Tracking Existing Factors Directly Affecting the Reproduction of Bumblebees: Current Knowledge
Simple Summary
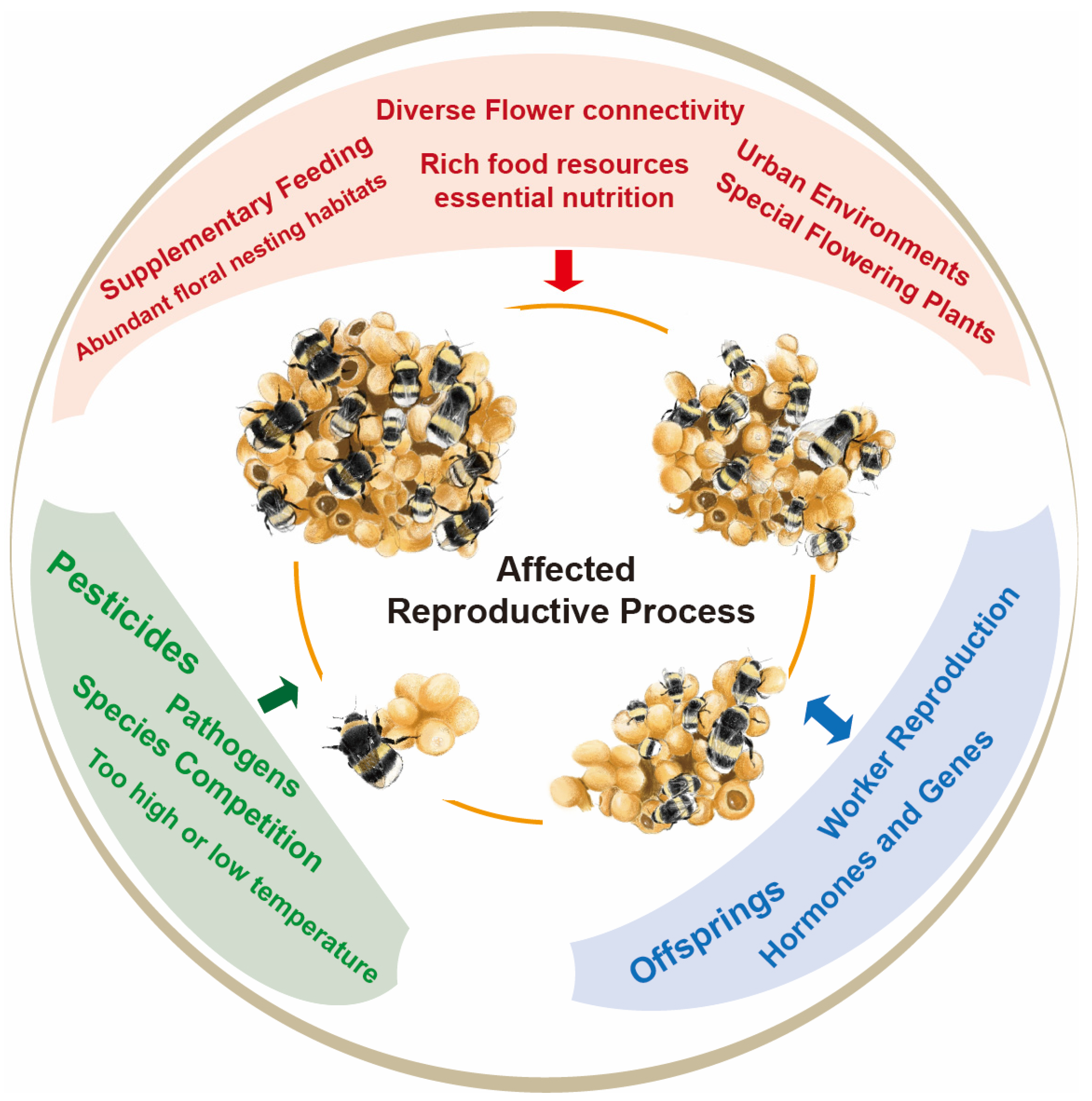
Abstract
1. Introduction
2. Impact of Environmental Factors on Reproduction of Bumblebee
2.1. Nesting Habitat and Flower Connectivity
2.2. Food Resources and Nutrition
2.3. Pesticides
2.4. Temperature
3. Impact of Biological Factors on Reproduction of Bumblebees
3.1. Species Competition
3.2. Different Castes of Bumblebees
3.3. Pathogens
3.4. Worker Reproduction
4. Hormones and Genes
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Plowright, R.C.; Laverty, T.M. The Ecology and Sociobiology of Bumble Bees. Annu. Rev. Entomol. 1984, 29, 175–199. [Google Scholar] [CrossRef]
- Röseler, P.-F.; van Honk, C.G.J. Castes and Reproduction in Bumblebees. In Social Insects: An Evolutionary Approach to Castes and Reproduction; Engels, W., Ed.; Springer: Berlin/Heidelberg, Germany, 1990; pp. 147–166. [Google Scholar]
- Duchateau, M.J.; Velthuis, H.H.W.; Boomsma, J.J. Sex ratio variation in the bumblebee Bombus terrestris. Behav. Ecol. 2004, 15, 71–82. [Google Scholar] [CrossRef]
- Bloch, G. Regulation of queen–worker conflict in bumble bee (Bombus terrestris) colonies. Proc. R. Soc. London. Ser. B Biol. Sci. 1999, 266, 2465–2469. [Google Scholar] [CrossRef]
- Duchateau, M.; Velthuis, H. Development and reproductive strategies in Bombus terrestris colonies. Behaviour 1988, 107, 186–207. [Google Scholar] [CrossRef]
- Goulson, D. Bumblebees: Behaviour, Ecology, and Conservation; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Velthuis, H.H.W.; Doorn, A.v. A century of advances in bumblebee domestication and the economic and environmental aspects of its commercialization for pollination. Apidologie 2006, 37, 421–451. [Google Scholar] [CrossRef]
- Treanore, E.; Barie, K.; Derstine, N.; Gadebusch, K.; Orlova, M.; Porter, M.; Purnell, F.; Amsalem, E. Optimizing Laboratory Rearing of a Key Pollinator, Bombus impatiens. Insects 2021, 12, 673. [Google Scholar] [CrossRef]
- Bowers, M.A. Resource Availability and Timing of Reproduction in Bumble Bee Colonies (Hymenoptera: Apidae). Environ. Entomol. 1986, 15, 750–755. [Google Scholar] [CrossRef]
- Schweiger, S.E.; Beyer, N.; Hass, A.L.; Westphal, C. Pollen and landscape diversity as well as wax moth depredation determine reproductive success of bumblebees in agricultural landscapes. Agric. Ecosyst. Environ. 2022, 326, 107788. [Google Scholar] [CrossRef]
- Persson, A.S.; Smith, H.G. Bumblebee colonies produce larger foragers in complex landscapes. Basic Appl. Ecol. 2011, 12, 695–702. [Google Scholar] [CrossRef]
- Persson, A.S.; Smith, H.G. Seasonal persistence of bumblebee populations is affected by landscape context. Agric. Ecosyst. Environ. 2013, 165, 201–209. [Google Scholar] [CrossRef]
- Geppert, C.; Hass, A.; Földesi, R.; Donkó, B.; Akter, A.; Tscharntke, T.; Batáry, P. Agri-environment schemes enhance pollinator richness and abundance but bumblebee reproduction depends on field size. J. Appl. Ecol. 2020, 57, 1818–1828. [Google Scholar] [CrossRef]
- Williams, N.M.; Regetz, J.; Kremen, C. Landscape-scale resources promote colony growth but not reproductive performance of bumble bees. Ecology 2012, 93, 1049–1058. [Google Scholar] [CrossRef]
- Rundlöf, M.; Persson, A.S.; Smith, H.G.; Bommarco, R. Late-season mass-flowering red clover increases bumble bee queen and male densities. Biol. Conserv. 2014, 172, 138–145. [Google Scholar] [CrossRef]
- Samuelson, A.E.; Gill, R.J.; Brown, M.J.F.; Leadbeater, E. Lower bumblebee colony reproductive success in agricultural compared with urban environments. Proc. R. Soc. B Biol. Sci. 2018, 285, 20180807. [Google Scholar] [CrossRef]
- Goulson, D.; Lepais, O.; O’connor, S.; Osborne, J.L.; Sanderson, R.A.; Cussans, J.; Goffe, L.; Darvill, B. Effects of land use at a landscape scale on bumblebee nest density and survival. J. Appl. Ecol. 2010, 47, 1207–1215. [Google Scholar] [CrossRef]
- Osborne, J.L.; Martin, A.P.; Shortall, C.R.; Todd, A.D.; Goulson, D.; Knight, M.E.; Hale, R.J.; Sanderson, R.A. Quantifying and comparing bumblebee nest densities in gardens and countryside habitats. J. Appl. Ecol. 2008, 45, 784–792. [Google Scholar] [CrossRef]
- Wilson, C.J.; Jamieson, M.A. The effects of urbanization on bee communities depends on floral resource availability and bee functional traits. PLoS ONE 2019, 14, e0225852. [Google Scholar] [CrossRef]
- Westphal, C.; Steffan-Dewenter, I.; Tscharntke, T. Mass flowering oilseed rape improves early colony growth but not sexual reproduction of bumblebees. J. Appl. Ecol. 2009, 46, 187–193. [Google Scholar] [CrossRef]
- Krama, T.; Krams, R.; Munkevics, M.; Willow, J.; Popovs, S.; Elferts, D.; Dobkeviča, L.; Raibarte, P.; Rantala, M.; Contreras-Garduño, J.; et al. Physiological stress and higher reproductive success in bumblebees are both associated with intensive agriculture. PeerJ 2022, 10, e12953. [Google Scholar] [CrossRef]
- Hemberger, J.; Witynski, G.; Gratton, C. Floral resource continuity boosts bumble bee colony performance relative to variable floral resources. Ecol. Entomol. 2022, 47, 703–712. [Google Scholar] [CrossRef]
- Malfi, R.L.; McFrederick, Q.S.; Lozano, G.; Irwin, R.E.; Adler, L.S. Sunflower plantings reduce a common gut pathogen and increase queen production in common eastern bumblebee colonies. Proc. R. Soc. B Biol. Sci. 2023, 290, 20230055. [Google Scholar] [CrossRef]
- Adler, L.S.; Barber, N.A.; Biller, O.M.; Irwin, R.E. Flowering plant composition shapes pathogen infection intensity and reproduction in bumble bee colonies. Proc. Natl. Acad. Sci. USA 2020, 117, 11559–11565. [Google Scholar] [CrossRef]
- Kämper, W.; Werner, P.K.; Hilpert, A.; Westphal, C.; Blüthgen, N.; Eltz, T.; Leonhardt, S.D. How landscape, pollen intake and pollen quality affect colony growth in Bombus terrestris. Landsc. Ecol. 2016, 31, 2245–2258. [Google Scholar] [CrossRef]
- Spiesman, B.J.; Bennett, A.; Isaacs, R.; Gratton, C. Bumble bee colony growth and reproduction depend on local flower dominance and natural habitat area in the surrounding landscape. Biol. Conserv. 2017, 206, 217–223. [Google Scholar] [CrossRef]
- Elliott, S.E. Surplus Nectar Available for Subalpine Bumble Bee Colony Growth. Environ. Entomol. 2009, 38, 1680–1689. [Google Scholar] [CrossRef]
- Requier, F.; Jowanowitsch, K.K.; Kallnik, K.; Steffan-Dewenter, I. Limitation of complementary resources affects colony growth, foraging behavior, and reproduction in bumble bees. Ecology 2020, 101, e02946. [Google Scholar] [CrossRef]
- Pelletier, L.; McNeil, J.N. The effect of food supplementation on reproductive success in bumblebee field colonies. Oikos 2003, 103, 688–694. [Google Scholar] [CrossRef]
- Klatt, B.K.; Nilsson, L.; Smith, H.G. Annual flowers strips benefit bumble bee colony growth and reproduction. Biol. Conserv. 2020, 252, 108814. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, H.; Mashilingi, S.K.; Jie, C.; Guo, B.; Guo, Y.; Hu, X.; Iqbal, S.; Wei, B.; Liu, Y.; et al. Bombus terrestris Prefer Mixed-Pollen Diets for a Better Colony Performance: A Laboratory Study. Insects 2024, 15, 285. [Google Scholar] [CrossRef]
- Vaudo, A.D.; Farrell, L.M.; Patch, H.M.; Grozinger, C.M.; Tooker, J.F. Consistent pollen nutritional intake drives bumble bee (Bombus impatiens) colony growth and reproduction across different habitats. Ecol. Evol. 2018, 8, 5765–5776. [Google Scholar] [CrossRef]
- Vaudo, A.D.; Stabler, D.; Patch, H.; Tooker, J.; Grozinger, C.; Wright, G. Bumble bees regulate their intake of essential protein and lipid pollen macronutrients. J. Exp. Biol. 2016, 219, 3962–3970. [Google Scholar] [CrossRef] [PubMed]
- Kriesell, L.; Hilpert, A.; Leonhardt, S.D. Different but the same: Bumblebee species collect pollen of different plant sources but similar amino acid profiles. Apidologie 2017, 48, 102–116. [Google Scholar] [CrossRef]
- Moerman, R.; Vanderplanck, M.; Fournier, D.; Jacquemart, A.-L.; Michez, D. Pollen nutrients better explain bumblebee colony development than pollen diversity. Insect Conserv. Divers. 2017, 10, 171–179. [Google Scholar] [CrossRef]
- Vaudo, A.D.; Patch, H.M.; Mortensen, D.A.; Tooker, J.F.; Grozinger, C.M. Macronutrient ratios in pollen shape bumble bee (Bombus impatiens) foraging strategies and floral preferences. Proc. Natl. Acad. Sci. USA 2016, 113, E4035–E4042. [Google Scholar] [CrossRef]
- Ruedenauer, F.A.; Spaethe, J.; Leonhardt, S.D. Hungry for quality—Individual bumblebees forage flexibly to collect high-quality pollen. Behav. Ecol. Sociobiol. 2016, 70, 1209–1217. [Google Scholar] [CrossRef]
- Kraus, S.; Gómez-Moracho, T.; Pasquaretta, C.; Latil, G.; Dussutour, A.; Lihoreau, M. Bumblebees adjust protein and lipid collection rules to the presence of brood. Curr. Zool. 2019, 65, 437–446. [Google Scholar] [CrossRef]
- Ren, C.-S.; Chang, Z.-M.; Han, L.; Chen, X.-S.; Long, J.-K. Higher Essential Amino Acid and Crude Protein Contents in Pollen Accelerate the Oviposition and Colony Foundation of Bombus breviceps (Hymenoptera: Apidae). Insects 2023, 14, 203. [Google Scholar] [CrossRef]
- Stabler, D.; Paoli, P.P.; Nicolson, S.W.; Wright, G.A. Nutrient balancing of the adult worker bumblebee (Bombus terrestris) depends on the dietary source of essential amino acids. J. Exp. Biol. 2015, 218, 793–802. [Google Scholar] [CrossRef]
- Moerman, R.; Vanderplanck, M.; Roger, N.; Declèves, S.; Wathelet, B.; Rasmont, P.; Fournier, D.; Michez, D. Growth Rate of Bumblebee Larvae is Related to Pollen Amino Acids. J. Econ. Entomol. 2015, 109, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Tasei, J.-N.; Aupinel, P. Nutritive value of 15 single pollens and pollen mixes tested on larvae produced by bumblebee workers (Bombus terrestris, Hymenoptera: Apidae). Apidologie 2008, 39, 397–409. [Google Scholar] [CrossRef]
- Nicolson, S.W.; Human, H. Chemical composition of the ‘low quality’pollen of sunflower (Helianthus annuus, Asteraceae). Apidologie 2013, 44, 144–152. [Google Scholar] [CrossRef]
- Roger, N.; Michez, D.; Wattiez, R.; Sheridan, C.; Vanderplanck, M. Diet effects on bumblebee health. J. Insect Physiol. 2017, 96, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Woodard, S.H.; Duennes, M.A.; Watrous, K.M.; Jha, S. Diet and nutritional status during early adult life have immediate and persistent effects on queen bumble bees. Conserv. Physiol. 2019, 7, coz048. [Google Scholar] [CrossRef] [PubMed]
- Pioltelli, E.; Guzzetti, L.; Ouled Larbi, M.; Labra, M.; Galimberti, A.; Biella, P. Landscape fragmentation constrains bumblebee nutritional ecology and foraging dynamics. Landsc. Urban Plan. 2024, 247, 105075. [Google Scholar] [CrossRef]
- Vaudo, A.D.; Tooker, J.F.; Grozinger, C.M.; Patch, H.M. Bee nutrition and floral resource restoration. Curr. Opin. Insect Sci. 2015, 10, 133–141. [Google Scholar] [CrossRef]
- Nicholson, C.C.; Knapp, J.; Kiljanek, T.; Albrecht, M.; Chauzat, M.-P.; Costa, C.; De la Rúa, P.; Klein, A.-M.; Mänd, M.; Potts, S.G.; et al. Pesticide use negatively affects bumble bees across European landscapes. Nature 2023, 628, 355–358. [Google Scholar] [CrossRef]
- Laycock, I.; Lenthall, K.M.; Barratt, A.T.; Cresswell, J.E. Effects of imidacloprid, a neonicotinoid pesticide, on reproduction in worker bumble bees (Bombus terrestris). Ecotoxicology 2012, 21, 1937–1945. [Google Scholar] [CrossRef]
- Chole, H.; de Guinea, M.; Woodard, S.H.; Bloch, G. Field-realistic concentrations of a neonicotinoid insecticide influence socially regulated brood development in a bumblebee. Proc. R. Soc. B Biol. Sci. 2022, 289, 20220253. [Google Scholar] [CrossRef]
- Whitehorn, P.R.; O’Connor, S.; Wackers, F.L.; Goulson, D. Neonicotinoid Pesticide Reduces Bumble Bee Colony Growth and Queen Production. Science 2012, 336, 351–352. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Zhou, Y.; Wang, F.; Wang, J.; Yao, X.; Imran, M.; Luo, S. Imidacloprid reduces the mating success of males in bumblebees. Sci. Total Environ. 2024, 928, 172525. [Google Scholar] [CrossRef]
- Elston, C.; Thompson, H.M.; Walters, K.F.A. Sub-lethal effects of thiamethoxam, a neonicotinoid pesticide, and propiconazole, a DMI fungicide, on colony initiation in bumblebee (Bombus terrestris) micro-colonies. Apidologie 2013, 44, 563–574. [Google Scholar] [CrossRef]
- Baron, G.L.; Jansen, V.A.A.; Brown, M.J.F.; Raine, N.E. Pesticide reduces bumblebee colony initiation and increases probability of population extinction. Nat. Ecol. Evol. 2017, 1, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Minnameyer, A.; Strobl, V.; Bruckner, S.; Camenzind, D.W.; Van Oystaeyen, A.; Wäckers, F.; Williams, G.R.; Yañez, O.; Neumann, P.; Straub, L. Eusocial insect declines: Insecticide impairs sperm and feeding glands in bumblebees. Sci. Total Environ. 2021, 785, 146955. [Google Scholar] [CrossRef] [PubMed]
- Laycock, I.; Cotterell, K.C.; O’Shea-Wheller, T.A.; Cresswell, J.E. Effects of the neonicotinoid pesticide thiamethoxam at field-realistic levels on microcolonies of Bombus terrestris worker bumble bees. Ecotoxicol. Environ. Saf. 2014, 100, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Dance, C.; Botías, C.; Goulson, D. The combined effects of a monotonous diet and exposure to thiamethoxam on the performance of bumblebee micro-colonies. Ecotoxicol. Environ. Saf. 2017, 139, 194–201. [Google Scholar] [CrossRef]
- Rundlöf, M.; Lundin, O. Can Costs of Pesticide Exposure for Bumblebees Be Balanced by Benefits from a Mass-Flowering Crop? Environ. Sci. Technol. 2019, 53, 14144–14151. [Google Scholar] [CrossRef]
- Siviter, H.; Brown, M.J.F.; Leadbeater, E. Sulfoxaflor exposure reduces bumblebee reproductive success. Nature 2018, 561, 109–112. [Google Scholar] [CrossRef]
- Linguadoca, A.; Rizzi, C.; Villa, S.; Brown, M.J.F. Sulfoxaflor and nutritional deficiency synergistically reduce survival and fecundity in bumblebees. Sci. Total Environ. 2021, 795, 148680. [Google Scholar] [CrossRef]
- Smagghe, G.; Deknopper, J.; Meeus, I.; Mommaerts, V. Dietary chlorantraniliprole suppresses reproduction in worker bumblebees. Pest Manag. Sci. 2013, 69, 787–791. [Google Scholar] [CrossRef]
- Bernauer, O.M.; Gaines-Day, H.R.; Steffan, S.A. Colonies of Bumble Bees (Bombus impatiens) Produce Fewer Workers, Less Bee Biomass, and Have Smaller Mother Queens Following Fungicide Exposure. Insects 2015, 6, 478–488. [Google Scholar] [CrossRef]
- Raine, N.E. Pesticide affects social behavior of bees. Science 2018, 362, 643–644. [Google Scholar] [CrossRef]
- Cueva del Castillo, R.; Sanabria-Urbán, S.; Serrano-Meneses, M.A. Trade-offs in the evolution of bumblebee colony and body size: A comparative analysis. Ecol. Evol. 2015, 5, 3914–3926. [Google Scholar] [CrossRef]
- Guiraud, M.; Cariou, B.; Henrion, M.; Baird, E.; Gérard, M. Higher developmental temperature increases queen production and decreases worker body size in the bumblebee Bombus terrestris. J. Hymenopt. Res. 2021, 88, 39–49. [Google Scholar] [CrossRef]
- Sepúlveda, Y.; Nicholls, E.; Schuett, W.; Goulson, D. Heatwave-like events affect drone production and brood-care behaviour in bumblebees. PeerJ 2024, 12, e17135. [Google Scholar] [CrossRef]
- Campion, C.; Rajamohan, A.; Dillon, M.E. Sperm can’t take the heat: Short-term temperature exposures compromise fertility of male bumble bees (Bombus impatiens). J. Insect Physiol. 2023, 146, 104491. [Google Scholar] [CrossRef]
- Amin, M.R.; Than, K.K.; Kwon, Y.J. Mating status of bumblebees, Bombus terrestris (Hymenoptera: Apidae) with notes on ambient temperature, age and virginity. Appl. Entomol. Zool. 2010, 45, 363–367. [Google Scholar] [CrossRef]
- Belsky, J.E.; Camp, A.A.; Lehmann, D.M. The Importance of Males to Bumble Bee (Bombus Species) Nest Development and Colony Viability. Insects 2020, 11, 506. [Google Scholar] [CrossRef]
- Cressman, A.; Amsalem, E. Impacts and mechanisms of CO2 narcosis in bumble bees: Narcosis depends on dose, caste and mating status and is not induced by anoxia. J. Exp. Biol. 2023, 226, jeb244746. [Google Scholar] [CrossRef]
- Yoon, H.J.; Lee, K.Y.; Kim, M.; Ahn, M.Y.; Park, I.G. Optimal cold temperature for the artificial hibernation of Bombus ignitus queen bumblebees. Int. J. Ind. Entomol. 2013, 26, 124–130. [Google Scholar] [CrossRef]
- Treanore, E.D.; Amsalem, E. Examining the individual and additive effects of cold storage and CO2 narcosis on queen survival and reproduction in bumble bees. J. Insect Physiol. 2022, 139, 104394. [Google Scholar] [CrossRef]
- Elbgami, T.; Kunin, W.E.; Hughes, W.O.H.; Biesmeijer, J.C. The effect of proximity to a honeybee apiary on bumblebee colony fitness, development, and performance. Apidologie 2014, 45, 504–513. [Google Scholar] [CrossRef]
- Goulson, D.; Sparrow, K.R. Evidence for competition between honeybees and bumblebees; effects on bumblebee worker size. J. Insect Conserv. 2009, 13, 177–181. [Google Scholar] [CrossRef]
- Thomson, D. Competitive interactions between the invasive European honeybee and native bumblebees. Ecology 2004, 85, 458–470. [Google Scholar] [CrossRef]
- Herbertsson, L.; Lindström, S.A.M.; Rundlöf, M.; Bommarco, R.; Smith, H.G. Competition between managed honeybees and wild bumblebees depends on landscape context. Basic Appl. Ecol. 2016, 17, 609–616. [Google Scholar] [CrossRef]
- Dafni, A.; Kevan, P.; Gross, C.L.; Goka, K. Bombus terrestris, pollinator, invasive and pest: An assessment of problems associated with its widespread introductions for commercial purposes. Appl. Entomol. Zool. 2010, 45, 101–113. [Google Scholar] [CrossRef]
- Dohzono, I.; Kunitake, Y.K.; Yokoyama, J.; Goka, K. Alien bumblebee affects native plant reproduction through interactions with native bumblebees. Ecology 2008, 89, 3082–3092. [Google Scholar] [CrossRef]
- Tsuchida, K.; Yamaguchi, A.; Kanbe, Y.; Goka, K. Reproductive Interference in an Introduced Bumblebee: Polyandry may Mitigate Negative Reproductive Impact. Insects 2019, 10, 59. [Google Scholar] [CrossRef]
- Kondo, N.I.; Yamanaka, D.; Kanbe, Y.; Kunitake, Y.K.; Yoneda, M.; Tsuchida, K.; Goka, K. Reproductive disturbance of Japanese bumblebees by the introduced European bumblebee Bombus terrestris. Naturwissenschaften 2009, 96, 467–475. [Google Scholar] [CrossRef]
- Yuan, X.; Muhammad, N.; Zhang, H.; Liang, C.; Huang, J.; An, J. Evaluation of reproductive disturbance to Chinese bumblebees by the European bumblebee, Bombus terrestris (Hymenoptera: Apidae). Acta Entomol. Sin. 2018, 61, 348–359. [Google Scholar] [CrossRef]
- Williams, P.H.; Osborne, J.L. Bumblebee vulnerability and conservation world-wide. Apidologie 2009, 40, 367–387. [Google Scholar] [CrossRef]
- Sarro, E.; Sun, P.; Mauck, K.; Rodriguez-Arellano, D.; Yamanaka, N.; Woodard, S.H. An organizing feature of bumble bee life history: Worker emergence promotes queen reproduction and survival in young nests. Conserv. Physiol. 2021, 9, coab047. [Google Scholar] [CrossRef]
- Woodard, S.H.; Bloch, G.; Band, M.R.; Robinson, G.E. Social regulation of maternal traits in nest-founding bumble bee (Bombus terrestris) queens. J. Exp. Biol. 2013, 216, 3474–3482. [Google Scholar] [CrossRef]
- Kerr, N.Z.; Crone, E.E.; Williams, N.M. Integrating vital rates explains optimal worker size for resource return by bumblebee workers. Funct. Ecol. 2019, 33, 467–478. [Google Scholar] [CrossRef]
- Sauter, A.; Brown, M.J.F. To copulate or not? The importance of female status and behavioural variation in predicting copulation in a bumblebee. Anim. Behav. 2001, 62, 221–226. [Google Scholar] [CrossRef][Green Version]
- Guo, Y.; Zhang, Q.; Hu, X.; Pang, C.; Li, J.; Huang, J. Mating Stimulates the Immune Response and Sperm Storage-Related Genes Expression in Spermathecae of Bumblebee (Bombus terrestris) Queen. Front. Genet. 2021, 12, 795669. [Google Scholar] [CrossRef]
- Amin, M.R.; Bussière, L.F.; Goulson, D. Effects of Male age and Size on Mating Success in the Bumblebee Bombus terrestris. J. Insect Behav. 2012, 25, 362–374. [Google Scholar] [CrossRef]
- Baer, B.; Schmid-Hempel, P.; Høeg, J.T.; Boomsma, J.J. Sperm length, sperm storage and mating system characteristics in bumblebees. Insectes Sociaux 2003, 50, 101–108. [Google Scholar] [CrossRef]
- Duvoisin, N.; Baer, B.; Schmid-Hempel, P. Sperm transfer and male competition in a bumblebee. Anim. Behav. 1999, 58, 743–749. [Google Scholar] [CrossRef]
- Gosterit, A.; Gurel, F. Male remating and its influences on queen colony foundation success in the bumblebee, Bombus terrestris. Apidologie 2016, 47, 828–834. [Google Scholar] [CrossRef]
- Fliszkiewicz, M.; Wilkaniec, Z.a. Fatty acids and amino acids in the fat body of bumblebee Bombus terrestris (L.) in diapausing and non-diapausing queens. J. Apic. Sci. 2007, 51, 55–63. [Google Scholar]
- Fliszkiewicz, M. Causes of the lack of diapause in bumble bee females (Bombus Latr., Apoidea). J. Apic. Sci. 2002, 46, 31–40. [Google Scholar]
- Beekman, M.; Stratum, P.v.; Veerman, A. Diapause in the bumblebee Bombus terrestris. Proc. Exper. & Appl. N. E. V. Amsterdam 1996, 7, 71–75. [Google Scholar]
- Beekman, M.; Van Stratum, P. Does the diapause experience of bumblebee queens Bombus terrestris affect colony characteristics? Ecol. Entomol. 2000, 25, 1–6. [Google Scholar] [CrossRef]
- Shykoff, J.A.; Schmid-Hempel, P. Parasites delay worker reproduction in bumblebees: Consequences for eusociality. Behav. Ecol. 1991, 2, 242–248. [Google Scholar] [CrossRef]
- Goulson, D.; O’connor, S.; Park, K.J. The impacts of predators and parasites on wild bumblebee colonies. Ecol. Entomol. 2018, 43, 168–181. [Google Scholar] [CrossRef]
- Fauser, A.; Sandrock, C.; Neumann, P.; Sadd, B.M. Neonicotinoids override a parasite exposure impact on hibernation success of a key bumblebee pollinator. Ecol. Entomol. 2017, 42, 306–314. [Google Scholar] [CrossRef]
- Brown, M.J.; Schmid-Hempel, R.; Schmid-Hempel, P. Strong context-dependent virulence in a host–parasite system: Reconciling genetic evidence with theory. J. Anim. Ecol. 2003, 72, 994–1002. [Google Scholar] [CrossRef]
- Brown, M.J.; Loosli, R.; Schmid-Hempel, P. Condition-dependent expression of virulence in a trypanosome infecting bumblebee. Oikos 2000, 91, 421–427. [Google Scholar] [CrossRef]
- van Der Steen, J.J. Infection and transmission of Nosema bombi in Bombus terrestris colonies and its effect on hibernation, mating and colony founding. Apidologie 2008, 39, 273–282. [Google Scholar] [CrossRef]
- Otti, O.; Schmid-HempelL, P. A field experiment on the effect of Nosema bombi in colonies of the bumblebee Bombus terrestris. Ecol. Entomol. 2008, 33, 577–582. [Google Scholar] [CrossRef]
- Otti, O.; Schmid-Hempel, P. Nosema bombi: A pollinator parasite with detrimental fitness effects. J. Invertebr. Pathol. 2007, 96, 118–124. [Google Scholar] [CrossRef]
- Rutrecht, S.T.; Brown, M.J.F. Differential virulence in a multiple-host parasite of bumble bees: Resolving the paradox of parasite survival? Oikos 2009, 118, 941–949. [Google Scholar] [CrossRef]
- Schmid-Hempel, P.; Durrer, S. Parasites, Floral Resources and Reproduction in Natural Populations of Bumblebees. Oikos 1991, 62, 342–350. [Google Scholar] [CrossRef]
- Imhoof, B.; Schmid-Hempel, P. Colony success of the bumble bee, Bombus terrestris, in relation to infections by two protozoan parasites, Crithidia bombi and Nosema bombi. Insectes Sociaux 1999, 46, 233–238. [Google Scholar] [CrossRef]
- Meeus, I.; Brown, M.J.F.; De Graaf, D.C.; Smagghe, G. Effects of Invasive Parasites on Bumble Bee Declines. Conserv. Biol. 2011, 25, 662–671. [Google Scholar] [CrossRef]
- Rutrecht, S.T.; Brown, M.J. The life-history impact and implications of multiple parasites for bumble bee queens. Int. J. Parasitol. 2008, 38, 799–808. [Google Scholar] [CrossRef]
- Pridal, A.; Sedlacek, I.; Marvanová, L. Microbiology of bumble bee larvae (Bombus terrestris L.) from laboratory rearing. Acta Univ. Agric. Silvic. Mendel. Brun. 1997, 45, 59–66. [Google Scholar]
- Meeus, I.; de Miranda, J.R.; de Graaf, D.C.; Wäckers, F.; Smagghe, G. Effect of oral infection with Kashmir bee virus and Israeli acute paralysis virus on bumblebee (Bombus terrestris) reproductive success. J. Invertebr. Pathol. 2014, 121, 64–69. [Google Scholar] [CrossRef]
- Fürst, M.A.; McMahon, D.P.; Osborne, J.L.; Paxton, R.J.; Brown, M.J. Disease associations between honeybees and bumblebees as a threat to wild pollinators. Nature 2014, 506, 364–366. [Google Scholar] [CrossRef]
- McMenamin, A.J.; Daughenbaugh, K.F.; Parekh, F.; Pizzorno, M.C.; Flenniken, M.L. Honey Bee and Bumble Bee Antiviral Defense. Viruses 2018, 10, 395. [Google Scholar] [CrossRef]
- McMenamin, A.J.; Flenniken, M.L. Recently identified bee viruses and their impact on bee pollinators. Curr. Opin. Insect Sci. 2018, 26, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Parmentier, L.; Smagghe, G.; de Graaf, D.C.; Meeus, I. Varroa destructor Macula-like virus, Lake Sinai virus and other new RNA viruses in wild bumblebee hosts (Bombus pascuorum, Bombus lapidarius and Bombus pratorum). J. Invertebr. Pathol. 2016, 134, 6–11. [Google Scholar] [CrossRef]
- Murray, E.A.; Burand, J.; Trikoz, N.; Schnabel, J.; Grab, H.; Danforth, B.N. Viral transmission in honey bees and native bees, supported by a global black queen cell virus phylogeny. Environ. Microbiol. 2019, 21, 972–983. [Google Scholar] [CrossRef]
- Graystock, P.; Goulson, D.; Hughes, W.O. The relationship between managed bees and the prevalence of parasites in bumblebees. PeerJ 2014, 2, e522. [Google Scholar] [CrossRef]
- Burnham, P.A.; Alger, S.A.; Case, B.; Boncristiani, H.; Hébert-Dufresne, L.; Brody, A.K. Flowers as dirty doorknobs: Deformed wing virus transmitted between Apis mellifera and Bombus impatiens through shared flowers. J. Appl. Ecol. 2021, 58, 2065–2074. [Google Scholar] [CrossRef]
- Alger, S.A.; Burnham, P.A.; Brody, A.K. Flowers as viral hot spots: Honey bees (Apis mellifera) unevenly deposit viruses across plant species. PLoS ONE 2019, 14, e0221800. [Google Scholar] [CrossRef] [PubMed]
- McMahon, D.P.; Fürst, M.A.; Caspar, J.; Theodorou, P.; Brown, M.J.F.; Paxton, R.J. A sting in the spit: Widespread cross-infection of multiple RNA viruses across wild and managed bees. J. Anim. Ecol. 2015, 84, 615–624. [Google Scholar] [CrossRef]
- Brown, M.J.F.; Schmid-Hempel, R.; Schmid-Hempel, P. Queen-controlled sex ratios and worker reproduction in the bumble bee Bombus hypnorum, as revealed by microsatellites. Mol. Ecol. 2003, 12, 1599–1605. [Google Scholar] [CrossRef] [PubMed]
- Amsalem, E.; Padilla, M.; Schreiber, P.M.; Altman, N.S.; Hefetz, A.; Grozinger, C.M. Do Bumble Bee, Bombus impatiens, Queens Signal their Reproductive and Mating Status to their Workers? J. Chem. Ecol. 2017, 43, 563–572. [Google Scholar] [CrossRef]
- Alaux, C.; Boutot, M.; Jaisson, P.; Hefetz, A. Reproductive plasticity in bumblebee workers (Bombus terrestris)—Reversion from fertility to sterility under queen influence. Behav. Ecol. Sociobiol. 2007, 62, 213–222. [Google Scholar] [CrossRef]
- Lopez-Vaamonde, C.; Brown, R.M.; Lucas, E.R.; Pereboom, J.J.M.; Jordan, W.C.; Bourke, A.F.G. Effect of the queen on worker reproduction and new queen production in the bumble bee Bombus terrestris. Apidologie 2007, 38, 171–180. [Google Scholar] [CrossRef]
- Padilla, M.; Amsalem, E.; Altman, N.; Hefetz, A.; Grozinger, C.M. Chemical communication is not sufficient to explain reproductive inhibition in the bumblebee Bombus impatiens. R. Soc. Open Sci. 2016, 3, 160576. [Google Scholar] [CrossRef]
- Bloch, G.; Hefetz, A. Reevaluation of the Role of Mandibular Glands in Regulation of Reproduction in Bumblebee Colonies. J. Chem. Ecol. 1999, 25, 881–896. [Google Scholar] [CrossRef]
- Bloch, G.; Hefetz, A. Regulation of reproduction by dominant workers in bumblebee (Bombus terrestris) queenright colonies. Behav. Ecol. Sociobiol. 1999, 45, 125–135. [Google Scholar] [CrossRef]
- Alaux, C.; Savarit, F.; Jaisson, P.; Hefetz, A. Does the queen win it all? Queen–worker conflict over male production in the bumblebee, Bombus terrestris. Naturwissenschaften 2004, 91, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Zanette, L.R.S.; Miller, S.D.L.; Faria, C.M.A.; Almond, E.J.; Huggins, T.J.; Jordan, W.C.; Bourke, A.F.G. Reproductive conflict in bumblebees and the evolution of worker policing. Evolution 2012, 66, 3765–3777. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, Y.; Zhang, H.; Breeze, T.D.; An, J. Worker-Born Males Are Smaller but Have Similar Reproduction Ability to Queen-Born Males in Bumblebees. Insects 2021, 12, 1008. [Google Scholar] [CrossRef]
- Huth-Schwarz, A.; León, A.; Vandame, R.; Moritz, R.F.A.; Kraus, F.B. Workers dominate male production in the neotropical bumblebee Bombus wilmattae (Hymenoptera: Apidae). Front. Zool. 2011, 8, 13. [Google Scholar] [CrossRef]
- Lopez-Vaamonde, C.; Koning, J.W.; Jordan, W.C.; Bourke, A.F.G. No evidence that reproductive bumblebee workers reduce the production of new queens. Anim. Behav. 2003, 66, 577–584. [Google Scholar] [CrossRef]
- Kreuter, K.; Bunk, E.; Lückemeyer, A.; Twele, R.; Francke, W.; Ayasse, M. How the social parasitic bumblebee Bombus bohemicus sneaks into power of reproduction. Behav. Ecol. Sociobiol. 2012, 66, 475–486. [Google Scholar] [CrossRef]
- Larrere, M.; Couillaud, F. Role of juvenile hormone biosynthesis in dominance status and reproduction of the bumblebee, Bombus terrestris. Behav. Ecol. Sociobiol. 1993, 33, 335–338. [Google Scholar] [CrossRef]
- Cnaani, J.; Robinson, G.E.; Bloch, G.; Borst, D.; Hefetz, A. The effect of queen-worker conflict on caste determination in the bumblebee Bombus terrestris. Behav. Ecol. Sociobiol. 2000, 47, 346–352. [Google Scholar] [CrossRef]
- Shpigler, H.Y.; Herb, B.; Drnevich, J.; Band, M.; Robinson, G.E.; Bloch, G. Juvenile hormone regulates brain-reproduction tradeoff in bumble bees but not in honey bees. Horm. Behav. 2020, 126, 104844. [Google Scholar] [CrossRef] [PubMed]
- Geva, S.; Hartfelder, K.; Bloch, G. Reproductive division of labor, dominance, and ecdysteroid levels in hemolymph and ovary of the bumble bee Bombus terrestris. J. Insect Physiol. 2005, 51, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Matsuyama, H.; Morita, N.; Ono, M. Caste differences in the association between dopamine and reproduction in the bumble bee Bombus ignitus. J. Insect Physiol. 2017, 103, 107–116. [Google Scholar] [CrossRef]
- Amsalem, E.; Malka, O.; Grozinger, C.; Hefetz, A. Exploring the role of juvenile hormone and vitellogenin in reproduction and social behavior in bumble bees. BMC Evol. Biol. 2014, 14, 45. [Google Scholar] [CrossRef]
- Awde, D.N.; Skandalis, A.; Richards, M.H. Vitellogenin expression corresponds with reproductive status and caste in a primitively eusocial bee. J. Insect Physiol. 2020, 127, 104113. [Google Scholar] [CrossRef]
- Sadd, B.M.; Barribeau, S.M.; Bloch, G.; de Graaf, D.C.; Dearden, P.; Elsik, C.G.; Gadau, J.; Grimmelikhuijzen, C.J.P.; Hasselmann, M.; Lozier, J.D.; et al. The genomes of two key bumblebee species with primitive eusocial organization. Genome Biol. 2015, 16, 76. [Google Scholar] [CrossRef]
- Sun, C.; Huang, J.; Wang, Y.; Zhao, X.; Su, L.; Thomas, G.W.C.; Zhao, M.; Zhang, X.; Jungreis, I.; Kellis, M.; et al. Genus-Wide Characterization of Bumblebee Genomes Provides Insights into Their Evolution and Variation in Ecological and Behavioral Traits. Mol. Biol. Evol. 2021, 38, 486–501. [Google Scholar] [CrossRef]
- Pozo, M.I.; Hunt, B.J.; Van Kemenade, G.; Guerra-Sanz, J.M.; Wäckers, F.; Mallon, E.B.; Jacquemyn, H. The effect of DNA methylation on bumblebee colony development. BMC Genom. 2021, 22, 73. [Google Scholar] [CrossRef]
- Amarasinghe, H.E.; Clayton, C.I.; Mallon, E.B. Methylation and worker reproduction in the bumble-bee (Bombus terrestris). Proc. R. Soc. B Biol. Sci. 2014, 281, 20132502. [Google Scholar] [CrossRef]
- Marshall, H.; Lonsdale, Z.N.; Mallon, E.B. Methylation and gene expression differences between reproductive and sterile bumblebee workers. Evol. Lett. 2019, 3, 485–499. [Google Scholar] [CrossRef]
- Amarasinghe, H.E.; Toghill, B.J.; Nathanael, D.; Mallon, E.B. Allele specific expression in worker reproduction genes in the bumblebee Bombus terrestris. PeerJ 2015, 3, e1079. [Google Scholar] [CrossRef]
- Cameron, S.A.; Sadd, B.M. Global Trends in Bumble Bee Health. Annu. Rev. Entomol. 2020, 65, 209–232. [Google Scholar] [CrossRef]
- Iwasaki, J.M.; Hogendoorn, K. Mounting evidence that managed and introduced bees have negative impacts on wild bees: An updated review. Curr. Res. Insect Sci. 2022, 2, 100043. [Google Scholar] [CrossRef]
- Williams, P.H. Mapping variations in the strength and breadth of biogeographic transition zones using species turnover. Proc. R. Soc. B Biol. Sci. 1996, 263, 579–588. [Google Scholar] [CrossRef]
- Huang, J.; An, J. Species diversity, pollination application and strategy for conservation of the bumblebees of China. Biodivers. Sci. 2018, 26, 486–497. [Google Scholar] [CrossRef]
- Williams, P.H.; Cameron, S.A.; Hines, H.M.; Cederberg, B.; Rasmont, P. A simplified subgeneric classification of the bumblebees (genus Bombus). Apidologie 2008, 39, 46–74. [Google Scholar] [CrossRef]
- Cameron, S.A.; Hines, H.M.; Williams, P.H. A comprehensive phylogeny of the bumble bees (Bombus). Biol. J. Linn. Soc. 2007, 91, 161–188. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Jiang, J.; Pang, Z.; Ma, W.; Jiang, Y.; Fu, Y.; Liu, Y. Tracking Existing Factors Directly Affecting the Reproduction of Bumblebees: Current Knowledge. Insects 2024, 15, 654. https://doi.org/10.3390/insects15090654
Zhao X, Jiang J, Pang Z, Ma W, Jiang Y, Fu Y, Liu Y. Tracking Existing Factors Directly Affecting the Reproduction of Bumblebees: Current Knowledge. Insects. 2024; 15(9):654. https://doi.org/10.3390/insects15090654
Chicago/Turabian StyleZhao, Xiaomeng, Jingxin Jiang, Zilin Pang, Weihua Ma, Yusuo Jiang, Yanfang Fu, and Yanjie Liu. 2024. "Tracking Existing Factors Directly Affecting the Reproduction of Bumblebees: Current Knowledge" Insects 15, no. 9: 654. https://doi.org/10.3390/insects15090654
APA StyleZhao, X., Jiang, J., Pang, Z., Ma, W., Jiang, Y., Fu, Y., & Liu, Y. (2024). Tracking Existing Factors Directly Affecting the Reproduction of Bumblebees: Current Knowledge. Insects, 15(9), 654. https://doi.org/10.3390/insects15090654






