Origins of Susceptibility to Insect Herbivores in High-Yielding Hybrid and Inbred Rice Genotypes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Insect Herbivores
2.3. Genotyping
2.3.1. Collection of Leaf Samples
2.3.2. DNA Extraction
2.3.3. Genotyping Using 384-Plex SNP Set on the BeadXpress Platform
2.4. Stemborer Antixenosis Experiments
2.5. Stemborer Antibiosis Experiments
2.6. Planthopper Antixenosis Experiments
2.7. Planthopper Antibiosis Experiments
2.8. Field Plot Experiment
2.9. Data Analyses
3. Results
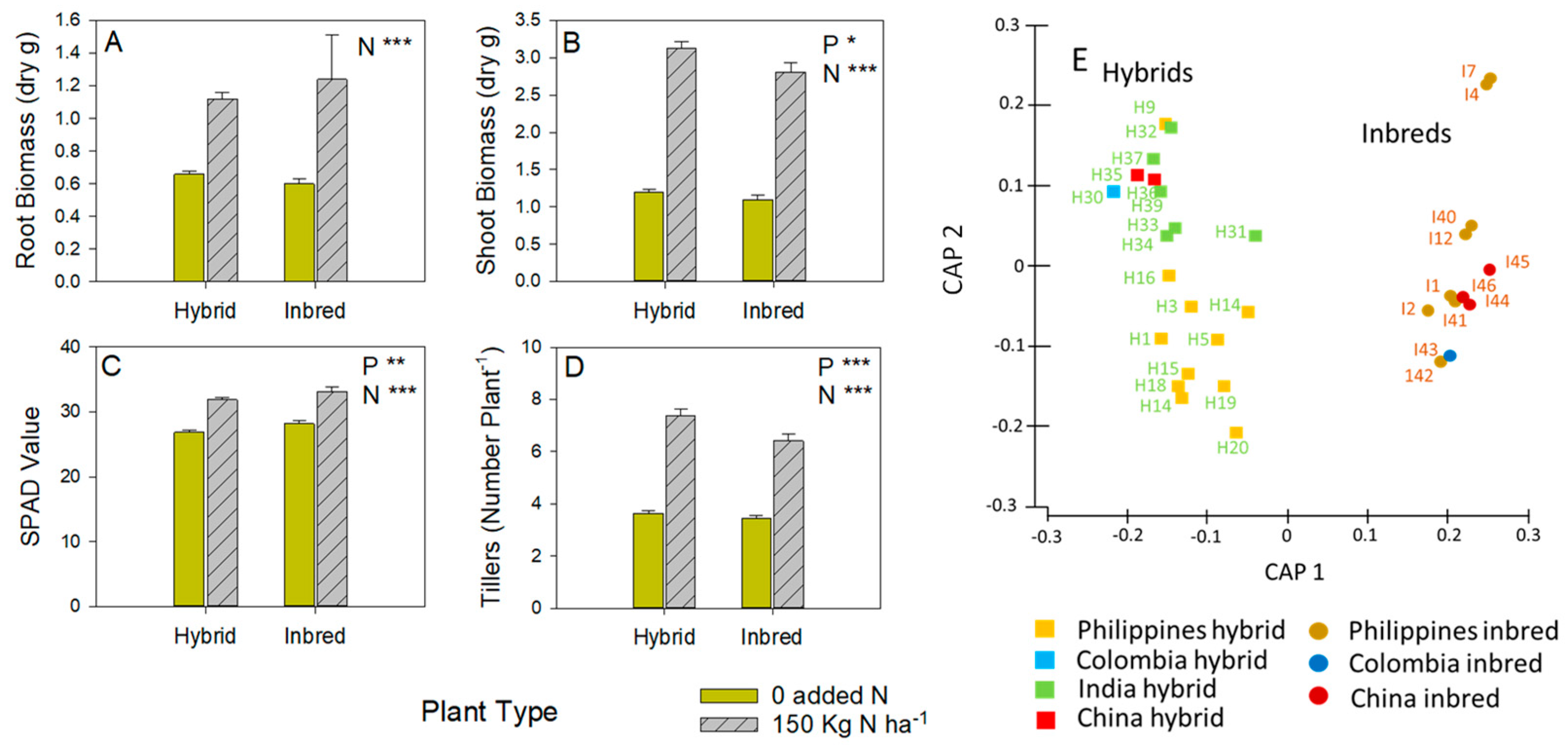
3.1. Plant Development and Genotyping
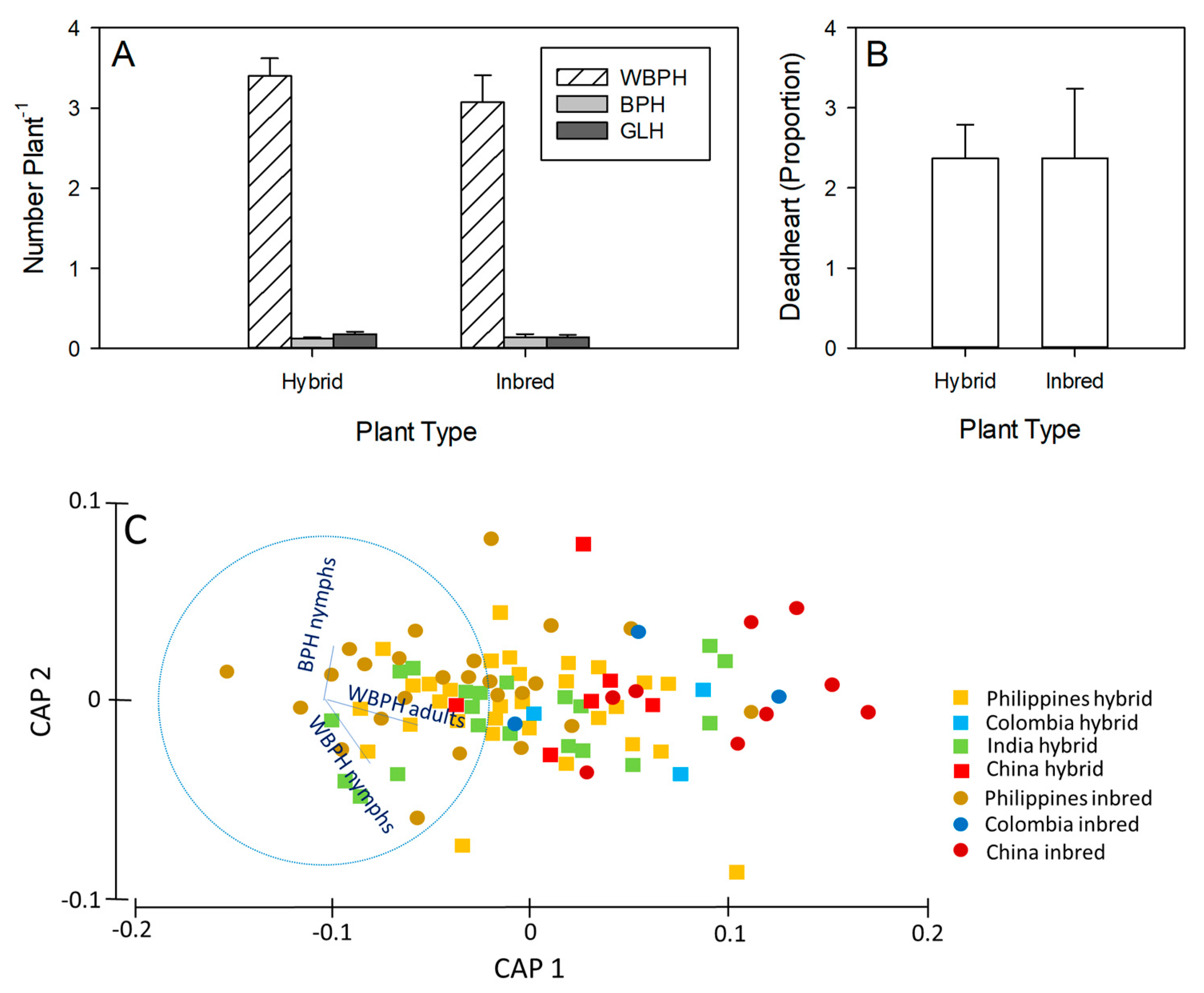
3.2. Stemborer Responses to Hybrid and Inbred Lines
3.3. Planthopper Responses to Hybrid and Inbred Lines
3.4. Pest Occurrence in Field Plots
4. Discussion
4.1. Hybrid and Inbred Rice Genotypes
4.2. Stemborer Responses to Hybrid and Inbred Genotypes
4.3. Planthopper Responses to Hybrid and Inbred Genotypes
4.4. Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Virmani, S.S.; Aquino, R.; Khush, G. Heterosis breeding in rice (Oryza sativa L.). Theor. Appl. Genet. 1982, 63, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Virmani, S.S. Prospects of hybrid rice in the tropics and subtropics. In Hybrid Rice Technology: New Developments and Future Prospects; Virmani, S.S., Ed.; International Rice Research Institute: Los Baños, Philippines, 1994; pp. 7–19. [Google Scholar]
- Richharia, R.H.; Misro, B.; Rao, R.K. Sterility in the rice hybrids and its significance. Euphytica 1962, 11, 137–142. [Google Scholar] [CrossRef]
- Yuan, L.-P. Development of hybrid rice to ensure food security. Rice Sci. 2014, 21, 1. [Google Scholar] [CrossRef]
- Horgan, F.G.; Crisol, E. Hybrid rice and insect herbivores in Asia. Entomol. Exp. Appl. 2013, 148, 1–19. [Google Scholar] [CrossRef]
- Spielman, D.J.; Kolady, D.E.; Ward, P.S. The prospects for hybrid rice in India. Food Secur. 2013, 5, 651–665. [Google Scholar] [CrossRef]
- Rumanti, I.; Purwoko, B.; Dewi, I.; Aswidinnoor, H.; Widyastuti, Y. Combining ability for yield and agronomic traits in hybrid rice derived from wild abortive, Gambiaca and Kalinga cytoplasmic male sterile lines. SABRAO J. Breed. Genet. 2017, 49, 69. [Google Scholar]
- Wan, J.; Yamaguchi, Y.; Kato, H.; Ikehashi, H. Two new loci for hybrid sterility in cultivated rice (Oryza sativa L.). Theor. Appl. Genet. 1996, 92, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Xinggui, L.; Virmani, S.; Yang, R. Advances in two-line hybrid rice breeding. In Advances in Hybrid Rice Technology; Virmani, S.S., Siddiq, E.A., Muralidharan, K., Eds.; International Rice Research Institute: Los Baños, Philippines, 1998; pp. 89–98. [Google Scholar]
- Bueno, C.S.; Lafarge, T. Maturity groups and growing seasons as key sources of variation to consider within breeding programs for high yielding rice in the tropics. Euphytica 2017, 213, 1–18. [Google Scholar] [CrossRef]
- Horgan, F.G.; Bernal, C.C.; Ramal, A.F.; Almazan, M.L.P.; Mundaca, E.A.; Crisol-Martínez, E. Heterosis for resistance to insect herbivores in a 3-line hybrid rice system. Insects 2024, 15, 164. [Google Scholar] [CrossRef]
- Zhou, G.; Chen, Y.; Yao, W.; Zhang, C.; Xie, W.; Hua, J.; Xing, Y.; Xiao, J.; Zhang, Q. Genetic composition of yield heterosis in an elite rice hybrid. Proc. Natl. Acad. Sci. USA 2012, 109, 15847–15852. [Google Scholar] [CrossRef]
- Mew, T.W.; Wang, F.M.; Wu, J.T.; Lin, K.R.; Khush, G.S. Disease and insect resistance in hybrid rice. In Hybrid Rice; International Rice Research Institute: Los Baños, Philippines, 1988; pp. 189–200. [Google Scholar]
- Horgan, F.G.; Crisol-Martínez, E.; Almazan, M.L.P.; Romena, A.; Ramal, A.F.; Ferrater, J.B.; Bernal, C.C. Susceptibility and tolerance in hybrid and pure-line rice varieties to herbivore attack: Biomass partitioning and resource-based compensation in response to damage. Ann. Appl. Biol. 2016, 169, 200–213. [Google Scholar] [CrossRef]
- Faiz, F.A.; Ijaz, M.; Awan, T.H.; Manzoor, Z.; Ahmed, M.; Wariach, N.M.; Zahid, M.A. Effect of wild abortive cytoplasm inducing male sterility on resistance/tolerance against brown planthopper and whitebacked planthopper in Basmati rice hybrids. J. Anim. Plant Sci. 2007, 17, 16–20. [Google Scholar]
- Sogawa, K.; Liu, G.-J.; Shen, J.-H. A review on the hyper-susceptibility of Chinese hybrid rice to insect pests. Chin. J. Rice Sci. 2003, 17, 23. [Google Scholar]
- Sogawa, K.; Liu, G.-J.; Zhou, J.; Han, X.; You, C.-B. Causal analysis on the whitebacked planthopper prevalence in Chinese hybrid rice Shanyou 63. Chin. J. Rice Sci. 2003, 17, 95. [Google Scholar]
- Zhu, Z.; Cheng, J.; Zuo, W.; Lin, X.; Guo, Y.; Jiang, Y.; Wu, X.; Teng, K.; Zhai, B.; Luo, J. Integrated management of rice stem borers in the Yangtze Delta, China. In Area-Wide Control of Insect Pests: From Research to Field Implementation; Vreysen, M.J.B., Robinson, A.S., Hendrichs, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 373–382. [Google Scholar]
- Horgan, F.G.; Bernal, C.C.; Ramal, A.F.; Almazan, M.L.P.; Mundaca, E.A.; Crisol-Martínez, E. Heterosis for interactions between insect herbivores and 3-Line hybrid rice under low and high soil nitrogen conditions. Insects 2024, 15, 416. [Google Scholar] [CrossRef] [PubMed]
- Abhilash Kumar, V.; Balachiranjeevi, C.; Bhaskar Naik, S.; Rekha, G.; Rambabu, R.; Harika, G.; Pranathi, K.; Hajira, S.; Anila, M.; Kousik, M. Marker-assisted pyramiding of bacterial blight and gall midge resistance genes into RPHR-1005, the restorer line of the popular rice hybrid DRRH-3. Mol. Breed. 2017, 37, 1–14. [Google Scholar] [CrossRef]
- Chen, Q.; Zeng, G.; Hao, M.; Jiang, H.; Xiao, Y. Improvement of rice blast and brown planthopper resistance of PTGMS line C815S in two-line hybrid rice through marker-assisted selection. Mol. Breed. 2020, 40, 21. [Google Scholar] [CrossRef]
- Fan, F.; Li, N.; Chen, Y.; Liu, X.; Sun, H.; Wang, J.; He, G.; Zhu, Y.; Li, S. Development of elite BPH-resistant wide-spectrum restorer lines for three and two line hybrid rice. Front. Plant Sci. 2017, 8, 986. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Xiao, Y.; Yu, J.; Li, J.; Meng, Q.; Qing, X.; Xiao, G. Pyramiding Xa21, Bph14, and Bph15 genes into the elite restorer line Yuehui9113 increases resistance to bacterial blight and the brown planthopper in rice. Crop Prot. 2019, 115, 31–39. [Google Scholar] [CrossRef]
- Wang, H.; Ye, S.; Mou, T. Molecular breeding of rice restorer lines and hybrids for brown planthopper (BPH) resistance using the Bph14 and Bph15 genes. Rice 2016, 9, 53. [Google Scholar] [CrossRef]
- Chen, J.-M.; Yu, X.-P.; Cheng, J.-A.; Lu, Z.-X.; Zheng, X.-S.; Xu, H.-X. Resistance screening and evaluation of newly-bred rice varieties (lines) to the rice brown planthopper, Nilaparvata lugens. Chin. J. Rice Sci. 2005, 19, 573. [Google Scholar]
- Bandong, J.P.; Litsinger, J.A. Rice crop stage susceptibility to the rice yellow stemborer Scirpophaga incertulas (Walker) (Lepidoptera: Pyralidae). Int. J. Pest Manag. 2005, 51, 37–43. [Google Scholar] [CrossRef]
- Bottrell, D.G.; Schoenly, K.G. Resurrecting the ghost of green revolutions past: The brown planthopper as a recurring threat to high-yielding rice production in tropical Asia. J. Asia-Pac. Entomol. 2012, 15, 122–140. [Google Scholar] [CrossRef]
- Hu, G.; Lu, F.; Zhai, B.-P.; Lu, M.-H.; Liu, W.-C.; Zhu, F.; Wu, X.-W.; Chen, G.-H.; Zhang, X.-X. Outbreaks of the brown planthopper Nilaparvata lugens (Stål) in the Yangtze River Delta: Immigration or local reproduction? PLoS ONE 2014, 9, e88973. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.M.; Jahan, M.; Islam, K.S. Impact of nitrogen, phosphorus and potassium on brown planthopper and tolerance of its host rice plants. Rice Sci. 2016, 23, 119–131. [Google Scholar] [CrossRef]
- Wu, J.; Ge, L.; Liu, F.; Song, Q.; Stanley, D. Pesticide-induced planthopper population resurgence in rice cropping systems. Annu. Rev. Entomol. 2020, 65, 409–429. [Google Scholar] [CrossRef] [PubMed]
- Kartohardjono, A.; Heinrichs, E.A. Populations of the brown planthopper, Nilaparvata lugens (Stål) (Homoptera: Delphacidae), and its predators on rice varieties with different levels of resistance. Environ. Entomol. 1984, 13, 359–365. [Google Scholar] [CrossRef]
- Salim, M.; Heinrichs, E.A. Impact of varietal resistance in rice and predation on the mortality of Sogatella furcifera (Horváth) (Homoptera: Delphacidae). Crop Prot. 1986, 5, 395–399. [Google Scholar] [CrossRef]
- Lou, Y.-G.; Ma, B.; Cheng, J.-A. Attraction of the parasitoid Anagrus nilaparvatae to rice volatiles induced by the rice brown planthopper Nilaparvata lugens. J. Chem. Ecol. 2005, 31, 2357–2372. [Google Scholar] [CrossRef]
- Horgan, F.G.; Romena, A.M.; Bernal, C.C.; Almazan, M.L.P.; Ramal, A.F. Differences between the strength of preference-performance coupling in two rice stemborers (Lepidoptera: Pyralidae, Crambidae) promotes coexistence at field-plot scales. Environ. Entomol. 2021, 50, 929–939. [Google Scholar] [CrossRef]
- Crisol, E.; Almazan, M.L.P.; Jones, P.W.; Horgan, F.G. Planthopper-rice interactions: Unequal stresses on pure-line and hybrid rice under similar experimental conditions. Entomol. Exp. Et Appl. 2013, 147, 18–32. [Google Scholar] [CrossRef]
- Stout, M.; Rice, W.; Ring, D. The influence of plant age on tolerance of rice to injury by the rice water weevil, Lissorhoptrus oryzophilus (Coleoptera: Curculionidae). Bull. Entomol. Res. 2002, 92, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Villegas, J.M.; Wilson, B.E.; Stout, M.J. Assessment of tolerance and resistance of inbred rice cultivars to combined infestations of rice water weevil and stemborers. Entomol. Exp. Et Appl. 2021, 169, 629–639. [Google Scholar] [CrossRef]
- Islam, Z.; Karim, A.N.M.R. Susceptibility of rice plants to stem borer damage at different growth stages and influence on grain yields. Bangladesh J. Entomol. 1999, 9, 121–130. [Google Scholar]
- Andama, J.B.; Mujiono, K.; Hojo, Y.; Shinya, T.; Galis, I. Nonglandular silicified trichomes are essential for rice defense against chewing herbivores. Plant Cell Environ. 2020, 43, 2019–2032. [Google Scholar] [CrossRef] [PubMed]
- Prasad, B.R.; Pasalu, I.; Raju, N.; Verma, N. Influence of nitrogen and rice varieties on population build up of brown planthopper, Nilaparvata lugens (Stal.). J. Entomol. Res. 2003, 27, 167–170. [Google Scholar]
- Rashid, M.M.; Jahan, M.; Islam, K.S. Effects of nitrogen, phosphorous and potassium on host-choice behavior of brown planthopper, Nilaparvata lugens (Stål) on rice cultivar. J. Insect Behav. 2017, 30, 1–15. [Google Scholar] [CrossRef]
- Jairin, J.; Phengrat, K.; Teangdeerith, S.; Vanavichit, A.; Toojinda, T. Mapping of a broad-spectrum brown planthopper resistance gene, Bph3, on rice chromosome 6. Mol. Breed. 2007, 19, 35–44. [Google Scholar] [CrossRef]
- Syobu, S.-I.; Mikuriya, H.; Yamaguchi, J.; Matsuzaki, M.; Matsumura, M. Fluctuations and factors affecting the wing-form ratio of the brown planthopper, Nilaparvata lugens Stål in rice fields. Jpn. J. Appl. Entomol. Zool. 2002, 46, 135–143. [Google Scholar] [CrossRef]
- Syobu, S.-I.; Otuka, A.; Matsumura, M. Annual fluctuations in the immigrant density of rice planthoppers, Sogatella furcifera and Nilaparvata lugens (Hemiptera: Delphacidae), in the Kyushu district of Japan, and associated meteorological conditions. Appl. Entomol. Zool. 2012, 47, 399–412. [Google Scholar] [CrossRef]
- Horgan, F.G.; Srinivasan, T.S.; Bentur, J.S.; Kumar, R.; Bhanu, K.V.; Sarao, P.S.; Chien, H.V.; Almazan, M.L.P.; Bernal, C.C.; Ramal, A.F.; et al. Geographic and research center origins of rice resistance to Asian planthoppers and leafhoppers: Implications for rice breeding and gene deployment. Agronomy 2017, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.; Thompson, W. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4326. [Google Scholar] [CrossRef] [PubMed]
- Thomson, M.J.; Zhao, K.; Wright, M.; McNally, K.L.; Rey, J.; Tung, C.-W.; Reynolds, A.; Scheffler, B.; Eizenga, G.; McClung, A. High-throughput single nucleotide polymorphism genotyping for breeding applications in rice using the BeadXpress platform. Mol. Breed. 2012, 29, 875–886. [Google Scholar] [CrossRef]
- Wright, M.H.; Tung, C.-W.; Zhao, K.; Reynolds, A.; McCouch, S.R.; Bustamante, C.D. ALCHEMY: A reliable method for automated SNP genotype calling for small batch sizes and highly homozygous populations. Bioinformatics 2010, 26, 2952–2960. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J. Permutational Multivariate Analysis of Variance (PERMANOVA). In WileyStatsRef: Statistics Reference Online; Balakrishnan, N., Colton, T., Everitt, B., Piegorsch, W.W., Ruggeri, F., Teugels, J.L., Eds.; John Wiley & Sons Inc.: Chichester, UK, 2014; pp. 1–15. [Google Scholar]
- Anderson, M.J.; Willis, T.J. Canonical Analysis of Principal Components: A useful method of constrained ordination for ecology. Ecology 2003, 84, 511–525. [Google Scholar] [CrossRef]
- Weir, B.S.; Cockerham, C.C. Estimating F-statistics for the analysis of population structure. Evolution 1984, 38, 1358–1370. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.C. Estimating hierarchical F-statistics. Evolution 1998, 52, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Goudet, J. Hierfstat, a package for R to compute and test variance components and F-statistics. Mol. Ecol. Notes 2005, 5, 184–186. [Google Scholar] [CrossRef]
- Huang, M. Hybrid breeding and cultivar diversity in rice production in China. Agric. Environ. Lett. 2022, 7, e20074. [Google Scholar] [CrossRef]
- Cohen, M.B.; Bernal, C.C.; Virmani, S.S. Do rice hybrids have heterosis for insect resistance? A study with Nilaparvata lugens (Hemiptera: Delphacidae) and Marasmia patnalis (Lepidoptera: Pyralidae). J. Econ. Entomol. 2003, 96, 1935–1941. [Google Scholar] [CrossRef]
- Yu, S.; Ali, J.; Zhang, C.; Li, Z.; Zhang, Q. Genomic breeding of green super rice varieties and their deployment in Asia and Africa. Theor. Appl. Genet. 2020, 133, 1427–1442. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q. Strategies for developing green super rice. Proc. Natl. Acad. Sci. USA 2007, 104, 16402–16409. [Google Scholar] [CrossRef]
- Jiang, M.; Cheng, J. Interactions between the striped stem borer Chilo suppressalis (Walk.) (Lep., Pyralidae) larvae and rice plants in response to nitrogen fertilization. J. Pest Sci. 2003, 76, 124–128. [Google Scholar] [CrossRef]
- Stout, M.J.; McCarter, K.; Villegas, J.M.; Wilson, B.E. Natural incidence of stem borer damage in U.S. rice varieties. Crop Prot. 2024, 177, 106565. [Google Scholar] [CrossRef]
- Matsumura, M.; Suzuki, Y. Direct and feeding-induced interactions between two rice planthoppers, Sogatella furcifera and Nilaparvata lugens: Effects on dispersal capability and performance. Ecol. Entomol. 2003, 28, 174–182. [Google Scholar] [CrossRef]
- Cao, T.-T.; Lü, J.; Lou, Y.-G.; Cheng, J.-A. Feeding-induced interactions between two rice planthoppers, Nilaparvata lugens and Sogatella furcifera (Hemiptera: Delphacidae): Effects on feeding and honeydew excretion. Environ. Entomol. 2013, 42, 1281–1291. [Google Scholar] [CrossRef]
- Tang, F.H.; Lenzen, M.; McBratney, A.; Maggi, F. Risk of pesticide pollution at the global scale. Nat. Geosci. 2021, 14, 206–210. [Google Scholar] [CrossRef]
- Tang, F.H.; Maggi, F. Pesticide mixtures in soil: A global outlook. Environ. Res. Lett. 2021, 16, 044051. [Google Scholar] [CrossRef]
- Maggi, F.; Tang, F.H. Estimated decline in global earthworm population size caused by pesticide residue in soil. Soil Secur. 2021, 5, 100014. [Google Scholar] [CrossRef]
- Widawsky, D.; Rozelle, S.; Jin, S.; Huang, J. Pesticide productivity, host-plant resistance and productivity in China. Agric. Econ. 1998, 19, 203–217. [Google Scholar]
- Claridge, M.; Den Hollander, J. Virulence to rice cultivars and selection for virulence in populations of the brown planthopper Nilaparvata lugens. Entomol. Exp. Appl. 1982, 32, 213–221. [Google Scholar] [CrossRef]
- Claridge, M.; Den Hollander, J.; Furet, I. Adaptations of brown planthopper (Nilaparvata lugens) populations to rice varieties in Sri Lanka. Entomol. Exp. Appl. 1982, 32, 222–226. [Google Scholar] [CrossRef]
- Kim, M.-S.; Ouk, S.; Jung, K.-H.; Song, Y.; Yang, J.-Y.; Cho, Y.-G. Breeding hybrid rice with genes resistant to diseases and insects using marker-assisted selection and evaluation of biological assay. Plant Breed. Biotechnol. 2019, 7, 272–286. [Google Scholar] [CrossRef]
- Li, C.-P.; Wu, D.-H.; Huang, S.-H.; Meng, M.; Shih, H.-T.; Lai, M.-H.; Chen, L.-J.; Jena, K.K.; Hechanova, S.L.; Ke, T.-J. The Bph45 gene confers resistance against brown planthopper in rice by reducing the production of limonene. Int. J. Mol. Sci. 2023, 24, 1798. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Wang, H.; Nie, L.; Tan, D.; Zhou, C.; Zhang, Q.; Li, Y.; Du, B.; Guo, J.; Huang, J. Bph30 confers resistance to brown planthopper by fortifying sclerenchyma in rice leaf sheaths. Mol. Plant 2021, 14, 1714–1732. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shi, S.; Guo, Q.; Nie, L.; Du, B.; Chen, R.; Zhu, L.; He, G. High-resolution mapping of a gene conferring strong antibiosis to brown planthopper and developing resistant near-isogenic lines in 9311 background. Mol. Breed. 2018, 38, 107. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, W.; Liu, H.; Zeng, Y.; Du, B.; Zhu, L.; He, G.; Chen, R. Marker assisted pyramiding of Bph6 and Bph9 into elite restorer line 93–11 and development of functional marker for Bph9. Rice 2017, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Bai, J.; Xie, H.; Wu, F.; Luo, X.; Jiang, S.; He, W.; Chen, L.; Cai, Q.; Xie, H. Breeding restorer lines of hybrid rice by pyramiding genes for resistance to white backed planthoppers and brown planthoppers. Chin. J. Rice Sci. 2019, 33, 421–428. [Google Scholar]
- He, Z.; Webster, S.; He, S.Y. Growth–defense trade-offs in plants. Curr. Biol. 2022, 32, R634–R639. [Google Scholar] [CrossRef]
- Kuang, Y.-H.; Fang, Y.-F.; Lin, S.-C.; Tsai, S.-F.; Yang, Z.-W.; Li, C.-P.; Huang, S.-H.; Hechanova, S.L.; Jena, K.K.; Chuang, W.-P. The impact of climate change on the resistance of rice near-isogenic lines with resistance genes against brown planthopper. Rice 2021, 14, 64. [Google Scholar] [CrossRef]
- Horgan, F.G.; Arida, A.; Ardestani, G.; Almazan, M.L.P. Elevated temperatures diminish the effects of a highly resistant rice variety on the brown planthopper. Sci. Rep. 2021, 11, 262. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Luo, P.Q.; Hu, F.Y.; Li, Y.; Sung, C.L.; Kuang, Y.H.; Lin, S.C.; Yang, Z.W.; Li, C.P.; Huang, S.H. Pyramiding BPH genes in rice maintains resistance against the brown planthopper under climate change. Pest Manag. Sci. 2024, 80, 1740–1750. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.B.; Alam, S.N.; Medina, E.B.; Bernal, C.C. Brown planthopper, Nilaparvata lugens, resistance in rice cultivar IR64: Mechanism and role in successful N. lugens management in Central Luzon, Philippines. Entomol. Exp. Et Appl. 1997, 85, 221–229. [Google Scholar] [CrossRef]
- Alam, S.; Cohen, M. Detection and analysis of QTLs for resistance to the brown planthopper, Nilaparvata lugens, in a doubled-haploid rice population. Theor. Appl. Genet. 1998, 97, 1370–1379. [Google Scholar] [CrossRef]
- Rubia, E.; Heong, K.; Zalucki, M.; Gonzales, B.; Norton, G. Mechanisms of compensation of rice plants to yellow stem borer Scirpophaga incertulas (Walker) injury. Crop Prot. 1996, 15, 335–340. [Google Scholar] [CrossRef]
- Rubia-Sanchez, E.; Suzuki, Y.; Miyamoto, K.; Watanabe, T. The potential for compensation of the effects of the brown planthopper Nilaparvata lugens Stål (Homoptera: Delphacidae) feeding on rice. Crop Prot. 1999, 18, 39–45. [Google Scholar] [CrossRef]
- Villegas, J.M.; Wilson, B.E.; Way, M.O.; Gore, J.; Stout, M.J. Tolerance to rice water weevil, Lissorhoptrus oryzophilus Kuschel (Coleoptera: Curculionidae), infestations among hybrid and inbred rice cultivars in the Southern U.S. Crop Prot. 2021, 139, 105368. [Google Scholar] [CrossRef]
- Horgan, F.G.; Romena, A.M.; Bernal, C.C.; Almazan, M.L.P.; Ramal, A.F. Stem borers revisited: Host resistance, tolerance, and vulnerability determine levels of field damage from a complex of Asian rice stemborers. Crop Prot. 2021, 142, 105513. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L. Global patterns of cereal diseases and the impacts of breeding for host plant resistance. In Achieving Durable Disease Resistance in Cereals; Burleigh Dodds Science Publishing: Cambridge, UK, 2021; pp. 1–11. [Google Scholar]
- Garrett, K.; Andersen, K.F.; Asche, F.; Bowden, R.; Forbes, G.; Kulakow, P.; Zhou, B. Resistance genes in global crop breeding networks. Phytopathology 2017, 107, 1268–1278. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horgan, F.G.; Almazan, M.L.P.; Bernal, C.C.; Dilla-Ermita, C.J.; Ardestani, G.; Mundaca, E.A.; Crisol-Martínez, E. Origins of Susceptibility to Insect Herbivores in High-Yielding Hybrid and Inbred Rice Genotypes. Insects 2024, 15, 608. https://doi.org/10.3390/insects15080608
Horgan FG, Almazan MLP, Bernal CC, Dilla-Ermita CJ, Ardestani G, Mundaca EA, Crisol-Martínez E. Origins of Susceptibility to Insect Herbivores in High-Yielding Hybrid and Inbred Rice Genotypes. Insects. 2024; 15(8):608. https://doi.org/10.3390/insects15080608
Chicago/Turabian StyleHorgan, Finbarr G., Maria Liberty P. Almazan, Carmencita C. Bernal, Christine Jade Dilla-Ermita, Goli Ardestani, Enrique A. Mundaca, and Eduardo Crisol-Martínez. 2024. "Origins of Susceptibility to Insect Herbivores in High-Yielding Hybrid and Inbred Rice Genotypes" Insects 15, no. 8: 608. https://doi.org/10.3390/insects15080608





