A Treatment for Rice Straw and Its Use for Mealworm (Tenebrio molitor L.) Feeding: Effect on Insect Performance and Diet Digestibility
Abstract
Simple Summary
Abstract
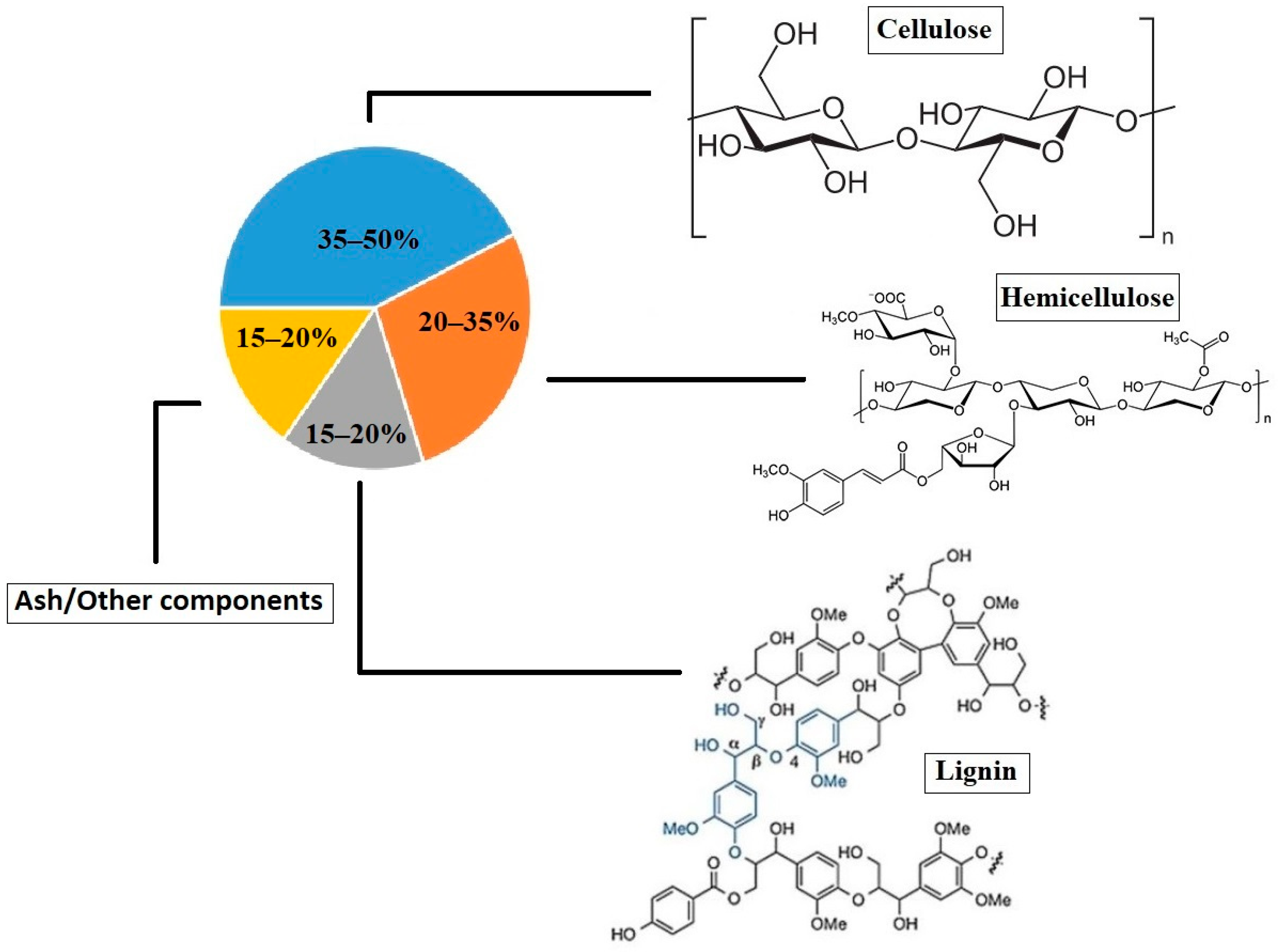
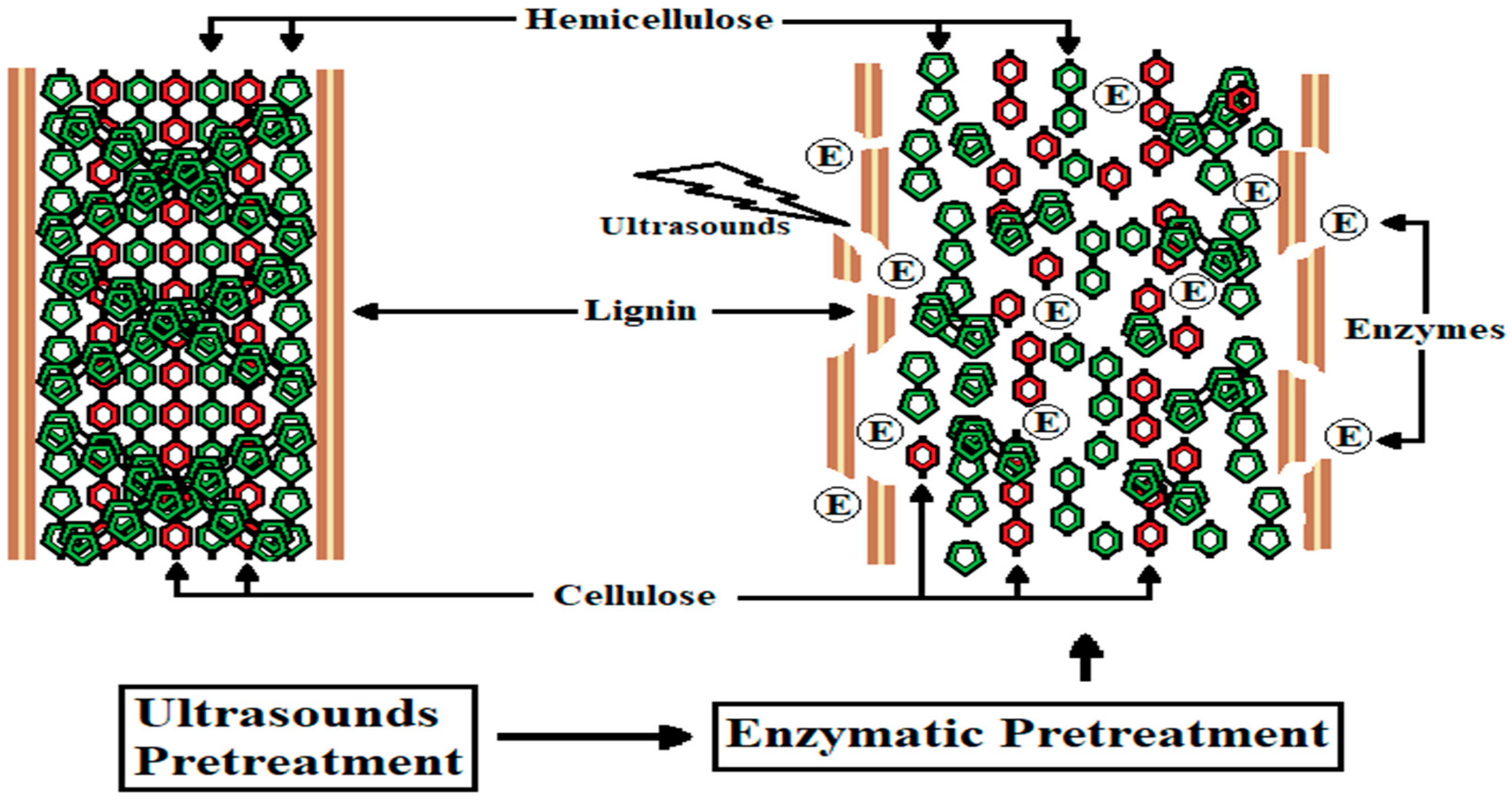
1. Introduction
2. Materials and Methods
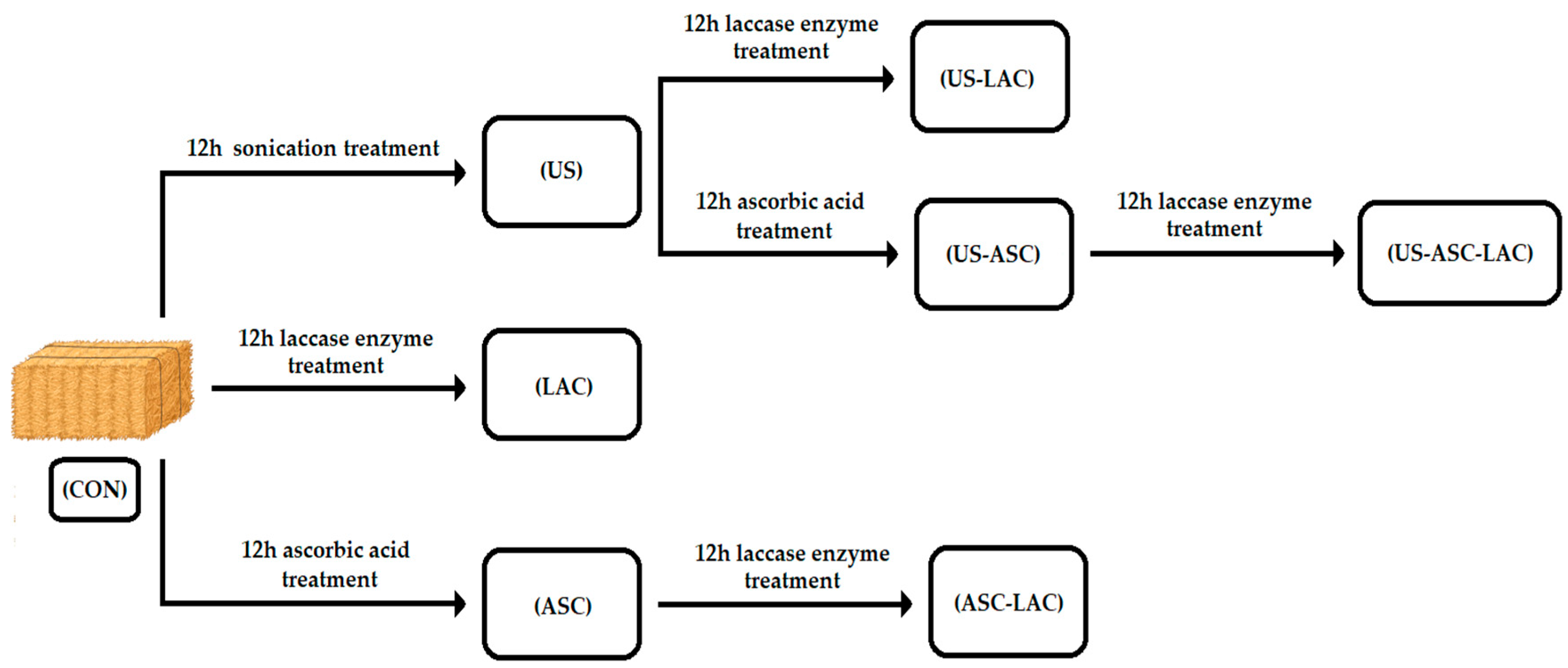
2.1. First Experiment: RS Treatment Method
2.1.1. Laccase Activity
2.1.2. Fiber Analysis Method
2.1.3. Soluble Solids Content
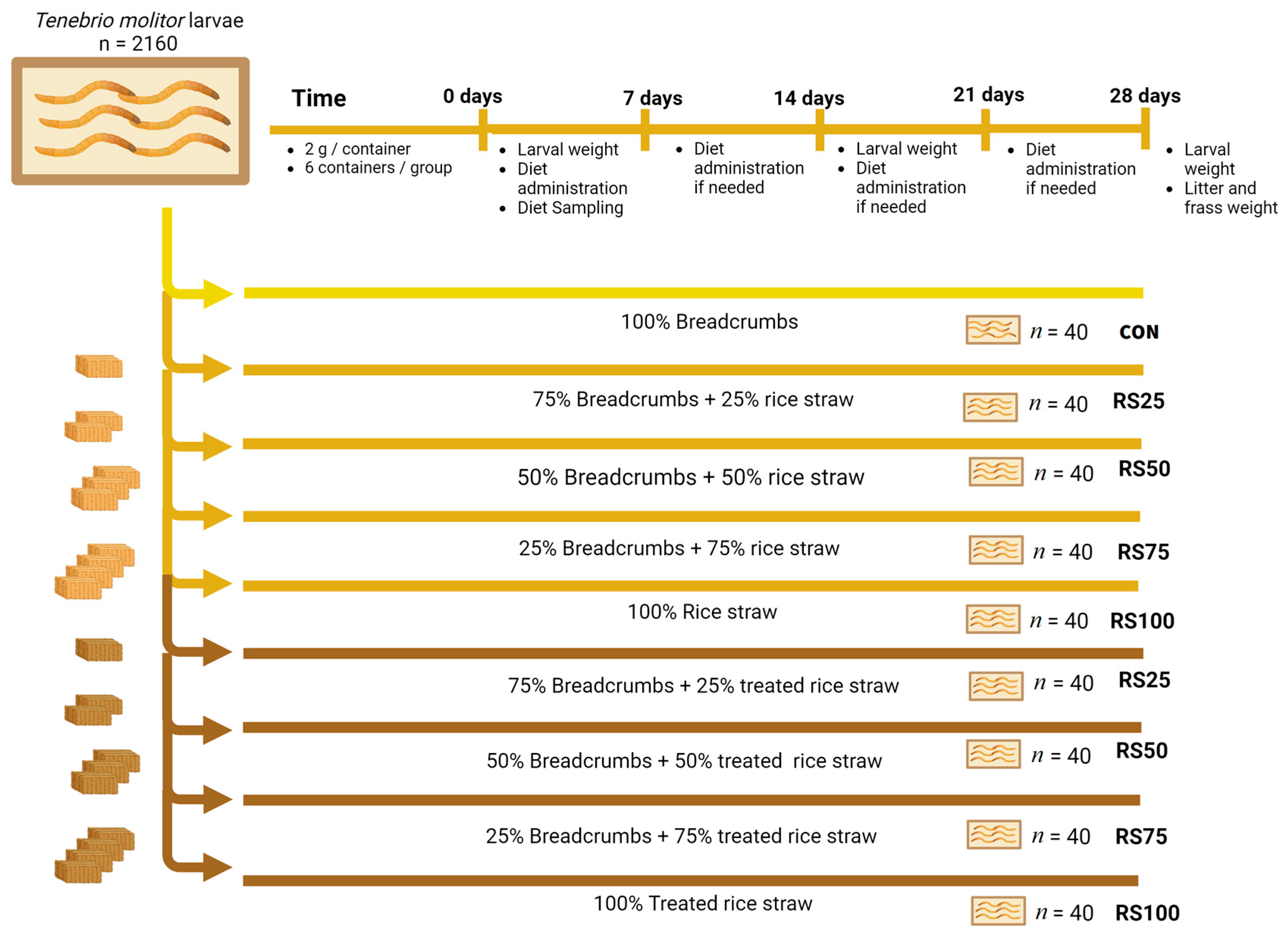
2.2. Second Experiment: Insect Rearing Method
2.2.1. Insect Rearing
2.2.2. Performance and Digestibility Test
2.2.3. Uric Acid Assay
2.2.4. Performance and Digestibility Calculation
2.3. Statistical Analysis
3. Results
3.1. RS Samples Fiber Analysis
3.2. Soluble Solid Content
3.3. Performance and Digestibility
4. Discussion
4.1. First Experiment: RS Treatment
4.2. Second Experiment: Insect Rearing
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stahel, W. The circular economy. Nature 2016, 531, 435–438. [Google Scholar] [CrossRef]
- Ruzene, D.; Silva, D.; Vicente, A.; Gonçalves, A.; Teixeira, J. An alternative application to the portuguese agro-industrial residue: Wheat straw. Appl. Biochem. Biotechnol. 2007, 147, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Goodman, B. Utilization of waste straw and husks from rice production: A review. J. Bioresour. Bioprod. 2020, 5, 143–162. [Google Scholar] [CrossRef]
- Ribó, M.; Albiach, R.; Pomares, F.; Canet, R. Alternativas de gestión de la paja de arroz en la albufera de valencia. Vida Rural 2017, 430, 56–60. [Google Scholar]
- Van Hung, N.; Maguyon-Detras, M.C.; Migo, M.V.; Quilloy, R.; Balingbing, C.; Chivenge, P.; Gummert, M. Rice straw overview: Availability, properties, and management practices. In Sustainable Rice Straw Management; Gummert, M., Van Hung, N., Chivenge, P., Douthwaite, B., Eds.; Springer Nature: Berlin/Heidelberg, Germany, 2019; pp. 1–13. [Google Scholar]
- Haghighi Mood, S.; Hossein Golfeshan, A.; Tabatabaei, M.; Salehi Jouzani, G.; Najafi, G.; Gholami, M.; Ardjmand, M. Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Renew. Sustain. Energy Rev. 2013, 27, 77–93. [Google Scholar] [CrossRef]
- Harun, S.; Geok, S.K. Effect of sodium hydroxide pretreatment on rice straw composition. Ind. J. Sci. Technol. 2016, 9, 1–9. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Wu, Y.; Liu, J. Effects of chemical treatments of rice straw on rumen fermentation characteristics, fibrolytic enzyme activities and populations of liquid- and solid-associated ruminal microbes in vitro. Anim. Feed Sci. Technol. 2008, 141, 1–14. [Google Scholar] [CrossRef]
- Iskalieva, A.; Yimmou, B.; Gogate, P.; Horvath, M.; Horvath, P.; Csoka, L. Cavitation assisted delignification of wheat straw: A review. Ultrason. Sonochem. 2012, 19, 984–993. [Google Scholar] [CrossRef]
- Boonterm, M.; Sunyadeth, S.; Dedpakdee, S.; Athichalinthorn, P.; Patcharaphun, S.; Mungkung, R.; Techapiesancharoenkij, R. Characterization and comparison of cellulose fiber extraction from rice straw by chemical treatment and thermal steam explosion. J. Clean. Prod. 2016, 134, 592–599. [Google Scholar] [CrossRef]
- Kaur, D.; Bhardwaj, N.; Lohchab, R. A study on pulping of rice straw and impact of incorporation of chlorine dioxide during bleaching on pulp properties and effluents characteristics. J. Clean. Prod. 2018, 170, 174–182. [Google Scholar] [CrossRef]
- Kurokochi, Y.; Sato, M. Steam treatment to enhance rice straw binderless board focusing hemicellulose and cellulose decomposition products. J. Wood Sci. 2020, 66, 7. [Google Scholar] [CrossRef]
- Bhat, M.; Shahi, N.; Lohani, U.; Kumar, A.; Malik, S. Influence of microwave-assisted chemical treatment on delignification of rice straw biomass. Environ. Conserv. J. 2021, 22, 87–95. [Google Scholar] [CrossRef]
- Cueva Z., L.L.; Griffin, G.J.; Ward, L.P.; Madapusi, S.; Shah, K.V.; Parthasarathy, R. A study of chemical pre-treatment and pyrolysis operating conditions to enhance biochar production from rice straw. J. Anal. Appl. Pyrol. 2022, 163, 105455. [Google Scholar] [CrossRef]
- Kanth, S.V.; Venba, R.; Madhan, B.; Chandrababu, N.K.; Sadulla, S. Cleaner tanning practices for tannery pollution abatement: Role of enzymes in eco-friendly vegetable tanning. J. Clean. Prod. 2009, 17, 507–515. [Google Scholar] [CrossRef]
- Shanavas, S.; Padmaja, G.; Moorthy, S.N.; Sajeev, M.S.; Sheriff, J.T. Process optimization for bioethanol production from cassava starch using novel eco-friendly enzymes. Biomass Bioenergy 2011, 35, 901–909. [Google Scholar] [CrossRef]
- Ramteke, P.W.; Maurice, N.G.; Joseph, B.; Wadher, B.J. Nitrile-converting enzymes: An eco-friendly tool for industrial biocatalysis. Biotechnol. Appl. Bioc. 2013, 60, 459–481. [Google Scholar] [CrossRef] [PubMed]
- Helal, G. Bioconversion of straw into improved fodder: Preliminary treatment of rice straw using mechanical, chemical and/or gamma irradiation. Mycobiology 2006, 34, 14. [Google Scholar] [CrossRef]
- Nunes, C.; Kunamneni, A. Chapter 7—Laccases—Properties and applications. In Enzymes in Human and Animal Nutrition; Nunes, C., Kumar, V., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 133–161. [Google Scholar]
- Janusz, G.; Pawlik, A.; Świderska-Burek, U.; Polak, J.; Sulej, J.; Jarosz-Wilkołazka, A.; Paszczyński, A. Laccase properties, physiological functions, and evolution. Int. J. Mol. Sci. 2020, 21, 966. [Google Scholar] [CrossRef]
- Backes, E.; Kato, C.; Corrêa, R.; Peralta Muniz Moreira, R.; Peralta, R.; Barros, L.; Ferreira, I.C.F.R.; Zanin, C.M.; Bracht, A.; Peralta, R.M. Laccases in food processing: Current status, bottlenecks and perspectives. Trends Food Sci. Technol. 2021, 115, 445–460. [Google Scholar] [CrossRef]
- Manzano-Nicolás, J.; Marín-Iniesta, F.; Taboada-Rodríguez, A.; García-Cánovas, F.; Tudela-Serrano, J.; Muñóz-Muñóz, J.L. Development of a method to measure laccase activity on methoxyphenolic food ingredients and isomers. Int. J. Biol. Macromol. 2020, 151, 1099–1107. [Google Scholar] [CrossRef]
- Hilgers, R.; Vincken, J.; Gruppen, H.; Kabel, M. Laccase/mediator systems: Their reactivity toward phenolic lignin structures. ACS Sustain. Chem. Eng. 2018, 6, 2037–2046. [Google Scholar] [CrossRef]
- Manzano-Nicolás, J.; Taboada-Rodríguez, A.; Teruel-Puche, J.; Marín-Iniesta, F.; García-Molina, F.; García-Cánovas, F. Enzymatic oxidation of oleuropein and 3-hydroxytyrosol by laccase, peroxidase, and tyrosinase. J. Food Biochem. 2021, 45, e13803. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Marshall, M.; Geib, S.; Tien, M.; Richard, T. Effects of laccase on lignin depolymerization and enzymatic hydrolysis of ensiled corn stover. Bioresour. Technol. 2012, 117, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Subhedar, P.; Gogate, P. Intensification of enzymatic hydrolysis of lignocellulose using ultrasound for efficient bioethanol production: A review. Ind. Eng. Chem. Res. 2013, 52, 11816–11828. [Google Scholar] [CrossRef]
- Dinh Vu, N.; Thi Tran, H.; Bui, N.; Duc Vu, C.; Viet Nguyen, H. Lignin and cellulose extraction from vietnam’s rice straw using ultrasound-assisted alkaline treatment method. Int. J. Polym. Sci. 2017, 2017, 1063695. [Google Scholar] [CrossRef]
- Flórez Pardo, L.; Parra Paz, A.; López Galán, J.; Figueroa Oviedo, J. Using a mediator system to increase the delignification of sugarcane residues with fungal enzymes. CTF-Cienc. Tecnol. Futuro 2015, 6, 81–91. [Google Scholar] [CrossRef][Green Version]
- He, L.; Zhang, Y.; Ding, M.; Li, M.; Ding, J.; Bai, S. Sustainable strategy for lignocellulosic crop wastes reduction by Tenebrio molitor Linnaeus (mealworm) and potential use of mealworm frass as a fertilizer. J. Clean. Prod. 2021, 325, 129301. [Google Scholar] [CrossRef]
- Montalbán, A.; Sánchez, C.J.; Hernández, F.; Schiavone, A.; Madrid, J.; Martínez-Miró, S. Effects of agro-industrial byproduct-based diets on the growth performance, digestibility, nutritional and microbiota composition of mealworm (Tenebrio molitor L.). Insects 2022, 13, 323. [Google Scholar] [CrossRef]
- Peng, B.; Su, Y.; Chen, Z.; Chen, J.; Zhou, X.; Benbow, M. Biodegradation of polystyrene by dark (Tenebrio obscurus) and yellow (Tenebrio molitor) mealworms (Coleoptera: Tenebrionidae). Environ. Sci. Technol. 2019, 53, 5256–5265. [Google Scholar] [CrossRef]
- Sánchez-Muros, M.; Barroso, F.; Manzano-Agugliaro, F. Insect meal as renewable source of food for animal feeding: A review. J. Clean. Prod. 2014, 65, 16–27. [Google Scholar] [CrossRef]
- Dreyer, M.; Hörtenhuber, S.; Zollitsch, W.; Jäger, H.; Schaden, L.; Gronauer, A.; Kral, I. Environmental life cycle assessment of yellow mealworm (Tenebrio molitor) production for human consumption in Austria—A comparison of mealworm and broiler as protein source. Int. J. Life Cycle Assess. 2021, 26, 2232–2247. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- van Broekhoven, S.; Oonincx, D.; van Huis, A.; van Loon, J. Growth performance and feed conversion efficiency of three edible mealworm species (Coleoptera: Tenebrionidae) on diets composed of organic by-products. J. Insect Physiol. 2015, 73, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, R.R. A simple spectrophotometric method for the direct determination of uric acid in avian excreta. Poult. Sci. 1983, 62, 2106–2108. [Google Scholar] [CrossRef] [PubMed]
- Oonincx, D.; van Broekhoven, S.; van Huis, A.; van Loon, J. Feed conversion, survival and development, and composition of four insect species on diets composed of food by-products. PLoS ONE 2015, 10, e0144601. [Google Scholar] [CrossRef]
- Waldbauer, G.P. The consumption and utilization of food by insects. Adv. Insect Phys. 1968, 5, 229–288. [Google Scholar]
- Nazar, M.; Xu, L.; Ullah, M.W.; Moradian, J.M.; Wang, Y.; Sethupathy, S.; Iqbal, B.; Nawaz, M.Z.; Zhu, D. Biological delignification of rice straw using laccase from Bacillus ligniniphilus L1 for bioethanol production: A clean approach for agro-biomass utilization. J. Clean. Prod. 2022, 360, 132171. [Google Scholar] [CrossRef]
- Sabarez, H.; Oliver, C.M.; Mawson, R.; Dumsday, G.; Singh, T.; Bitto, N.; McSweeney, C.; Augustin, M.A. Synergism between ultrasonic pretreatment and white rot fungal enzymes on biodegradation of wheat chaff. Ultrason. Sonochem. 2014, 21, 2084–2091. [Google Scholar] [CrossRef]
- Deng, Z.; Xia, A.; Liao, Q.; Zhu, X.; Huang, Y.; Fu, Q. Laccase pretreatment of wheat straw: Effects of the physicochemical characteristics and the kinetics of enzymatic hydrolysis. Biotechnol. Biofuels 2019, 12, 159. [Google Scholar] [CrossRef]
- Muryanto, M.; Muharramah, R.; Falah, S.; Hidayat, A. Enzymatic biodelignification of corncob by laccase (LAC) from Cerrena Sp.B.Md.T.A.1. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1011, 012030. [Google Scholar] [CrossRef]
- Sheng, Y.; Tan, X.; Gu, Y.; Zhou, X.; Tu, M.; Xu, Y. Effect of ascorbic acid assisted dilute acid pretreatment on lignin removal and enzyme digestibility of agricultural residues. Renew. Energy 2021, 163, 732–739. [Google Scholar] [CrossRef]
- Ratnakumar, A.; Samarasekara, B.A.M.P.; Amarasinghe, S.D.A.; Karunanayake, L. Effect of sonication time on cellulose nanofiber disintegration from locally available rice straw. Macromol. Symp. 2022, 402, 2100425. [Google Scholar] [CrossRef]
- Yang, S.S.; Chen, Y.D.; Kang, J.H.; Xie, T.R.; He, L.; Xing, D.F.; Ren, N.Q.; Ho, S.H.; Wu, W.M. Generation of high-efficient biochar for dye adsorption using frass of yellow mealworms (larvae of Tenebrio molitor Linnaeus) fed with wheat straw for insect biomass production. J. Clean. Prod. 2019, 227, 33–47. [Google Scholar] [CrossRef]
- Mancini, S.; Fratini, F.; Turchi, B.; Mattioli, S.; Dal Bosco, A.; Tuccinardi, T.; Nozic, S.; Paci, G. Former foodstuff products in Tenebrio molitor rearing: Effects on growth, chemical composition, microbiological load, and antioxidant status. Animals 2019, 9, 484. [Google Scholar] [CrossRef] [PubMed]
- Baldacchino, F.; Spagnoletta, A.; Lamaj, F.; Vitale, M.L.; Verrastro, V. First optimization of tomato pomace in diets for Tenebrio molitor (L.) (Coleoptera: Tenebrionidae). Insects 2023, 14, 854. [Google Scholar] [CrossRef]
- Kröncke, N.; Benning, R. Influence of dietary protein content on the nutritional composition of mealworm larvae (Tenebrio molitor L.). Insects 2023, 14, 261. [Google Scholar] [CrossRef]
- Montalbán, A.; Martínez-Miró, S.; Schiavone, A.; Madrid, J.; Hernández, F. Growth performance, diet digestibility, and chemical composition of mealworm (Tenebrio molitor L.) fed agricultural by-products. Insects 2023, 14, 824. [Google Scholar] [CrossRef]
- Przemieniecki, S.W.; Kosewska, A.; Kosewska, O.; Purwin, C.; Lipiński, K.; Ciesielski, S. Polyethylene, polystyrene and lignocellulose wastes as mealworm (Tenebrio molitor L.) diets and their impact on the breeding condition, biometric parameters, metabolism, and digestive microbiome. Sci. Total Environ. 2022, 832, 154758. [Google Scholar] [CrossRef]
- Qi, W.; Chen, C.L.; Wang, J.Y. Reducing sugar-producing bacteria from guts of Tenebrio molitor Linnaeus (yellow mealworm) for lignocellulosic waste minimization. Microbes Environ. 2011, 26, 354–359. [Google Scholar] [CrossRef]
- Rho, M.; Lee, K. Geometric analysis of nutrient balancing in the mealworm beetle, Tenebrio molitor L. (Coleoptera: Tenebrionidae). J. Insect Physiol. 2014, 71, 37–45. [Google Scholar] [CrossRef]
- Rahimi, H.; Sattler, M.; Hossain, M.; Rodrigues, J. Boosting landfill gas production from lignin-containing wastes via termite hindgut microorganism. Waste Manag. 2020, 105, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Alvira, P.; Moreno, A.D.; Ibarra, D.; Sáez, F.; Ballesteros, M. Improving the fermentation performance of Saccharomyces cerevisiae by laccase during ethanol production from steam-exploded wheat straw at high-substrate loadings. Biotechnol. Prog. 2012, 29, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Rico, A.; Rencoret, J.; del Río, J.; Martínez, A.; Gutiérrez, A. Pretreatment with laccase and a phenolic mediator degrades lignin and enhances saccharification of eucalyptus feedstock. Biotechnol. Biofuels 2014, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.D.; Ibarra, D.; Alvira, P.; Tomás-Pejó, E.; Ballesteros, M. Exploring laccase and mediators behavior during saccharification and fermentation of steam-exploded wheat straw for bioethanol production. J. Chem. Technol. Biotechnol. 2015, 91, 1816–1825. [Google Scholar] [CrossRef]
| Diets | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ingredients (g/100 g DM) | CON | RS25 | RS50 | RS75 | RS100 | TRS25 | TRS50 | TRS75 | TRS100 |
| Breadcrumbs | 86.1 | 61.1 | 36.1 | 11.1 | 0.0 | 61.1 | 36.1 | 11.1 | 0.0 |
| Rice straw | 0.0 | 25.0 | 50.0 | 75.0 | 86.1 | 0.0 | 0.0 | 0.0 | 0.0 |
| Treated rice straw | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 25.0 | 50.0 | 75.0 | 86.1 |
| Brewer’s yeast | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Supplement 1 | 3.4 | 3.4 | 3.4 | 3.4 | 3.4 | 3.4 | 3.4 | 3.4 | 3.4 |
| Linoleic acid | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Calculated composition (g/100 g DM) | |||||||||
| Crude protein | 16.9 | 14.3 | 11.7 | 9.1 | 8.0 | 14.3 | 11.7 | 9.1 | 8.0 |
| Starch | 67.8 | 48.1 | 28.5 | 8.8 | 0.1 | 48.1 | 28.5 | 8.8 | 0.1 |
| Neutral detergent fiber (NDF) | 13.0 | 24.8 | 36.6 | 48.4 | 53.6 | 21.5 | 30.0 | 38.5 | 42.2 |
| Fibrillar Composition (%) | SSC (°Brix) | |||||
|---|---|---|---|---|---|---|
| Rice Straw Sample | NDF 1 | ADF | ADL | Hemicellulose | Cellulose | |
| CON 2 | 62.2 ± 4.8 a | 38.4 ± 2.1 a | 3.5 ± 0.8 ab | 23.8 ± 2.8 a | 34.9 ± 2.8 a | 0.0 ± 0.0 h |
| ASC | 56.3 ± 4.8 b | 35.8 ± 2.2 b | 2.9 ± 0.7 cde | 20.6 ± 3.2 b | 32.8 ± 2.4 ab | 0.6 ± 0.0 e |
| LAC | 51.8 ± 4.9 bc | 32.0 ± 5.8 cd | 2.6 ± 0.4 e | 19.9 ± 3.5 b | 29.3 ± 2.9 cd | 1.3 ± 0.0 a |
| US | 62.2 ± 4.0 a | 39.2 ± 2.8 a | 3.9 ± 0.9 a | 23.1 ± 2.0 a | 35.3 ± 3.2 a | 0.4 ± 0.0 g |
| US-ASC | 55.6 ± 3.4 b | 34.9 ± 1.7 bc | 3.3 ± 1.1 bc | 20.8 ± 2.0 b | 31.6 ± 1.6 bc | 0.5 ± 0.0 f |
| US-LAC | 53.6 ± 6.6 bc | 33.5 ± 4.6 bc | 3.0 ± 0.9 bcde | 20.2 ± 2.3 b | 30.4 ± 4.8 bc | 1.1 ± 0.0 c |
| ASC-LAC | 53.8 ± 4.5 b | 33.4 ± 2.2 bc | 3.2 ± 1.1 bcd | 20.4 ± 2.6 b | 30.2 ± 3.1 cd | 1.2 ± 0.0 b |
| US-ASC-LAC | 49.0 ± 5.7 c | 30.4 ± 3.8 d | 2.7 ± 0.7 de | 18.6 ± 2.3 b | 27.7 ± 4.0 d | 0.9 ± 0.0 d |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Item | Inclusion Level % (L) | p-Value 1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 (CON) | 25 | 50 | 75 | 100 | Straw Type (T) | L | T × L | F-Value | |
| Initial ind. weight (mg) | 0.754 | 0.099 | 0.847 | 1.09 | |||||
| RS | 63.5 ± 0.3 | 63.7 ± 0.4 | 63.7 ± 0.6 | 64.3 ± 0.3 | 63.8 ± 0.3 | ||||
| TRS | 63.5 ± 0.3 | 63.9 ± 0.9 | 64.0 ± 0.8 | 64.2 ± 0.5 | 63.6 ± 0.8 | ||||
| Final ind. weight (mg) | <0.001 | <0.001 2 | 0.080 | 28.58 | |||||
| RS | 133.9 ± 6.8 a | 98.8 ± 8.6 bc | 99.6 ± 7.8 b | 93.2 ± 3.6 bc | 85.1 ± 9.3 c | ||||
| TRS | 133.9 ± 6.8 a | 116.1 ± 4.5 b | 110.3 ± 6.3 b | 105.6 ± 4.6 b | 89.7 ± 9.8 c | ||||
| Total weight gain (mg) | <0.001 | <0.001 2 | 0.100 | 27.38 | |||||
| RS | 70.4 ± 7.0 a | 35.1 ± 8.9 bc | 35.7 ± 7.7 b | 28.9 ± 3.8 bc | 21.3 ± 9.2 c | ||||
| TRS | 70.4 ± 7.0 a | 52.3 ± 4.8 b | 46.3 ± 6.7 b | 41.4 ± 5.0 b | 25.8 ± 10.4 c | ||||
| Total intake (mg/larvae) | 0.236 | <0.001 2 | <0.001 | 1527.9 | |||||
| RS | 199.7 ± 1.8 a | 97.4 ± 2.8 b | 47.8 ± 4.5 c | 37.1 ± 1.9 d | 35.3 ± 5.9 d | ||||
| TRS | 199.7 ± 1.8 a | 85.3 ± 2.4 b | 39.5 ± 3.3 c | 43.1 ± 4.4 c | 43.3 ± 5.1 c | ||||
| FCR (g/g) | 0.009 | <0.001 2 | 0.057 | 11.17 | |||||
| RS | 3.0 ± 0.2 a | 3.0 ± 0.5 a | 1.4 ± 0.2 b | 1.5 ± 0.2 b | 2.2 ± 1.2 ab | ||||
| TRS | 3.0 ± 0.2 a | 1.7 ± 0.1 bc | 0.9 ± 0.1 d | 1.1 ± 0.2 cd | 2.3 ± 0.8 ab | ||||
| ECI (%) | 0.001 | <0.001 2 | 0.004 | 14.84 | |||||
| RS | 33.5 ± 3.0 b | 34.4 ± 5.9 b | 72.2 ± 13.1 a | 67.7 ± 11.8 a | 59.8 ± 32.6 ab | ||||
| TRS | 33.5 ± 3.0 b | 57.8 ± 4.4 b | 98.8 ± 14.0 a | 95.1 ± 19.8 a | 49.3 ± 16.3 b | ||||
| Pupae (%) | <0.001 | <0.001 2 | 0.243 | 12.5 | |||||
| RS | 14.0 ± 5.7 a | 16.0 ± 4.1 a | 5.5 ± 3.2 b | 2.0 ± 3.2 b | 2.5 ± 1.7 b | ||||
| TRS | 14.0 ± 5.7 b | 23.0 ± 5.9 a | 14.0 ± 4.1 b | 6.5 ± 2.8 b | 7.5 ± 3.5 b | ||||
| Mortality (%) | 0.747 | <0.001 2 | 0.014 | 4.8 | |||||
| RS | 0.5 ± 1.1 | 1.0 ± 2.24 | 0.5 ± 1.1 | 6.0 ± 4.1 | 4.0 ± 4.5 | ||||
| TRS | 0.5 ± 1.1 b | 1.0 ± 1.3 b | 1.5 ± 2.2 b | 1.0 ± 1.3 b | 6.9 ± 2.0 a | ||||
| Uric acid (mg/mg excreta) | 0.921 | <0.001 2 | 0.583 | 13.73 | |||||
| RS | 0.07 ± 0.01 ab | 0.08 ± 0.02 a | 0.05 ± 0.01 b | 0.03 ± 0.01 b | 0.02 ± 0.01 b | ||||
| TRS | 0.07 ± 0.01 a | 0.07 ± 0.01 a | 0.04 ± 0.01 b | 0.03 ± 0.01 b | 0.03 ± 0.01 b | ||||
| AD (%) | 0.072 | <0.001 2 | 0.001 | 36.56 | |||||
| RS | 74.7 ± 1.9 b | 87.9 ± 2.8 a | 54.6 ± 3.1 c | 53.2 ± 5.3 c | 35.8 ± 4.0 d | ||||
| TRS | 74.7 ± 1.9 a | 81.9 ± 2.9 a | 73.6 ± 10.3 a | 49.6 ± 10.3 b | 43.4 ± 11.7 b | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saura-Martínez, J.; Montalbán, A.; Manzano-Nicolás, J.; Taboada-Rodríguez, A.; Hernández, F.; Marín-Iniesta, F. A Treatment for Rice Straw and Its Use for Mealworm (Tenebrio molitor L.) Feeding: Effect on Insect Performance and Diet Digestibility. Insects 2024, 15, 631. https://doi.org/10.3390/insects15080631
Saura-Martínez J, Montalbán A, Manzano-Nicolás J, Taboada-Rodríguez A, Hernández F, Marín-Iniesta F. A Treatment for Rice Straw and Its Use for Mealworm (Tenebrio molitor L.) Feeding: Effect on Insect Performance and Diet Digestibility. Insects. 2024; 15(8):631. https://doi.org/10.3390/insects15080631
Chicago/Turabian StyleSaura-Martínez, Jorge, Ana Montalbán, Jesús Manzano-Nicolás, Amaury Taboada-Rodríguez, Fuensanta Hernández, and Fulgencio Marín-Iniesta. 2024. "A Treatment for Rice Straw and Its Use for Mealworm (Tenebrio molitor L.) Feeding: Effect on Insect Performance and Diet Digestibility" Insects 15, no. 8: 631. https://doi.org/10.3390/insects15080631
APA StyleSaura-Martínez, J., Montalbán, A., Manzano-Nicolás, J., Taboada-Rodríguez, A., Hernández, F., & Marín-Iniesta, F. (2024). A Treatment for Rice Straw and Its Use for Mealworm (Tenebrio molitor L.) Feeding: Effect on Insect Performance and Diet Digestibility. Insects, 15(8), 631. https://doi.org/10.3390/insects15080631








