Slit in a Nest Site Influences the Nest Site Selection in Cavity Nesting Ant Colonies
Abstract
Simple Summary
Abstract
1. Introduction
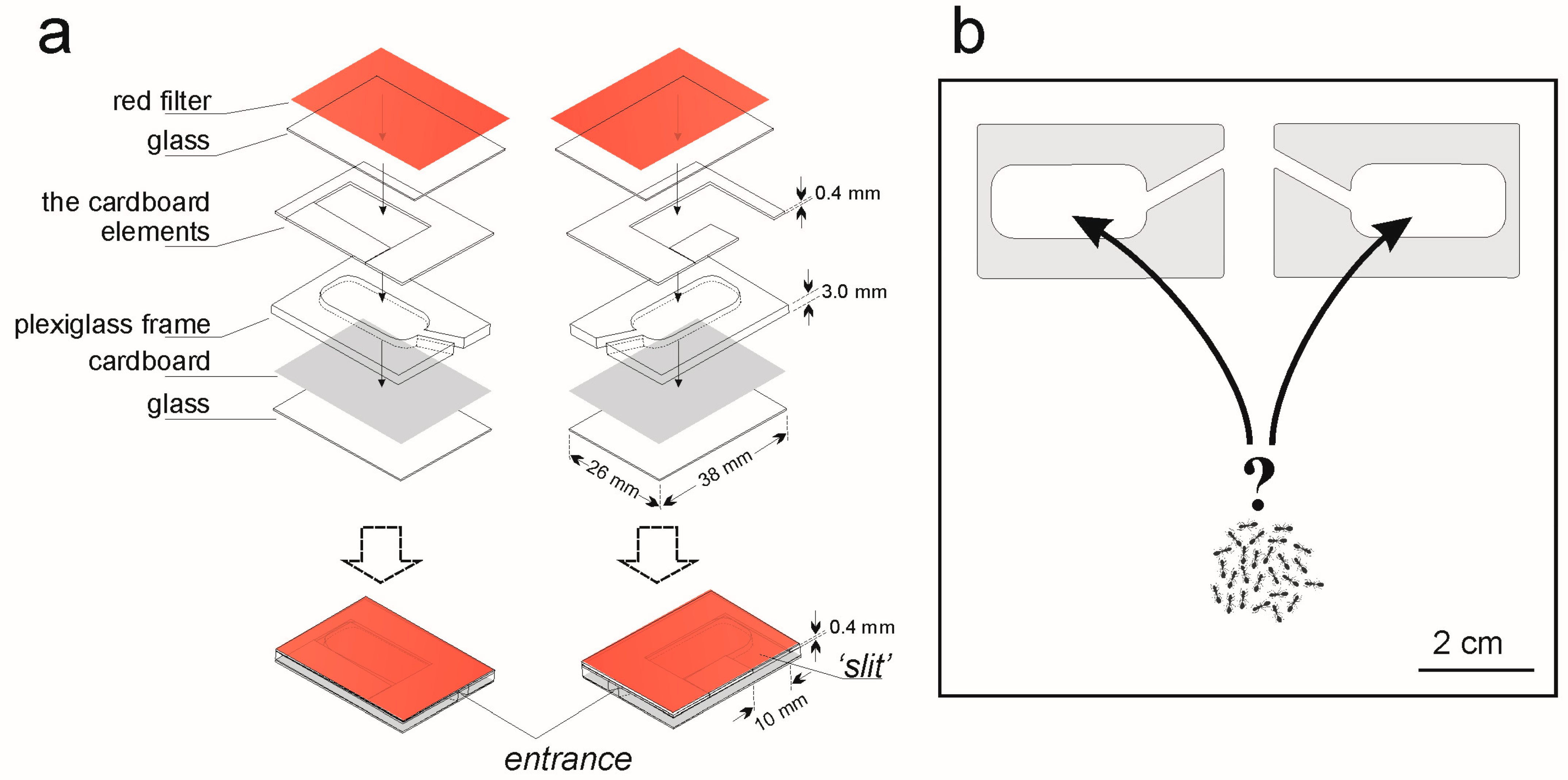
2. Materials and Methods
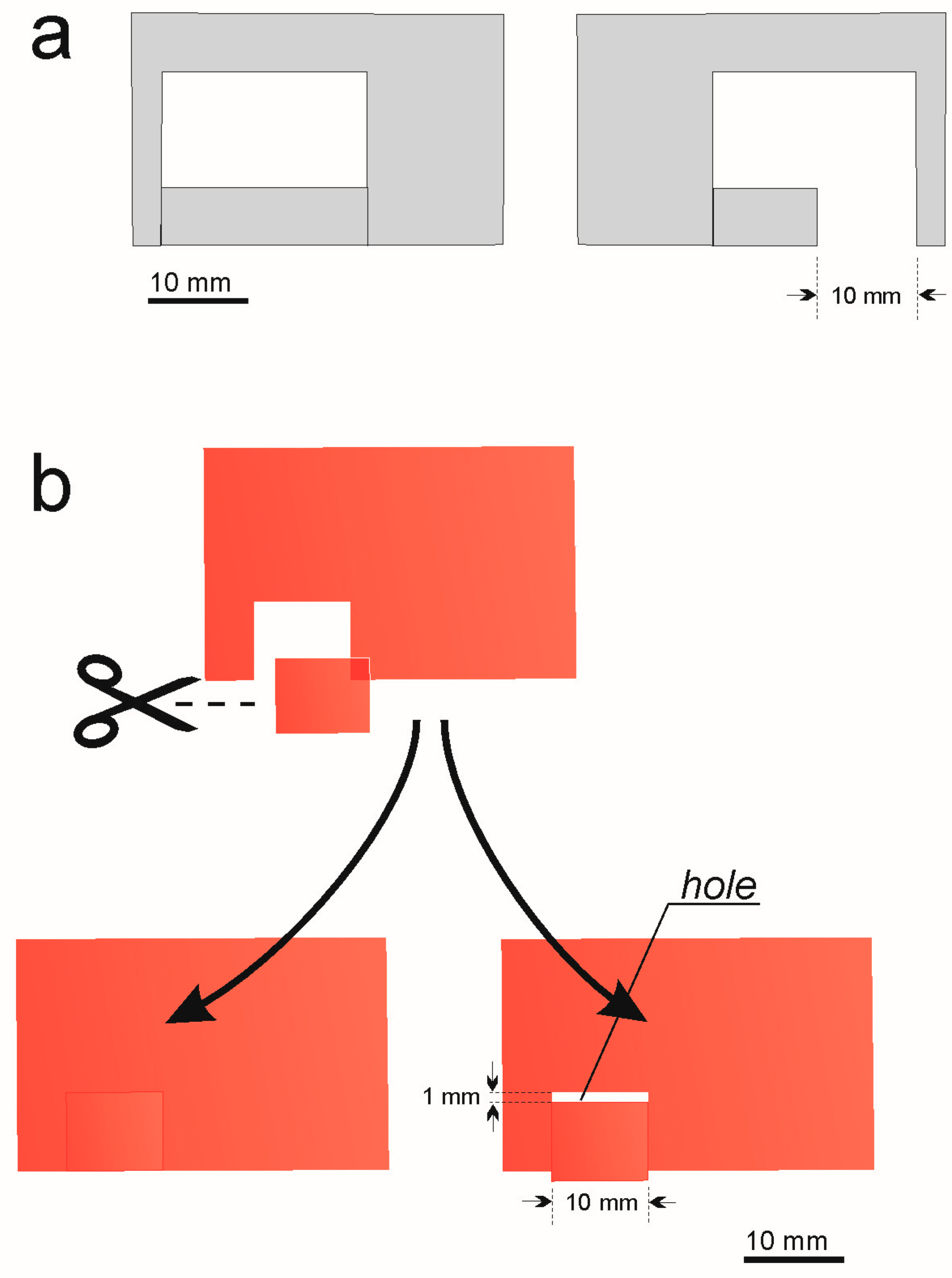
- Experiment 1. If a ‘slit’ in the nest side influences the nest site selection
- Experiment 2. If a ‘slit’ covered by food foil influences the nest site selection
- Experiment 3. If a hole in the red foil covering the nest site influences the nest site selection
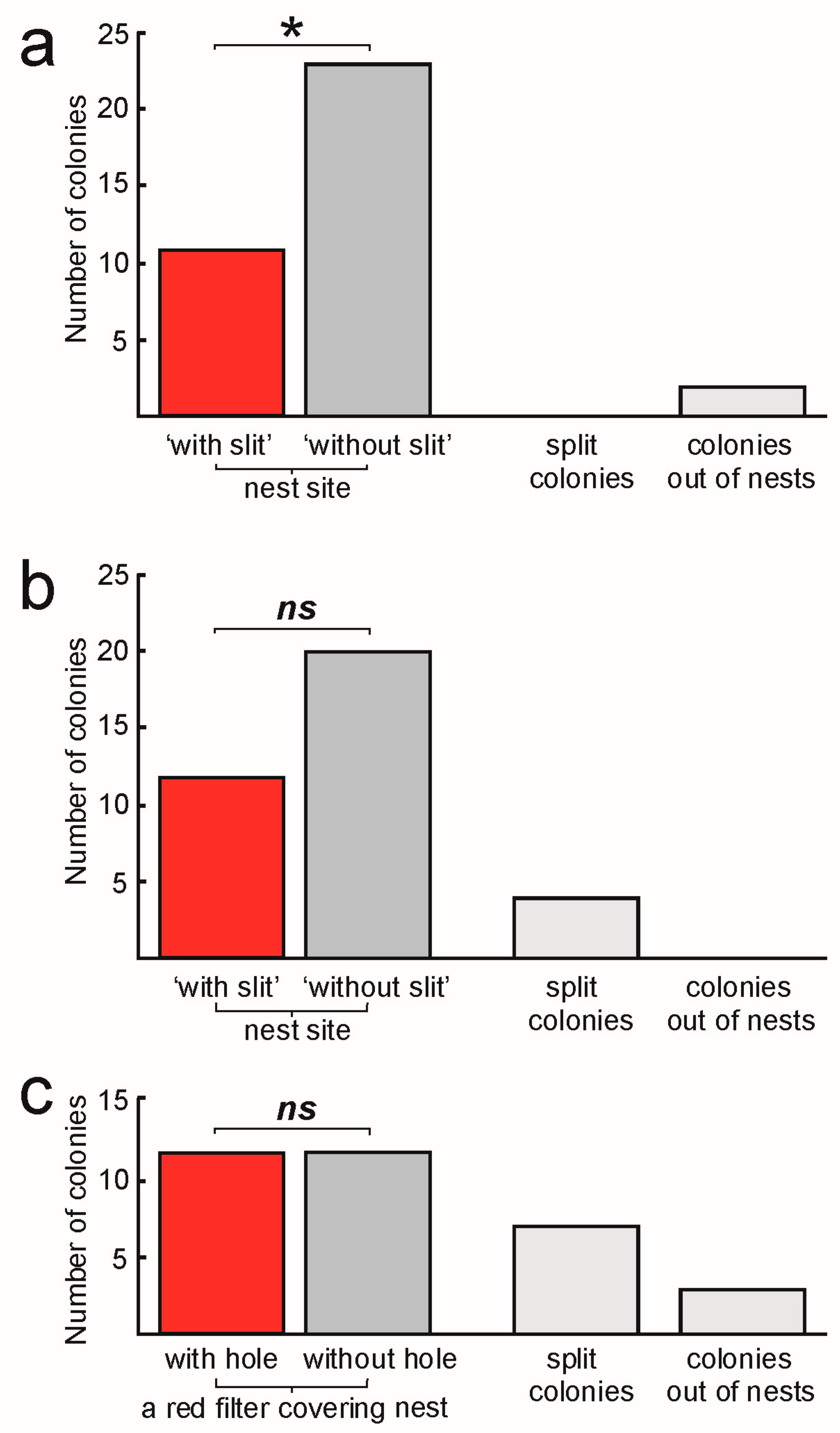
3. Results
- Experiment 1. If a ‘slit’ in the nest side influences the nest site selection
- Experiment 2. If a ‘slit’ covered by food foil influences the nest site selection
- Experiment 3. If a hole in the red foil covering the nest site influences the nest site selection
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bolton, B. An Online Catalog of the Ants of the World. Available online: https://antcat.org (accessed on 23 May 2024).
- Hölldobler, B.; Wilson, E.O. The Ants; Harvard University Press: Cambridge, MA, USA, 1990. [Google Scholar]
- Lach, L.; Parr, C.L.; Abbot, P. (Eds.) Ant Ecology; Oxford University Press: Oxford, UK, 2010; p. 402. [Google Scholar]
- Parker, J.; Kronauer, D.J.C. How ants shape biodiversity. Curr. Biol. 2021, 31, R1208–U1221. [Google Scholar] [CrossRef] [PubMed]
- Blüthgen, N.; Feldhaar, H. Food and shelter: How resources influence ant ecology. In Ant Ecology; Lach, L., Parr, C.L., Abbott, K.L., Eds.; Oxford University Press: Oxford, UK, 2010; pp. 115–136. [Google Scholar]
- Seifert, B. The Ants of Central and North Europe; Lutra: Tauer, Germany, 2018; p. 408. [Google Scholar]
- Stukalyuk, S.; Gilev, A.; Antonov, I.; Netsvetov, M. Size of nest complexes, the size of anthills, and infrastructure development in 4 species of red wood ants (Formica rufa, F. polyctena, F. aquilonia, F. lugubris) (Hymenoptera; Formicidae). Turkish J. Zool. 2021, 45, 464–478. [Google Scholar] [CrossRef]
- Jimenez-Soto, E.; Philpott, S.M. Size matters: Nest colonization patterns for twig-nesting ants. Ecol. Evol. 2015, 5, 3288–3298. [Google Scholar] [CrossRef]
- Lebas, C.; Galkowski, C.; Blatrix, R.; Wegnez, P. Ants of Britain and Europe; Bloomsbury Wildlife: London, UK, 2019; p. 415. [Google Scholar]
- Heinze, J. Life in a nutshell—Social evolution in formicoxenine ants. In Life Cycles in Social Insects: Behaviour, Ecology and Evolution; St. Petersburg University Press ed.; Kipyatkov, V.E., Ed.; St. Petersburg University Press: St. Petersburg, Russia, 2006; pp. 49–61. [Google Scholar]
- Pratt, S.C.; Pierce, N.E. The cavity-dwelling ant Leptothorax curvispinosus uses nest geometry to discriminate between potential homes. Anim. Behav. 2001, 62, 281–287. [Google Scholar] [CrossRef]
- Franks, N.R.; Mallon, E.B.; Bray, H.E.; Hamilton, M.J.; Mischler, T.C. Strategies for choosing between alternatives with different attributes: Exemplified by house-hunting ants. Anim. Behav. 2003, 65, 215–223. [Google Scholar] [CrossRef]
- Dornhaus, A.; Franks, N.R.; Hawkins, R.M.; Shere, H.N.S. Ants move to improve: Colonies of Leptothorax albipennis emigrate whenever they find a superior nest site. Anim. Behav. 2004, 67, 959–963. [Google Scholar] [CrossRef]
- Pratt, S.C. Nest Site Choice in Social Insects. In Encyclopedia of Animal Behavior, Volume 2; Breed, M.D., Moore, J., Eds.; Academic Press: Oxford, UK, 2010; pp. 534–540. [Google Scholar]
- Franks, N.R.; Hardcastle, K.A.; Collins, S.; Smith, F.D.; Sullivan, K.M.E.; Robinson, E.J.H.; Sendova-Franks, A.B. Can ant colonies choose a far-and-away better nest over an in-the-way poor one? Anim. Behav. 2008, 76, 323–334. [Google Scholar] [CrossRef]
- Sasaki, T.; Pratt, S.C. Ants learn to rely on more informative attributes during decision-making. Biol. Lett. 2013, 9, 20130667. [Google Scholar] [CrossRef]
- Myczko, Ł.; Dylewski, Ł.; Chrzanowski, A.; Sparks, T.H. Acorns of invasive Northern Red Oak (Quercus rubra) in Europe are larval hosts for moths and beetles. Biol. Invasions 2017, 19, 2419–2425. [Google Scholar] [CrossRef]
- Novais, S.M.A.; DaRocha, W.D.; Calderon-Cortes, N.; Quesada, M. Wood-boring beetles promote ant nest cavities: Extended effects of a twig-girdler ecosystem engineer. Basic Appl. Ecol. 2017, 24, 53–59. [Google Scholar] [CrossRef]
- Mitrus, S. Acorn ants may create and use two entrances to the nest cavity. Insects 2021, 12, 912. [Google Scholar] [CrossRef] [PubMed]
- Giannetti, D.; Schifani, E.; Castracani, C.; Spotti, F.A.; Mori, A.; Grasso, D.A. The introduced oak Quercus rubra and acorn-associated arthropods in Europe: An opportunity for both carpophagous insects and their ant predators. Ecol. Entomol. 2022, 47, 515–526. [Google Scholar] [CrossRef]
- Giannetti, D.; Schifani, E.; Castracani, C.; Spotti, F.A.; Mori, A.; Grasso, D.A. Different nest entrance architecture by Colobopsis and Temnothorax ants colonizing oak galls. Insectes Sociaux 2022, 69, 383–388. [Google Scholar] [CrossRef]
- Mitrus, S. Colony size and number of workers outside the nest site of the acorn ant Temnothorax crassispinus under artificial conditions. Fragmenta Naturae 2023, 56, 26–33. [Google Scholar] [CrossRef]
- Czechowski, W.; Radchenko, A.; Czechowska, W.; Vepsäläinen, K. The Ants of Poland with Reference to the Myrmecofauna of Europe; Museum and Institute of Zoology of the Polish Academy of Sciences, Natura optima dux Foundation: Warszawa, Poland, 2012. [Google Scholar]
- Białas, B.; Granieczny, P.; Pędzisz, A.; Mitrus, S. Colony size, density and type of nesting sites of the ant Temnothorax crassispinus (Hymenoptera: Formicidae). Opole Sci. Soc. Nat. J. 2011, 44, 185–191. [Google Scholar]
- Hunt, E.R.; O’Shea-Wheller, T.; Albery, G.F.; Bridger, T.H.; Gumn, M.; Franks, N.R. Ants show a leftward turning bias when exploring unknown nest sites. Biol. Lett. 2014, 10, 20140945. [Google Scholar] [CrossRef]
- Foitzik, S.; Strätz, M.; Heinze, J. Ecology, life history and resource allocation in the ant, Leptothorax nylanderi. J. Evol. Biol. 2003, 16, 670–680. [Google Scholar] [CrossRef] [PubMed]
- TIBCO Software Inc. Statistica (data analysis software system), version 13; TIBCO Software Inc.: Palo Alto, CA, USA, 2017. [Google Scholar]
- Debout, G.; Schatz, B.; Elias, M.; Mckey, D. Polydomy in ants: What we know, what we think we know, and what remains to be done. Biol. J. Linn. Soc. 2007, 90, 319–348. [Google Scholar] [CrossRef]
- Strätz, M.; Heinze, J. Colony structure and sex allocation ratios in the ant Temnothorax crassispinus. Insectes Sociaux 2004, 51, 372–377. [Google Scholar] [CrossRef]
- Franks, N.R.; Dornhaus, A.; Metherell, B.G.; Nelson, T.R.; Lanfear, S.A.J.; Symes, W.S. Not everything that counts can be counted: Ants use multiple metrics for a single nest trait. P. Roy. Soc. B-Biol. Sci. 2006, 273, 165–169. [Google Scholar] [CrossRef]
- Giannetti, D.; Castracani, C.; Spotti, F.A.; Mori, A.; Grasso, D.A. Gall-Colonizing Ants and Their Role as Plant Defenders: From ‘Bad Job’ to ‘Useful Service’. Insects 2019, 10, 392. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruszka, A.; Rolski, M.; Marczak, M.; Mitrus, S. Slit in a Nest Site Influences the Nest Site Selection in Cavity Nesting Ant Colonies. Insects 2024, 15, 638. https://doi.org/10.3390/insects15090638
Gruszka A, Rolski M, Marczak M, Mitrus S. Slit in a Nest Site Influences the Nest Site Selection in Cavity Nesting Ant Colonies. Insects. 2024; 15(9):638. https://doi.org/10.3390/insects15090638
Chicago/Turabian StyleGruszka, Anna, Mateusz Rolski, Mariia Marczak, and Sławomir Mitrus. 2024. "Slit in a Nest Site Influences the Nest Site Selection in Cavity Nesting Ant Colonies" Insects 15, no. 9: 638. https://doi.org/10.3390/insects15090638
APA StyleGruszka, A., Rolski, M., Marczak, M., & Mitrus, S. (2024). Slit in a Nest Site Influences the Nest Site Selection in Cavity Nesting Ant Colonies. Insects, 15(9), 638. https://doi.org/10.3390/insects15090638





