Simple Summary
The red imported fire ant, Solenopsis invicta Buren, is a highly invasive species. Some native ants may show aggression towards S. invicta and thus suppress S. invicta invasion. However, only a few such native ants have been identified, and the available data are largely limited to their behaviors. Here, we not only observed the aggression levels between native ants and S. invicta, but also investigated their abundance in various habitats, the seasonal dynamics of their abundance, and their spatial distribution in habitats with S. invicta. Two ant species, Monomorium chinense and Nylanderia bourbonica, were demonstrated to show aggression towards S. invicta and could kill a large proportion of S. invicta. However, another ant, Iridomyrmex anceps, did not show aggression. M. chinense and N. bourbonica are more abundant in green belts (e.g., lawns) and grasslands relative to woodland and farmland. Their abundance is quite low in the early months of the year but increases in later parts of the season. M. chinense and N. bourbonica are restricted to marginal habitats, but after control of S. invicta, they can rebound rapidly within a few weeks. M. chinense and N. bourbonica have the potential to suppress S. invicta invasion in some habitats.
Abstract
Native ants have long been considered for their potential to suppress the red imported fire ant, Solenopsis invicta Buren (Hymenoptera: Formicidae), a highly invasive and destructive species. However, the knowledge in this field is limited to behavioral observations of a few related native ants. In this study, by setting up a series of ant combinations of three native ants, i.e., Monomorium chinense Santschi, the robust crazy ant Nylanderia bourbonica Forel, and Iridomyrmex anceps Roger, with S. invicta, we observed the aggression levels and mortality rates. Using baited vials, we also investigated the abundance of native ants in four types of habitats in Eastern China that are preferred by S. invicta (woodland, green belts on roadsides, grassland, and farmland), as well as their seasonal abundance when co-existing with S. invicta and their spatial distribution before and after control of S. invicta. We found that M. chinense and N. bourbonica show a degree of aggression towards S. invicta and can kill substantial proportions of S. invicta under laboratory conditions, but I. anceps does not. Both M. chinense and N. bourbonica can occur in each type of habitat investigated and are more abundant in green belts (particularly lawns with turf) and grasslands relative to other habitats. In grasslands with S. invicta, M. chinense maintained a low density before early July; however, its abundance increased thereafter and reached a peak in September. N. bourbonica also had a low density early in the season and increased steadily from April. Its abundance began to decrease substantially from November. In grasslands invaded by S. invicta, both M. chinense and N. bourbonica were restricted to sites close to the margins before S. invicta was controlled; however, they spread to a larger range within a few weeks after control of S. invicta. In conclusion, M. chinense and N. bourbonica have the potential to suppress S. invicta invasion in habitats that are abundant with these two native ants.
1. Introduction
The red imported fire ant, Solenopsis invicta Buren (Hymenoptera: Formicidae), is one of the most widespread, abundant, and destructive invasive ants globally [1]. This ant has a broad range of negative, and often severe, impacts on agriculture, forestry, wildlife, infrastructure, and human health and lifestyle [2,3,4,5]. Native to South America [6], S. invicta was introduced to the United States between 1933 and 1945 and has since spread to the Caribbean, Oceania, Eastern Asia, and recently, to Europe [7,8]. In mainland China, since the first report of its presence in 2004 [9], S. invicta has spread to at least 620 counties or districts in 12 provinces [10] and has likely spread across a larger range due to human activities and climatic warming [11,12]. Various habitats, such as farmlands, forests, plant nurseries, grasslands, wetlands, and urban green spaces, can be infested by this ant [5,13], leading to serious economic and ecosystem service losses and biodiversity reduction in some regions [5]. Due to its multifaceted impacts, S. invicta is ranked as one of the most harmful invasive insect species in China, particularly in agro-ecosystems [14].
Native ants are generally vulnerable to S. invicta invasion and are often displaced if S. invicta thrives [2,5,15,16], primarily because of the high population density and the formidable competitive abilities of this ant [17]. However, some native ants can coexist with S. invicta, such as Monomorium minimum Buckley, Paratrechina terricola Buckley, and certain species of Pheidole and Forelius in the USA [18] and Tapinoma melanocephalum Fabricius, Monomorium chinense Santschi, and some species of Pheidole and Paratrechina in China [19,20,21]. Specifically, some (e.g., M. minimum) can invade S. invicta worker-defended colonies, prey upon broods, or prevent S. invicta workers from leaving the nest for foraging [22,23]. They may adopt certain protective tactics when encountering S. invicta, such as running away rapidly and releasing chemicals (venoms) that are deleterious to S. invicta [21]. For these reasons, a few native ants are believed to be capable of suppressing S. invicta (re)invasion to a certain degree, and it has been suggested that they be integrated into S. invicta control programs [22,23,24]. However, except for the few native ant species mentioned above, the potential that most native ants may hold for suppressing S. invicta remains unknown. As ants are one of the most diverse, abundant, and widespread animal groups in the world [25], some native ant species, undiscovered so far, are likely to be able to successfully persist with S. invicta.
With this in mind, it is crucial to learn where these ants reside and about their abundance in various habitats. Understanding their success in the habitats preferred by S. invicta would help us to evaluate their effectiveness within these habitats. However, such information is quite limited, even for the native ants that have been shown to be aggressive towards S. invicta (e.g., [23]).
In this study, we investigated the native ants that co-occur with S. invicta in Eastern China. This research was carried out in Eastern China because we had previously identified native ants in this region during our field investigations and during the implementation of S. invicta control programs. Moreover, Eastern China is one of the regions experiencing S. invicta expansion [10] and is being invaded by this ant on a larger scale [11,12]. Here, we first surveyed the aggressive interactions between three native ants and S. invicta. Then, we investigated their abundance in four types of habitats. Next, we performed a one-year investigation of the seasonal dynamics of native ants in a grassland, and their spatial distribution before and after the removal of S. invicta in the grassland. Our aims were to identify native ants that are aggressive towards S. invicta to determine their overall local distribution and population dynamics in the habitats that are preferred by S. invicta (i.e., grassland) and, based on these results, to evaluate their potential for suppressing S. invicta as a biotic factor to be included in S. invicta control programs.
2. Materials and Methods
2.1. Aggressive Interactions between Native Ants and S. invicta
This experiment was performed by combining native ants with S. invicta in different ways. Levels of aggressive interactions were assayed, and the resulting levels of mortality were observed.
2.1.1. Ant Collection
Based on our knowledge of the ant community in Eastern China, we selected three native ant species for aggression assays: M. chinense, the robust crazy ant Nylanderia bourbonica Forel, and Iridomyrmex anceps Roger. Their workers and S. invicta workers were collected using baited vials from one grassland and adjacent lawns located in Redwood Town in Longyou County, Zhejiang Province, China. The collected ants were kept in plastic containers (16 cm × 11 cm × 7 cm) coated with talcum powder and maintained in a climate-controlled chamber (25 °C ± 2 °C and 75% ± 10% RH with a photoperiod of 14:10 L:D). The ants were fed ham sausage (Wangzhongwang, Shuanghui Industry Group Co., Ltd., Luohe, Henan Province, China; the same below). They were used for assays within 24 h after collection. The assays were performed under laboratory conditions at temperatures of 25–27 °C.
2.1.2. Dyad Interactions
As differently sized ants may show different interaction outcomes [26], we used large-, medium-, and small-sized S. invicta workers (head width > 1.35 mm, 1.00–1.35 mm, and <1.00 mm, respectively) separately for the assays, with each being paired with workers of each of the three native ants mentioned above. Within each native ant species, all of the workers had a similar body size: M. chinense, 1.3–1.7 mm; N. bourbonica, 2.3–3.3 mm; and I. anceps, 3.0–4.0 mm. The assay methods were similar to those described by Buczkowski and Bennett [27]. Briefly, we placed one native ant and one S. invicta in a 1.5 mL Axygen centrifuge tube, immediately settled the tube horizontally, and started to observe their behaviors. The observation lasted 5 min for each ant pair, during which we recorded the timepoint of the ants initiating physical contact, the duration of the contact, and the aggression score (levels) of the contact. The aggression scores were determined by referring to Grangier et al. [28] with some modifications, including 1. ants make short (≤1 s) antennal contact; 2. ants make long (>1 s) antennal contact; 3. one or both ants in the pair exhibit threatening postures by opening the mandibles or curling the gaster, or bite quickly; and 4. the ants fight by prolonged biting and eventually stinging. The score was recorded as “0” if either ant alone or both avoided contacting the other throughout the trial. As one trial ended, the maximum score (i.e., a number from 0 to 4) attained in the trial was determined. Thirty trials (replicates) were performed for each type of native ant and S. invicta combination. The effects of S. invicta body size on the aggression scores were analyzed; the means were compared between the combinations with different S. invicta sizes and between the combinations with different native ants.
2.1.3. Symmetrical Group-on-Group Interactions
For each native ant species, 1, 3, 5, and 10 workers were released separately into talcum-powder-coated plastic containers (4.2 cm in diameter, 6.8 cm high). After 5 min, the same number of medium-sized S. invicta workers were introduced to the containers, forming four types of ant combinations: 1 vs. 1, 3 vs. 3, 5 vs. 5, and 10 vs. 10. Here we elected to use medium-sized S. invicta workers because they are the primary force for defense, carrying larger venom arsenals, longer stings than small-sized workers [29], and greater in number than large-sized workers. At 12, 24, and 36 h, the numbers of dead ants (without visible movement of the legs) or dying ants (unable to stand) were recorded for each partnership. We performed 30 trials for each combination, taking every 10 trials as one replicate (i.e., there were three replicates in total). Within each replicate, the number of dead/dying ants were pooled for each partnership, and their percentages (mortality rates) in the pooled initial number of introduced ants (i.e., 10, 30, 50, and 100 ants) were calculated. The mortality rates of the native ants and S. invicta at 36 h after treatment initiation were used to analyze the relations with the ant combination (factor 1) and species of native ant (factor 2).
2.1.4. Asymmetrical Group-on-One Interactions
All the methods of this assay were similar to those described above for the symmetrical group-on-group interactions, except that only one medium-sized S. invicta worker was used in each combination. Additionally, the combination with 10 native ants was set up for M. chinense due to its smaller size to balance the biomass disparity with the S. invicta worker by providing a greater number of M. chinense.
2.2. Abundance of Native Ants in Different Types of Habitats
2.2.1. Location and Habitats
The location of this investigation was in Redwood Town and an adjacent 1.3 km × 1.1 km region (29.072–29.084° N; 119.229–119.254° E) where S. invicta was introduced unintentionally before 2016 and was chemically controlled by the local government from late August 2019 to March 2020. The control consisted of two steps. Applying Kai Rui® (baits, 0.73% hydramethylnon, BASF, Ludwigshafen, Germany) first to the margin of S. invicta mounds at a dosage of 20 g per mound, after 3–4 days a mixture of 5% imidacloprid and 40% chlorpyrifos was poured into nests. This method can reduce the contact of native ants to chemicals and, thus, be perceived to be safe to them. Our investigations were performed in four types of habitats, namely woodland, green belt on the roadside, grassland, and farmland, after the removal of S. invicta in the corresponding habitats. To reduce unexpected impacts from S. invicta, for each type of habitat, only the sites with a previous infestation of 225–300 S. invicta nests per ha were selected. No active S. invicta mounds were found in the selected sites.
The selected woodland was located on a hill approximately 1 km in perimeter and approximately 50 m in altitude. The trees in this woodland were mostly <4 m in height, spaced 3–6 m apart, and dominated by Cinnamomum camphora (L.) Presl. and Ilex chinensis Sims. Abundant natural shrubs and perennial grasses were present as ground-cover vegetation. The green belt on the roadside neighboring the hill was 3–8 m in width and approximately 300 m in length. The vegetation in the belt was dominated by Cynodon dactylon (L.) Pars. The belt was decorated with rows of shrubs (e.g., Buxus megistophylla H. Lév.). For the grassland habitat, which was scattered within the study location, three sites were selected for investigation, with one (approximately 28 ha) at the southeastern margin of the town, one (10 m × 800 m) adjacent to local farmland, and one (9.24 ha) surrounded by roads and factory buildings, with each dominated by Imperata cylindrica (L.) Beauv. as ground vegetation. The farmland (50 m × 600 m) was approximately 130 m to the north of the hill described above and was previously used to grow rice. It had been abandoned for at least two years prior to our study.
2.2.2. Ant Sampling
For each type of habitat, sampling began approximately seven months after the control of S. invicta, i.e., from June to October 2020, except for one grassland site, where sampling began on 16 September 2019, nearly three weeks after S. invicta control had been carried out. By July 2022, four to six samplings had been obtained for each type of habitat, occurring from June to October. Vials (25 mL) baited with a piece of ham sausage (approximately 1 g) were used for the sampling, and 20–185 such vials were used at each site, depending on the size of the sampled sites. The baited vials were placed on the ground at a space of 10 m and were collected after 30 min by quickly capping them to trap any ants inside. To ensure baiting efficiency, the samplings were performed between 1600 and 1800 h, when the ants were speculated to be actively foraging.
2.2.3. Ant Identification and Sorting
The collected ants were identified by referring to the ants’ morphological features, as described by Wu and Wang [30], Zhou [31], and Zhou and Chen [32]. Some ants were identified as belonging to genera only due to a lack of available references. The identification of species/genera was confirmed by cloning a 710 bp fragment of ant mitochondrial COI [33], followed by sequencing (Tsingke Biotech, Hangzhou, China) and blasting the sequence in the National Center for Biotechnology Information (NCBI) database. The sequences have been submitted to GenBank, as shown in Table S1. The number of vials used for trapping the ants (with at least one ant) and the number of ants in each vial were recorded for each batch (one batch was defined as the sampling performed in one type of habitat on one sampling date), and their corresponding percentages in the batch were calculated. Using these percentages, the abundance of native ant species was compared between habitat types and between sampling dates to identify the differential distribution of the major native ants in the local ecosystem, particularly those aggressive towards S. invicta.
2.3. Seasonal Dynamics of Native Ants Coexisting with S. invicta
After finding that grassland is one of the major habitats of M. chinense and N. bourbonica, the two native ants that are aggressive towards S. invicta, we further investigated their seasonal dynamics in one S. invicta-invaded grassland plot (ca. 10 m wide, 460 m long) located in the middle of Zhejiang Province, adjacent to the east of Jinhua New Energy Vehicle Town. The plot had a north–south orientation, was neighbored in the west by a ditch (ca. 2 m width), and was neighbored in the east by a few ponds, cultivated farmlands, and abandoned farmlands. There were 40–70 S. invicta mounds on the plot (87–152 mounds per ha.), which varied with the season. The vegetation was at a medium density, dominated by the Poaceae and Asteraceae families, and at heights of mostly <80 cm. The investigation began in mid-February 2023 and ended in mid-February 2024. Ant sampling was performed every 7–10 d from mid-February to mid-April, monthly from May to October 2023, twice in November 2023, and once in mid-February 2024. No sampling was performed from December 2023 to January 2024, which was the winter season. The investigation was not replicated in this plot in 2024, as S. invicta had to be controlled in early March 2024 in an eradication program of this pest by the local government.
We obtained a total of 18 ant samplings in the plot, adopting the sampling method described above for the investigations in different habitats. For each sampling, 46 vials baited with ham sausage were used, placed every 10 m, and retrieved after 30 min. S. invicta, M. chinense, N. bourbonica, and other ant species in the vials were counted, and the percentages of vials with trapped ants were calculated with respect to each native ant species.
The percentage of baits that trapped N. bourbonica increased steadily from early April to early June 2023, and the number of this ant reached >50 in a few vials (see Results). We considered it likely that this trend might also occur in other grasslands, representing a typical population feature of N. bourbonica under local conditions. To clarify this, we selected another grassland plot (ca. 20 m wide, 160 m long; S. invicta density ca. 88–128 mounds per ha.) on the western side of the first grassland plot and performed the same investigation from 10 June 2023 to 30 December 2023. The soil type of this plot was mineral, and the vegetation was also dominated by the Poaceae and Asteraceae families, with a much higher plant density. Eight ant samplings were obtained, and for each sampling, 30 baited vials were used, which were arranged in two rows with 15 vials in each row.
In addition, we also investigated the native ants in nine locations in Zhejiang and Jiangxi provinces, where measures had not been taken to control S. invicta or control measures had been taken but this ant was not absolutely removed. We selected these locations randomly, with the aim of collecting supporting information showing that M. chinense and N. bourbonica can coexist with S. invicta in different regions and still increase their population later in the season, e.g., from August to October, as shown in previous investigations.
2.4. Spatial Distribution of Native Ants Coexisting with S. invicta
Alongside the abundance (in different habitats) and seasonal dynamics (in major habitats), the spatial distribution of native ants within S. invicta-invaded habitats can also be quite informative regarding their ecological responses toward S. invicta. In 2021, we investigated this on the banks of the Dongyangjiang River (N 119°74′, E 29°09′) in mid-Zhejiang. The banks are trapezoid shaped in cross-section, 600 m long in the east–west orientation, with one road of ca. 4 m width at its top. On the northern and southern sides of the road, there is one slope each, which are ca. 18 m and 8 m in width, respectively. The dominant vegetation is turf and grasses of various families. Samplings of ants were performed on three dates, namely 3 September, 18 September, and 9 October, i.e., 1 d before, 2 weeks after, and 5 weeks after control of S. invicta, respectively. The control of S. invicta was carried out on 4 September using the same chemical method stated in Section 2.2.1. On each sampling date, 360 baited vials were used for trapping ants, with 180 vials for each slope, which were arranged in three rows (60 vials per row): one row on the upper side of the slope, one row on the lower side of the slope, and one in the middle. Within each row, the vials were placed on the ground every ca. 7.5 m. The other methods were the same as described above.
2.5. Data Analysis
SPSS 25 [34] was used for the data analysis. The Kolmogorov–Smirnov test and Levene’s test were used to test the normality and the variance homogeneity of the aggression score data, respectively. For data with a normal distribution and equal variance, a one-way analysis of variance (ANOVA) was performed to analyze the effect of S. invicta body size and native ant species on the aggression level, and the means were compared using Tukey’s multiple comparison test. For aggression score data with a non-normal distribution or without equal variance, the Kruskal–Wallis H test was performed, and means were compared using the Mann–Whitney U test. The same methods were used to analyze data on ant mortality from group-on-group and group-on-one interactions to discover relations of mortality with the number of ants (ant combination) and the species of native ants. The significance level was 0.05.
For the proportion data of baits trapping ants 1 d before, 2 weeks after, and 5 weeks after S. invicta control, chi-square tests were used to analyze the differences among these dates. For the number data of trapped ants, an ANOVA was used to test the associations with sampling date, and Tukey’s test was used to compare means. The number data were square-root transformed prior to analysis. The significance level was 0.05.
3. Results
3.1. Aggression Interactions between Native Ants and S. invicta
3.1.1. Dyad Interactions
S. invicta body size had a significant effect on the scores of the aggression interactions between S. invicta and N. bourbonica (H = 8.536, df = 2, p = 0.014; Kruskal–Wallis H test) and between S. invicta and M. chinense (H = 6.327, df = 2, p = 0.042) but did not for interactions between S. invicta and I. anceps (H = 3.661, df = 2, p = 0.160). In pairs with N. bourbonica or I. anceps, the highest score was observed for those interacting with large-sized S. invicta, reaching 3.45 and 3.40, respectively, and the score declined with the size of S. invicta. However, in pairs with M. chinense, the highest score (2.70) was observed in those interacting with medium-sized S. invicta (Table 1). Overall, pairs with N. bourbonica and those with I. anceps had similar scores, irrespective of S. invicta size, and they had higher scores than those with M. chinense, which statistically reached a significant level when interacting with large or small S. invicta (p < 0.05, Mann–Whitney U test; Table 1).

Table 1.
Scores (mean ± SE) for aggression observed in dyad interactions between native ants and Solenopsis invicta of different body sizes.
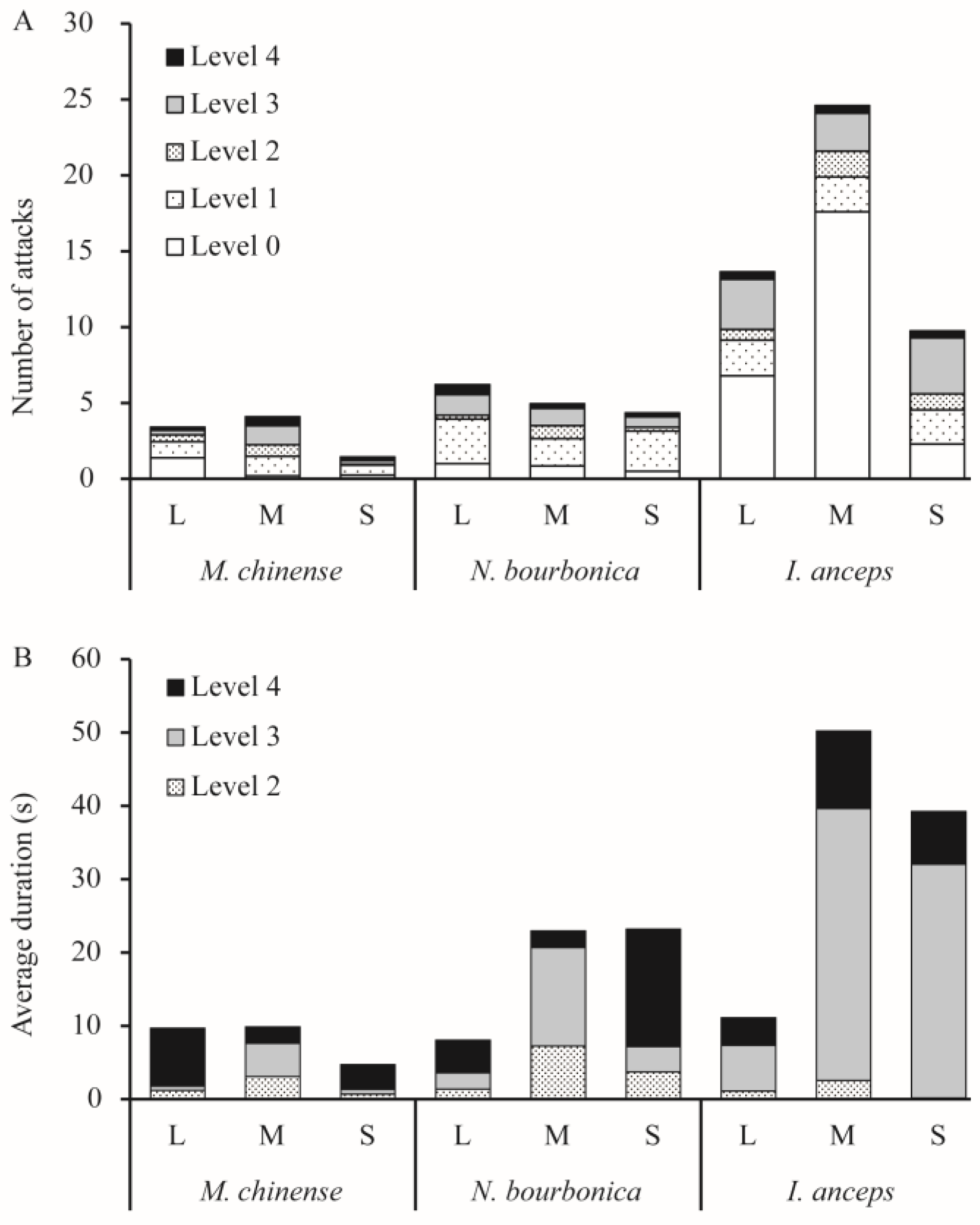
When meeting S. invicta, N. bourbonica typically run rapidly, exhibiting few avoidance or defensive behaviors. They showed on average only 1–2 aggressive acts of level 3 (threatening or biting quickly) and level 4 (fighting) (Figure 1A) evoked by S. invicta. Fights between N. bourbonica and small-sized S. invicta lasted for 16 s on average (Figure 1B). However, with medium-sized S. invicta, the fight lengths shortened to only approximately 2 s, and the threatening (of S. invicta) extended to 13.5 s (Figure 1B). When interacting with large-sized S. invicta, both fights and threatening were shortened to less than 5 s. Aggressive acts (levels 1–4) were mostly initiated within 100 s after initiation of treatment, irrespective of the body size of S. invicta (Figure 2).
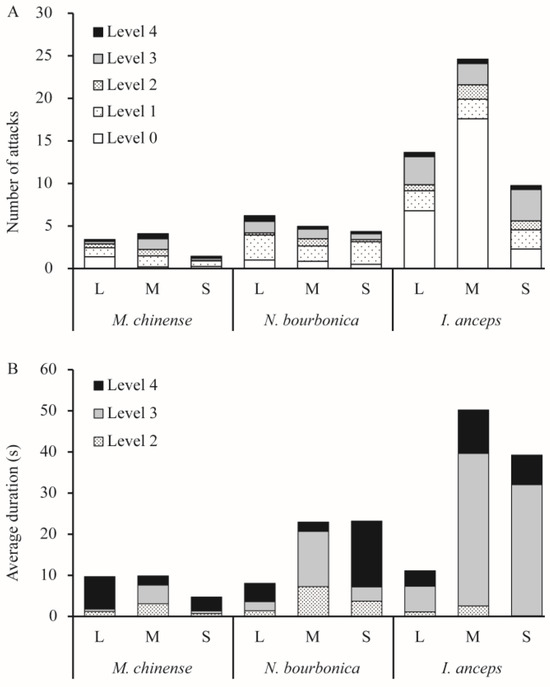
Figure 1.
Number of each level of aggression act (A) and the average duration of levels 2–4 (B) observed in dyad interactions between Solenopsis invicta and native ants. Level 0—avoidance, level 1—ants make short (≤1 s) antennal contact, level 2—ants make long (>1 s) antennal contact, level 3—ants exhibit threatening postures or bite quickly, and level 4—fighting.
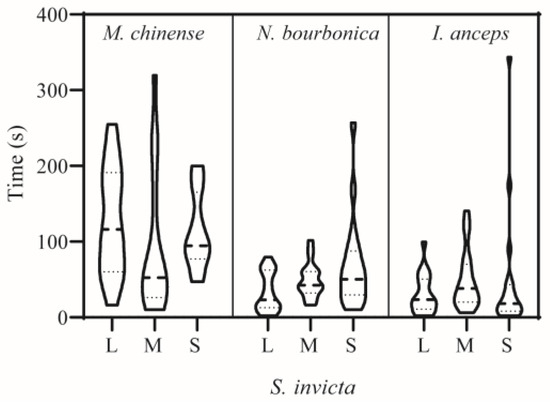
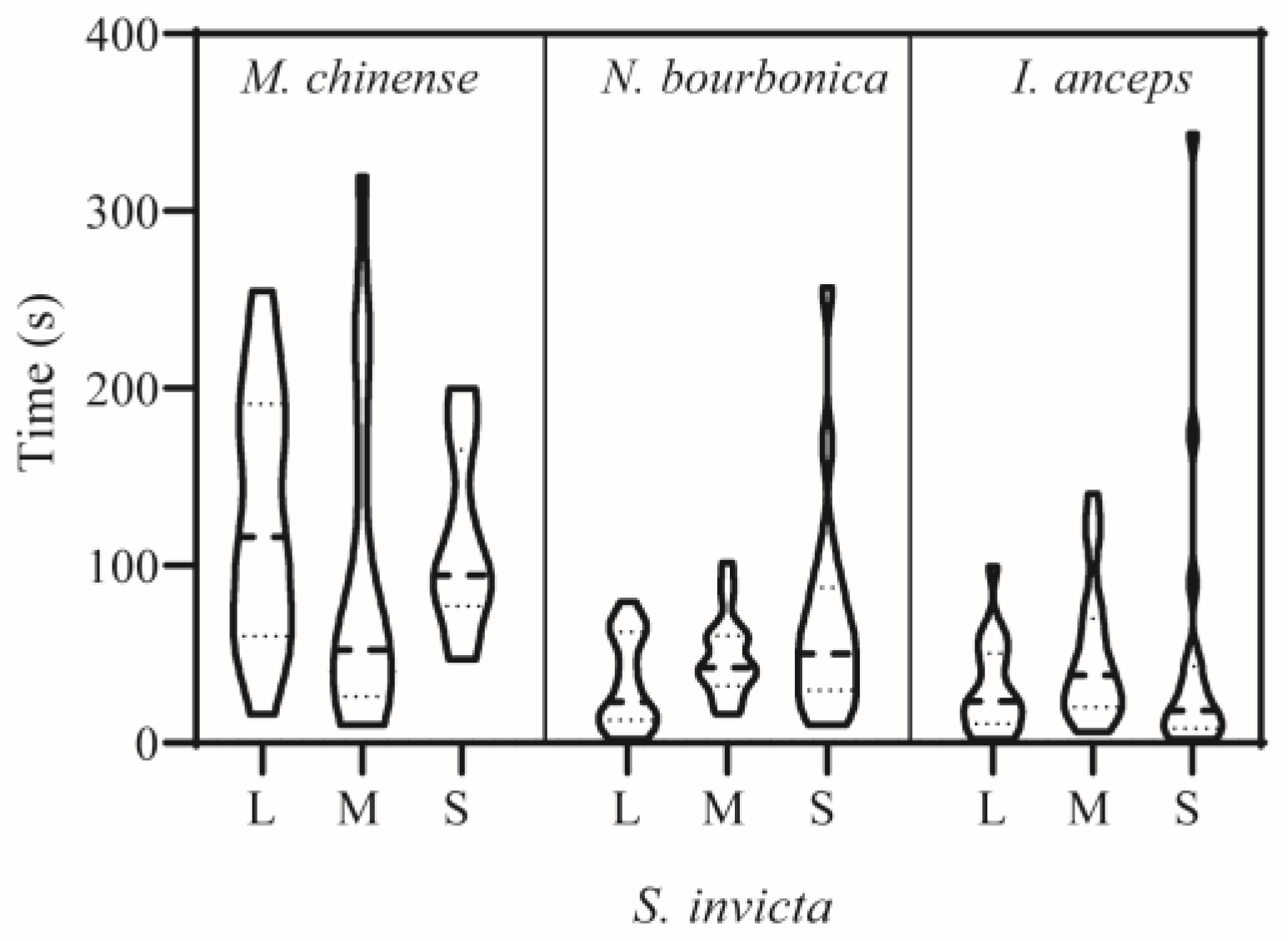
Figure 2.
Time of the first attack in dyad interactions between Solenopsis invicta and native ants. L, M, and S stand for large-, medium-, and small-sized S. invicta used in the trials, respectively. Refer to the note in Figure 1 for information on aggression levels. All repetitions are represented for all ant pairs displaying aggressive acts from levels 1–4.
Notably, most S. invicta avoided M. chinense, although the latter is much smaller in body size and is evidently slower than the former. We observed very few interactive acts of levels 2–4 (i.e., from making >1 s antennal contacts to fighting) in pairs with large- or small-sized S. invicta; the number of acts increased a little with medium-sized S. invicta (Figure 1A). Most of the observed acts lasted <5 s, and a few acts extended to nearly 8 s with large-sized S. invicta (Figure 1B). The time for initiation of acts was variable and often late (Figure 2).
I. anceps generally moved around rapidly and avoided contact with S. invicta, particularly when paired with large- or medium-sized S. invicta. In contrast, S. invicta typically exhibited a threatening posture (level 3) toward I. anceps 2.5–3.7 times on average in 5 min (Figure 1A), and the threatening behavior lasted much longer (>30 s) when the S. invicta was medium or small in size (Figure 1B). In such pairs, fights (level 4) could also take place, which lasted for 7–11s on average. Most acts were initiated within 100 s, irrespective of the body size of S. invicta (Figure 2).
3.1.2. Symmetrical Group-on-Group Interactions
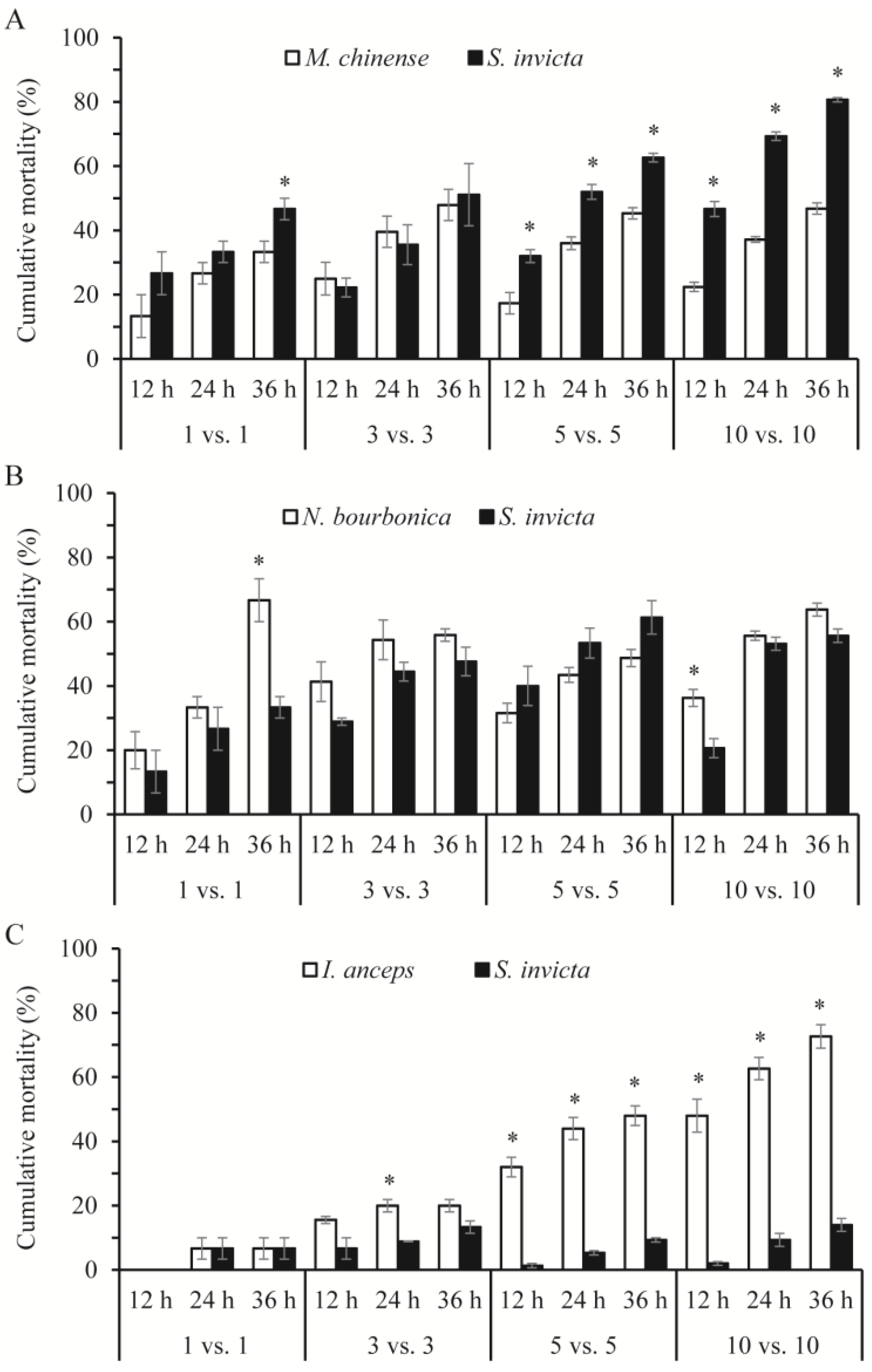
In most cases, the mortality rates of the native ants and S. invicta were related significantly or nearly significantly to the number of ants and the species of the native ant under observation (Table 2). With M. chinense, S. invicta suffered higher mortality than M. chinense (Figure 3A), although S. invicta initiated nearly all of the aggressive acts. Strikingly, in the 5 vs. 5 and 10 vs. 10 combinations with M. chinense, approximately 65% and 80% of S. invicta died (including dying individuals, the same below) within 36 h, which was a significantly rate higher than M. chinense (5 vs. 5: Z = −1.993, p = 0.046; 10 vs. 10: Z = −1.993, p = 0.046). In combinations of S. invicta and N. bourbonica, over half of both died in some trials, and their mortality rates did not differ significantly (p > 0.05), except in the 1 vs. 1 combination at 36 h and the 10 vs. 10 combination at 12 h, where significantly more N. bourbonica died (Z = −2.023, p = 0.043; and Z = −1.964, p = 0.050, respectively; Figure 3B). I. anceps died at higher proportions than S. invicta, as their number increased to 5–10, particularly in 10 vs. 10, where nearly 75% of I. anceps but only 15% of S. invicta had died by 36 h (Figure 3C).

Table 2.
Relations of the mortality rates of native ants and Solenopsis invicta at 36 h after treatment with the number of ants and species of native ant (factors) in group-on-group interaction tests.
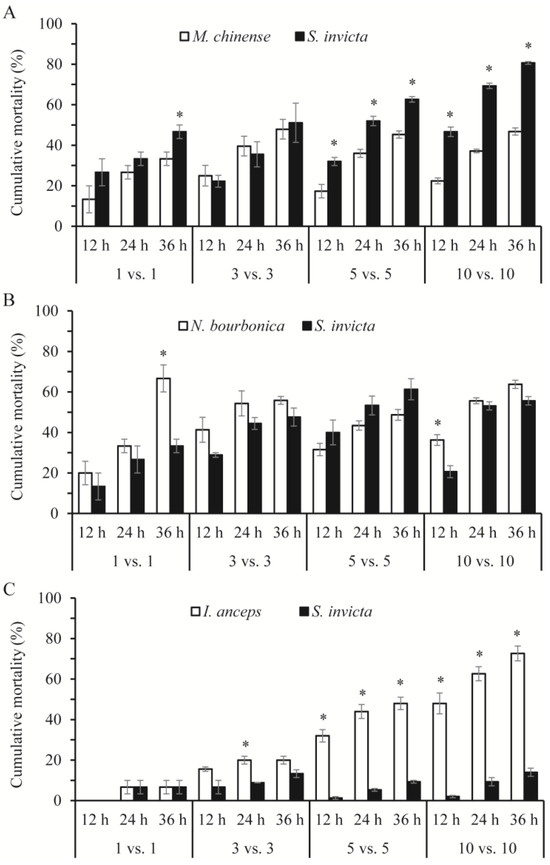
Figure 3.
Cumulative mortality of native ants and Solenopsis invicta at 12, 14, and 36 h in the group-on-group interaction tests, where groups of 1, 3, 5, and 10 native ants were placed with the same number of S. invicta. (A), Monomorium chinense + S. invicta; (B), Nylanderia bourbonica + S. invicta; and (C), Iridomyrmex anceps + S. invicta. The asterisk indicates a significant difference in mortality between the native ants and S. invicta (p < 0.05, Mann–Whitney U test).
3.1.3. Asymmetrical Group-on-One Interactions
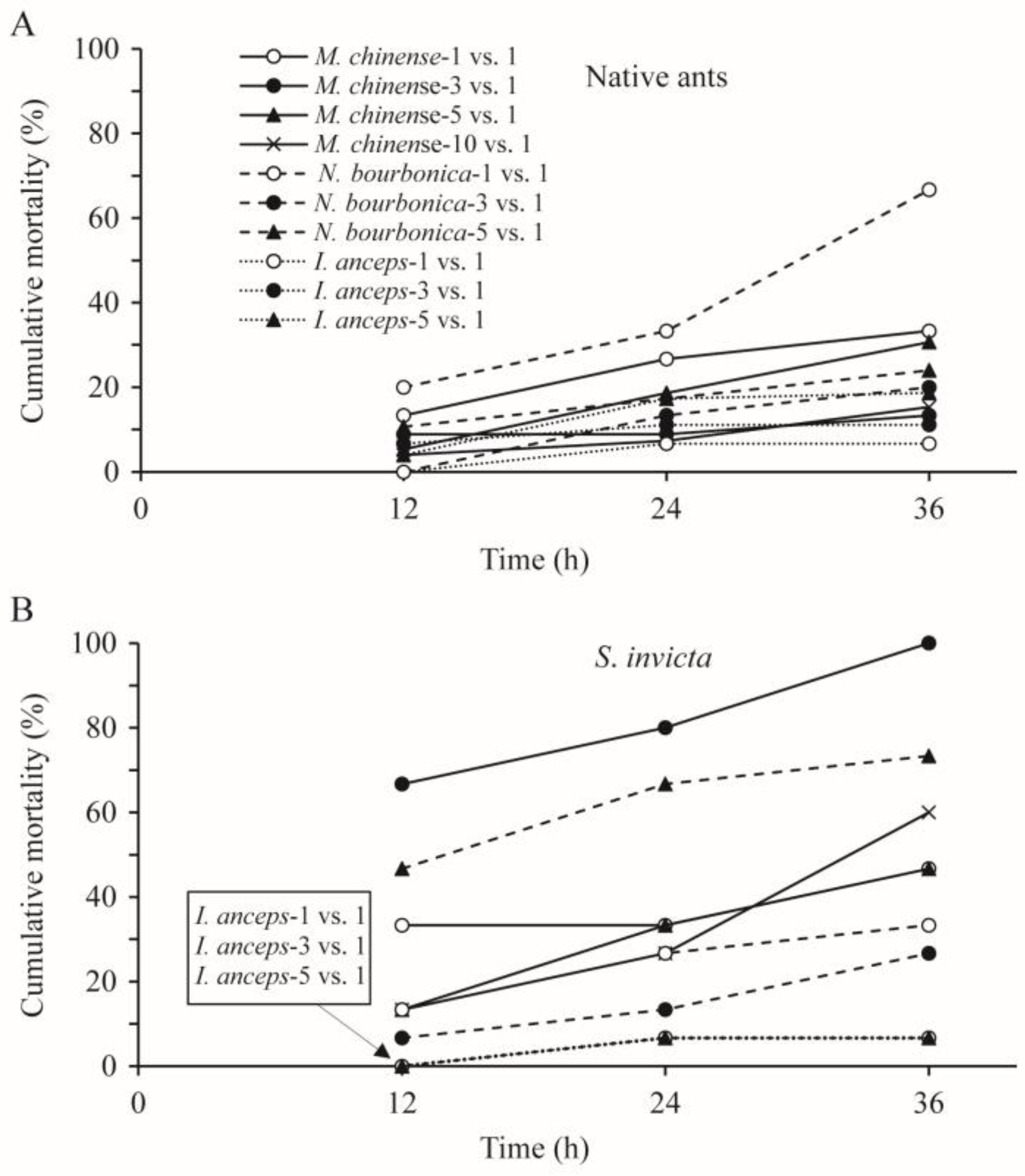
The cumulative mortality of each native ant species was mostly below 30% at 36 h after treatment (Figure 4A). The mortality of M. chinense related significantly with the number of ants used (p < 0.05; Table 3); nearly one-third of this type of ant died in the 1 vs. 1 and 5 vs. 1 trials, which was significantly higher than the mortality in the 3 vs. 1 and 10 vs. 1 trials (13–15%; p < 0.05). This relation was nearly significant for N. bourbonica and I. anceps (p = 0.059, and p = 0.057, respectively; Table 3). Nearly 70% of N. bourbonica were killed in the 1 vs. 1 combination (Figure 4A).
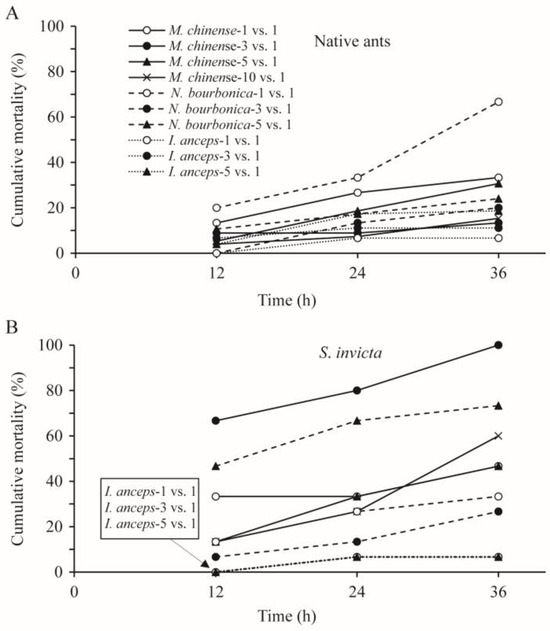
Figure 4.
Cumulative mortality of native ants (A) and Solenopsis invicta (B) at 12, 14, and 36 h in the group-on-one interaction tests, where groups of 1, 3, 5, and 10 native ants were placed independently with an individual S. invicta.

Table 3.
Relations of mortality rates of native ants and Solenopsis invicta at 36 h after treatment with the number of ants and species of native ants (factors) subjected to group-on-one interaction tests.
The mortality rate of S. invicta was significantly related to the number of N. bourbonica, but not to the number of M. chinense and I. anceps (Table 3). In trials with five N. bourbonica or three M. chinense, nearly 75% and 100% of S. invicta were killed within 36 h, respectively, which was significantly higher than the corresponding native ant (24% and 13%, respectively; p < 0.05). Considerable S. invicta mortality was also observed (27–60% within 36 h) in all of the other trials using N. bourbonica and M. chinense. In contrast, in all of the combinations with I. anceps, <10% of S. invicta died (Figure 4B).
Both at 1 vs. 1 and 3 vs. 1, the mortality of the native ants depended significantly on their species (p < 0.05). More N. bourbonica were killed than the other two native ants (Figure 4A). The relation with native ant species was not significant for 5 vs. 1 (p = 0.223; Table 3). For each combination, the mortality of S. invicta was significantly related to the species of native ant (p < 0.05; Table 3). More S. invicta were killed, as mentioned above, by N. bourbonica at 5 vs. 1 and by M. chinense at 3 vs. 1, while fewer were killed by I. anceps in all combinations.
3.2. Abundance of Native Ants in Various Habitats
Using baited vials, we collected a total of 17 batches with 121,139 native ants from the woodlands, road green belts on roadsides, grasslands, and farmland. They belonged to 10 species and three subfamilies, Myrmicinae, Dolichoderinae, and Formicinae (Table 4). M. chinense, N. bourbonica, and I. anceps were captured in 27.9%, 24.8%, and 19.5% of the 1590 baits that trapped ants, accounting for 41.1%, 10.9%, and 16.6% of the total ants trapped, respectively. We also found many other native ants, including Tetramorium caespitum L., Ochetellus glaber Mayr, and one Pheidole species, which were captured by 12.3%, 9.4%, and 5.0% of the baits, respectively, and Nylanderia flavipes Smith, Pristomyrmex punctatus Smith, another Pheidole species, and one Crematogaster species, each captured by <1% of the baits (Table 4).

Table 4.
Taxon composition and relative abundance of native ants collected from a location after the removal of Solenopsis invicta (Redwood Town and nearby areas, Longyou, Zhejiang, China, mid-September 2019 to late July 2022).
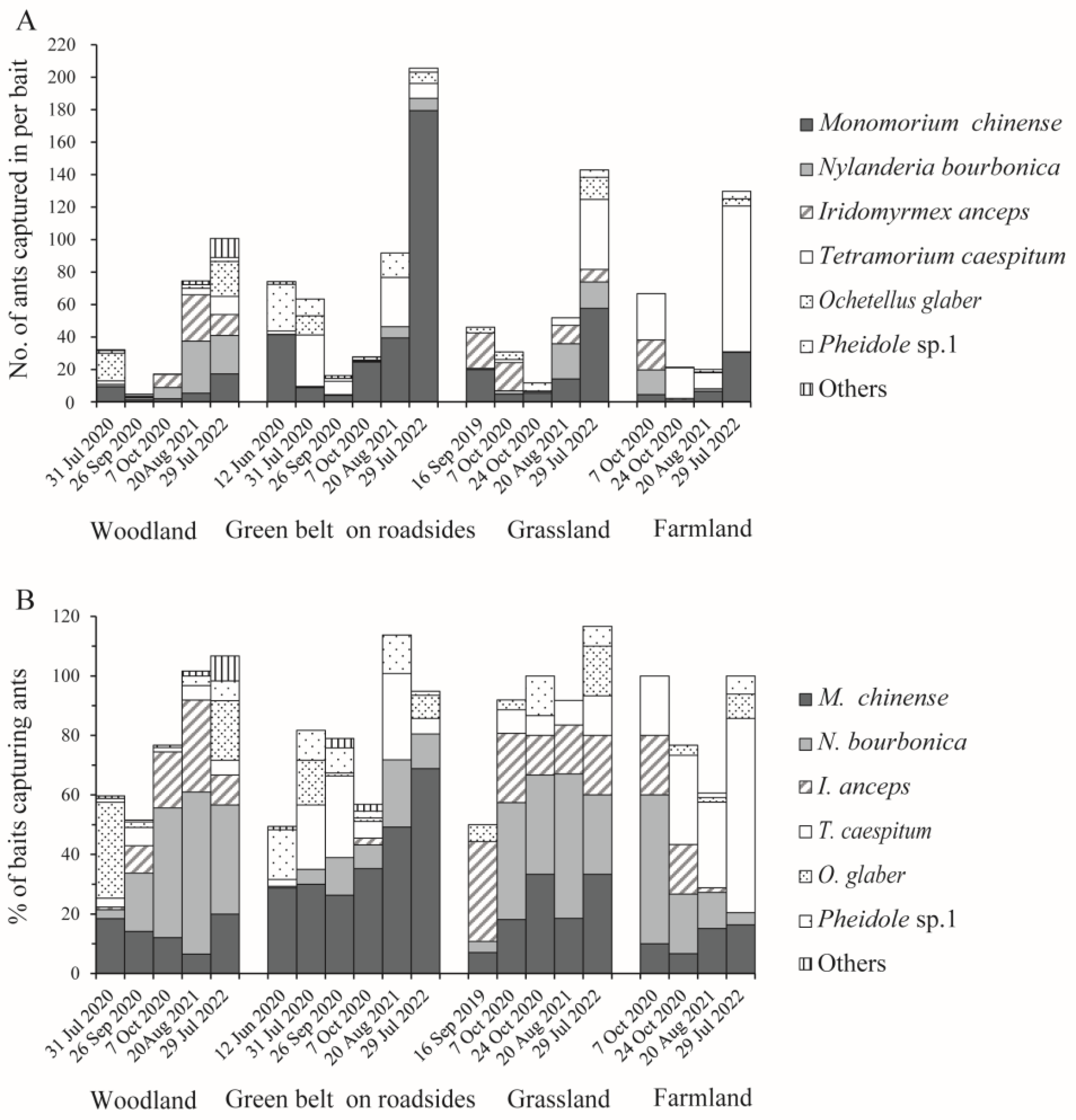
Within batches, M. chinense, T. caespitum, N. bourbonica, and I. anceps were more abundant than the other ants, which accounted for 30.7%, 21.0%, 18.0%, and 15.7%, on average, of the total number of ants trapped, and 23.0%, 13.3%, 25.6%, and 11.6% of the total number of baits that trapped ants, respectively. More M. chinense were found in the green belt on roadsides, reaching >40 individuals per bait in one-half (3/6) of the batches (Figure 5A). In contrast, both N. bourbonica and I. anceps tended to be more abundant in woodlands and grasslands (and I. anceps was rarely found in the road green belt), and T. caespitum occurred dominantly in farmland (Figure 5A,B). When comparing between batches, M. chinense and N. bourbonica were more abundant in the later batches relative to the earlier batches (Figure 5A).
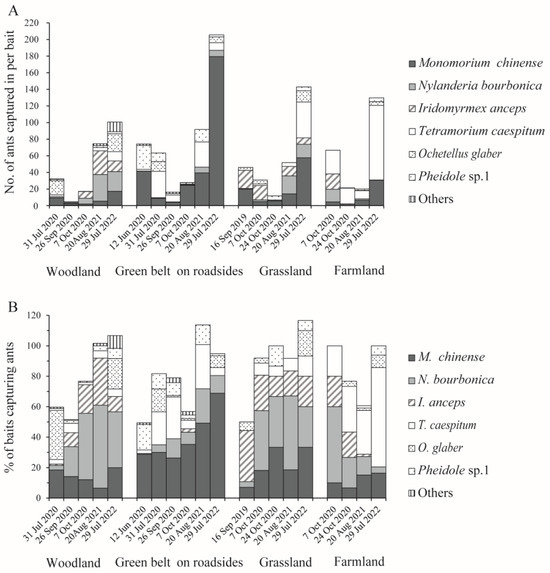
Figure 5.
The average number of native ants trapped per bait (A) and the percentage of baits that trapped them (B) in the four types of habitats under investigation.
3.3. Seasonal Dynamics of Native Ants Coexisting with S. invicta
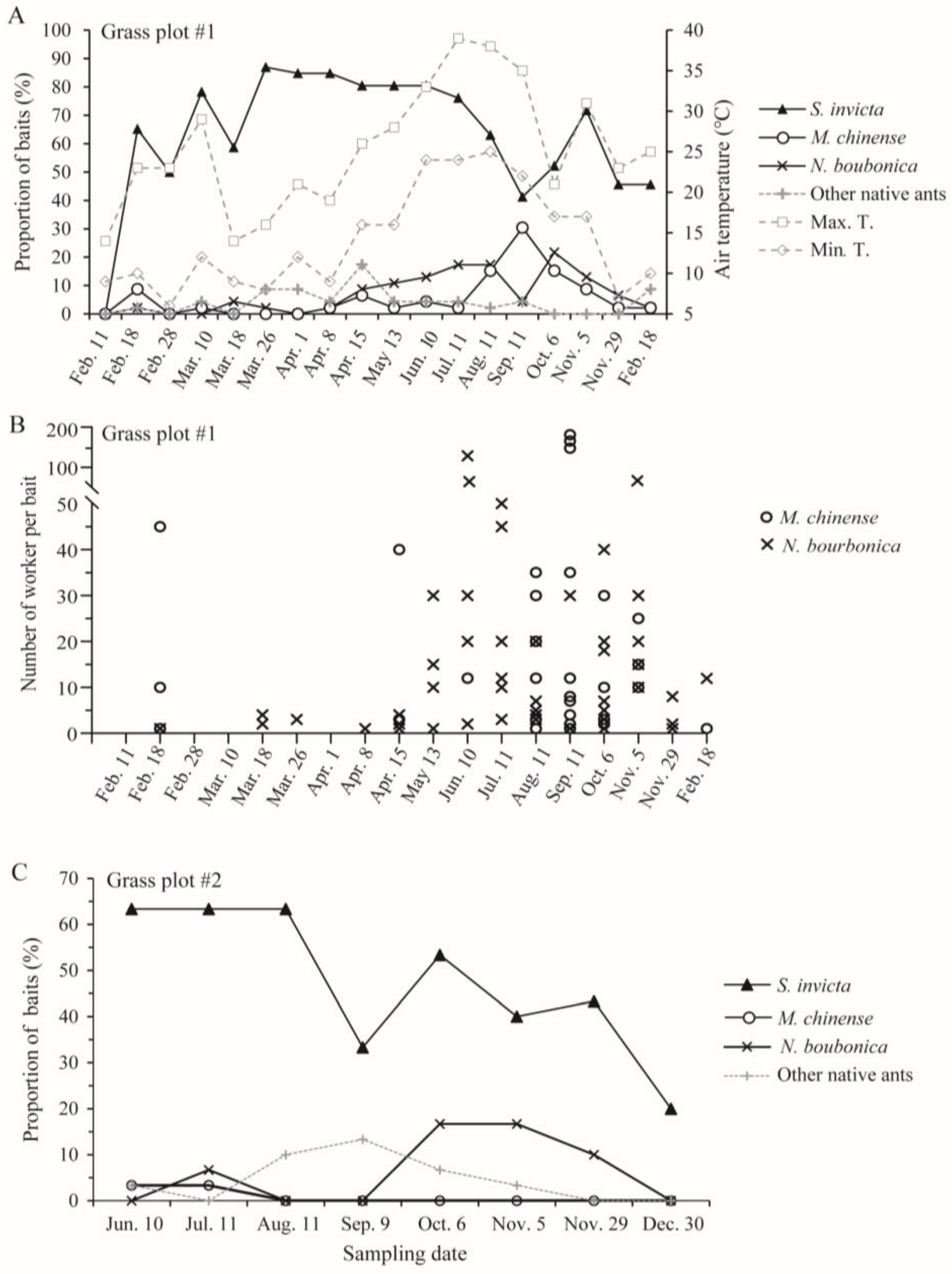
From 18 February 2023 to 18 February 2024, we trapped a large number of S. invicta in the investigated grassland plot (#1) in at least 40% of the baited traps (Figure 6A,B; no ants were trapped on 11 February 2023 due to low winter temperatures). Both M. chinense and N. bourbonica were trapped on at least two-thirds of the 18 sampling dates. M. chinense was not abundant before early July, and its peak occurred from mid-August to early October, when it was trapped by 30% of the baits. N. bourbonica was not abundant before mid-April (trapped by <10% baits), while it increased steadily after this, becoming trapped in ca. 20% of baits from July to October. Very low N. bourbonica trapping rates occurred on 11 September for unknown reasons. Both M. chinense and N. bourbonica decreased from early November (Figure 6A,B).
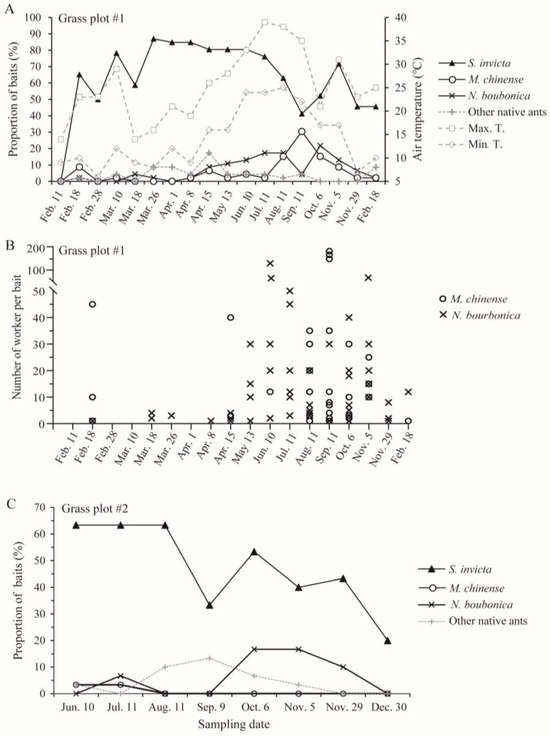
Figure 6.
Seasonal dynamics of native ants coexisting with S. invicta in two grass plots near Jinhua New Energy Vehicle Town, as revealed by the proportions of baits trapping different ants (A,C) and the number of the two native ants (M. chinense and N. bourbonica) in the vials in one grass plot (B).
In the other grassland plot (#2), S. invicta was trapped by 33–63% of the baits from early June to late November. Few M. chinense were trapped. N. bourbonica were trapped at much higher rates, occurring in nearly 20% of the baits during October and early November and decreasing from late November (Figure 6C). Combined with the results from plot #1, we found that both M. chinense and N. bourbonica may vary greatly between different grasslands in their abundance.
Both M. chinense and N. bourbonica were found in other locations, again with great variance in their abundance, and each could coexist with S. invicta (Table 5). Even at locations heavily invaded by S. invicta (trapped by 100% of baits), M. chinense and N. bourbonica could be present and could occur simultaneously with S. invicta in the same baits. These two native ants could also occur simultaneously in the same baits.

Table 5.
Percentage of baits trapping various ants in some habitats of Zhejiang (ZJ) and Jiangxi (JX) provinces invaded by S. invicta.
3.4. Spatial Distribution of Native Ants Coexisting with S. invicta
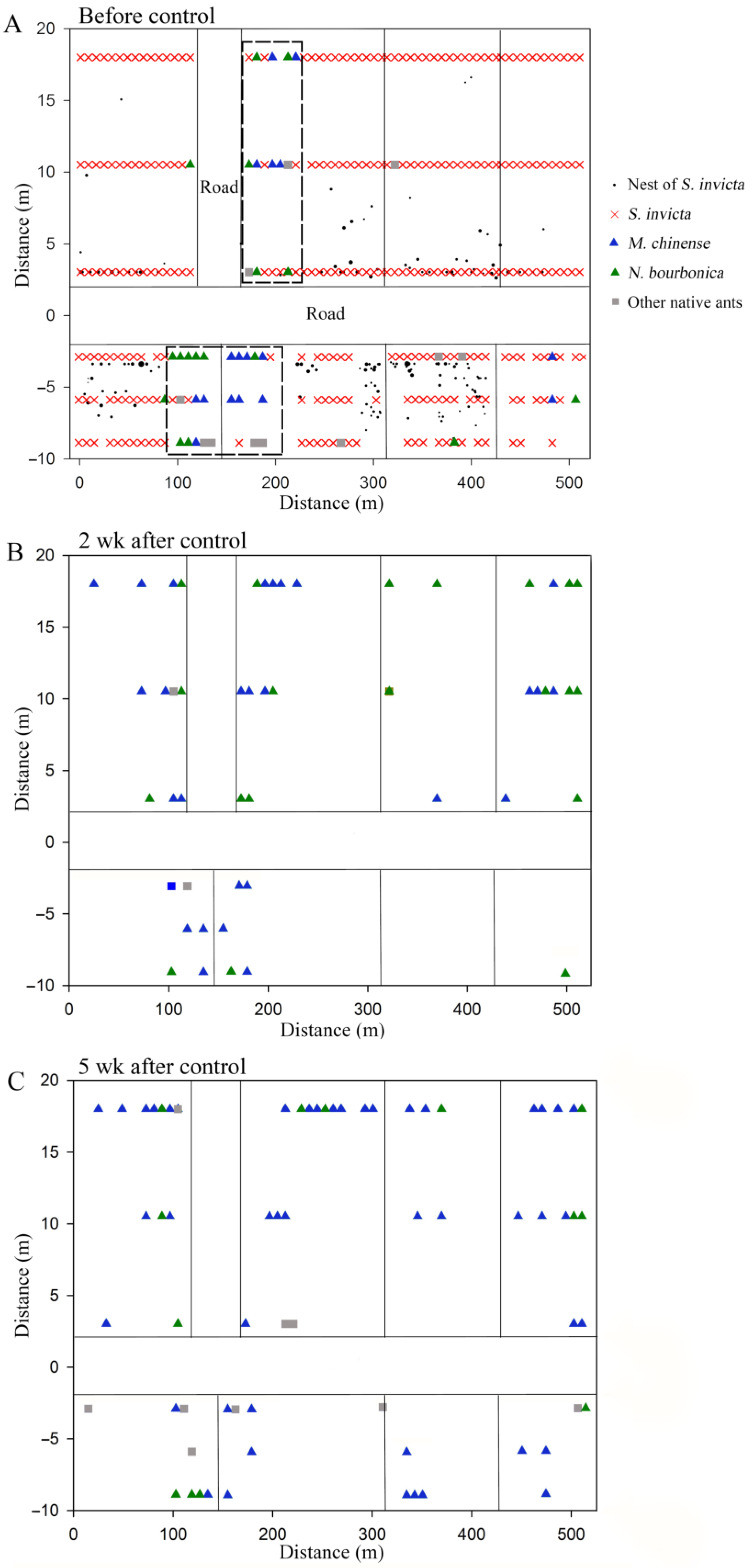
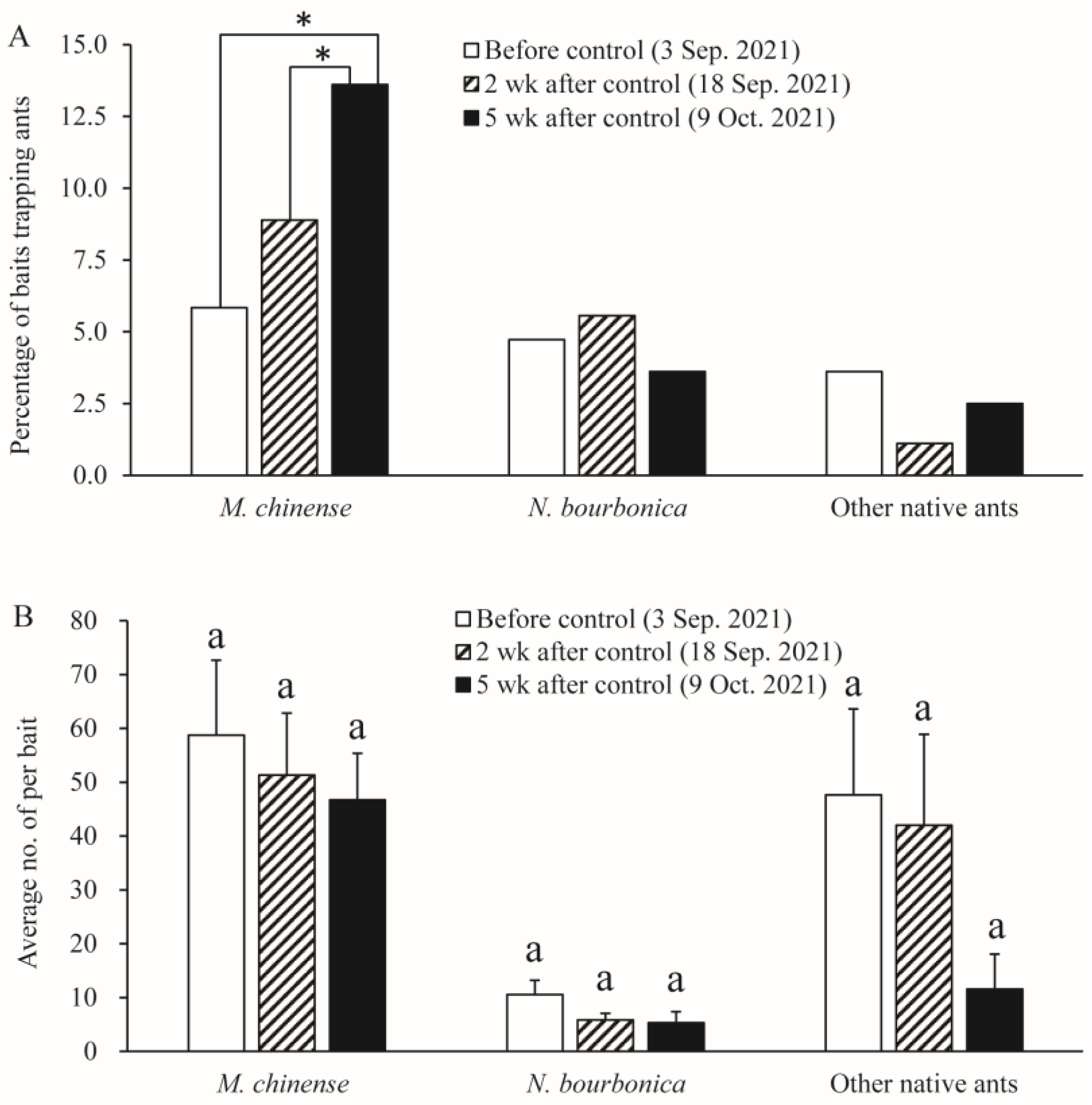
We found six native ant species in the grassland plot present with S. Invicta, namely M. chinense, N. bourbonica, O. glaber, T. caespitum, Pheidole sp., and Solenopsis sp., with the former two species being dominant. Before the control of S. invicta, most native ants were restricted to sites close to the margins of the investigated plots, and a majority of M. chinense and N. bourbonica were distributed in only two patches (indicated by the dashed rectangle in Figure 7A). However, 2 weeks and 5 weeks after S. invicta control, both M. chinense and N. bourbonica spread to a larger range (Figure 7B,C). Particularly, M. chinense was trapped by 13.6% of the baits 5 weeks after control, which was significantly higher (5.8%) than before control (χ2 = 12.406, df = 1, p < 0.001, chi-square test) and the percentage (8.9%) at 2 weeks after S. invicta removal (χ2 = 4.020, df = 1, p = 0.029; Figure 8A). Moreover, the number of M. chinense trapped per bait did not decline significantly despite the spatial dilution of the population (Figure 8B).
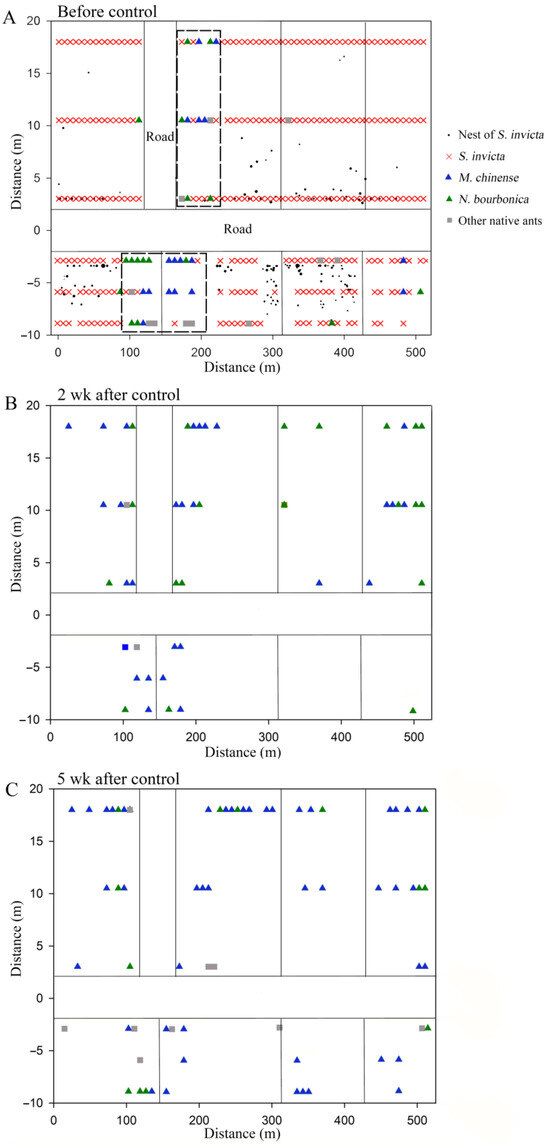
Figure 7.
Spatial distribution of native ant species before control of S. invicta (A) and 2 weeks (B) and 5 weeks (C) after control of S. invicta in the grasslands on the banks of the Dongyangjiang River in Jinghua, Zhejiang province. The dashed rectangle in (A) indicates the major patches with M. chinense and N. bourbonica. Note that except for the parts specifically labeled, the vertical lines in the distribution map represent cement roads with a width of approximately 3 m, and the size of the black solid circles in (A) corresponds to the actual size of the red imported fire ant nests.
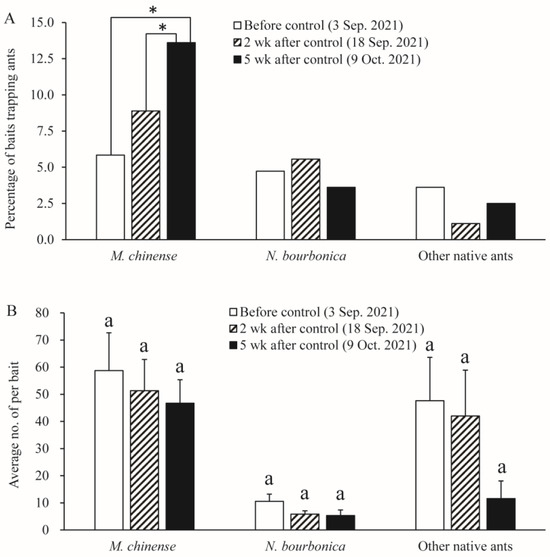
Figure 8.
Abundance of native ants before control of S. invicta and 2 weeks and 5 weeks after control of S. invicta in the grasslands on the banks of the Dongyangjiang River in Jinghua, Zhejiang province. This was revealed by the proportions of baits trapping different ants (A) and the number of ants per bait (B). The asterisk indicates a significant difference between dates. Bars with same letter indicate no significant difference from each other (Tukey’s test, p > 0.05).
4. Discussion
Native ants have long attracted attention for their potential role in S. invicta control, but available data are largely limited to the behavioral performances of related ants, with little regarding their distribution and seasonal dynamics. Here, we not only identified the ants that show aggression towards S. invicta, but also determined the type of habitats abundant with them, their seasonal abundance in the preferred habitat, and their spatial distribution before and after S. invicta removal. This, to our knowledge, is one of the few comprehensive studies in this field, and the first report in Eastern China, a region heavily invaded by S. invicta in some habitats.
We showed that, as M. chinense, N. bourbonica, and I. anceps encounter S. invicta in a limited space, threatening and fights may take place (Table 1; Figure 1). As a result, a substantial proportion of S. invicta can be killed by M. chinense and N. bourbonica (Figure 3A,B and Figure 4B). Moreover, M. chinense and N. bourbonica are abundant in some habitats (Table 4; Figure 5), where they are able to maintain populations instead of being displaced by S. invicta (Figure 6 and Figure 7). This suggests that, if S. invicta arrives at sites it prefers (e.g., grassland and lawn) that are abundant with M. chinense and N. bourbonica, its establishment and subsequent local expansion might be delayed or reduced.
M. chinense showed impressive aggression during this study, as irrespective of its much smaller body size and lower movement speed relative to S. invicta, it often fought with large S. invicta (lasting for 8 s, as observed in dyad interactions, Figure 1B) and killed more S. invicta than itself was killed by S. invicta (Figure 3A). M. chinense needed only three individuals to kill one S. invicta (Figure 4B). This result was in accordance with Chen et al. [23], who reported that M. chinense can kill S. invicta through chemical defense (venom). Our experimental design differed from Chen et al. [23]. We used combinations of different numbers of ants, allowing us to observe more aggression-related detail in the behaviors, whereas Chen et al. [21] used an equal biomass of the two ants. Combining the results from other reports (e.g., [21]), it appears that M. chinense shows aggression or defensiveness as a crucial tactic to cope with S. invicta. Interestingly, similar findings have also been reported for M. chinense when encountering the Argentine ant, Linepithema humile Mayr, another invasive ant species [35]. Thus, the success of M. chinense in habitats invaded by alien ants might be a common, if not ubiquitous, phenomenon.
The behavior of M. chinense described above might be enhanced in groups (this is often observed in this ant, possibly due to intraspecific cooperation). As indicated in the trials of the 10 vs. 10 combination, where M. chinense often stayed in groups with little movement, S. invicta suffered a higher mortality relative to the combinations with fewer ants, i.e., 5 vs. 5 (Figure 3A). On the other hand, the staying-together behavior of M. chinense might induce certain responses in S. invicta. For instance, in the survey with three (fewer) M. chinense and one S. invicta, the S. invicta ant may have chosen to attack more often, thereby suffering a higher mortality rate. In contrast, in the surveys with five or ten (more) M. chinense and one S. invicta, the S. invicta possibly reduced its attacks and thus suffered a lower mortality rate (Figure 4B). In other words, the outcomes of M. chinense and S. invicta interactions were probably highly density dependent.
N. bourbonica occurs in various habitats, particularly woodlands and grasslands (Figure 5), and is often found in the vicinity and even on the surface of S. invicta mounds (based on observation). This is in line with previous reports that N. bourbonica may be a dominant species in local ant communities invaded by S. invicta [36,37]. In fact, N. bourbonica, native to Southeastern Asia and introduced to other regions (such as parts of North America and the West Indies [36]) is an invasive species itself and is considered a widespread household and agricultural pest in some regions [38,39,40]. There are several factors related to its success, for example, the ability to adapt to frequent disturbance and marginal habitats [41] and the use of chemicals when fighting with other ants (based on observation). However, N. bourbonica has also been reported to be a weak competitor to S. invicta in some regions [37]. Thus, the performances of N. bourbonica, and its interactions with S. invicta, may differ in different habitats or regions. Other species of Nylanderia, some of which have been reported to be able to take advantage of spaces not yet occupied by S. invicta [42], were not found in our study.
I. anceps showed no aggression and was sensitive to S. invicta attack (Figure 3C). This was consistent with our observations that I. anceps rarely occurs in the vicinity of S. invicta mounds (based on observation), and with another report that Iridomyrmex spp. generally occurs in lower numbers in the areas invaded by invasive ants, e.g., Anoplolepis gracilipes Smith, L. humile, and Pheidole megacephala Fabricius [15]. However, some Iridomyrmex species have been documented as having a strong competitive ability, and regions rich with them may be resistant to ant invasions ([1], and the references therein). In Australia, for example, Iridomyrmex spp. are thought to have limited the spread of invasive ants [43,44,45] due to their widespread distribution, high abundance, activity levels, and behavioral aggression [46]. Some Iridomyrmex ants can recover rapidly after the removal of S. invicta [47]. Thus, considering that Iridomyrmex often dominate warm, open habitats in terms of both number and function ([43] and this study), some of its species may have the potential to suppress S. invicta invasion, although this has not been found yet in China.
All of the other native ants that we found, including T. caespitum, O. glaber, and the two Pheidole species, did not show evident aggression (unpublished data). Despite this, attention needs to be given to Pheidole ants, as some have been reported to be very aggressive, and even to kill or prey upon invasive ants [21,48,49], or show superiority in exploitative competition over invasive ants [50]. In addition, some Pheidole species are ubiquitous in the regions invaded by S. invicta or other ants [51,52,53,54]. Thus, Pheidole ants of interest could exist elsewhere.
Native ants were present near the margins of plots before S. invicta control (Figure 7), and some (e.g., M. chinense) rebounded rapidly after control (Figure 8A), implying that marginal areas may serve as a refuge for native ants. We suggest giving more research focus to habitat margins for their significance to native ants. Moreover, the heterogeneity within habitats should also be analyzed because some of the variables could have important effects on native ants’ abundance, including disturbance, micro-climate, soil type, vegetation cover, and the partition of food resources [55,56,57,58,59].
It is well known that both M. chinense and N. bourbonica have a broad distribution range in China. They exist in (at least) Eastern, Middle, and Southern China [30,59,60], and specifically, M. chinense has been reported to coexist with S. invicta in different regions [20,23,61,62] and habitats ([63] and this study). Thus, this could be an example of the coexistence of native ants with invasive ants, which has been reported previously at the regional scale [64].
In conclusion, we identified two native ants, M. chinense and N. bourbonica, that show aggression towards S. invicta and possess the potential to suppress S. invicta invasion. They could be integrated with supplemental control methods that are under development, such as botanical insecticides and pathogens [65,66,67]. Our study is one of the few involving the interspecific interactions of native ants with S. invicta, and we hope our results will enrich the knowledge in this field. Further research is needed to understand the behavior of M. chinense and N. bourbonica at the colony level, their dynamics during different stages of S. invicta invasion, and as mentioned above, the effects of within-habitat factors. Future research may focus on grasslands and lawns, which are favored by S. invicta and which probably contain an abundance of M. chinense and/or N. bourbonica.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/insects15080582/s1: Table S1: Information about NCBI GenBank accession numbers of ant species in this study.
Author Contributions
Conceptualization, M.N. and M.J.; methodology, M.N., X.Y. and M.J.; investigation, M.N., X.Y., Y.Z. and Y.W.; data curation, M.N. and X.Y.; writing—original draft preparation, M.N.; writing—review and editing, M.J.; supervision, M.J.; funding acquisition, M.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Pioneer and Leading Goose R&D Program of Zhejiang (Grant No. 2023C02025).
Data Availability Statement
Data are contained within the article or Supplementary Materials. The original contributions presented in the study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
The data that support the findings of this study are available upon reasonable request from the first and corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Holway, D.A.; Lach, L.; Suarez, A.V.; Tsutsui, N.D.; Case, T.J. The causes and consequences of ant invasions. Annu. Rev. Ecol. Evol. Syst. 2002, 33, 181–233. [Google Scholar] [CrossRef]
- Porter, S.D.; Savignano, D.A. Invasion of polygyne fire ants decimates native ants and disrupts arthropod community. Ecology 1990, 71, 2095–2106. [Google Scholar] [CrossRef]
- Jemal, A.; Hughes-Jones, M. A review of the red imported fire ant (Solenopsis invicta Buren) and its impacts on plant, animal, and human health. Prev. Vet. Med. 1993, 17, 19–32. [Google Scholar] [CrossRef]
- Allen, C.R.; Epperson, D.M.; Garmestani, A.S. Red imported fire ant impacts on wildlife: A decade of research. Am. Midl. Nat. 2004, 152, 88–103. [Google Scholar] [CrossRef]
- Wang, L.; Xu, Y.J.; Zeng, L.; Lu, Y.Y. Impact of the red imported fire ant Solenopsis invicta Buren on biodiversity in South China: A review. J. Integr. Agr. 2019, 18, 788–796. [Google Scholar] [CrossRef]
- Buren, W.F.; Allen, G.E.; Whitcomb, W.H.; Lennartz, F.E.; Williams, R.N. Zoogeography of the imported fire ants. J. N. Y. Entomol. 1974, 82, 113–124. [Google Scholar]
- Wetterer, J.K. Exotic spread of Solenopsis invicta Buren (Hymenoptera: Formicidae) beyond North America. Sociobiology 2013, 60, 50–55. [Google Scholar] [CrossRef]
- Menchetti, M.; Schifani, E.; Alicata, A.; Cardador, L.; Sbrega, E.; Toro-Delgado, E.; Vila, R. The invasive ant Solenopsis invicta is established in Europe. Curr. Biol. 2023, 33, R896–R897. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Lu, Y.Y.; He, X.F.; Zhang, W.Q.; Liang, G.W. Identification of red imported fire ant Solenopsis invicta to invade mainland China and infestation in Wuchaun, Guangdong. Chin. J. Appl. Entomol. 2005, 42, 144–148, (In Chinese with English abstract). [Google Scholar]
- Ministry of Agriculture and Rural Affairs of the People’s Republic of China (MARA of China). List of Administrative Regions for the Distribution of Agricultural Plant Quarantine Pests in China. Available online: http://www.moa.gov.cn/govpublic/ZZYGLS/202309/t20230904_6435699.htm (accessed on 14 July 2024).
- Wang, L.; Chen, K.W.; Feng, X.D.; Wang, X.L.; Lu, Y.Y. Long-term prediction of red imported fire ant (Solenopsis invicta Buren) expansion in mainland China. J. Environ. Entomol. 2022, 44, 339–344, (In Chinese with English abstract). [Google Scholar]
- Li, M.; Zhao, H.X.; Xian, X.Q.; Zhu, J.Q.; Chen, B.X.; Jia, T.; Wang, R.; Liu, W.X. Geographical distribution pattern and ecological niche of Solenopsis invicta Buren in China under climate change. Diversity 2023, 15, 607. [Google Scholar] [CrossRef]
- Du, C.J.; Wang, L.; Lu, Y.Y.; Jia, C.J.; Lin, X.P.; Xu, S.C.; Wen, X.J.; Wang, C. Research advances in occurrence characteristics and monitoring and control strategies of Solenopsis invicta (Hymenoptera: Formicidae) in forests, grasslands, wetlands and urban green spaces. Acta Entomol. Sin. 2023, 66, 1128–1138, (In Chinese with English abstract). [Google Scholar]
- Wan, F.H.; Yang, N.W. Invasion and management of agricultural alien insects in China. Annu. Rev. Entomol. 2016, 61, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Wittman, S.E. Impacts of invasive ants on native ant communities (Hymenoptera: Formicidae). Myrmecol. News 2014, 19, 111–123. [Google Scholar]
- Wang, L.; Zeng, L.; Xu, Y.Y.; Lu, Y.Y. Prevalence and management of Solenopsis invicta in China. NeoBiota 2020, 54, 89–124. [Google Scholar] [CrossRef]
- Morrison, L.W. Mechanisms of interspecific competition among an invasive and two native fire ants. Oikos 2000, 90, 238–252. [Google Scholar] [CrossRef]
- Callcott, A.M.A.; Oi, D.H.; Collins, H.L.; Williams, D.F.; Lockley, T.C. Seasonal studies of an isolated red imported fire ant (Hymenoptera: Formicidae) population in eastern Tennessee. Environ. Entomol. 2000, 29, 788–794. [Google Scholar] [CrossRef]
- Song, Z.D.; Lu, Y.Y.; Xu, Y.J.; Huang, J.; Liang, G.W.; Zeng, L. Dynamic of native ants on the lawn with the invasion of Solenopsis invicta Buren. Acta Ecol. Sin. 2010, 39, 1287–1295, (In Chinese with English abstract). [Google Scholar]
- Qi, G.J.; Huang, Y.F.; Cen, Y.J.; Lu, L.H. Effects of the red imported fire ant, Solenopsis invicta Buren, on the structure and diversity of the ant community in a human disturbed area. Chin. J. Appl. Entomol. 2015, 52, 1368–1375, (In Chinese with English abstract). [Google Scholar]
- Chen, Y.; Kafle, L.; Shih, C. Interspecific competition between Solenopsis invicta and two native ant species, Pheidole fervens and Monomorium chinense. J. Econ. Entomol. 2011, 104, 614–621. [Google Scholar] [CrossRef]
- Rao, A.; Vinson, S.B. Invasion of red imported fire ant nests by selected predatory ants: Prospects of utilizing native ants in fire ant management. Southwest. Entomol. 2002, 25, 61–70. [Google Scholar]
- Rao, A.; Vinson, S.B. Ability of resident ants to destruct small colonies of Solenopsis invicta (Hymenoptera: Formicidae). Environ. Entomol. 2004, 33, 587–598. [Google Scholar] [CrossRef]
- Calixto, A.A.; Harris, M.K.; Barr, C. Resurgence and persistence of Dorymyrmex flavus after reduction of Solenopsis invicta Buren with a broadcast bait. Environ. Entomol. 2007, 36, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Bolton, B. An Online Catalog of the Ants of the World. Available online: https://antcat.org (accessed on 14 April 2024).
- Nowbahari, E.; Fénéron, R.; Malberbe, M. Effect of body size on aggression in the ant, Cataglyphis niger (Hymenoptera: Formicidae). Aggress. Behav. 1999, 25, 369–379. [Google Scholar] [CrossRef]
- Buczkowski, G.; Bennett, G.W. Aggressive interactions between the introduced Argentine ant, Linepithema humile and the native odorous house ant, Tapinoma sessile. Biol. Invasions 2008, 10, 1001–1011. [Google Scholar] [CrossRef]
- Grangier, J.; Le Breton, J.; Dejean, A.; Orivel, J. Coexistence between Cyphomyrmex ants and dominant populations of Wasmannia auropunctata. Behav. Process. 2007, 74, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Haight, K.L. Worker size and nest defense in Solenopsis invicta (Hymenoptera: Formicidae). Ann. Entomol. Soc. Am. 2010, 103, 678–682. [Google Scholar] [CrossRef]
- Wu, J.; Wang, C.L. The Ants of China; China Forestry Publishing House: Beijing, China, 1995; pp. 1–214. (In Chinese) [Google Scholar]
- Zhou, S.Y. The Ants in Guangxi; Guangxi Normal University Press: Guangxi, China, 2001; pp. 1–255. (In Chinese) [Google Scholar]
- Zhou, S.Y.; Chen, Z.L. Ecological Illustrated of Common Ant Species from China; Henan Science and Technology Press: Henan, China, 2020; pp. 1–321. (In Chinese) [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome coxidase subunit I from diverse metazoan invertebrates. Molecu. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- SPSS. Base 25.0 User’s Guide; SPSS: Chicago, IL, USA, 2017. [Google Scholar]
- Park, S.H.; Kim, I.; Kim, C.J.; Choi, M.B. Settlement and population competition assessments of invasive Argentine ants (Linepithema humile) in South Korea. Bioinvasions Rec. 2023, 12, 545–554. [Google Scholar] [CrossRef]
- Wetterer, J.K. New-World spread of the Old-World robust crazy ant, Nylanderia bourbonica (Forel) (Hymenoptera: Formicidae). Sociobiology 2022, 69, e7343. [Google Scholar] [CrossRef]
- Roldán, E.L.; Beuzelin, J.M.; VanWeelden, M.T.; Cherry, R.H. Abundance of the sugarcane borer (Lepidoptera: Crambidae) and foraging ants (Hymenoptera: Formicidae) in sugarcane grown on organic and mineral soils in Florida. Environ. Entomol. 2020, 49, 473–481. [Google Scholar] [CrossRef]
- Klotz, J.H.; Mangold, J.R.; Vail, K.M.; Davis, L.R.; Patterson, R.S. A survey of the urban pest ants (Hymenoptera: Formicidae) of peninsular Florida. Fla. Entomol. 1995, 78, 109–118. [Google Scholar] [CrossRef]
- Gotzek, D.; Brady, S.G.; Kallal, R.J.; LaPolla, J.S. The Importance of using multiple approaches for identifying emerging invasive species: The case of the rasberry crazy ant in the United States. PLoS ONE 2012, 7, e45314. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.L.; Lucky, A. Non-native and invasive Nylanderia crazy ants (Hymenoptera: Formicidae) of the world: Integrating genomics to enhance taxonomic preparedness. Ann. Entomol. Soc. Am. 2020, 113, 318–336. [Google Scholar] [CrossRef]
- Williams, J.L.; Zhang, Y.M.M.; Lloyd, M.W.; LaPolla, J.S.; Schultz, T.R.; Lucky, A. Global domination by crazy ants: Phylogenomics reveals biogeographical history and invasive species relationships in the genus Nylanderia (Hymenoptera: Formicidae). Syst. Entomol. 2020, 45, 730–744. [Google Scholar] [CrossRef]
- Vandermeer, J.; Flores, J.; Longmeyer, J.; Perfecto, I. Spatiotemporal foraging dynamics of Solenopsis invicta (Hymenoptera: Formicidae) and its potential effects on the spatial structure of interspecific competition. Environ. Entomol. 2022, 51, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.N. Functional groups and patterns of organization in North American ant communities: A comparison with Australia. J. Biogeogr. 1997, 24, 433–460. [Google Scholar] [CrossRef]
- Hoffmann, B.D. Impact of an introduced ant on native rain forest invertebrates. Oecologia 1999, 120, 595. [Google Scholar] [CrossRef] [PubMed]
- Walters, A.C.; Mackay, D.A. Importance of large colony size for successful invasion by Argentine ants (Hymenoptera: Formicidae): Evidence for biotic resistance by native ants. Austral Ecol. 2005, 30, 395–406. [Google Scholar] [CrossRef]
- Andersen, A.N. A classification of Australian ant communities, based on functional groups which parallel plant life-forms in relation to stress and disturbance. J. Biogeogr. 1995, 22, 15–29. [Google Scholar] [CrossRef]
- McNaught, M.K.; Wylie, F.R.; Harris, E.J.; Alston, C.L.; Burwell, C.J.; Jennings, C. Effect of broadcast baiting on abundance patterns of red imported fire ants (Hymenoptera: Formicidae) and key local ant genera at long-term monitoring sites in Brisbane, Australia. J. Econ. Entomol. 2014, 107, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Le Breton, J.; Orivel, J.; Chazeau, J.; Dejean, A. Unadapted behaviour of native, dominant ant species during the colonization of an aggressive, invasive ant. Ecol. Res. 2007, 22, 107–114. [Google Scholar] [CrossRef]
- Abril, S.; Gómez, C. Aggressive behaviour of the two European Argentine ant supercolonies (Hymenoptera: Formicidae) towards displaced native ant species of the northeastern Iberian Peninsula. Myrmecol. News 2011, 14, 99–106. [Google Scholar]
- Perfecto, I.; Vandermeer, J. Discovery dominance tradeoff: The case of Pheidole subarmata and Solenopsis geminata (Hymenoptera: Formicidae) in neotropical pastures. Environ. Entomol. 2011, 40, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Morrison, L.W.; Porter, S.D. Positive association between densities of the red imported fire ant, Solenopsis invicta (Hymenoptera: Formicidae), and generalized ant and arthropod diversity. Environ. Entomol. 2003, 32, 548–554. [Google Scholar] [CrossRef]
- Carpintero, S.; Reyes-López, J. The role of competitive dominance in the invasive ability of the Argentine ant (Linepithema humile). Biol. Invasions 2008, 10, 25–35. [Google Scholar] [CrossRef]
- Cerda, X.; Angulo, E.; Caut, S.; Courchamp, F. Ant community structure on a small Pacific island: Only one native species living with the invaders. Biol. Invasions 2012, 14, 323–339. [Google Scholar] [CrossRef]
- Moyano, M.; Feener, D.H. Nest relocation in the ant Pheidole dentata. Insectes Soc. 2014, 61, 71–81. [Google Scholar] [CrossRef]
- Ward, D. Ecological partitioning and invasive ants (Hymenoptera: Formicidae) in a tropical rain forest ant community from Fiji. Pac. Sci. 2008, 62, 473. [Google Scholar] [CrossRef]
- Roura-Pascual, N.; Bas, J.M.; Hui, C. The spread of the Argentine ant: Environmental determinants and impacts on native ant communities. Biol. Invasions 2010, 12, 2399–2412. [Google Scholar] [CrossRef]
- LeBrun, E.G.; Plowes, R.M.; Gilbert, L.E. Imported fire ants near the edge of their range: Disturbance and moisture determine prevalence and impact of an invasive social insect. J. Anim. Ecol. 2012, 81, 884–895. [Google Scholar] [CrossRef] [PubMed]
- Gippet, J.M.M.W.; George, L.; Bertelsmeier, C. Local coexistence of native and invasive ant species is associated with micro-spatial shifts in foraging activity. Biol. Invasions 2022, 24, 761–773. [Google Scholar] [CrossRef]
- Chen, Y.Z. Phylogenetic Study of Lasiini and Plagiolepidini Based on Morphological and Molecular Data. Master’s Thesis, Guangxi Normal University, Guilin, China, 2020. [Google Scholar]
- Silva, T.S.R.; Hamer, M.T.; Guénard, B. A checklist of Nylanderia (Hymenoptera: Formicidae: Formicinae) from Hong Kong and Macao SARs, with an illustrated identification key for species in Southeast China and Taiwan. Zootaxa 2023, 5301, 501–539. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.H.; Mao, R.Q.; Zhang, R.J. Comparisons of foraging activities and competitive interactions between the red imported fire ant (Hymenoptera: Formicidae) and two native ants under high soil-surface temperatures. Sociobiology 2007, 50, 1165–1175. [Google Scholar]
- Yan, D.; Lu, Z.X.; Wang, Q.; Yu, X.Y.; Zhang, N.N.; Li, Q.; Chen, Y.Q. Effects of invasion intensity of the red imported fire ant, Solenopsis invicta (Hymenoptera: Formicidae), on the discovery-dominance trade-off of species of native ant communities. Acta Entomol. Sinica 2020, 63, 334–342, (In Chinese with English abstract). [Google Scholar]
- Harada, Y.; Matsumoto, Y.; Maeda, S.; Oyama, A.; Yamane, S. Comparison of ant fauna among different habitats of Yaku-shima Island, southern Japan. Bull. Biogeogra. Soc. Jpn. 2009, 64, 125–134. [Google Scholar]
- Berman, M.; Andersen, A.N.; Hély, C.; Gaucherel, C. Overview of the distribution, habitat association and impact of exotic ants on native ant communities in New Caledonia. PLoS ONE 2013, 8, e67245. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.R.; Zhang, S.Q.; Zheng, X.B.; Deng, J.F.; Yang, J.Q.; Liang, Y.L.; Li, Z.Q.; Yue, X.L.; Lu, Y.Y.; Xu, Y.J.; et al. Efficacy of matrine, rotenone, and pyrethrin against red imported fire ant Solenopsis invicta (Hymenoptera: Formicidae) and their impact on aquatic organisms. Environ. Entomol. 2022, 51, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Nong, X.Q.; Wang, G.J.; Wang, Y.Y.; Zhang, L.; Gao, Q.H.; Yu, Y.H. Potential and prospect of Beauveria bassiana and Metarhizium anisopliae as biological pesticides for the control of red fire ants. Chin. J. Biol. Control 2023, 39, 453–461, (In Chinese with English abstract). [Google Scholar]
- Wu, S.Y.; Tang, H.T.; Zhang, C.; Tang, F.X.; Lin, J.H.; Wang, Y.X.; Chen, L.H.; Hou, Y.M. Potential of entomopathogenic nematode-infected insect cadavers for the biocontrol of the red imported fire ant Solenopsis invicta. Pest Manag. Sci. 2023, 79, 4383–4389. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).