - | - |
| Precipitation Seasonality (bio15) | 7.8 | 2.1 |
| Precipitation of Wettest Quarter (bio16, mm) | - | - |
| Precipitation of Driest Quarter (bio17, mm) | 3.7 | 4 |
| Precipitation of Warmest Quarter (bio18, mm) | 5.1 | 15.5 |
| Precipitation of Coldest Quarter (bio19, mm) | 0.9 | 1.1 |
| Elevation (bio20, m) | 1 | 3.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Zhao, J.; Hu, C.; Ma, J.; Deng, C.; Ma, L.; Qie, X.; Yuan, X.; Yan, X. Predicting the Current and Future Distribution of Monolepta signata (Coleoptera: Chrysomelidae) Based on the Maximum Entropy Model. Insects 2024, 15, 575. https://doi.org/10.3390/insects15080575
Liu Q, Zhao J, Hu C, Ma J, Deng C, Ma L, Qie X, Yuan X, Yan X. Predicting the Current and Future Distribution of Monolepta signata (Coleoptera: Chrysomelidae) Based on the Maximum Entropy Model. Insects. 2024; 15(8):575. https://doi.org/10.3390/insects15080575
Chicago/Turabian StyleLiu, Qingzhao, Jinyu Zhao, Chunyan Hu, Jianguo Ma, Caiping Deng, Li Ma, Xingtao Qie, Xiangyang Yuan, and Xizhong Yan. 2024. "Predicting the Current and Future Distribution of Monolepta signata (Coleoptera: Chrysomelidae) Based on the Maximum Entropy Model" Insects 15, no. 8: 575. https://doi.org/10.3390/insects15080575





