Mapping the Distribution of Curculio davidi Fairmaire 1878 under Climate Change via Geographical Data and the MaxEnt Model (CMIP6)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Species Distribution Data
2.2. Environmental Variables
2.3. Modelling Process
2.4. Classification of Suitable Grades
3. Results
3.1. Model Result Verification
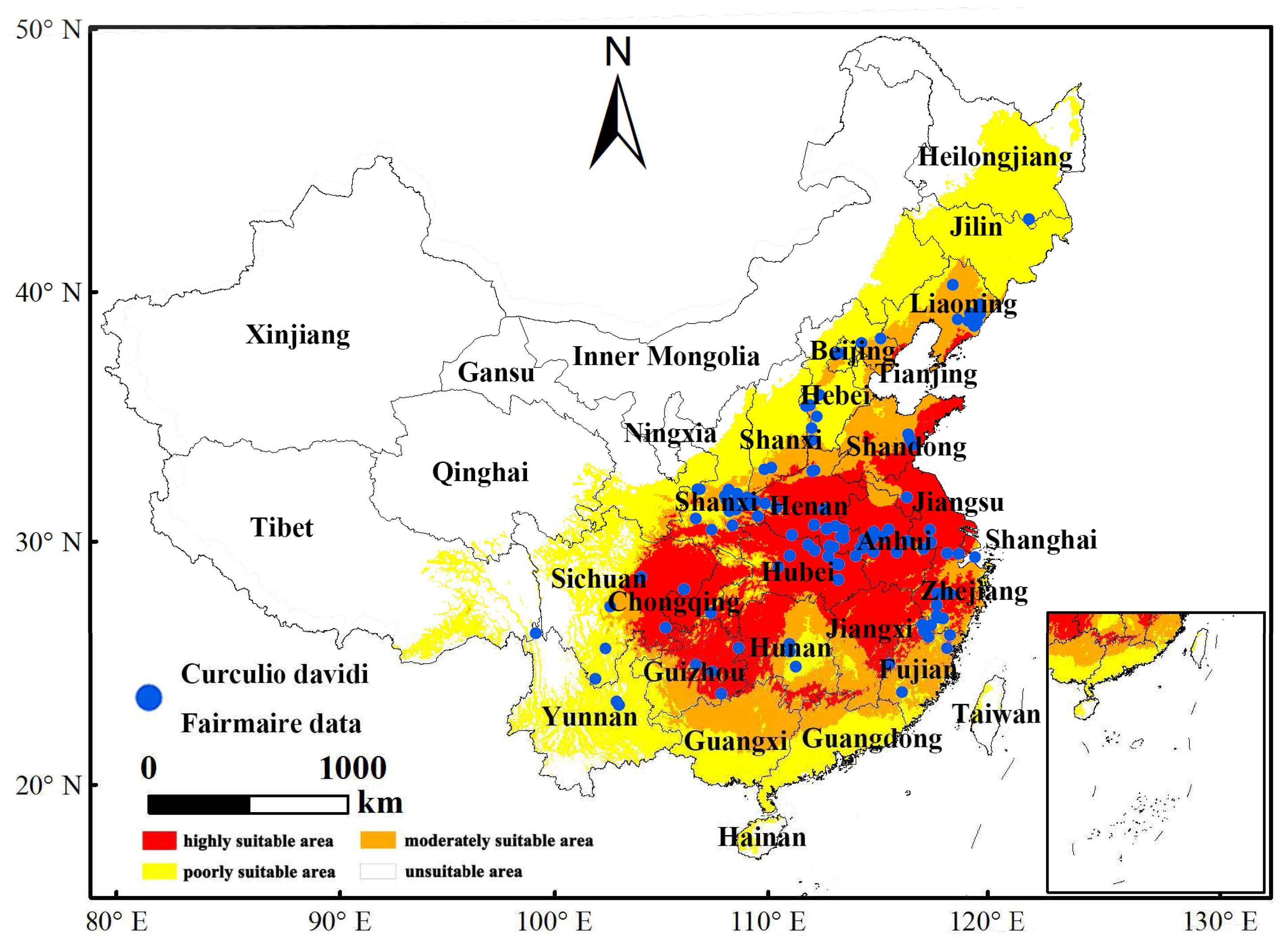
3.2. Current Distribution Forecast
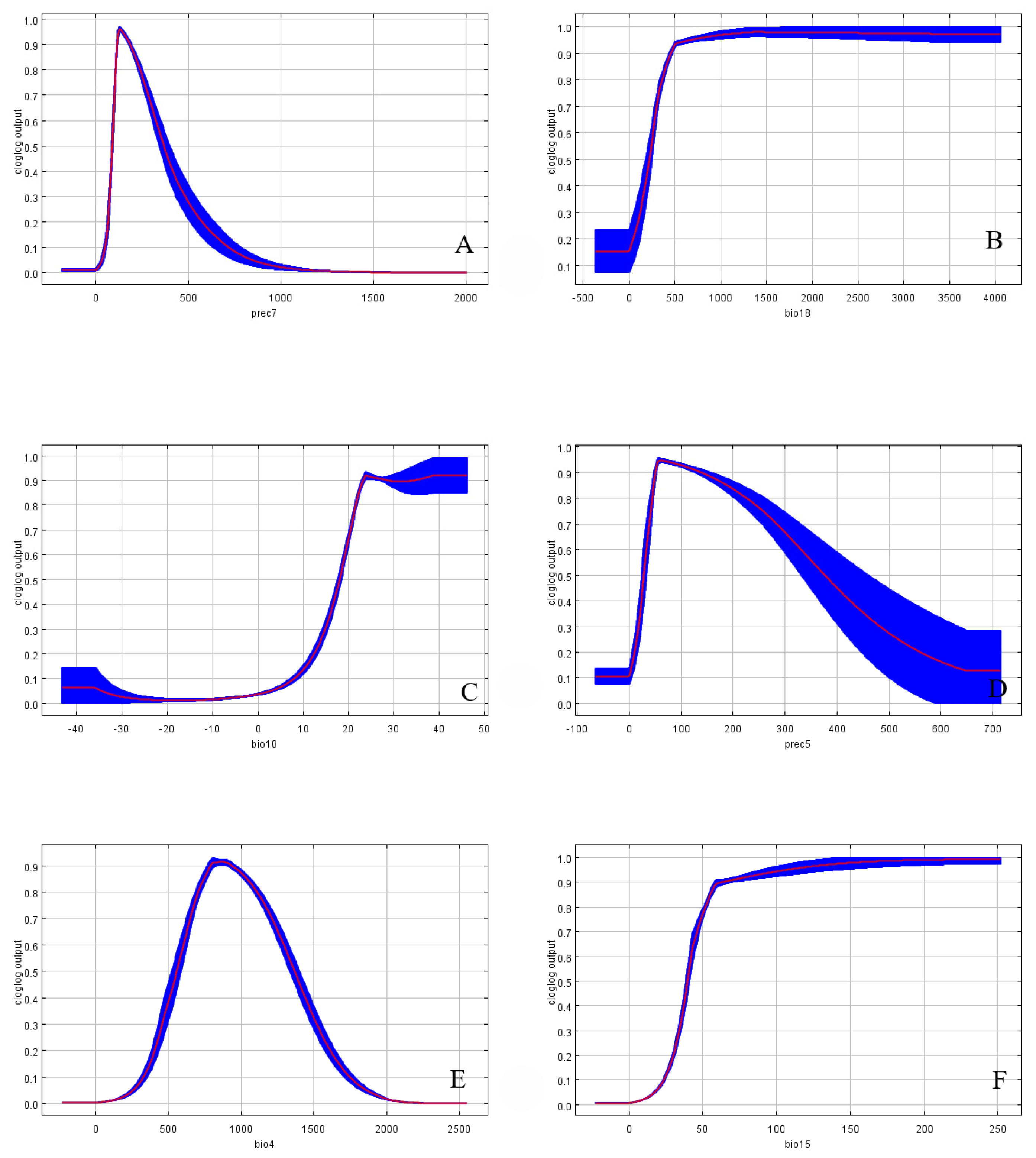
3.3. Environmental Variable Analysis
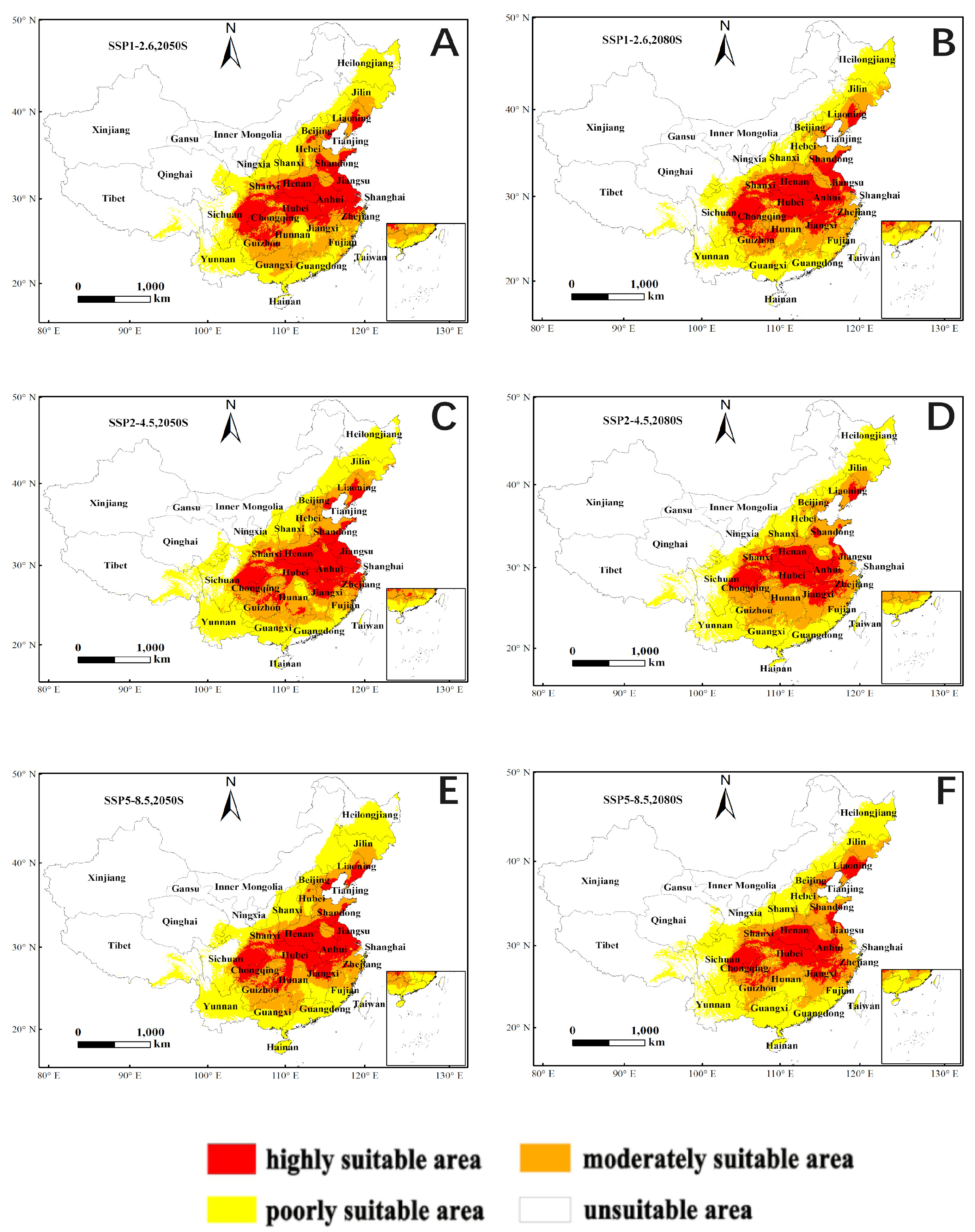
3.4. Future Distribution Forecast
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, J.; Wei, X.; Liu, Y.; Min, Q.; Liu, R.; Zhang, W.; Guo, C. Extraction of Chestnut Forest Distribution based on Multi-temporal Remote Sensing Observations. Remote Sens. Technol. Appl. 2020, 35, 1226–1236. [Google Scholar]
- Kan, L.; Li, Q.; Xie, S.; Ouyang, J. Resource distribution and nutritional quality difference of Chinese chestnuts. Sci. Technol. Food Ind. 2016, 37, 396–400. [Google Scholar]
- Caliskan, S.; Göltaş, M.; Aslan, V.; Özer, G.; Tandoğan, M.; Sezgin, G.; Cebeci, H. Variation in fruit traits and infestation ratios in natural sweet chestnut (Castanea sativa) populations under chestnut weevil (Curculio elephas) damage. Biologia 2020, 75, 2287–2294. [Google Scholar] [CrossRef]
- Chen, C.; Xie, Y.; Zhou, X.; Wang, X.; Wang, Y. Virulence of three strains of Beauveria bassiana infected Curculio davidi Fairmaire. J. Environ. Entomol. 2017, 39, 198–206. [Google Scholar]
- Higaki, M.; Toyama, M. Evidence for reversible change in intensity of prolonged diapause in the chestnut weevil Curculio sikkimensis. J. Insect Physiol. 2012, 58, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Wu, P.; Xu, L.; Pan, Y. Analysis of COI Genetic Sequence Polymorphisms of Curculio bimaculatu. J. Southwest. For. Univ. 2018, 38, 110–116. [Google Scholar]
- Tong, J. Observation on Biological Characteristics and Pollution-Free Control of Chestnut Elephant in Dechang Area; Sichuan Agricultural University: Ya’an, Chian, 2019. [Google Scholar]
- Wang, C.; Zhang, L.; Guo, A.; Li, X.; Liu, W.; Zhuang, L.; Lu, M.; Lü, H.; Bao, Y. Long-term Meteorological Prediction Model on the Occurrence and Development of Rice Leaf Roller Based on Atmospheric Circulation. J. Appl. Meteorol. Sci. 2019, 30, 565–576. [Google Scholar]
- Peterson, A.T. Uses and Requirements of Ecological Niche Models and Related Distributional Models. Biodivers. Inform. 2006, 3, 59–72. [Google Scholar] [CrossRef]
- Zhu, G.; Liu, G.; Bu, W.; Gao, Y. Ecological niche modeling and its applications in biodiversity conservation. Biodivers. Sci. 2013, 21, 90–98. [Google Scholar]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of maxent for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Yackulic, C.B.; Chandler, R.; Zipkin, E.F.; Royle, J.A.; Nichols, J.D.; Campbell Grant, E.H.; Veran, S.; O’Hara, R.B. Presence-only modelling using MAXENT: When can we trust the inferences? Methods Ecol. Evol. 2013, 4, 236–243. [Google Scholar] [CrossRef]
- Fourcade, Y.; Engler, J.O.; Rodder, D.; Secondi, J. Mapping species distributions with MAXENT using a geographically biased sample of presence data: A performance assessment of methods for correcting sampling bias. PLoS ONE 2014, 9, e97122. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, Y.; Chen, D.; Guo, X.; Li, Q.; Wang, M. Analysis of the potential distribution of the Asian citrus psyllid, Diaphorina citri Kuwayama in Southwest China using the maxent model. Plant Prot. 2021, 47, 84–90+96. [Google Scholar]
- Wu, S.; Zhu, T.; Qiao, T. Projections of Yunnan pine moth Dendrolimus houi in Sichuan Province under future climate change based on species distribution model. Plant Prot. 2021, 48, 882–890. [Google Scholar]
- Shangke, S.U.; Jianguo, D.; Bin, C.; Hongjian, T.; Wen, Y.; Like, D.; Peng, D.; Weiwei, Y.U.; Wenjia, H.U. Impact of climate change on the potential habitat distributions of eight pelagic fishes in the coastal waters of China. Acta Ecol. Sin. 2022, 42, 4834–4846. [Google Scholar]
- Hortal, J.; Roura-Pascual, N.; Sanders, N.J.; Rahbek, C. Understanding (insect) species distributions across spatial scales. Ecography 2010, 33, 51–53. [Google Scholar] [CrossRef]
- Yang, X.-Q.; Kushwaha, S.P.S.; Saran, S.; Xu, J.; Roy, P.S. Maxent modeling for predicting the potential distribution of medicinal plant, Justicia adhatoda L. in Lesser Himalayan foothills. Ecol. Eng. 2013, 51, 83–87. [Google Scholar] [CrossRef]
- Harris, R.M.B.; Grose, M.R.; Lee, G.; Bindoff, N.L.; Porfirio, L.L.; Fox-Hughes, P. Climate projections for ecologists. Wiley Interdiscip. Rev. Clim. Chang. 2014, 5, 621–637. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. Enmeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Khan, A.M.; Li, Q.; Saqib, Z.; Khan, N.; Habib, T.; Khalid, N.; Majeed, M.; Tariq, A. Maxent Modelling and Impact of Climate Change on Habitat Suitability Variations of Economically Important Chilgoza Pine (Pinus gerardiana Wall.) in South Asia. Forests 2022, 13, 715. [Google Scholar] [CrossRef]
- Gao, T.; Xu, Q.; Liu, Y.; Zhao, J.; Shi, J. Predicting the Potential Geographic Distribution of Sirex nitobei in China under Climate Change Using Maximum Entropy Model. Forests 2021, 12, 151. [Google Scholar] [CrossRef]
- Dai, Y.; Jin, T.; Xu, H.; Wang, D.; Wang, L. Study on the suitablearea of Camellia luteoflora Y.K.Li in China based on maxent model. J. Sichuan Univ. Nat. Sci. Ed. 2021, 58, 10. [Google Scholar]
- Shcheglovitova, M.; Anderson, R.P. Estimating optimal complexity for ecological niche models: A jackknife approach for species with small sample sizes. Ecol. Model. 2013, 269, 9–17. [Google Scholar] [CrossRef]
- Ji, H.; Ren, R.; Peng, M.; Hui, N.; Liu, C.J. Genetic structure of Curculio Davidi Fairmaire population on a phosphorus site in subtropical geology. Henan Norm. Univ. (Nat. Sci. Ed.) 2022, 50, 130–138. [Google Scholar]
- Filgueiras, C.C.; Willett, D.S. The Lesser Chestnut Weevil (Curculio sayi): Damage and Management with Biological Control Using Entomopathogenic Fungi and Entomopathogenic Nematodes. Insects 2022, 13, 1097. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Song, X.; Wang, Q.; Deng, X.; Ma, X.; Zhou, Q.; Shen, G.; Guo, A. Control Effect on Curculio davidi and Safety Evaluation of Three Kinds of Pesticides. J. Northeast. For. Univ. 2015, 43, 98–102. [Google Scholar]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Qin, J.L.; Yang, X.H.; Yang, Z.W.; Luo, J.T.; Lei, X.F. New technology for using meteorological information in forest insect pest forecast and warning systems. Pest Manag. Sci. 2017, 73, 2509–2518. [Google Scholar] [CrossRef] [PubMed]
- Perulli, G.D.; Boini, A.; Morandi, B.; Grappadelli, L.C.; Manfrini, L. The effects of chestnut orchard microclimate on burr development. Sci. Hortic. 2023, 319, 112183. [Google Scholar] [CrossRef]
- Small, G.E.; Pringle, C.M. Deviation from strict homeostasis across multiple trophic levels in an invertebrate consumer assemblage exposed to high chronic phosphorus enrichment in a Neotropical stream. Oecologia 2010, 162, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Mcfeeters, B.J.; Frost, P.C. Temperature and the effects of elemental food quality on Daphnia. Freshw. Biol. 2011, 56, 1447–1455. [Google Scholar] [CrossRef]
- Han, W.X.; Fang, J.Y.; Reich, P.B.; Ian Woodward, F.; Wang, Z.H. Biogeography and variability of eleven mineral elements in plant leaves across gradients of climate, soil and plant functional type in China. Ecol. Lett. 2011, 14, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Kang, H.Z.; Du, H.M.; Hu, H.B.; Zhou, J.B.; Hou, J.L.; Zhou, X.; Liu, C.J. Stoichiometric traits of oriental oak (Quercus variabilis) acorns and their variations in relation to environmental variables across temperate to subtropical China. Ecol. Res. 2012, 27, 765–773. [Google Scholar] [CrossRef]
- Yu, Q.; Chen, Q.; Elser, J.J.; He, N.; Wu, H.; Zhang, G.; Wu, J.; Bai, Y.; Han, X. Linking stoichiometric homoeostasis with ecosystem structure, functioning and stability. Ecol. Lett. 2010, 13, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhou, X.; Small, G.E.; Sterner, R.; Kang, H.; Liu, C. Energy storage and C: N:P variation in a holometabolous insect (Curculio davidi Fairmaire) larva across a climate gradient. J. Insect Physiol. 2013, 59, 408–415. [Google Scholar] [CrossRef] [PubMed]
- De la Vega, G.J.; Schilman, P.E. Using eco-physiological traits to understand the realized niche: The role of desiccation tolerance in Chagas disease vectors. Oecologia 2017, 185, 607–618. [Google Scholar] [CrossRef] [PubMed]




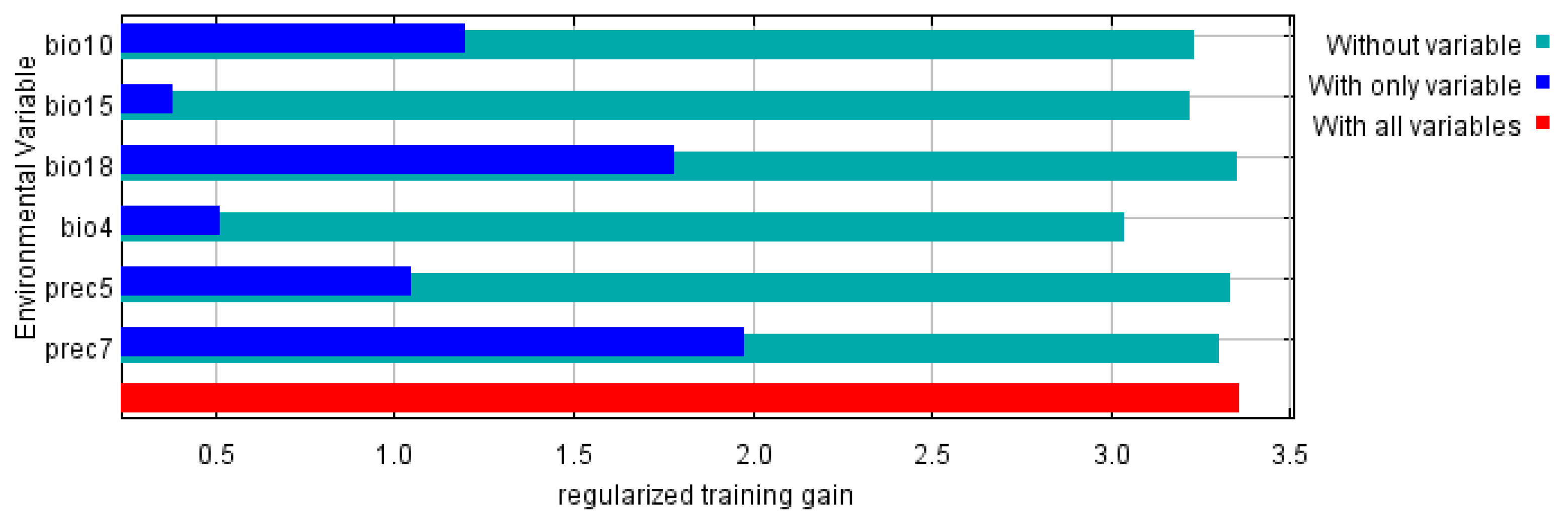
| Variable | Percent Contribution (%) | Permutation Importance (%) |
|---|---|---|
| Precipitation from July (Prec7) | 42.3 | 25.3 |
| Precipitation of warmest quarter (Bio18) | 22.3 | 3.4 |
| Temperature seasonality (standard deviation × 100) (Bio4) | 21.7 | 28.3 |
| Precipitation Seasonality (Coefficient of Variation) (Bio15) | 7.2 | 8.2 |
| Mean Temperature of Warmest Quarter (Bio10) | 4.1 | 28.9 |
| Precipitation from May (Prec5) | 2.4 | 5.8 |
| Province | High Suitable Area (km2) | Percentage of High Suitable Areas in Province (%) | Percentage of High Suitable Areas in China (%) |
|---|---|---|---|
| Gansu | 68 | 0.02 | 0.10 |
| Hebei | 72 | 0.04 | 0.11 |
| Shanxi | 183 | 0.12 | 0.28 |
| Yunnan | 208 | 0.05 | 0.32 |
| Shanghai | 265 | 4.18 | 0.41 |
| Guangxi | 271 | 0.11 | 0.42 |
| Liaoning | 341 | 0.23 | 0.52 |
| Guangdong | 358 | 0.20 | 0.55 |
| Fujian | 899 | 0.74 | 1.38 |
| Shanxi | 1881 | 0.91 | 2.88 |
| Zhejiang | 3150 | 3.09 | 4.83 |
| Shandong | 3255 | 2.06 | 4.99 |
| Chongqing | 3650 | 4.43 | 5.59 |
| Hunan | 4887 | 2.31 | 7.49 |
| Guizhou | 5031 | 2.86 | 7.71 |
| Jiangsu | 5107 | 4.76 | 7.83 |
| Jiangxi | 5506 | 3.30 | 8.44 |
| Anhui | 6918 | 4.94 | 10.60 |
| Sichuan | 7256 | 1.49 | 11.12 |
| Henan | 7519 | 4.50 | 11.52 |
| Hubei | 8426 | 4.53 | 12.91 |
| China | 65,251 | - | 0.68 |
| Environmental Variables | Suitable Range | Optimum Value |
|---|---|---|
| Prec7/mm | 107.22~324.13 | 103.13 |
| Bio18/mm | 305.02~1498.07 | 490.35 |
| Bio10/°C | 20.55~29.90; 29.90~39.95 | 23.58 |
| Prec5/mm | 44.08~299.32 | 55.15 |
| Bio4/°C | 674.2~1301.02 | 798.1 |
| Bio15/mm | 45.68~250.36 | 58.63 |
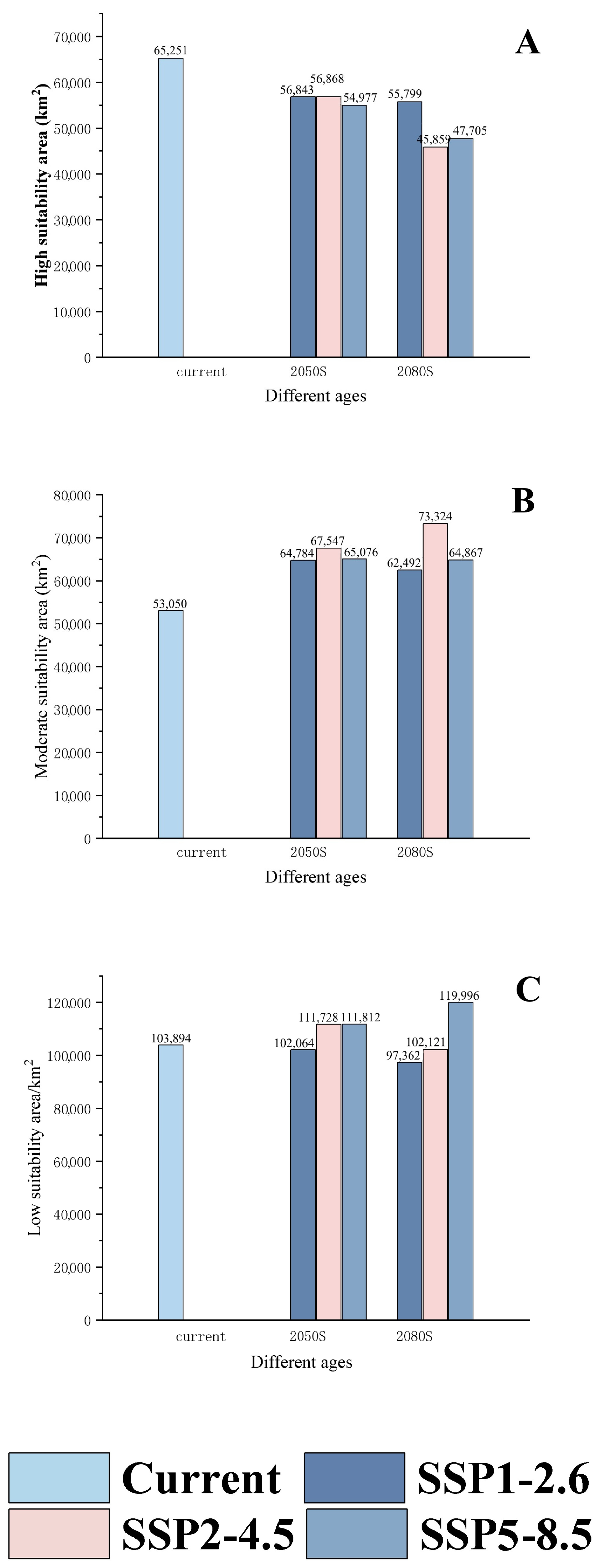
| Predicted Area (km2) | Comparison with Current Distribution (%) | ||||||
|---|---|---|---|---|---|---|---|
| Decade | Scenarios | Poor Suitable Area | Moderate Suitable Area | High Suitable Area | Poor Suitable Area | Moderate Suitable Area | High Suitable Area |
| current | 103,894 | 53,050 | 65,251 | ||||
| 2050 s | SSP1-2.6 | 102,064 | 64,784 | 56,843 | −1.76 | 22.12 | −12.89 |
| SSP2-4.5 | 111,728 | 67,547 | 56,868 | 7.54 | 27.33 | −12.85 | |
| SSP5-8.5 | 111,812 | 65,076 | 54,977 | 7.62 | 22.67 | −15.75 | |
| 2080 s | SSP1-2.6 | 97,362 | 62,492 | 55,799 | −6.29 | 17.80 | −14.49 |
| SSP2-4.5 | 102,121 | 73,324 | 45,859 | −1.71 | 38.22 | −29.72 | |
| SSP5-8.5 | 119,996 | 64,867 | 47,705 | 15.5 | 22.28 | −26.89 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Wei, X.; Wang, Z.; Peng, Y.; Liu, B.; Zhuo, Z. Mapping the Distribution of Curculio davidi Fairmaire 1878 under Climate Change via Geographical Data and the MaxEnt Model (CMIP6). Insects 2024, 15, 583. https://doi.org/10.3390/insects15080583
Wu J, Wei X, Wang Z, Peng Y, Liu B, Zhuo Z. Mapping the Distribution of Curculio davidi Fairmaire 1878 under Climate Change via Geographical Data and the MaxEnt Model (CMIP6). Insects. 2024; 15(8):583. https://doi.org/10.3390/insects15080583
Chicago/Turabian StyleWu, Junhao, Xinju Wei, Zhuoyuan Wang, Yaqin Peng, Biyu Liu, and Zhihang Zhuo. 2024. "Mapping the Distribution of Curculio davidi Fairmaire 1878 under Climate Change via Geographical Data and the MaxEnt Model (CMIP6)" Insects 15, no. 8: 583. https://doi.org/10.3390/insects15080583





