Field Exploration for Colony Selection: Evaluating Hygienic Behavior in Apis cerana indica Colonies
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Pin-Killed Brood Assay
2.3. Colony Growth Parameters
2.4. Statistical Analysis
3. Result
3.1. Descriptive Statistics
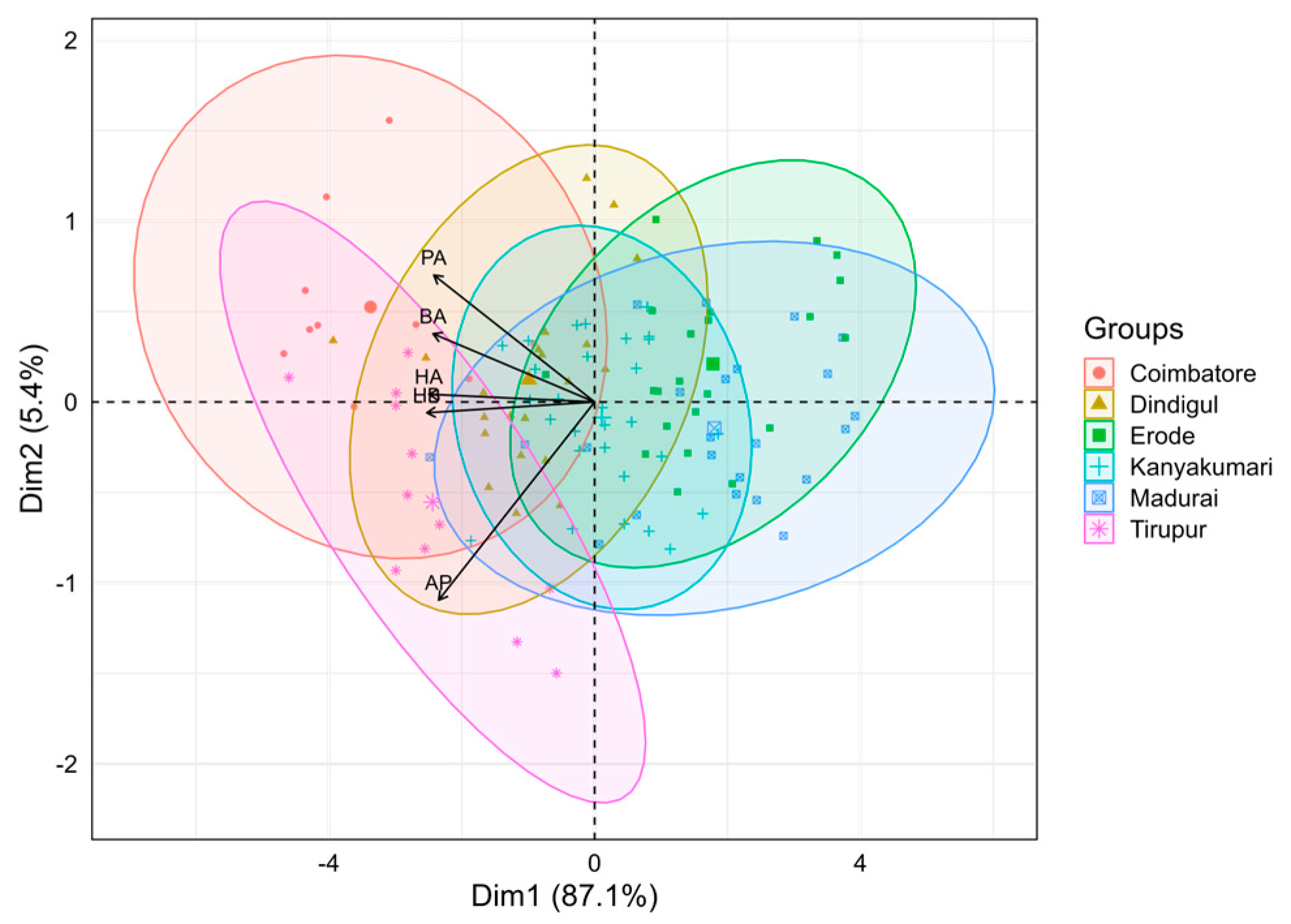
3.2. Principal Component Analysis
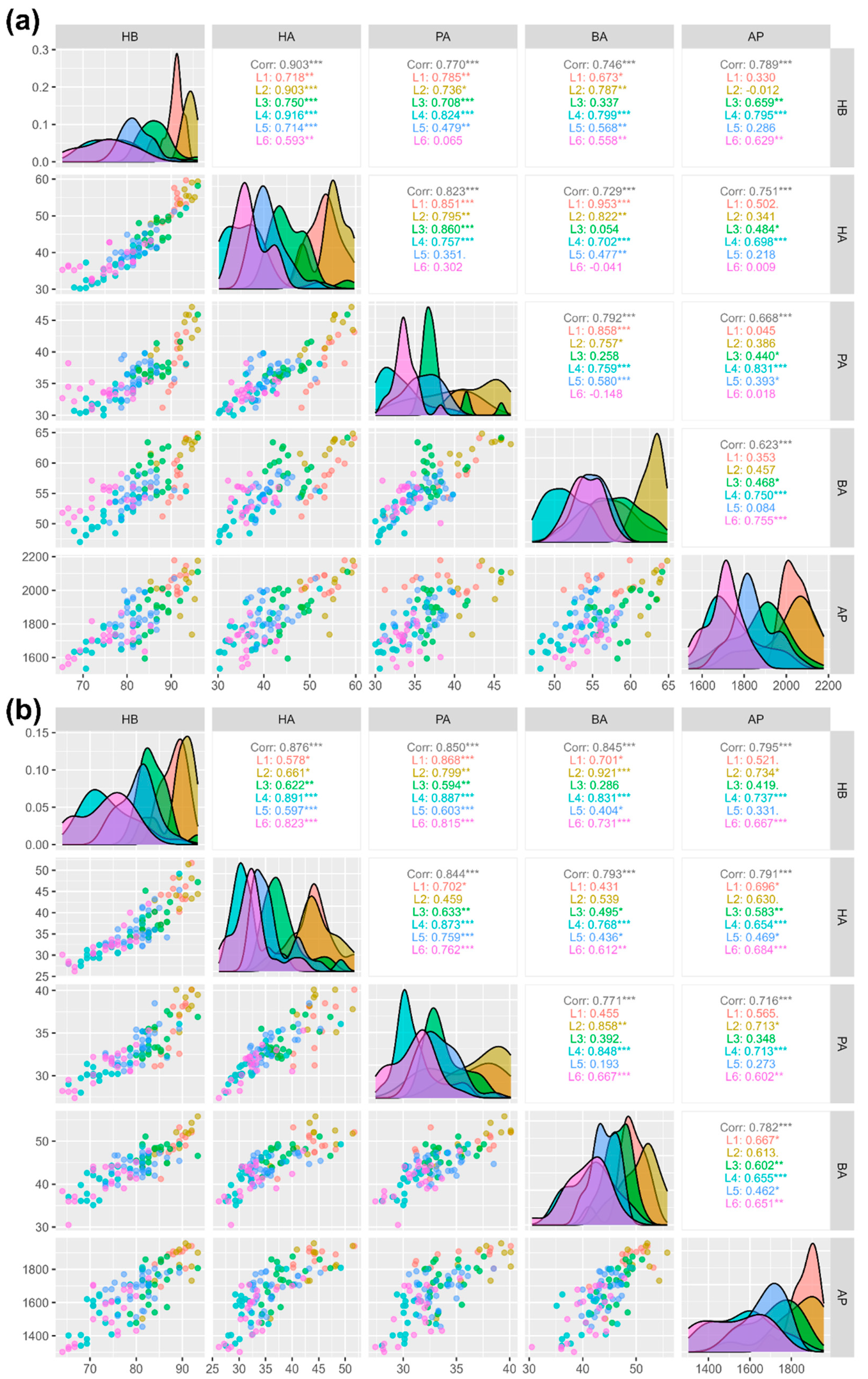
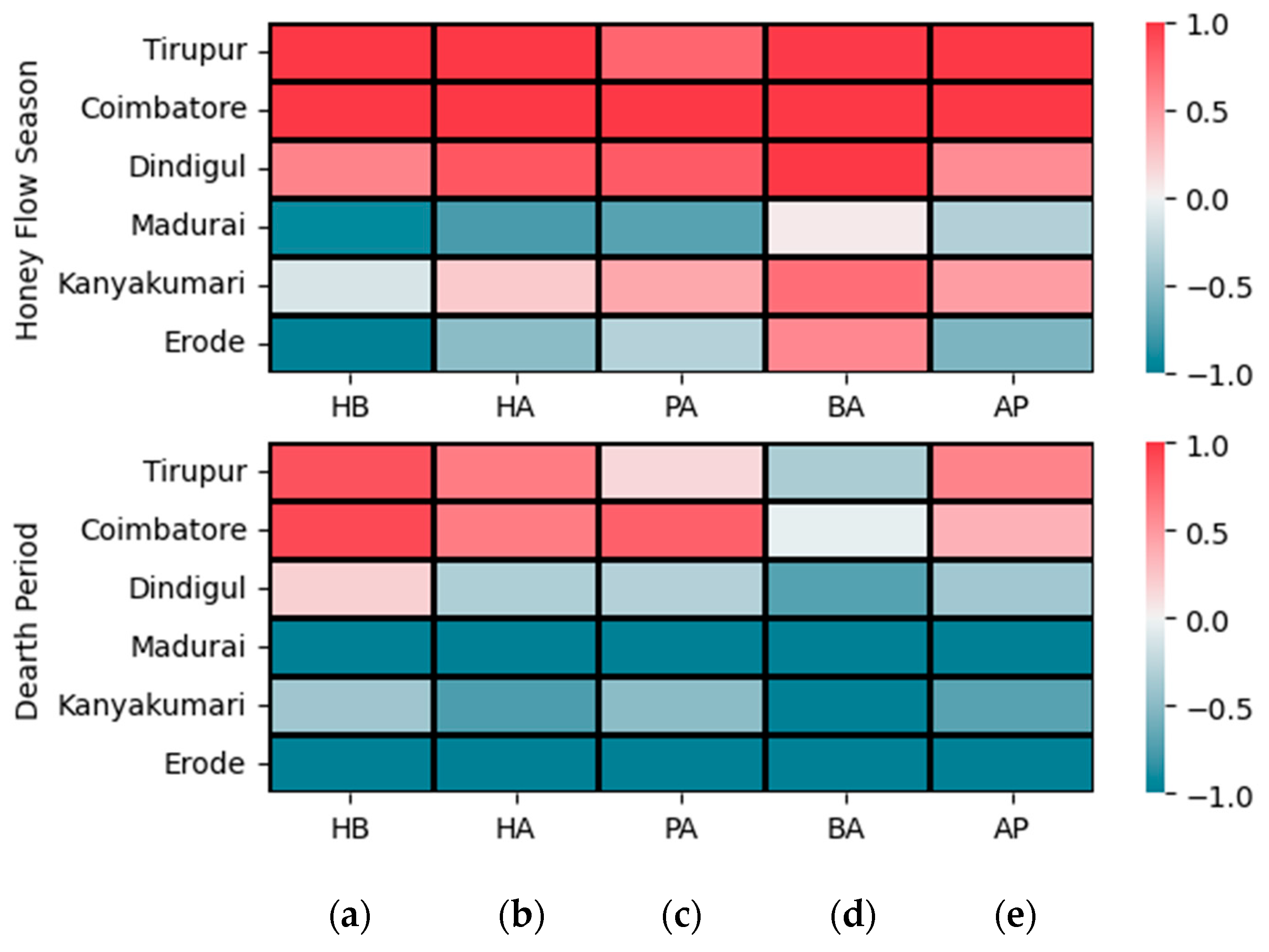
3.3. Correlation Analysis
3.4. Analysis of Variance
3.4.1. Hygienic Behavior
3.4.2. Honey Area
3.4.3. Pollen Area
3.4.4. Brood Area
3.4.5. Adult Population
3.5. Colony Selection Based on the Selection Index
3.6. Hierarchical Agglomerative Clustering (HAC)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iwasaki, J.M.; Hogendoorn, K. How protection of honey bees can help and hinder bee conservation. Curr. Opin. Insect Sci. 2021, 46, 112–118. [Google Scholar] [CrossRef]
- Winston, M.L. The Biology of the Honey Bee; Harvard University Press: Cambridge, MA, USA, 1991. [Google Scholar]
- Iqbal, J.; Manzoor, M.; Boruah, T.; Wani, S.H.; Wachkoo, A.A. Sex and Caste Determination and Life Cycle of Honey Bees. In Honey Bees, Beekeeping and Bee Products; CRC Press: Boca Raton, FL, USA, 2024; pp. 23–35. [Google Scholar]
- Ruttner, H. Technical recommendations for methods of evaluating performance of bee colonies. In Controlled Mating and Selection of the Honey Bee; Ruttner, F., Ed.; Apimondia Publishing House: Bucharest, Romania, 1972; pp. 87–92. [Google Scholar]
- Cremer, S.; Armitage, S.A.; Schmid-Hempel, P. Social immunity. Curr. Biol. 2007, 17, R693–R702. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, G.; Pannebakker, B.; Gengler, N.; Nguyen, B.K.; Francis, F. Drawbacks and benefits of hygienic behavior in honey bees (Apis mellifera L.): A review. J. Apic. Res. 2017, 56, 366–375. [Google Scholar] [CrossRef]
- Spivak, M.; Danka, R.G. Perspectives on hygienic behavior in Apis mellifera and other social insects. Apidologie 2021, 52, 1–16. [Google Scholar] [CrossRef]
- Simone-Finstrom, M. Social immunity and the superorganism: Behavioral defenses protecting honey bee colonies from pathogens and parasites. Bee World 2017, 94, 21–29. [Google Scholar] [CrossRef]
- Rothenbuhler, W.C. Behaviour genetics of nest cleaning in honey bees. I. Responses of four inbred lines to disease-killed brood. Anim. Behav. 1964, 12, 578–583. [Google Scholar] [CrossRef]
- Spivak, M.; Reuter, G.S. Resistance to American foulbrood disease by honey bee colonies Apis mellifera bred for hygienic behavior. Apidologie 2001, 32, 555–565. [Google Scholar] [CrossRef]
- Boecking, O.; Spivak, M. Behavioral defenses of honey bees against Varroa jacobsoni Oud. Apidologie 1999, 30, 141–158. [Google Scholar] [CrossRef]
- Büchler, R.; Berg, S.; Le Conte, Y. Breeding for resistance to Varroa destructor in Europe. Apidologie 2010, 41, 393–408. [Google Scholar] [CrossRef]
- Gilliam, M.; Taber, S., III; Lorenz, B.J.; Prest, D.B. Factors affecting development of chalkbrood disease in colonies of honey bees, Apis mellifera, fed pollen contaminated with Ascosphaera apis. J. Invertebr. Pathol. 1988, 52, 314–325. [Google Scholar] [CrossRef]
- Gilliam, M.; Taber, S., III; Richardson, G.V. Hygienic behavior of honey bees in relation to chalkbrood disease. Apidologie 1983, 14, 29–39. [Google Scholar] [CrossRef]
- Rinderer, T.E.; Harris, J.W.; Hunt, G.J.; De Guzman, L.I. Breeding for resistance to Varroa destructor in North America. Apidologie 2010, 41, 409–424. [Google Scholar] [CrossRef]
- Park, O. Disease resistance and American foulbrood. Am. Bee J. 1936, 77, 20–25. [Google Scholar]
- Pull, C.D.; Ugelvig, L.V.; Wiesenhofer, F.; Grasse, A.V.; Tragust, S.; Schmitt, T.; Brown, M.J.; Cremer, S. Destructive disinfection of infected brood prevents systemic disease spread in ant colonies. eLife 2018, 7, e32073. [Google Scholar] [CrossRef] [PubMed]
- Wilson-Rich, N.; Spivak, M.; Fefferman, N.H.; Starks, P.T. Genetic, individual, and group facilitation of disease resistance in insect societies. Annu. Rev. Entomol. 2009, 54, 405–423. [Google Scholar] [CrossRef] [PubMed]
- Spivak, M.; Gilliam, M. Hygienic behaviour of honey bees and its application for control of brood diseases and Varroa: Part II. Studies on hygienic behaviour since the Rothenbuhler era. Bee World 1998, 79, 169–186. [Google Scholar] [CrossRef]
- Spivak, M. Honey bee hygienic behavior and defense against Varroa jacobsoni. Apidologie 1996, 27, 245–260. [Google Scholar] [CrossRef]
- Invernizzi, C.; Zefferino, I.; Santos, E.; Sánchez, L.; Mendoza, Y. Multilevel assessment of grooming behavior against Varroa destructor in Italian and Africanized honey bees. J. Apic. Res. 2022, 54, 321–327. [Google Scholar] [CrossRef]
- Masterman, R.; Smith, B.; Spivak, M. Brood odor discrimination abilities in hygienic honey bees (Apis mellifera L.) using proboscis extension reflex conditioning. J. Insect Behav. 2000, 13, 87–101. [Google Scholar] [CrossRef]
- Ali, H.; Iqbal, J.; Raweh, H.S.; Alqarni, A.S. Proboscis behavioral response of four honey bee Apis species towards different concentrations of sucrose, glucose, and fructose. Saudi J. Biol. Sci. 2021, 28, 3275–3283. [Google Scholar] [CrossRef]
- Senapathi, D.; Fründ, J.; Albrecht, M.; Garratt, M.P.; Kleijn, D.; Pickles, B.J.; Potts, S.G.; An, J.; Andersson, G.K.; Bänsch, S. Wild insect diversity increases inter-annual stability in global crop pollinator communities. Proc. R. Soc. B 2021, 288, 20210212. [Google Scholar] [CrossRef] [PubMed]
- Kamaraj, N.; Rasappan, K. Temporal and Spatial Foraging Activity of Indian Honey Bee (Apis cerana indica F.) at Different Migratory Sites. Sociobiology 2024, 71, e9733. [Google Scholar] [CrossRef]
- Partap, U. The Pollination Role of Honeybees. In Honeybees of Asia; Hepburn, H.R., Radloff, S.E., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 227–255. [Google Scholar]
- Tewarson, N.; Singh, A.; Engels, W. Reproduction of Varroa jacobsoni in colonies of Apis cerana indica under natural and experimental conditions. Apidologie 1992, 23, 161–171. [Google Scholar] [CrossRef]
- Lin, Z.; Page, P.; Li, L.; Qin, Y.; Zhang, Y.; Hu, F.; Neumann, P.; Zheng, H.; Dietemann, V. Go east for better honey bee health: Apis cerana is faster at hygienic behavior than A. mellifera. PLoS ONE 2016, 11, e0162647. [Google Scholar] [CrossRef] [PubMed]
- Aruna, R.; Srinivasan, M.; Selvarajan, R.; Subramanian, S.; Thakur, R. Epidemiology of the Thai Sacbrood Virus Disease Attacking Indian Honey Bee Apis cerana indica F. and Morphological Characterization of the Virus Particle using Transmission Electron Microscope. Madras Agric. J. 2016, 103, 51–56. [Google Scholar]
- Mohan Rao, K.; Katna, S.; Rana, B.S.; Rana, R. Thai sacbrood and sacbrood viruses versus European foulbrood of hive bees in India—A review. J. Apic. Res. 2015, 54, 192–199. [Google Scholar] [CrossRef]
- Rana, B.; Garg, I.; Khurana, S.; Verma, L.; Agrawal, H. Thai sacbrood virus of honeybees (Apis cerana indica F.) in north-west Himalayas. Indian J. Virol. 1986, 2, 127–131. [Google Scholar]
- Büchler, R.; Andonov, S.; Bernstein, R.; Bienefeld, K.; Costa, C.; Du, M.; Gabel, M.; Given, K.; Hatjina, F.; Harpur, B.A. Standard methods for rearing and selection of Apis mellifera queens 2.0. J. Apic. Res. 2024, 23, 1–57. [Google Scholar] [CrossRef]
- Costa, C.; Büchler, R.; Berg, S.; Bienkowska, M.; Bouga, M.; Bubalo, D.; Charistos, L.; Le Conte, Y.; Drazic, M.; Dyrba, W. A Europe-wide experiment for assessing the impact of genotype-environment interactions on the vitality and performance of honey bee colonies: Experimental design and trait evaluation. J. Apic. Sci. 2012, 56, 147–158. [Google Scholar] [CrossRef]
- Anjali, J.; Amritha, V.; Anitha, N.; Vijayasree, V.; Gopinath, P.P. Evaluation of first-generation Indian bee, Apis cerana indica colonies raised from breeder colonies by grafting method. J. Entomol. Zool. Stud. 2023, 11, 54–59. [Google Scholar]
- Rinderer, T.E. Bee Genetics and Breeding; Academic Press: Cambridge, MA, USA, 1986. [Google Scholar]
- Devi, S.; Rana, K.; Negi, N.; Sharma, H.K.; Thakur, M. Seasonal incidence of brood diseases in hives of Asian honey bee (Apis cerana F.) correlates with colony and weather parameters. Pharma Innov. J. 2021, 10, 1945–1951. [Google Scholar]
- Uzunov, A. Biological and Productive Characteristics of native honey bee (Apis mellifera Macedonica) on the Territory of Republic of Macedonia. Ph.D. Thesis, Faculty for Agricultural Sciences and Food, Skopje, North Macedonia, 2013. [Google Scholar]
- Hatjina, F.; Costa, C.; Büchler, R.; Uzunov, A.; Drazic, M.; Filipi, J.; Charistos, L.; Ruottinen, L.; Andonov, S.; Meixner, M.D. Population dynamics of European honey bee genotypes under different environmental conditions. J. Apic. Res. 2014, 53, 233–247. [Google Scholar] [CrossRef]
- Ali, H.; Alqarni, A.S.; Iqbal, J.; Owayss, A.A.; Raweh, H.S.; Smith, B.H. Effect of season and behavioral activity on the hypopharyngeal glands of three honey bee Apis mellifera L. races under stressful climatic conditions of central Saudi Arabia. J. Hymenopt. Res. 2019, 25, 85–101. [Google Scholar] [CrossRef]
- Güler, A.; Toy, H. Relationship between dead pupa removal and season and productivity of honey bee (Apis mellifera, Hymenoptera: Apidae) colonies. Turk. J. Vet. Anim. Sci. 2013, 37, 462–467. [Google Scholar] [CrossRef]
- Panasiuk, B.; Skowronek, W.; Gerula, D. Effect of period of the season and environmental conditions on rate of cleaning cells with dead brood. J. Apic. Sci. 2009, 53, 95–103. [Google Scholar]
- Bigio, G.; Schürch, R.; Ratnieks, F.L. Hygienic behavior in honey bees (Hymenoptera: Apidae): Effects of brood, food, and time of the year. J. Econ. Entomol. 2013, 106, 2280–2285. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.R. Organization of work in the honeybee: A compromise between division of labour and behavioural flexibility. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Momot, J.P.; Rothenbuhler, W.C. Behaviour genetics of nest cleaning in honeybees. VI. Interactions of age and genotype of bees, and nectar flow. J. Apic. Res. 1971, 10, 11–21. [Google Scholar] [CrossRef]
- Robinson, G.E. Regulation of division of labor in insect societies. Annu. Rev. Entomol. 1992, 37, 637–665. [Google Scholar] [CrossRef]
- Athreya, S.; Reddy, M. Variation of hygienic behaviour (nest cleaning behaviour) in honey bee, Apis cerana indica F. in different eco habitats of South India. Curr. Biot. 2013, 7, 101–104. [Google Scholar]
- Rasolofoarivao, H.; Delatte, H.; Raveloson-Ravaomanarivo, L.H.; Reynaud, B.; Clémencet, J. Assessing hygienic behavior of Apis mellifera unicolor (Hymenoptera: Apidae), the endemic honey bee from Madagascar. Genet. Mol. Res. 2015, 14, 5879–5889. [Google Scholar] [CrossRef]
- Rosenkranz, P.; Tewarson, N.C.; Singh, A.; Engels, W. Differential hygienic behaviour towards Varroa jacobsoni in capped worker brood of Apis cerana depends on alien scent adhering to the mites. J. Apic. Res. 1993, 32, 89–93. [Google Scholar] [CrossRef]
- Spivak, M.; Reuter, G.S. Performance of hygienic honey bee colonies in a commercial apiary. Apidologie 1998, 29, 291–302. [Google Scholar] [CrossRef]
- Seltzer, R.; Kahanov, P.; Kamer, Y.; Hetzroni, A.; Bieńkowska, M.; Hefetz, A.; Soroker, V. The payoffs and tradeoffs of hygienic behavior: A five year field study on a local population of honey bees. J. Apic. Res. 2022, 61, 492–501. [Google Scholar] [CrossRef]
- Masaquiza, D.; Vargas, J.; Ortíz, N.; Salazar, R.; Curbelo, L.; Pérez, A.; Arenal, A. Hygienic behavior of Apis mellifera and its relationship with Varroa destructor infestation and honey production in the central highlands of Ecuador. Insects 2021, 12, 966. [Google Scholar] [CrossRef]
- Maucourt, S.; Fortin, F.; Robert, C.; Giovenazzo, P. Genetic parameters of honey bee colonies traits in a Canadian selection program. Insects 2020, 11, 587. [Google Scholar] [CrossRef] [PubMed]
- Spivak, M.; Gilliam, M. Facultative expression of hygienic behaviour of honey bees in relation to disease resistance. J. Apic. Res. 1993, 32, 147–157. [Google Scholar] [CrossRef]
- Bailey, L.; Gibbs, A.; Woods, R. Sacbrood virus of the larval honey bee (Apis mellifera Linnaeus). Virology 1964, 23, 425–429. [Google Scholar] [CrossRef]
- Awad, A.M.; Owayss, A.A.; Iqbal, J.; Raweh, H.S.; Alqarni, A.S. GIS approach for determining the optimum spatiotemporal plan for beekeeping and honey production in hot-arid subtropical ecosystems. J. Econ. Entomol. 2019, 112, 1032–1042. [Google Scholar] [CrossRef]
- Danner, N.; Keller, A.; Härtel, S.; Steffan-Dewenter, I. Honey bee foraging ecology: Season but not landscape diversity shapes the amount and diversity of collected pollen. PLoS ONE 2017, 12, e0183716. [Google Scholar] [CrossRef]




| Source | Df | Residual | HB | HA | PA | BA | AP | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F Value | F Value | F Value | F Value | F Value | ||||||||
| Season | 1 | 222 | 17.61 | 0.07 | 157.92 | 0.42 | 94.14 | 0.30 | 564.21 | 0.72 | 131.86 | 0.37 |
| Location | 5 | 222 | 93.61 | 0.68 | 82.17 | 0.65 | 47.39 | 0.52 | 51.78 | 0.54 | 43.25 | 0.49 |
| Season × Location | 5 | 222 | 0.28 | 0.01 | 4.02 | 0.08 | 2.23 | 0.05 | 3.42 | 0.07 | 0.09 | 0.00 |
| Colony ID | Selection Index | Rank |
|---|---|---|
| T4 | 107.6779 | 1 |
| C2 | 104.3092 | 2 |
| C7 | 99.05145 | 3 |
| C9 | 98.61222 | 4 |
| C10 | 92.61824 | 5 |
| C4 | 91.99659 | 6 |
| D10 | 91.68568 | 7 |
| C8 | 81.35574 | 8 |
| T6 | 71.77229 | 9 |
| T12 | 71.70287 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haran, R.; Sumathi, E.; Iqbal, J.; Krupesh, S.; Parthasarathi, G.; Vijay, S.; Saminathan, V.R.; Srinivasan, M.R.; Kokiladevi, E.; Jayakanthan, M.; et al. Field Exploration for Colony Selection: Evaluating Hygienic Behavior in Apis cerana indica Colonies. Insects 2024, 15, 598. https://doi.org/10.3390/insects15080598
Haran R, Sumathi E, Iqbal J, Krupesh S, Parthasarathi G, Vijay S, Saminathan VR, Srinivasan MR, Kokiladevi E, Jayakanthan M, et al. Field Exploration for Colony Selection: Evaluating Hygienic Behavior in Apis cerana indica Colonies. Insects. 2024; 15(8):598. https://doi.org/10.3390/insects15080598
Chicago/Turabian StyleHaran, Ramkumar, Ettiappan Sumathi, Javaid Iqbal, Sivakumar Krupesh, Ganesan Parthasarathi, Settu Vijay, Vangili Ramasami Saminathan, Madapuji Rajagopalan Srinivasan, Eswaran Kokiladevi, Mannu Jayakanthan, and et al. 2024. "Field Exploration for Colony Selection: Evaluating Hygienic Behavior in Apis cerana indica Colonies" Insects 15, no. 8: 598. https://doi.org/10.3390/insects15080598
APA StyleHaran, R., Sumathi, E., Iqbal, J., Krupesh, S., Parthasarathi, G., Vijay, S., Saminathan, V. R., Srinivasan, M. R., Kokiladevi, E., Jayakanthan, M., & Zeshan, A. (2024). Field Exploration for Colony Selection: Evaluating Hygienic Behavior in Apis cerana indica Colonies. Insects, 15(8), 598. https://doi.org/10.3390/insects15080598






