Identification of Diptera Puparia in Forensic and Archeo-Funerary Contexts
Abstract
:Simple Summary
Abstract
1. Introduction
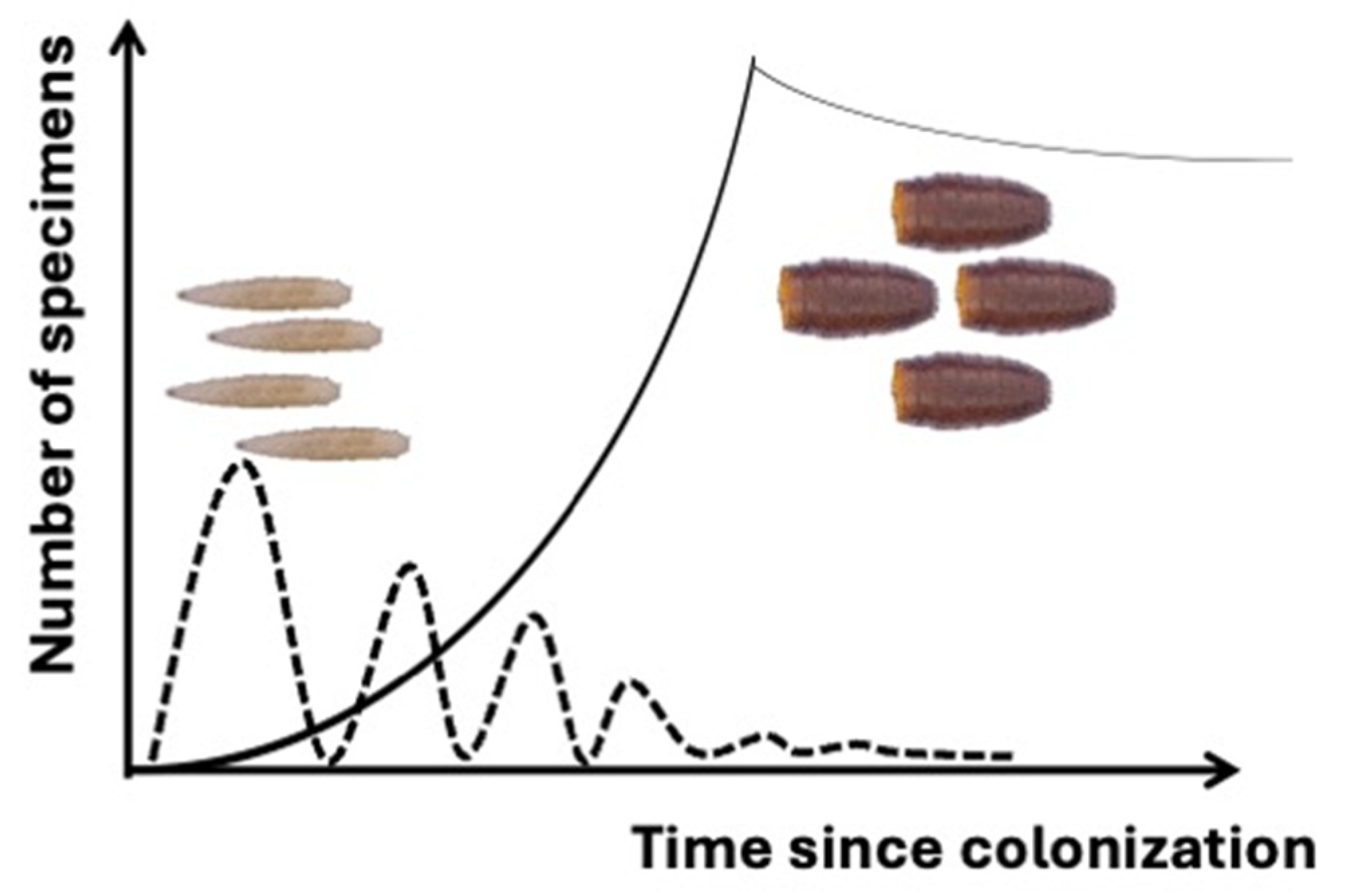
2. Definition, Structure, and Formation of Puparia
3. Morphology-Based Approach
4. DNA-Based Approach
4.1. Fragmentation and Chemical Modifications
4.2. PCR Inhibition
5. Chemical Identification of Puparia
5.1. Cuticular Hydrocarbons
- The lack of a comprehensive database of fly puparia CHC signatures;
- Changes in puparia CHC signatures over time, which, while useful for aging puparia, may affect identification precision;
- The requirement for specific and expensive equipment, as well as trained personnel, further complicates widespread adoption.
5.2. Lipidis
6. Methodology Remarks
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tilstone, W.J.; Hastrup, M.L.; Hald, C. Fisher’s Techniques of Crime Scene Investigation, First International Edition; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Smith, K.G.V. A Manual of Forensic Entomology; Cornell University Press: Ithaca, NY, USA, 1986. [Google Scholar]
- Amendt, J.; Richards, C.S.; Campobasso, C.P.; Zehner, R.; Hall, M.J.R. Forensic entomology: Applications and limitations. Forensic Sci. Med. Pathol. 2011, 7, 379–392. [Google Scholar] [CrossRef] [PubMed]
- de Carvahlo, L.M.L. Toxicology and forensic entomology. In Current Concept in Forensic Entomology; Amendt, J., Goff, M.L., Grassberger, M., Campobasso, C.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 163–178. [Google Scholar]
- Marchetti, D.; Arena, E.; Boschi, I.; Vanin, S. Human DNA extraction from empty puparia. Forensic Sci. Int. 2013, 229, e26–e29. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Singh, P.; Tuccia, F.; Pradelli, J.; Giordani, G.; Vanin, S. DNA characterization from gut content of larvae of Megaselia scalaris (Diptera, Phoridae). Sci. Justice 2019, 59, 654–659. [Google Scholar] [CrossRef]
- Miller, M.L.; Lord, W.D.; Goff, M.L.; Donnelly, B.; McDonough, E.T.; Alexis, J.C. Isolation of amitriptyline and nortriptyline from fly puparia (Phoridae) and beetle exuviae (Dermestidae) associated with mummified human remains. J. Forensic Sci. 1994, 39, 1305–1313. [Google Scholar] [CrossRef]
- Grassberger, M.; Reiter, C. Effect of temperature on Lucilia sericata (Diptera: Calliphoridae) development with special reference to the isomegalen- and isomorphen-diagram. Forensic Sci. Int. 2001, 120, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Richards, C.S.; Paterson, I.D.; Villet, M.H. Estimating the age of immature Chrysomya albiceps (Diptera: Calliphoridae), correcting for temperature and geographical latitude. Int. J. Leg. Med. 2008, 122, 271–279. [Google Scholar] [CrossRef]
- Charabidze, D.; Gosselin, M.; Hedouin, V. Use of necrophagous insects as evidence of cadaver relocation: Myth or reality? PeerJ 2017, 5, e3506. [Google Scholar] [CrossRef]
- Vanin, S.; Tasinato, P.; Ducolin, G.; Terranova, C.; Zancaner, S.; Montisci, M.; Ferrara, P.; Turchetto, M. Use of Lucilia species (Diptera: Calliphoridae) for forensic investigations in Southern Europe. Forensic Sci Int. 2008, 177, 37–41. [Google Scholar] [CrossRef]
- Lo Pinto, S.; Giordani, G.; Tuccia, F.; Ventura, F.; Vanin, S. First records of Synthesiomyia nudiseta (Diptera: Muscidae) from forensic cases in Italy. Forensic Sci. Inter. 2017, 276, e1–e7. [Google Scholar] [CrossRef]
- Turchetto, M.; Vanin, S. Forensic Entomology and Climatic Change. Forensic Sci. Inter. 2004, 146, 207–209. [Google Scholar] [CrossRef]
- The International Code of Zoological Nomenclature. Available online: https://www.iczn.org/the-code/the-international-code-of-zoological-nomenclature/ (accessed on 1 July 2024).
- Grzywacz, A.; Hall, M.J.; Pape, T.; Szpila, K. Muscidae (Diptera) of forensic importance-an identification key to third instar larvae of the Western Palaearctic region and a catalogue of the muscid carrion community. Int. J. Legal Med. 2017, 131, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Rozkošný, R.; Gregor, F.; Pont, A.C. The European Fanniidae (Diptera). Acta Sci. Nat. Brno 1997, 31, 1–80. [Google Scholar]
- Szpila, K. Key for the identification of third instars of European blowflies (Diptera: Calliphoridae) of forensic importance. In Current Concepts in Forensic Entomology; Amendt, J., Goff, M.L., Campobasso, P.C., Grassberger, M., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 43–56. [Google Scholar]
- Szpila, K.; Richet, R.; Pape, T. Third instar larvae of flesh flies (Diptera: Sarcophagidae) of forensic importance—Critical review of characters and key for European species. Parasitol. Res. 2015, 114, 2279–2289. [Google Scholar] [CrossRef] [PubMed]
- Erzinçlioğlu, Y.Z. Immature stages of British Calliphora and Cynomya, with a re-evaluation of the taxonomic characters of larval Calliphoridae (Diptera). J. Nat. Hist. 1985, 19, 69–96. [Google Scholar] [CrossRef]
- Skidmore, P. The Biology of the Muscidae of the World; Springer: Dordrecht, The Netherlands, 1985. [Google Scholar]
- Giordani, G.; Grzywacz, A.; Vanin, S. Characterization and identification of puparia of Hydrotaea Robineau-Desvoidy, 1830 (Diptera: Muscidae) from forensic and archaeological contexts. J. Med. Entomol. 2018, 56, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Giordani, G.; Tuccia, F.; Floris, I.; Vanin, S. First record of Phormia regina (Meigen, 1826) (Diptera: Calliphoridae) from mummies at the Sant’Antonio Abate Cathedral of Castelsardo, Sardinia, Italy. PeerJ 2018, 6, e4176. [Google Scholar] [CrossRef] [PubMed]
- Giordani, G.; Tuccia, F.; Zoppis, S.; Vecchiotti, C.; Vanin, S. Record of Leptometopa latipes (Diptera: Milichiidae) from a human cadaver in the Mediterranean area. Forensic Sci. Res. 2018, 3, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Giordani, G.; Tuccia, F.; Martín-Vega, D.; Angell, C.S.; Pradelli, J.; Vanin, S. Morphological and molecular characterization of puparia of Piophilidae species of forensic relevance. Med. Vet. Entomol. 2023, 37, 339–358. [Google Scholar] [CrossRef] [PubMed]
- Giordani, G.; Vanin, S. Morphological characterization of puparia and molecular analysis of Heleomyza serrata (Linnaeus, 1758) (Diptera: Heleomyzidae): A species of potential forensic interest. Eur. J. Zool. 2023, 90, 604–613. [Google Scholar] [CrossRef]
- Scaramozzino, P.L.; Di Giovanni, F.; Loni, A.; Gisondi, S.; Lucchi, A.; Cerretti, P. Tachinid (Diptera, Tachinidae) parasitoids of Lobesia botrana (Denis & Schiffermüller, 1775) (Lepidoptera, Tortricidae) and other moths. ZooKeys 2020, 934, 111–140. [Google Scholar]
- Wells, J.; Stevens, J. Application of DNA-based methods in forensic entomology. Annu. Rev. Entomol. 2008, 53, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Mazzanti, M.; Alessandrini, F.; Tagliabracci, A.; Wells, J.D.; Campobasso, C.P. DNA degradation and genetic analysis of empty puparia: Genetic identification limits in forensic entomology. Forensic Sci. Int. 2010, 195, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Moore, H.E.; Pechal, J.L.; Benbow, M.E.; Drijfhout, F.P. The potential use of cuticular hydrocarbons and multivariate analysis to age empty puparial cases of Calliphora vicina and Lucilia sericata. Sci. Rep. 2017, 7, 1933. [Google Scholar] [CrossRef] [PubMed]
- Martín-Vega, D.; Hall, M.J.; Simonsen, T.J. Resolving confusion in the use of concepts and terminology in intrapuparial development studies of cyclorrhaphous Diptera. J. Med. Entomol. 2016, 53, 1249–1251. [Google Scholar] [CrossRef] [PubMed]
- Vanin, S.; Huchet, J. Forensic Entomology and Funerary Archaeoentomology. In Taphonomy of Human Remains: Analysis of the Death and the Depositional Environments; Schotsmans, E., Marquez-Grant, N., Forbes, S., Eds.; John Wiley & Sons Ltd.: Oxford, UK, 2017; pp. 176–186. [Google Scholar]
- Martín-Vega, D.; Baz, A.; Díaz-Aranda, L.M. The immature stages of the necrophagous fly, Prochyliza nigrimana: Comparison with Piophila casei and medicolegal considerations (Diptera: Piophilidae). Parasitol. Res. 2012, 111, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakopulu, E. Dipterous remains and archaeological interpretation. J. Arch. Sci. 2004, 31, 1675–1684. [Google Scholar] [CrossRef]
- Amendt, J.; Campobasso, C.P.; Gaudry, E.; Reiter, C.; Leblanc, H.N.; Hall, M.J. Best practice in forensic entomology-standards and guidelines. Int. J. Leg. Med. 2007, 121, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Kenward, H. Northern Regional Review of Environmental Archaeology: Invertebrates in Archaeology in the North of England: Environmental Studies Report; English Heritage Research Department, Fort Cumberland: Swindon, UK, 2009. [Google Scholar] [CrossRef]
- Buckland, P.I.; Buckland, P.C.; Olsson, F. Paleoentomology: Insects and other arthropods in environmental archaeology. In Encyclopedia of Global Archaeology; Smith, C., Ed.; Springer: New York, NY, USA, 2014. [Google Scholar]
- King, G.A.; Gilbert, M.T.; Willerslev, E.; Collins, M.J.; Kenward, H. Recovery of DNA from archaeological insect remains: First results, problems and potential. J. Archaeol. Sci. 2009, 36, 1179–1183. [Google Scholar] [CrossRef]
- Turchetto, M.; Vanin, S. Climate change and Forensic entomology. In Current Concepts in Forensic Entomology; Amendt, J., Campobasso, C.P., Goff, M.L., Grassberger, M., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 327–352. [Google Scholar]
- Prado e Castro, C.; García, M.D. First record of Chrysomya megacephala (Fabricius, 1794) (Diptera, Calliphoridae) from Portugal. Graellsia 2009, 65, 75–77. [Google Scholar] [CrossRef]
- Ebejer, M.J. The ocurrence of Chrysomya megacephala (Fabricius) (Diptera: Brachycera) in Malta and records of other Calliphoridae from Maltese Island. Entomol. Mon. Mag. 2007, 143, 165–170. [Google Scholar]
- Martínez-Sánchez, A.; Ivorra, T.; Roberts, L.C.; Giner, S.; Beringola, L.M.; Cano, P.M.; Rojo, S. First record of Chrysomya megacephala (Diptera: Calliphoridae) on human corpses from Europe. In Proceedings of the 19th Meeting European Association of Forensic Entomology, Kolymbari, Greece, 17–20 May 2023. [Google Scholar]
- Hochkirch, A.; Casino, A.; Penev, L.; Allen, D.; Tilley, L.; Georgiev, T.; Gospodinov, K.; Barov, B. European Red List of Insect Taxonomists; Publication Office of the European Union: Luxembourg, 2022. [Google Scholar]
- Tuccia, F.; Giordani, G.; Vanin, S. A general review of the most common COI primers for Calliphoridae identification in forensic entomology. Forensic Sci. Int. Genet. 2016, 24, e9–e11. [Google Scholar] [CrossRef] [PubMed]
- Tuccia, F.; Giordani, G.; Vanin, S. A combined protocol for identification of maggots of forensic interest. Sci. Justice 2016, 56, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.; Ratnasingham, S.; De Waard, J.R. Barcoding animal life: Cytochrome C Oxidase subunit 1 divergences among closely related species. Proc. Roy. Soc. Lond. Ser. B Biol. Sci. 2003, 270, S96–S99. [Google Scholar] [CrossRef] [PubMed]
- Gilarriortua, M.; Salona Bordas, M.I.; Kohnemann, S.; Pfeiffer, H.; De Pancorbo, M.M. Molecular differentiation of central European blowfly species (Diptera, Calliphoridae) using mitochondrial and nuclear genetic markers. Forensic Sci. Int. 2014, 242, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial Cytochrome C Oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotech. 1994, 3, 294–299. [Google Scholar]
- Yeo, D.; Srivathsan, A.; Meier, R. Longer is not always better: Optimizing barcode length for large-scale species discovery and identification. Syst. Biol. 2020, 69, 999–1015. [Google Scholar] [CrossRef] [PubMed]
- Grzywacz, A.; Wyborska, D.; Piwczyński, M. DNA barcoding allows identification of European Fanniidae (Diptera) of forensic interest. For. Sci. Int. 2017, 278, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Jordaens, K.; Sonet, G.; Richet, R.; Dupont, E.; Braet, Y.; Desmyter, S. Identification of forensically important Sarcophaga species (Diptera: Sarcophagidae) using the mitochondrial COI gene. Int. J. Leg. Med. 2013, 127, 491–504. [Google Scholar] [CrossRef]
- Gilarriortua, M.; Salona-Bordas, M.I.; Caine, L.M.; Pinheiro, F.; De Pancorbo, M.M. Mitochondrial and nuclear DNA approaches for reliable identification of Lucilia (Diptera, Calliphoridae) species of forensic interest from Southern Europe. For. Sci. Int. 2015, 257, 393–397. [Google Scholar] [CrossRef]
- Boheme, P.; Amendt, J.; Zehner, R. The use of COI barcodes for molecular identification of forensically important fly species in Germany. Parasitol. Res. 2011, 110, 2325–2332. [Google Scholar] [CrossRef]
- Gilarriortua, M.; Salona Bordas, M.I.; Caine, L.M.; Pinheiro, F.; De Pancorbo, M.M. Cytochrome b as a useful tool for the identification of blowflies of forensic interest (Diptera, Calliphoridae). Forensic Sci. Int. 2013, 228, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, H.P.A.; Uribe, S.S.I.; Lopez, R.A. Analysis of mitochondrial DNA sequences (Cytb and ND1) in Lucilia eximia (Diptera: Calliphoridae). Rev. Colomb. Entomol. 2011, 37, 273–278. [Google Scholar] [CrossRef]
- Zehner, R.; Amendt, J.; Schütt, S.; Sauer, J.; Krettek, R.; Povolný, D. Genetic identification of forensically important flesh flies (Diptera: Sarcophagidae). Int. J. Leg. Med. 2004, 118, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Bortolini, S.; Giordani, G.; Tuccia, F.; Maistrello, L.; Vanin, S. Do longer sequences improve the accuracy of identification of forensically important Calliphoridae species? PeerJ 2018, 6, e5962. [Google Scholar] [CrossRef]
- McDonagh, L.M.; Stevens, J.R. The molecular systematics of blowflies and screwworm flies (Diptera: Calliphoridae) using 28S rRNA, COX1 and EF-1α: Insights into the evolution of dipteran parasitism. Parasitology 2011, 138, 1760–1777. [Google Scholar] [CrossRef] [PubMed]
- Yusseff-Vanegas, S.Z.; Agnarsson, I. DNA-barcoding of forensically important blow flies (Diptera: Calliphoridae) in the Caribbean Region. PeerJ 2017, 5, e3516. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zha, L.; Yan, W.; Li, P.; Cai, J.; Wu, L. Identification of forensically important sarcophagid flies (Diptera: Sarcophagidae) in China based on COI and Period gene. Int. J. Leg. Med. 2014, 128, 221–228. [Google Scholar] [CrossRef]
- Schoofs, K.R.; Krzeminska Ahmadzai, U.; Goodwin, W. Analysis of the complete mitochondrial genomes of two forensically important blowfly species: Lucilia caesar and Lucilia illustris. Mitochondrial DNA B Resour. 2018, 3, 1114–1116. [Google Scholar] [CrossRef]
- Tautz, D.; Arctander, P.; Minelli, A.; Thomas, R.H.; Volger, A.P. A plea for DNA taxonomy. Trends Ecol. Evol. 2003, 18, 70–74. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Hebert, P.D.N. BOLD: The Barcode of Life Data System (www.barcodinglife.org). Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef]
- Glocke, I.; Meyer, M. Extending the spectrum of DNA sequences retrieved from ancient bones and teeth. Genome Res. 2017, 27, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Reiss, R.A. Ancient DNA from ice age insects: Proceed with caution. Quat. Sci. Rev. 2006, 25, 1877–1893. [Google Scholar] [CrossRef]
- Austin, J.J.; Smith, A.B.; Thomas, R.H. Paleontology in a molecular world: The search for authentic ancient DNA. Tree 1997, 12, 303–306. [Google Scholar] [PubMed]
- Fulton, T.L.; Stiller, M. 2012. PCR amplification, cloning, and sequencing of ancient DNA. Mol. Biol. 2012, 840, 111–119. [Google Scholar]
- Hofreiter, H.; Serre, D.; Poinar, H.N.; Kuch, M.; Pääbo, S. Ancient DNA. Nat. Rev. Genet. 2001, 2, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Hofreiter, M.; Jaenicke, V.; Serre, D.; Haeseler, A.V.; Pääbo, S. DNA sequences from multiple amplifications reveal artifacts induced by cytosine deamination in ancient DNA. Nucleic Acids Res. 2001, 29, 4793–4799. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.I.; Chamberlain, A.T.; Riley, M.S.; Stringer, C.; Collins, M.J. The thermal history of human fossils and the likelihood of successfull DNA amplification. J. Hum. Evol. 2003, 45, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Handt, O.; Höss, M.; Krings, M.; Pääbo, S. Ancient DNA: Methodological challenges. Experientia 1994, 50, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.W.; Overballe-Petersen, S.; Ermini, L.; Sarkissian, C.D.; Haile, J.; Hellstrom, M.; Spens, J.; Thomsen, P.F.; Bohmann, K.; Cappellini, E.; et al. Ancient and modern environmental DNA. Philos. Trans. R. Soc. Lond. B Biol. Sci 2015, 370, 20130383. [Google Scholar] [CrossRef]
- Yang, D.Y.; Watt, K. Contamination controls when preparing archaeological remains for ancient DNA analysis. J. Archaeol. Sci. 2005, 32, 331–336. [Google Scholar] [CrossRef]
- Wilson, I.G. Inhibition and facilitation of nucleic acid amplification. Appl. Environ. Microbiol. 1997, 63, 3741–3751. [Google Scholar] [CrossRef]
- Benbow, M.E.; Tomberlin, J.K.; Tarone, A.M. Carrion Ecology, Evolution, and Their Applications; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Rahi, G.S.; Adams, J.L.; Yuan, J.; Devone, D.J.; Lodhi, K.M. Whole human blood DNA degradation associated with artificial ultraviolet and solar radiations as a function of exposure time. Forensic Sci. Int. 2021, 319, 110674. [Google Scholar] [CrossRef] [PubMed]
- Mcgregor, D.P.; Forster, S.; Steven, J.; Adair, J.; Leary, S.E.C.; Leslie, D.L.; Harris, W.J.; Titball, R.W. Simultaneous detection of microorganisms in soil suspension based on PCR amplification of bacterial 16S rRNA fragments. BioTechniques 1996, 21, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Kemp, B.M.; Monroe, C.; Smith, D.G. Repeat silica extraction: A simple technique for the removal of PCR inhibitors from DNA extracts. J. Arch. Sci. 2006, 33, 1680–1689. [Google Scholar] [CrossRef]
- Golebiowski, M. Comparison of free fatty acids composition of cuticular lipids of Calliphora vicina larvae and pupae. Lipids 2012, 47, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Li, K.; Zhu, J.; Zhu, G.; Hu, C. Cuticular hydrocarbon composition in pupal exuviae for taxonomic differentiation of six necrophagous flies. J. Med. Entomol. 2007, 44, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Golebiowski, M.; Cerkowniak, M.; Bogus, M.I.; Wloka, E.; Przybysz, E.; Stepnowski, P. Developmental changes in the sterol composition and the glycerol content of cuticular and internal lipids of three species of flies. Chem. Biodivers. 2013, 10, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Golebiowski, M.; Urbanek, A.; Oleszczak, A.; Dawgul, M.; Kamysz, W.; Boguś, M.I.; Stepnowski, P. The antifungal activity of fatty acids of all stages of Sarcophaga carnaria L. (Diptera: Sarcophagidae). Microbiol. Res. 2014, 169, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Frederickx, C.; Dekeirsschieter, J.; Brostaux, Y.; Wathelet, J.P.; Verheggen, F.J.; Haubruge, E. Volatile organic compounds released by blowfly larvae and pupae: New perspectives in forensic entomology. Forensic Sci. Int. 2012, 219, 215–220. [Google Scholar] [CrossRef]
- Kranz, W.; Carroll, C.; Dixon, D.A.; Goodpaster, J.V.; Picard, C.J. Factors affecting species identifications of blow fly pupae based upon chemical profiles and multivariate statistics. Insects 2017, 8, 43. [Google Scholar] [CrossRef]
- Giordani, G.; Whitmore, D.; Vanin, S. A New, non-invasive methodology for the molecular identification of adult Sarcophagidae from collections. Insects 2023, 14, 635. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanin, S.; Tuccia, F.; Pradelli, J.; Carta, G.; Giordani, G. Identification of Diptera Puparia in Forensic and Archeo-Funerary Contexts. Insects 2024, 15, 599. https://doi.org/10.3390/insects15080599
Vanin S, Tuccia F, Pradelli J, Carta G, Giordani G. Identification of Diptera Puparia in Forensic and Archeo-Funerary Contexts. Insects. 2024; 15(8):599. https://doi.org/10.3390/insects15080599
Chicago/Turabian StyleVanin, Stefano, Fabiola Tuccia, Jennifer Pradelli, Giuseppina Carta, and Giorgia Giordani. 2024. "Identification of Diptera Puparia in Forensic and Archeo-Funerary Contexts" Insects 15, no. 8: 599. https://doi.org/10.3390/insects15080599
APA StyleVanin, S., Tuccia, F., Pradelli, J., Carta, G., & Giordani, G. (2024). Identification of Diptera Puparia in Forensic and Archeo-Funerary Contexts. Insects, 15(8), 599. https://doi.org/10.3390/insects15080599







