Impact of Neem Seed Extract on Mortality, Esterase and Glutathione-S-Transferase Activities in Thai Polyvoltine Hybrid Silkworm, Bombyx mori L.
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Silkworm Larvae Rearing
2.2. Neem Seed Extract Preparation
2.3. Toxicity Bioassay
2.4. Detoxification Enzyme Activity Estimation
2.4.1. Enzyme Extraction
2.4.2. Estimation of Enzyme Activities and Protein Content
2.5. Data Analysis
3. Results
3.1. Effects of Thai Neem Seed Crude Extract on Thai Hybrid Silkworm Larvae
3.1.1. Effect on Silkworm Larvae Mortality
3.1.2. Toxicity of Neem Seed Extract on Silkworm Larvae
3.2. Estimation of Detoxification Enzyme Activity
3.2.1. Estimation of Esterase Enzyme (EST)
3.2.2. Estimation of Glutathione-S-Transferase Enzyme (GST)
3.2.3. Total Protein Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Panthee, S.; Paudel, A.; Hamamoto, H.; Sekimizu, K. Advantages of the Silkworm as an Animal Model for Developing Novel Antimicrobial Agents. Front. Microbiol. 2017, 8, 242960. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, S.M.B.D.; Salvador, R.B.; Barros, M.V.; de Francisco, A.C.; Guedes, G. Circularity of Brazilian Silk: Promoting a Circular Bioeconomy in the Production of Silk Cocoons. J. Environ. Manag. 2021, 296, 113373. [Google Scholar] [CrossRef] [PubMed]
- Chand, S.; Chand, S.; Raula, B. Usage of Silkworm Materials in Various Ground of Science and Research. J. Nat. Fibers 2022, 20, 2139328. [Google Scholar] [CrossRef]
- Tengratanaprasert, S. Ubon Ratchathani 60-35, a Bi x Polyvoltine Silkworm Hybrid. Thai Agri. Res. 1993, 11, 127–132. Available online: https://li01.tci-thaijo.org/index.php/thaiagriculturalresearch/article/view/241614 (accessed on 22 June 2024).
- Ruth, L.; Ghatak, S.; Subbarayan, S.; Choudhury, B.N.; Gurusubramanian, G.; Kumar, N.S.; Bin, T. Influence of Micronutrients on the Food Consumption Rate and Silk Production of Bombyx mori (Lepidoptera: Bombycidae) Reared on Mulberry Plants Grown in a Mountainous Agro-Ecological Condition. Front. Physiol. 2019, 10, 372893. [Google Scholar] [CrossRef] [PubMed]
- Krajnc, A.U.; Bakonyi, T.; Andó, I.; Kurucz, E.; Solymosi, N.; Pongrac, P.; Berčič, R.L. The Effect of Feeding with Central European Local Mulberry Genotypes on the Development and Health Status of Silkworms and Quality Parameters of Raw Silk. Insects 2022, 13, 836. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, H.; He, F.; Li, X.; Tan, H.; Zeng, D. Combined Toxicity of Chlorantraniliprole, Lambda-Cyhalothrin, and Imidacloprid to the Silkworm Bombyx mori (Lepidoptera: Bombycidae). Environ. Sci. Pollut. Res. 2018, 25, 22598–22605. [Google Scholar] [CrossRef] [PubMed]
- Jyothi, N.B.; Prashant, N.B.; Maribashetty, V.G.; Radhakrishna, P.G. Effect of pesticide residue in soil on silkworm, Bombyx mori L.—Survey analysis. Int. J. Ind. Entomol. 2019, 38, 31–37. [Google Scholar] [CrossRef]
- Santorum, M.; Costa, R.M.; Dos Reis, G.H.; Dos Santos, D.C. Novaluron Impairs the Silk Gland and Productive Performance of Silkworm Bombyx mori (Lepidoptera: Bombycidae) Larvae. Chemosphere 2020, 239, 124697. [Google Scholar] [CrossRef]
- Lu, Z.; Ye, W.; Feng, P.; Dai, M.; Bian, D.; Ren, Y.; Zhu, Q.; Mao, T.; Su, W.; Li, F.; et al. Low Concentration Acetamiprid-Induced Oxidative Stress Hinders the Growth and Development of Silkworm Posterior Silk Glands. Pestic. Biochem. Physiol. 2021, 174, 104824. [Google Scholar] [CrossRef]
- Wang, W.; Su, Y.; Liu, X.; Qi, R.; Li, F.; Li, B.; Sun, H. Low Concentration of Indoxacarb Interferes with the Growth and Development of Silkworm by Damaging the Structure of Midgut Cells. Pestic. Biochem. Physiol. 2023, 195, 105567. [Google Scholar] [CrossRef]
- Bora, D.; Khanikor, B.; Gogoi, H. Plant based pesticides: Green Environment with Special Reference to Silkworms. Pestic. Adv. Chem. Bot. Pestic. 2012, 8, 171–206. [Google Scholar] [CrossRef]
- Keosaeng, K.; Songoen, W.; Yooboon, T.; Bullangpoti, V.; Pluempanupat, W. Insecticidal Activity of Isolated Gingerols and Shogaols from Zingiber officinale Roscoe Rhizomes against Spodoptera spp. (Lepidoptera: Noctuidae). Nat. Prod. Res. 2022, 37, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Adusei, S.; Azupio, S. Neem: A Novel Biocide for Pest and Disease Control of Plants. J. Chem. 2022, 1, 6778554. [Google Scholar] [CrossRef]
- Schmutterer, H. Properties and Potential of Natural Pesticides from the Neem Tree, Azadirachta Indica. Annu. Rev. Entomol. 1990, 35, 271–297. [Google Scholar] [CrossRef]
- Boeke, S.J.; Van Loon, J.J.A.; Van Huis, A.; Kossou, D.K.; Dicke, M. The Use of Plant Material to Protect Stored Leguminous Seeds against Seed Beetles: A Review; (No. 2001-31); Backhuys: Oegstgeest, The Netherlands, 2001; Available online: https://edepot.wur.nl/282994 (accessed on 10 December 2023).
- Thacker, J.R.M. An Introduction to Arthropod Pest Control; Choice Reviews Online 2003; Cambridge University Press: Cambridge, UK, 2002; Volume 40, pp. 40–5220. Available online: https://shorturl.asia/AoI0m (accessed on 10 December 2023).
- Isman, M.B. Botanical Insecticides, Deterrents, and Repellents in Modern Agriculture and an Increasingly Regulated World. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef]
- Muhammad, A.; Kashere, M.A. Neem, Azadirachta Indica L. (A. Juss): An Eco-friendly Botanical Insecticide for Managing Farmers’ Insects Pest Problems—A Review. FUDMA J. Sci. 2021, 4, 484–491. [Google Scholar] [CrossRef]
- Mordue, A.J.; Nisbet, A.J. Azadirachtin from the Neem Tree Azadirachta indica: Its Action against Insects. An. Da Soc. Entomológica Do Bras. 2000, 29, 615–632. [Google Scholar] [CrossRef]
- Sakthivel, N.; Qadri, S.M.H. Impact of Insecticides and Botanicals on Population Build-up of Predatory Coccinellids in Mulberry. J. Biopestic. 2010, 3, 85. Available online: http://www.jbiopest.com/users/lw8/efiles/Sakthivel_N.pdf (accessed on 6 January 2024).
- Zhang, J.; Liu, H.; Sun, Z.; Xie, J.; Zhong, G.; Yi, X. Azadirachtin Induced Apoptosis in the Prothoracic Gland in Bombyx mori and a Pronounced Release Effect in Sf9 Cells. Int. J. Biol. Sci. 2017, 13, 1532–1539. [Google Scholar] [CrossRef]
- Li, X.; Schuler, M.A.; Berenbaum, M.R. Molecular Mechanisms of Metabolic Resistance to Synthetic and Natural Xenobiotics. Annu. Rev. Entomol. 2007, 52, 231–253. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Zhou, X.; Huang, Y.; Zhang, W.; Chen, S. Characterization of the Role of Esterases in the Biodegradation of Organophosphate, Carbamate, and Pyrethroid Pesticides. J. Hazard. Mater. 2021, 411, 125026. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, H.; Tian, Z.; Su, X.; Li, Y.; Ye, X.; Zhou, Y.; Zheng, S.; Liu, J.; Zhang, Y. The Determination of Plutella xylostella (L.) GSTs (GSTs) Involved in the Detoxification Metabolism of Tolfenpyrad. Pest Manag. Sci. 2020, 76, 4036–4045. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Kang, Z.; Ren, F.; Zhou, Y.; Guo, P. Effects of Quercetin on the Growth and Expression of Immune-Pathway-Related Genes in Silkworm (Lepidoptera: Bombycidae). J. Insect Sci. 2020, 20, 23. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Yamada, N. Identification of a Diazinon-Metabolizing Glutathione S-Transferase in the Silkworm, Bombyx mori. Sci. Rep. 2016, 6, 30073. [Google Scholar] [CrossRef] [PubMed]
- Senthil-Nathan, S. Physiological and Biochemical Effect of Neem and Other Meliaceae Plants Secondary Metabolites against Lepidopteran Insects. Front. Physiol. 2013, 4, 359. [Google Scholar] [CrossRef] [PubMed]
- Després, L.; David, J.-P.; Gallet, C. The Evolutionary Ecology of Insect Resistance to Plant Chemicals. Trends Ecol. Evol. 2007, 22, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Bian, D.; Ren, Y.; Ye, W.; Dai, M.; Li, F.; Wei, J.; Sun, H.; Li, B. Evaluation of Tolerance to λ-Cyhalothrin and Response of Detoxification Enzymes in Silkworms Reared on Artificial Diet. Ecotoxicol. Environ. Saf. 2022, 232, 113232. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, Z.; Li, M.; Fang, Y.; Qu, J.; Mao, T.; Chen, J.; Li, F.; Sun, H.; Li, B. Responses of Detoxification Enzymes in the Midgut of Bombyx mori after Exposure to Low-Dose of Acetamiprid. Chemosphere 2020, 251, 126438. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, D.; Wang, G.; He, Q.; Song, Y.; Jiang, Y.; Xia, Q.; Zhao, P. Adaptive Changes in Detoxification Metabolism and Transmembrane Transport of Bombyx mori Malpighian Tubules to Artificial Diet. Int. J. Mol. Sci. 2023, 24, 9949. [Google Scholar] [CrossRef]
- Nonsrirach, T.; Homhuk, P.; Promma, S.; Sumida, M.; Sutthikhum, V. Comparison of Filament Sizes from Outermost to Innermost Layers of Cocoon in Fourteen Thai Polyvoltine Silkworm, Bombyx mori, Strains. Int. J. Wild Silkmoth Silk 2020, 22, 35–41. Available online: https://www.jstage.jst.go.jp/article/ijwss/22/0/22_35/_pdf/-char/ja (accessed on 17 October 2023).
- Tabassam, S.M.; Iqbal, Z.; Jabbar, A.; Sindhu, Z.-u.-D.; Chattha, A.I. Efficacy of Crude Neem Seed Kernel Extracts against Natural Infestation of Sarcoptes scabiei Var. Ovis. J. Ethnopharmacol. 2008, 115, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Xin, X.; Liu, Z.-X.; Wang, J.; Zhang, R.; Gui, Z.-Z. Transcriptional Response of Detoxifying Enzyme Genes in Bombyx mori under Chlorfenapyr Exposure. Pestic. Biochem. Physiol. 2021, 177, 104899. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Sun, S.; Yang, X.; Yan, H.; Wang, K.; Ba, X.; Wang, H. Sublethal Effects of Neonicotinoid Insecticides on the Development, Body Weight and Economic Characteristics of Silkworm. Toxics 2023, 11, 402. [Google Scholar] [CrossRef] [PubMed]
- Finney, D.J. A Statistical Treatment of the Sigmoid Response Curve. Probit Analysis; Cambridge University Press: London, UK, 1971; pp. 1–318. [Google Scholar]
- Phairiron, A. Expression of Esterase Enzyme Activity on Nang Noi Silkworm Larvae, Bombyx mori L. against Carbosulfan. Int. J. Wild Silkmoth Silk 2011, 16, 55–62. [Google Scholar]
- Yooboon, T.; Pengsook, A.; Ratwatthananon, A.; Pluempanupat, W.; Bullangpoti, V. A Plant-Based Extract Mixture for Controlling Spodoptera litura (Lepidoptera: Noctuidae). Chem. Biol. Technol. Agric. 2019, 6, 5. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, Z.; Wang, J.M.; Sun, S.-s.; Wang, B.B.; Jin, Y.; Shen, W.D.; Li, B. Changes in the Activity and the Expression of Detoxification Enzymes in Silkworms (Bombyx mori) after Phoxim Feeding. Pestic. Biochem. Physiol. 2013, 105, 13–17. [Google Scholar] [CrossRef]
- Simplício, A.L.; Coroadinha, A.S.; Gilmer, J.F.; Lamego, J. A Methodology for Detection and Quantification of Esterase Activity. In Capillary Electrophoresis of Biomolecules. Methods in Molecular Biology; Volpi, N., Maccari, F., Eds.; Humana Press: Totowa, NJ, USA, 2013; Volume 984, pp. 309–319. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases: The First Enzymatic Step in Mercapturic Acid Formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Karunarathne, P.; Pocquet, N.; Labbé, P.; Milesi, P. BioRssay: An R Package for Analyses of Bioassays and Probit Graphs. Parasites Vectors 2022, 15, 35. [Google Scholar] [CrossRef]
- Husseini, M.M.E. Effect of the Fungus, Beauveria bassiana (Balsamo) Vuillemin, on the Beet Armyworm, Spodoptera exigua (Hübner) Larvae (Lepidoptera: Noctuidae), under Laboratory and Open Field Conditions. Egypt. J. Biol. Pest Control. 2019, 29, 52. [Google Scholar] [CrossRef]
- Gao, Y.-P.; Luo, M.; Wang, X.-P.; He, X.Z.; Lu, W.; Zheng, X.-L. Pathogenicity of Beauveria bassiana PfBb and Immune Responses of a Non-Target Host, Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2022, 13, 914. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.; Cotter, S.C.; Reeson, A.; Pell, J.K. Melanism and Disease Resistance in Insects. Ecol. Lett. 2001, 4, 637–649. [Google Scholar] [CrossRef]
- Sun, S.; Chen, Q.; Chen, G.; Chen, Z.; Wang, K.; Wang, H. Toxicity of Nitenpyram to Silkworm (Bombyx mori L.) And Its Potential Mechanisms. Chemosphere 2023, 311, 137026. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.R.; Barreiros, L.; Oliveira, R.F.; Cruz, A.; Prudêncio, C.; Oliveira, A.I.; Pinho, C.; Santos, N.; Morgado, J. Chemistry, Bioactivities, Extraction and Analysis of Azadirachtin: State-of-the-Art. Fitoterapia 2019, 134, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Islas, J.F.; Acosta, E.; G-Buentello, Z.; Delgado-Gallegos, J.L.; Moreno-Treviño, M.G.; Escalante, B.; Moreno-Cuevas, J.E. An Overview of Neem (Azadirachta indica) and Its Potential Impact on Health. J. Funct. Foods 2020, 74, 104171. [Google Scholar] [CrossRef]
- Tulashie, S.K.; Adjei, F.; Abraham, J.; Addo, E. Potential of Neem Extracts as Natural Insecticide against Fall Armyworm (Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae). Case Stud. Chem. Environ. Eng. 2021, 4, 100130. [Google Scholar] [CrossRef]
- Sharath, M.; Narayanaswamy, K.C.; Gowda, M. Efficacy of Pesticides against Yellow Mite in Mulberry and its Residual Toxicity on Silkworm. Mysore J. Agric. Sci. 2022, 56, 315–324. Available online: https://www.uasbangalore.edu.in/images/2022-2nd-Issue/39.pdf (accessed on 5 May 2024).
- Bandyopadhyay, U.K.; Chatterjee, S.; Maji, C.; Bindroo, B.B. Efficacy of Plant Oils against Leaf Webber (Glyphodes pyloalis Walker) on Mulberry (Morus alba L.) and Biosafety to Silkworm. Ann. Plant Prot. Sci. 2013, 21, 53–56. Available online: https://shorturl.asia/U7SQu (accessed on 5 May 2024).
- Yeshika, M.P.; Banuprakash, K.G.; Mohan, K.M. Field Evaluation of Novel Insecticides against Maconellicoccus hirsutus Green in Mulberry Ecosystem and their Safety to Silkworm Bombyx mori L. J. Entomol. Zool. Stud. 2020, 8, 1067–1072. [Google Scholar] [CrossRef]
- Murugan, K. Toxicity and biological effects of neem limonoids on diamondback moth, Plutella xylostella. In Proceedings of the Sixth International Workshop on Management of the Diamondback Moth and Other Crucifer Insect Pests; Srinivasan, R., Shelton, A.M., Collins, H.L., Eds.; AVRDC-World Vegetable Center: Tainan, Taiwan, 2011; Volume 11, pp. 164–171. Available online: https://shorturl.asia/2e6wv (accessed on 5 May 2024).
- Koul, O.; Multani, J.S.; Goomber, S.; Daniewski, W.M.; Berlozecki, S. Activity of Some Nonazadirachtin Limonoids from Azadirachta Indica against Lepidopteran Larvae. Aust. J. Entomol. 2004, 43, 189–195. [Google Scholar] [CrossRef]
- de Oliveira Lima, V.; Braghini, A.; de Paula, F.C.; Souza, J.M.R.; Figueiredo, G.P.; Vacari, A.M.; Vacari, A.M. Toxicity of botanical insecticides at different developmental stages of the coffee leaf miner, Leucoptera coffeella (Lepidoptera: Lynetiidae), and their side effects on predator Chrysoperla externa (Neuroptera: Chrysopidae). Crop Prot. 2024, 181, 106678. [Google Scholar] [CrossRef]
- Xu, S.; Hao, Z.; Li, Y.; Zhou, Y.; Shao, R.; Chen, R.; Zheng, M.; Xu, Y.; Wang, H. Biochemical Toxicity and Transcriptome Aberration Induced by Dinotefuran in Bombyx mori. Environ. Pollut. 2022, 307, 119562. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, E. Introduction to Biotransformation (metabolism). In Hayes’ Handbook of Pesticide Toxicology, 3rd ed.; Hayes, W.J., Ed.; Elsevier: Amsterdam, Netherlands, 2010; Volume 1, pp. 865–875. [Google Scholar] [CrossRef]
- Yao, J.; Zhu, Y.C.; Adamczyk, J.J.; Luttrell, R. Influences of Acephate and Mixtures with Other Commonly Used Pesticides on Honey Bee (Apis mellifera) Survival and Detoxification Enzyme Activities. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2018, 209, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Bullangpoti, V.; Wajnberg, E.; Audant, P.; Feyereisen, R. Antifeedant Activity of Jatropha gossypifolia and Melia azedarach Senescent Leaf Extracts on Spodoptera frugiperda (Lepidoptera: Noctuidae) and Their Potential Use as Synergists. Pest Manag. Sci. 2012, 68, 1255–1264. [Google Scholar] [CrossRef]
- Feng, R.; Chen, W.; Isman, M.B. Synergism of Malathion and Inhibition of Midgut Esterase Activities by an Extract from Melia toosendan (Meliaceae). Pestic. Biochem. Physiol. 1995, 53, 34–41. [Google Scholar] [CrossRef]
- Miao, Z.; Xiong, C.; Cao, X.; Shan, T.; Jin, Q.; Jiang, H. Genome-Wide Identification, Classification, and Expression Profiling of Serine Esterases and Other Esterase-Related Proteins in the Tobacco Hornworm, Manduca sexta. Insect Sci. 2022, 30, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Gajger, I.T.; Dar, S.A. Plant Allelochemicals as Sources of Insecticides. Insects 2021, 12, 189. [Google Scholar] [CrossRef]
- Vechia, J.F.D.; Leeuwen, T.V.; Rossi, G.D.; Andrade, D.J. The Role of Detoxification Enzymes in the Susceptibility of Brevipalpus californicus Exposed to Acaricide and Insecticide Mixtures. Pestic. Biochem. Physiol. 2021, 175, 104855. [Google Scholar] [CrossRef]
- Simpson, S.J.; Simpson, C.L. The Mechanisms of Nutritional Compensation by Phytophagous Insects. In Insect-Plant Interactions, 1st ed.; Bernays, E.A., Ed.; CRC Press: Boca Raton, FL, USA, 1989; pp. 111–160, eBook ISBN 9780203711736. [Google Scholar] [CrossRef]
- Duan, H.; Yu, L.; Tian, F.; Zhai, Q.; Fan, L.; Chen, W. Gut Microbiota: A Target for Heavy Metal Toxicity and a Probiotic Protective Strategy. Sci. Total Environ. 2020, 742, 140429. [Google Scholar] [CrossRef]
- Mao, T.; Li, F.; Fang, Y.; Wang, H.; Chen, J.; Li, M.; Lu, Z.; Qu, J.; Li, J.; Hu, J.; et al. Effects of Chlorantraniliprole Exposure on Detoxification Enzyme Activities and Detoxification-Related Gene Expression in the Fat Body of the Silkworm, Bombyx mori. Ecotoxicol. Environ. Saf. 2019, 176, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-E.; Ma, H.-J.; Feng, D.-D.; Lai, X.-F.; Chen, Z.-M.; Xu, M.-Y.; Yu, Q.-Y.; Zhang, Z. Induction of Detoxification Enzymes by Quercetin in the Silkworm. J. Econ. Entomol. 2012, 105, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Enayati, A.A.; Ranson, H.; Hemingway, J. Insect Glutathione Transferases and Insecticide Resistance. Insect Mol. Biol. 2005, 14, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yang, X.; Dai, J.; Li, Y.; Veeran, S.; Lin, J.; Shu, B. Effects of Azadirachtin on Detoxification-Related Gene Expression in the Fat Bodies of the Fall Armyworm, Spodoptera frugiperda. Environ. Sci. Pollut. Res. 2022, 30, 42587–42595. [Google Scholar] [CrossRef]
- Song, X.; Pei, L.; Zhang, Y.; Chen, X.; Zhong, Q.; Ji, Y.-H.; Tang, J.; Feng, F.; Li, B. Functional Diversification of Three Delta-Class Glutathione S-Transferases Involved in Development and Detoxification in Tribolium castaneum. Insect Mol. Biol. 2020, 29, 320–336. [Google Scholar] [CrossRef]
- Gui, Z.Z.; Kim, B.Y.; Lee, K.S.; Wei, Y.D.; Guo, X.; Sohn, H.D.; Jin, B.R. Glutathione S-Transferases from the Larval Gut of the Silkworm Bombyx mori: cDNA Cloning, Gene Structure, Expression and Distribution. Eur. J. Entomol. 2008, 105, 567–574. [Google Scholar] [CrossRef]
- Hirowatari, A.; Nagaoka, S.; Yamada, N.; Yamamoto, K. Identifying a Sigma Class Glutathione S-Transferase 2 from the Silkworm Bombyx mori. J. Insect Biotechnol. Sericology 2017, 86, 1–7. [Google Scholar] [CrossRef]
- Nikou, D.; Ranson, H.; Hemingway, J. An Adult-Specific CYP6 P450 Gene Is Overexpressed in a Pyrethroid-Resistant Strain of the Malaria Vector, Anopheles gambiae. Gene 2003, 318, 91–102. [Google Scholar] [CrossRef]
- El-Ashram, D.; Olfat, E.A.; Enas, M.E. Impacts of emamectin benzoate and lemon oil on silkworm Bombyx mori (Lepidoptera: Bombycidae). Egypt. J. Plant Prot. Res. Inst. 2022, 5, 318–327. Available online: http://www.ejppri.eg.net/pdf/v5n4/2.pdf (accessed on 14 May 2024).
- Yu, Q.; Lu, C.; Li, W.-L.; Xiang, Z.; Zhang, Z. Annotation and Expression of Carboxylesterases in the Silkworm, Bombyx mori. BMC Genom. 2009, 10, 553. [Google Scholar] [CrossRef]
- Guengerich, F.P. Mechanisms of Cytochrome P450-Catalyzed Oxidations. ACS Catal. 2018, 8, 10964–10976. [Google Scholar] [CrossRef]
- Aurade, R.M.; Gull, A.; Padhan, D.; Chandrakanth, N.; Jayaram, H.K.; Satish, K.; Moorthy, S.M.; Doss, S.G. Biochemical Analysis of Defensive Enzymes in the Hemolymph of Bivoltine Silkworm Breeds of Bombyx mori (Lepidoptera: Bombycidae). Biologia 2024, 13, 1–11. [Google Scholar] [CrossRef]
- Cui, S.; Wang, L.; Ma, L.; Geng, X. P450-Mediated Detoxification of Botanicals in Insects. Phytoparasitica 2016, 44, 585–599. [Google Scholar] [CrossRef]
- Liu, S.; Liang, Q.-M.; Huang, Y.-J.; Yuan, X.; Zhou, W.; Qiao, F.; Cheng, J.; Gurr, G.M.; Zhu, Z.-R. Cloning, Functional Characterization, and Expression Profiles of NADPH-Cytochrome P450 Reductase Gene from the Asiatic Rice Striped Stem Borer, Chilo Suppressalis (Lepidoptera: Pyralidae). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2013, 16, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.V.; Flück, C.E. NADPH P450 Oxidoreductase: Structure, Function, and Pathology of Diseases. Pharmacol. Ther. 2013, 138, 229–254. [Google Scholar] [CrossRef]
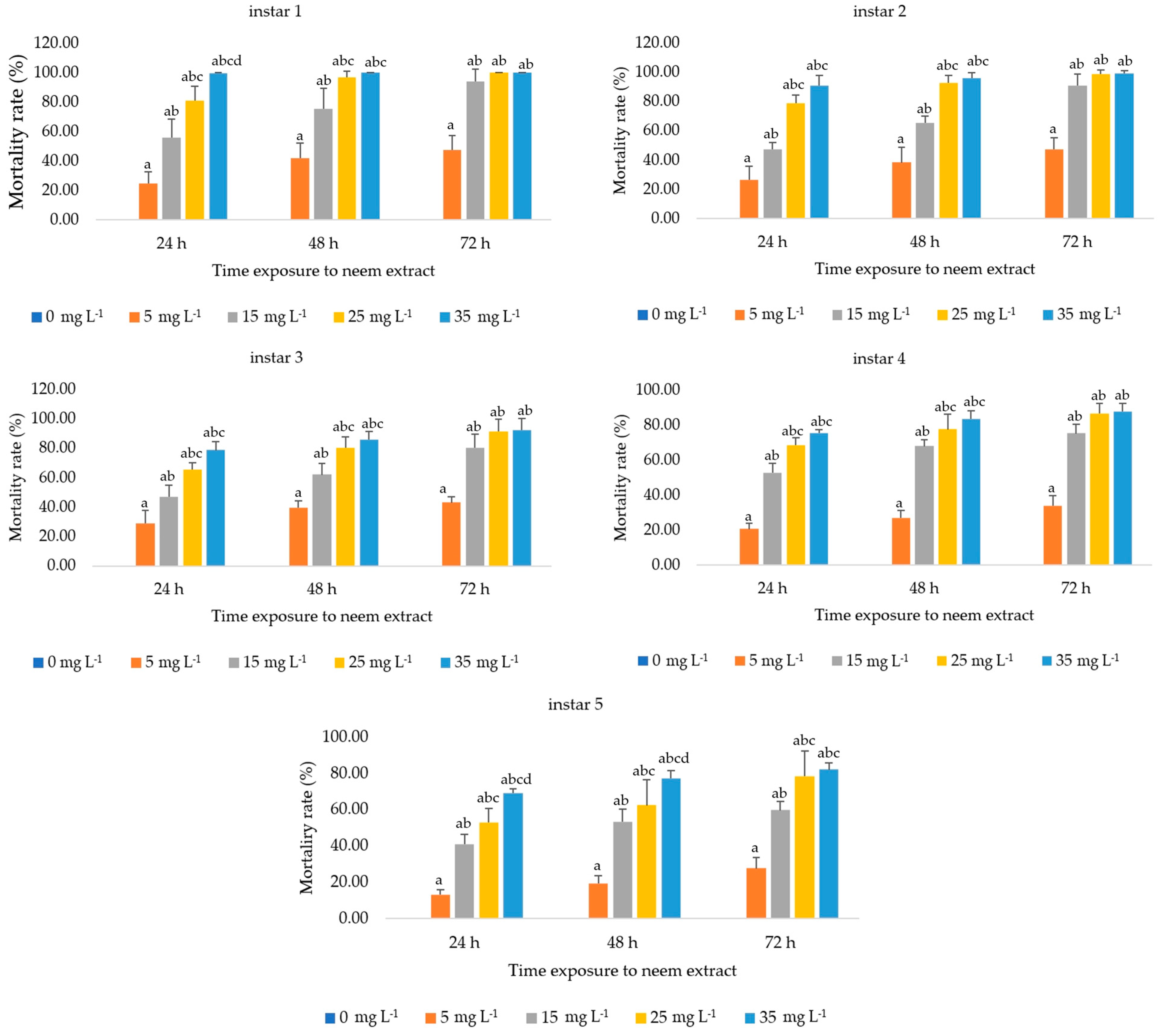
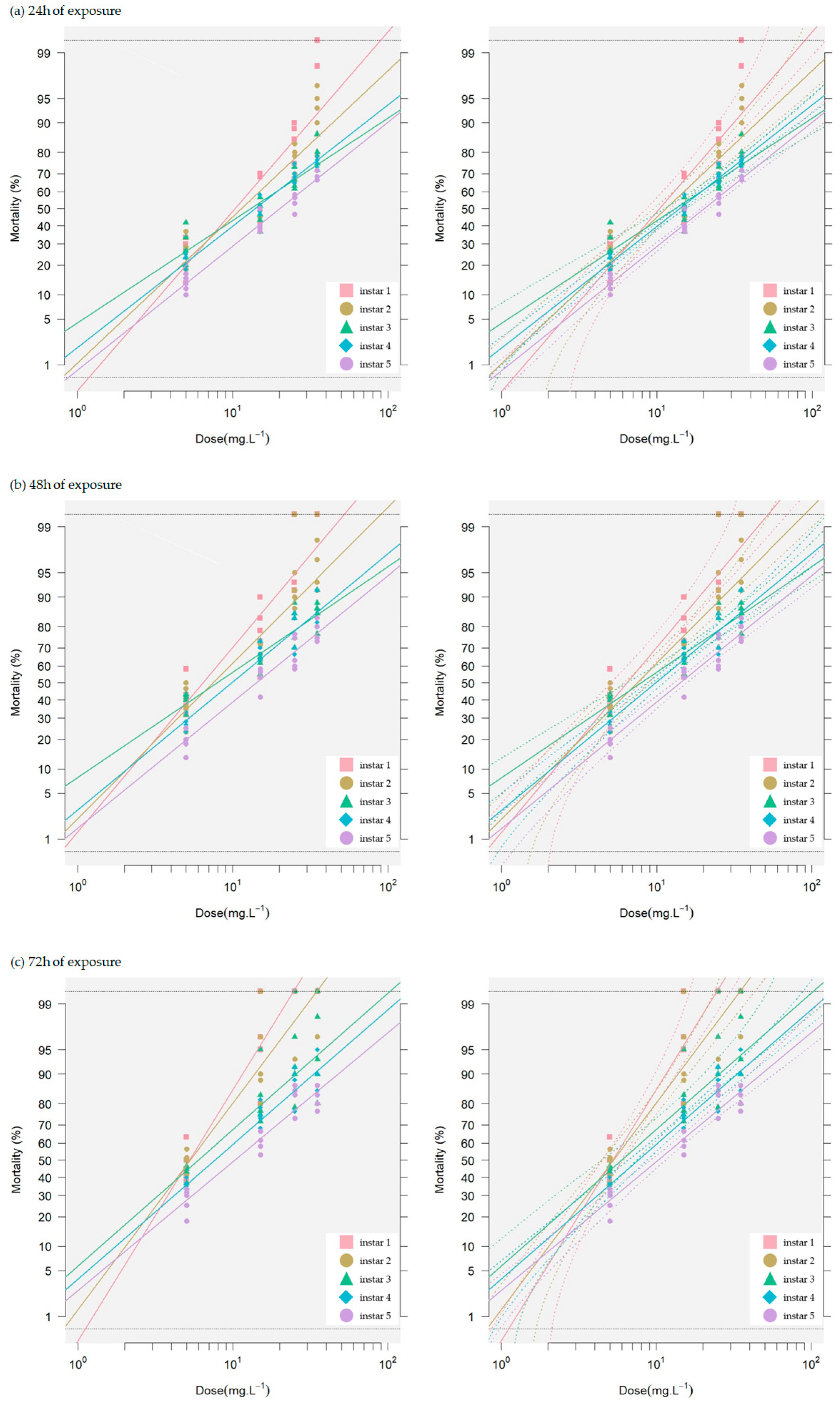



| Larval Stage (Instar) | Time Exposure (h) | Slope ± SE | Intercept ± SE | Toxicity Value of Neem Extract (mg L−1) | ||
|---|---|---|---|---|---|---|
| LC10 (CI) | LC50 (CI) | LC90 (CI) | ||||
| 1 | 24 | 2.68 ± 0.34 | (−2.7252) ± 0.40 | 3.46 (1.50–14.00) | 10.00 (3.61–63.00) | 31.00 (8.64–281.00) |
| 48 | 2.76 ± 0.34 | (−2.2296) ± 0.36 | 2.21 (1.13–6.70) | 6.43 (2.67–28.00) | 19.00 (6.27–118.00) | |
| 72 | 3.74 ± 0.38 | (−2.6904) ± 0.34 | 2.38 (1.43–5.22) | 5.23 (2.74–14.00) | 12.00 (5.24–39.00) | |
| 2 | 24 | 2.19 ± 0.24 | (−2.309 ± 0.30 | 2.95 (1.42–9.50) | 11.00 (4.26–55.00) | 44.00 (13.00–318.00) |
| 48 | 2.32 ± 0.25 | (−2.0278) ± 0.29 | 2.10 (1.11–5.69) | 7.47 (3.14–29.00) | 27.00 (8.87–152.00) | |
| 72 | 3.07 ± 0.31 | (−2.223) ± 0.30 | 2.02 (1.21–4.41) | 5.29 (2.69–15.00) | 14.00 (5.96–50.00) | |
| 3 | 24 | 1.53 ± 0.16 | (−1.7105) ± 0.20 | 1.91 (1.01–5.07) | 13.00 (4.95–60.00) | 90.00 (24.00–700.00) |
| 48 | 1.57 ± 0.15 | (−1.4100) ± 0.18 | 1.21 (0.73–2.54) | 7.88 (3.51–26.00) | 51.00 (17.00–277.00) | |
| 72 | 2.03 ± 0.26 | (−1.5737) ± 0.30 | 1.39 (0.74–4.06) | 5.96 (2.36–29.00) | 25.00 (7.45–211.00) | |
| 4 | 24 | 1.81 ± 0.13 | (−2.0759) ± 0.16 | 2.74 (1.72–5.03) | 14.00 (7.23–33.00) | 71.00 (30.00–216.00) |
| 48 | 1.91 ± 0.14 | (−1.8987) ± 0.17 | 2.10 (1.32–3.93) | 9.83 (5.06–24.00) | 46.00 (19.00–148.00) | |
| 72 | 2.00 ± 0.15 | (−1.7668) ± 1.77 | 1.75 (1.12–3.18) | 7.61 (4.03–18.00) | 33.00 (14.00–104.00) | |
| 5 | 24 | 1.85 ± 0.13 | (−2.4166) ± 0.17 | 4.10 (2.40–8.32) | 20.00 (9.71–53.00) | 99.00 (39.00–338.00) |
| 48 | 1.89 ± 0.13 | (−2.1709) ± 0.16 | 2.96 (1.81–5.70) | 14.00 (7.13–35.00) | 68.00 (28.00–218.00) | |
| 72 | 1.92 ± 0.14 | (−1.9420) ± 0.17 | 2.21 (1.39–4.12) | 10.00 (5.30–25.00) | 48.00 (20.00–152.00) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rattanapan, A.; Sujayanont, P. Impact of Neem Seed Extract on Mortality, Esterase and Glutathione-S-Transferase Activities in Thai Polyvoltine Hybrid Silkworm, Bombyx mori L. Insects 2024, 15, 591. https://doi.org/10.3390/insects15080591
Rattanapan A, Sujayanont P. Impact of Neem Seed Extract on Mortality, Esterase and Glutathione-S-Transferase Activities in Thai Polyvoltine Hybrid Silkworm, Bombyx mori L. Insects. 2024; 15(8):591. https://doi.org/10.3390/insects15080591
Chicago/Turabian StyleRattanapan, Ajin, and Patcharawan Sujayanont. 2024. "Impact of Neem Seed Extract on Mortality, Esterase and Glutathione-S-Transferase Activities in Thai Polyvoltine Hybrid Silkworm, Bombyx mori L." Insects 15, no. 8: 591. https://doi.org/10.3390/insects15080591






