Comprehensive Screening and Validation of Stable Internal Reference Genes for Accurate qRT-PCR Analysis in Holotrichia parallela under Diverse Biological Conditions and Environmental Stresses
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Treatment Methods
2.2. RNA Isolation and Reverse Transcription
2.3. Selection of Reference Genes and Primer Formulation
2.4. Fluorescent Quantitative PCR Reaction Conditions
2.5. Assessment of Reference Gene Stability
2.6. Validation of Selected Reference Genes
3. Results
3.1. Assessment of Primer Efficiency and Specificity in Amplification
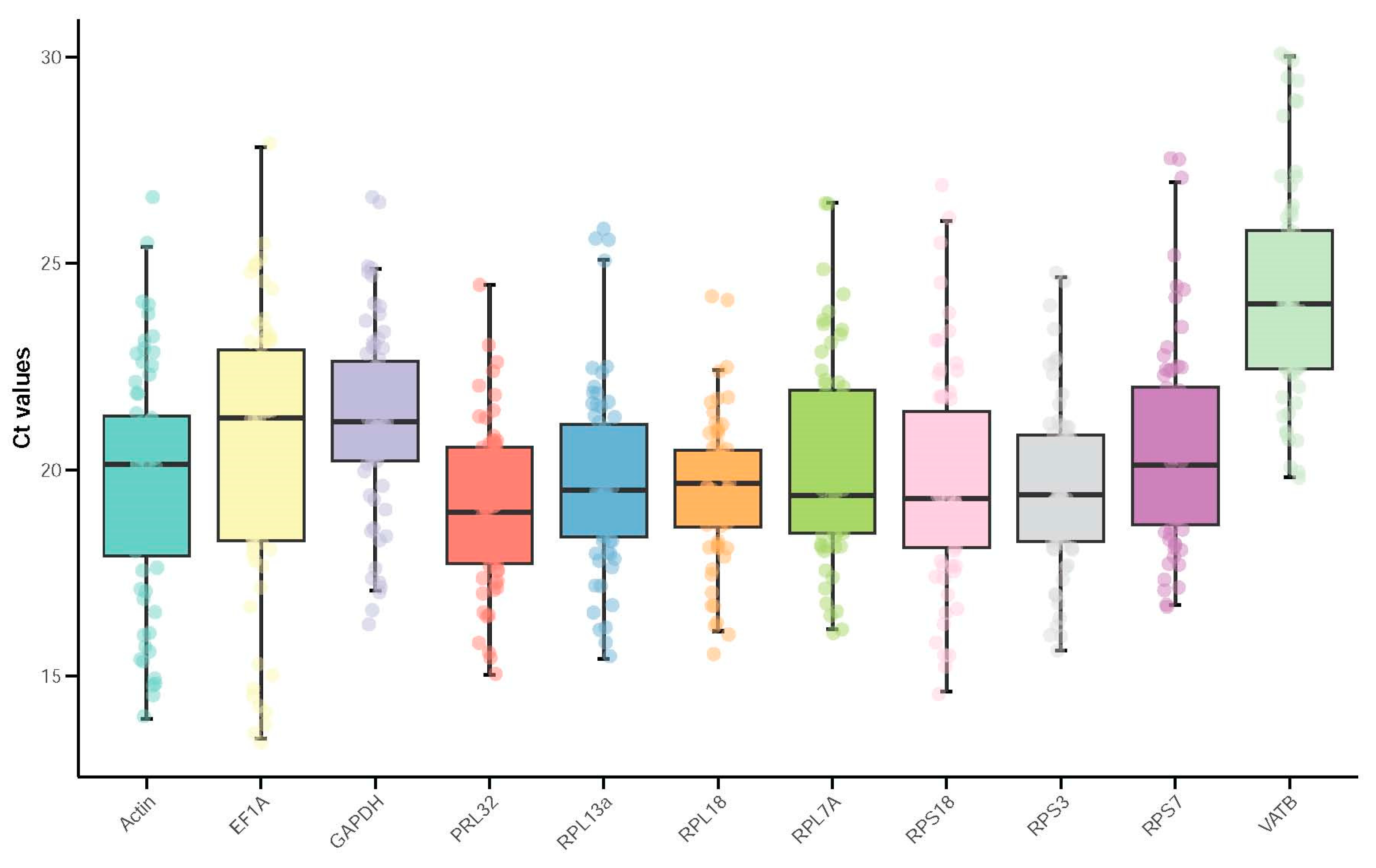
3.2. Expression Profile of Reference Genes
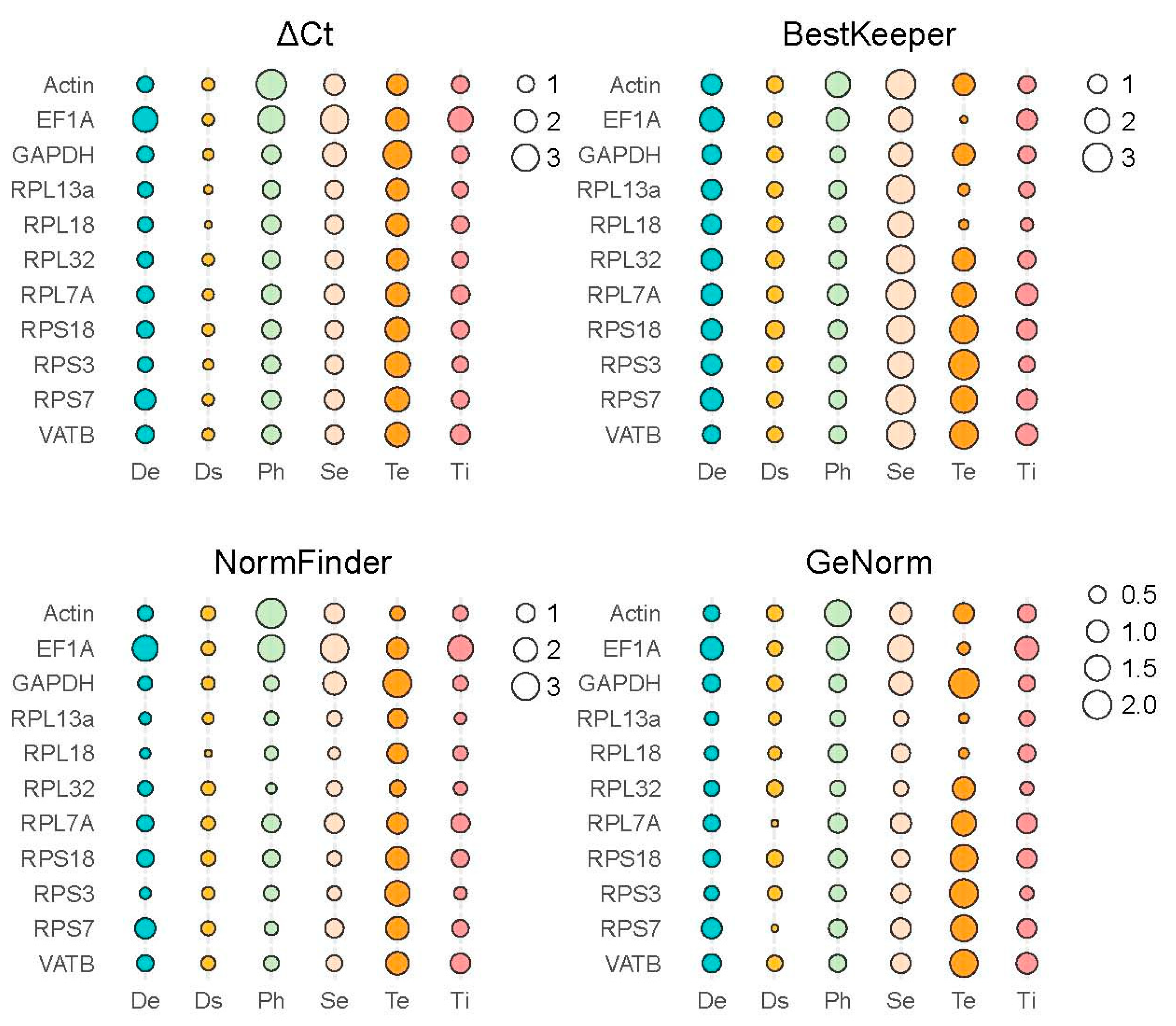
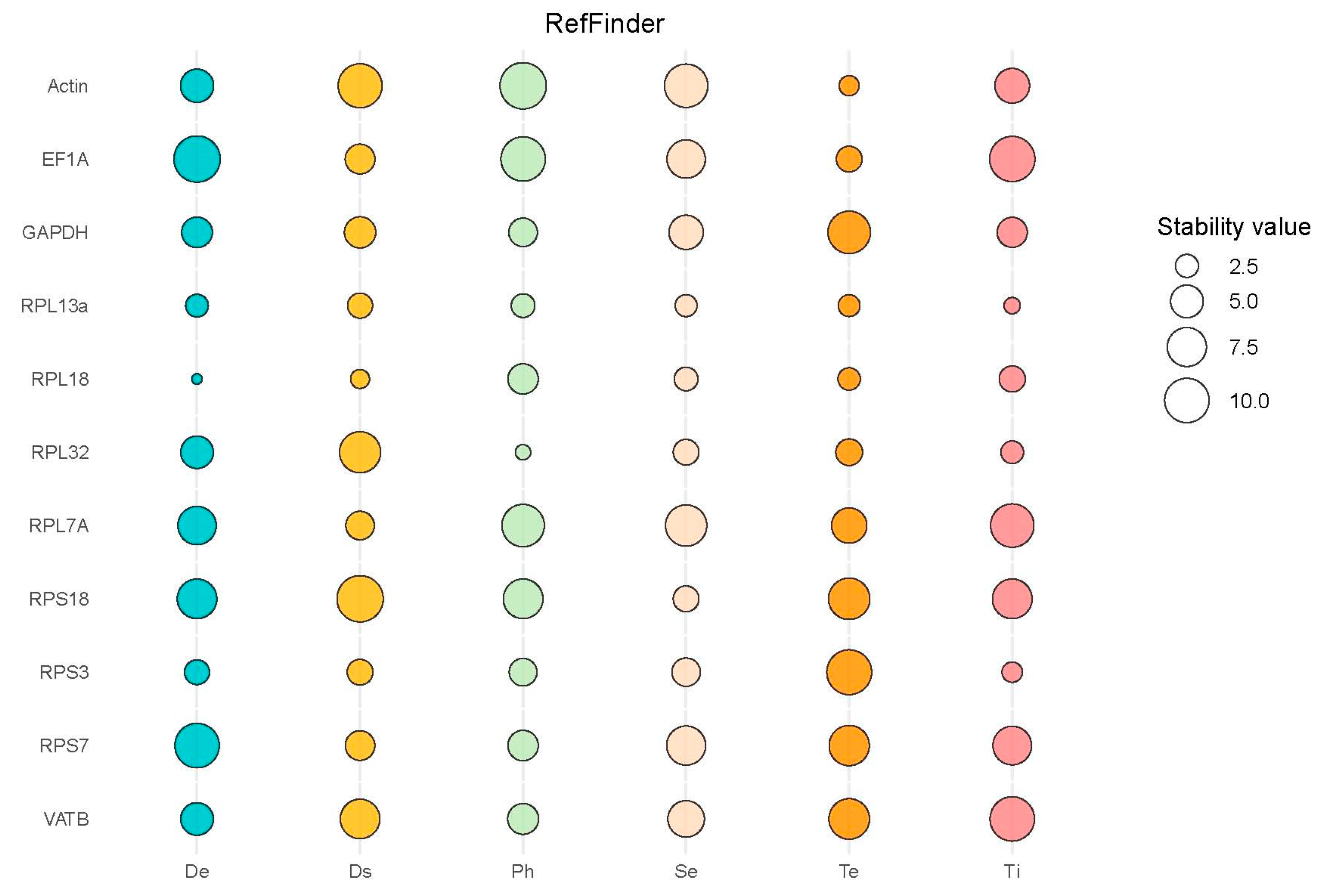
3.3. Identifying Reference Genes with Consistent Expression Stability
3.4. Optimal Number of Reference Genes
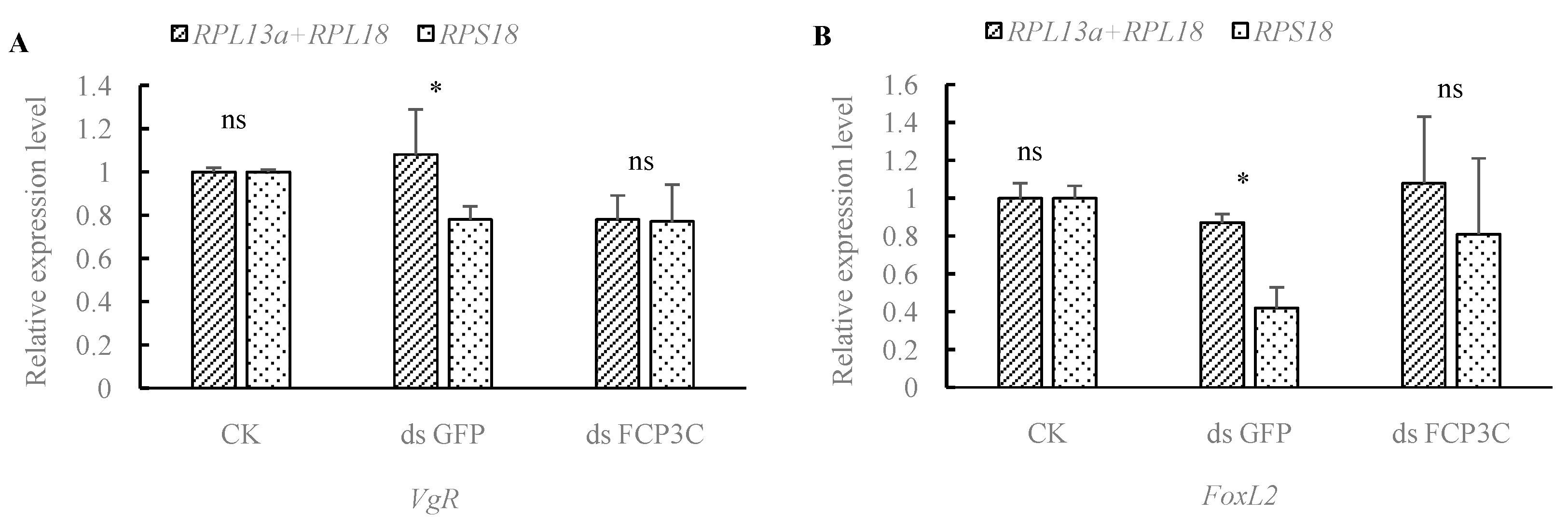
3.5. The Influence of Housekeeping Genes on Quantitative PCR Data Interpretation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034. [Google Scholar] [CrossRef]
- Thellin, O.; Zorzi, W.; Lakaye, B.; De Borman, B.; Coumans, B.; Hennen, G.; Grisar, T.; Igout, A.; Heinen, E. Housekeeping genes as internal standards: Use and limits. J. Biotechnol. 1999, 75, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, L.; Mauriat, M.; Guénin, S.; Pelloux, J.; Lefebvre, J.F.; Louvet, R.; Rusterucci, C.; Moritz, T.; Guerineau, F.; Bellini, C. The lack of a systematic validation of reference genes: A serious pitfall undervalued in reverse transcription-polymerase chain reaction (RT-PCR) analysis in plants. Plant Biotechnol. J. 2008, 6, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Qiao, H.; Liu, S.; Yuan, X.; Xu, C. Transcriptome-based selection and validation of reference genes for gene expression in Goji Fruit Fly (Neoceratitis asiatica Becker) under developmental stages and five abiotic stresses. Int. J. Mol. Sci. 2023, 24, 451. [Google Scholar] [CrossRef]
- Dong, X.M.; Zhang, W.; Zhang, S.B. Selection and validation of reference genes for quantitative real-time PCR analysis of development and tissue-dependent flower color formation in Cymbidium lowianum. Int. J. Mol. Sci. 2022, 23, 738. [Google Scholar] [CrossRef]
- Shi, C.; Yang, F.; Zhu, X.; Du, E.; Yang, Y.; Wang, S.; Wu, Q.; Zhang, Y. Evaluation of housekeeping genes for quantitative real-time PCR analysis of Bradysia odoriphaga (Diptera: Sciaridae). Int. J. Mol. Sci. 2016, 17, 1034. [Google Scholar] [CrossRef]
- Hruz, T.; Wyss, M.; Docquier, M.; Pfaffl, M.W.; Masanetz, S.; Borghi, L.; Verbrugghe, P.; Kalaydjieva, L.; Bleuler, S.; Laule, O.; et al. RefGenes: Identification of reliable and condition specific reference genes for RT-qPCR data normalization. BMC Genom. 2011, 12, 156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cui, Z.; Zhang, N.; Xie, G.; Wang, W.; Chen, L. Electrophysiological and behavioral responses of Holotrichia parallela to volatiles from peanut. Insects 2021, 12, 158. [Google Scholar] [CrossRef]
- Li, E.T.; Zhang, S.; Li, K.B.; Nyamwasaa, I.; Li, J.Q.; Li, X.F.; Qin, J.H.; Yin, J. Efficacy of entomopathogenic nematode and Bacillus thuringiensis combinations against Holotrichia parallela (Coleoptera: Scarabaeidae) larvae. Biol. Control 2021, 152, 104469. [Google Scholar] [CrossRef]
- Liu, Q.; Li, J.; Xu, X.; Sun, C.; Kang, Y.; Zhou, H.; Hu, D.; Ma, J.; Li, S. The preliminary study on grub control with Rhabditis (Oscheius) spp in peanut fields. Acta Agric. Boreali Sin. 2007, 22, 250–253. [Google Scholar] [CrossRef]
- Toepfer, S.; Li, H.; Pak, S.G.; Son, K.M.; Ryang, Y.S.; Kang, S.I.; Han, R.; Holmes, K. Soil insect pests of cold temperate zones of East Asia, including DPR Korea: A review. J. Pest Sci. 2014, 87, 567–595. [Google Scholar] [CrossRef]
- Zhang, M.; Yin, J.; Li, K.; Cao, Y. Research progress on the occurrences of white grub and its control. China Plant Prot. 2014, 34, 20–28. [Google Scholar]
- Pei, G.; Ma, S.; Liu, J.; Liu, B. Effects of different cultivation patterns on grubs occurrence and yield in soybean fields. Soybean Bull 2010, 4, 19–20. [Google Scholar]
- Liu, S.; Li, K.; Yin, J.; Cao, Y. Review of the researches on biological control of grubs. Chin. J. Biol. Control 2008, 24, 168–173. [Google Scholar] [CrossRef]
- Koo, J.; Palli, S.R. Recent advances in understanding of the mechanisms of RNA interference in insects. Insect Mol. Biol. 2024, 1–14. [Google Scholar] [CrossRef]
- Bargmann, C.I. High-throughput reverse genetics: RNAi screens in Caenorhabditis elegans. Genome Biol. 2001, 2, 1005. [Google Scholar] [CrossRef]
- De Schutter, K.; Taning, C.N.T.; Van Daele, L.; Van Damme, E.J.; Dubruel, P.; Smagghe, G. RNAi-based biocontrol products: Market status, regulatory aspects, and risk assessment. Front. Insect Sci. 2022, 1, 818037. [Google Scholar] [CrossRef] [PubMed]
- Li, E.T.; Wu, H.J.; Qin, J.H.; Luo, J.; Li, K.B.; Cao, Y.Z.; Zhang, S.; Peng, Y.; Yin, J. Involvement of Holotrichia parallela odorant-binding protein 3 in the localization of oviposition sites. Int. J. Biol. Macromol. 2023, 242, 124744. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Qin, J.; Feng, H.; Li, J.; Li, X.; Nyamwasa, I.; Cao, Y.; Ruan, W.; Li, K.; Yin, J. Immune-related genes of the larval Holotrichia parallela in response to entomopathogenic nematodes Heterorhabditis beicherriana LF. BMC Genom. 2021, 22, 192. [Google Scholar] [CrossRef]
- Gong, Z.; Zhang, J.; Li, Y.; Li, H.; Zhang, Z.; Qin, Y.; Jiang, Y.; Duan, Y.; Li, T.; Miao, J.; et al. Identification of potential gene targets for suppressing oviposition in Holotrichia parallela using comparative transcriptome analysis. Int. J. Mol. Sci. 2023, 24, 13138. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, Z.R.; Wu, H.; Fu, C.R.; Li, Y.Z.; Lu, X.J.; Guo, W. RNA interference-mediated functional characterization of Group I chitin deacetylases in Holotrichia parallela Motschulsky. Pestic. Biochem. Physiol. 2021, 173, 104770. [Google Scholar] [CrossRef]
- Shakeel, M.; Rodriguez, A.; Tahir, U.B.; Jin, F. Gene expression studies of reference genes for quantitative real-time PCR: An overview in insects. Biotechnol. Lett. 2018, 40, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.H.; Hu, J.R.; Zhang, Y.J. Research progress on reference genes of insect for quantitative real-time reverse transcription PCR (RT-qPCR). Univers. J. Agric. Res. 2015, 3, 211–219. [Google Scholar] [CrossRef]
- Lü, J.; Yang, C.; Zhang, Y.; Pan, H. Selection of reference genes for the normalization of RT-qPCR data in gene expression studies in insects: A systematic review. Front. Physiol. 2018, 9, 1560. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Ju, Q.; Qu, M.; Ye, M.; Wang, L.; Zhao, Z.; Du, L. The research on artificial rearing of Holotrichia parallela and its susceptive to insecticides. J. Peanut Sci. 2008, 37, 46–48. [Google Scholar] [CrossRef]
- Xie, M.; Zhong, Y.; Lin, L.; Zhang, G.; Su, W.; Ni, W.; Qu, M.; Chen, H. Transcriptome analysis of Holotrichia oblita reveals differentially expressed unigenes related to reproduction and development under different photoperiods. Comp. Biochem. Physiol. D 2022, 42, 100959. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Zhong, Y.; Lin, L.; Zhang, G.; Su, W.; Ni, W.; Qu, M.; Chen, H. Evaluation of reference genes for quantitative real-time PCR normalization in the scarab beetle Holotrichia oblita. PLoS ONE 2020, 15, e0240972. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper–Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef]
- Silver, N.; Best, S.; Jiang, J.; Thein, S.L. Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol. Biol. 2006, 7, 33. [Google Scholar] [CrossRef]
- Xie, F.; Wang, J.; Zhang, B. RefFinder: A web-based tool for comprehensively analyzing and identifying reference genes. Funct. Integr. Genom. 2023, 23, 125. [Google Scholar] [CrossRef]
- Bustin, S.A.; Beaulieu, J.-F.; Huggett, J.; Jaggi, R.; Kibenge, F.S.; Olsvik, P.A.; Penning, L.C.; Toegel, S. MIQE precis: Practical implementation of minimum standard guidelines for fluorescence-based quantitative real-time PCR experiments. BMC Mol. Biol. 2010, 11, 74. [Google Scholar] [CrossRef]
- Radonić, A.; Thulke, S.; Mackay, I.M.; Landt, O.; Siegert, W.; Nitsche, A. Guideline to reference gene selection for quantitative real-time PCR. Biochem. Biophys. Res. Commun. 2004, 313, 856–862. [Google Scholar] [CrossRef]
- Kozera, B.; Rapacz, M. Reference genes in real-time PCR. J. Appl. Genet. 2013, 54, 391–406. [Google Scholar] [CrossRef]
- Chervoneva, I.; Li, Y.; Schulz, S.; Croker, S.; Wilson, C.; Waldman, S.A.; Hyslop, T. Selection of optimal reference genes for normalization in quantitative RT-PCR. BMC Bioinform. 2010, 11, 253. [Google Scholar] [CrossRef]
- Huggett, J.; Dheda, K.; Bustin, S.; Zumla, A. Real-time RT-PCR normalisation; strategies and considerations. Genes Immun. 2005, 6, 279–284. [Google Scholar] [CrossRef]
- Wong, M.L.; Medrano, J.F. Real-time PCR for mRNA quantitation. Biotechniques 2005, 39, 75–85. [Google Scholar] [CrossRef]
- Yi, J.; Wang, S.; Wang, Z.; Wang, X.; Li, G.; Zhang, X.; Pan, Y.; Zhao, S.; Zhang, J.; Zhou, J.J.; et al. Identification of candidate carboxylesterases associated with odorant degradation in Holotrichia parallela antennae based on transcriptome analysis. Front. Physiol. 2021, 12, 674023. [Google Scholar] [CrossRef]
- Shu, C.; Tan, S.; Yin, J.; Soberón, M.; Bravo, A.; Liu, C.; Geng, L.; Song, F.; Li, K.; Zhang, J. Assembling of Holotrichia parallela (dark black chafer) midgut tissue transcriptome and identification of midgut proteins that bind to Cry8Ea toxin from Bacillus thuringiensis. Appl. Microbiol. Biotechnol. 2015, 99, 7209–7218. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, X.; Liu, Z.; Lu, X.; Guo, W. Identification and functional analysis of two potential RNAi targets for chitin degradation in Holotrichia parallela Motschulsky (Insecta Coleoptera). Pestic. Biochem. Physiol. 2022, 188, 105257. [Google Scholar] [CrossRef]
- Zhao, D.; Guo, W.; Li, S.; Li, R.; Xu, D.; Lu, X. Identification of a new peritrophic membrane protein from larval Holotrichia parallela (Coleoptera: Motschulsky). Molecules 2014, 19, 17799–17809. [Google Scholar] [CrossRef] [PubMed]
- Lord, J.C.; Hartzer, K.; Toutges, M.; Oppert, B. Evaluation of quantitative PCR reference genes for gene expression studies in Tribolium castaneum after fungal challenge. J. Microbiol. Meth. 2010, 80, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Yang, X.; Siegfried, B.D.; Zhou, X. A comprehensive selection of reference genes for RT-qPCR analysis in a predatory lady beetle, Hippodamia convergens (Coleoptera: Coccinellidae). PLoS ONE 2015, 10, e0125868. [Google Scholar] [CrossRef]
- Xie, J.; Liu, T.; Khashaveh, A.; Yi, C.; Liu, X.; Zhang, Y. Identification and evaluation of suitable reference genes for RT-qPCR analysis in Hippodamia variegata (Coleoptera: Coccinellidae) under different biotic and abiotic conditions. Front. Physiol. 2021, 12, 669510. [Google Scholar] [CrossRef]
- Yang, C.; Pan, H.; Noland, J.E.; Zhang, D.; Zhang, Z.; Liu, Y.; Zhou, X. Selection of reference genes for RT-qPCR analysis in a predatory biological control agent, Coleomegilla maculata (Coleoptera: Coccinellidae). Sci. Rep. 2015, 5, 18201. [Google Scholar] [CrossRef]
- Yang, C.; Preisser, E.L.; Zhang, H.; Liu, Y.; Dai, L.; Pan, H.; Zhou, X. Selection of reference genes for RT-qPCR analysis in Coccinellas eptempunctata to assess un-intended effects of RNAi transgenic plants. Front. Plant Sci. 2016, 7, 1672. [Google Scholar] [CrossRef]
- Yang, X.; Pan, H.; Yuan, L.; Zhou, X. Reference gene selection for RT-qPCR analysis in Harmonia axyridis, a global invasive lady beetle. Sci. Rep. 2018, 8, 2689. [Google Scholar] [CrossRef]
- Chen, H.; Qu, M.; Ali, F.; Lin, L.; Xie, M.; Zhang, G.; Su, W. Expression analysis of odorant-binding protein genes and chemosensory protein genes in Anomala corpulenta Motschulsky (Coleoptera: Scarabaeidae). J. Kans. Entomol. Soc. 2019, 92, 376–389. [Google Scholar] [CrossRef]
- Wang, L.; Yang, C.; Liu, Q.; Zhang, X.; Mei, X.; Zhang, T.; Ning, J. Validation and evaluation of reference genes for quantitative real-time PCR analysis in Mythimna loreyi (Lepidoptera: Noctuidae). Insects 2024, 15, 185. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Q.; Guo, P.; Gao, Z.; Chen, D.; Zhang, T.; Ning, J. Evaluation of reference genes for quantitative real-time PCR analysis in the Bean Bug, Riptortuspedestris (Hemiptera: Alydidae). Insects 2023, 14, 960. [Google Scholar] [CrossRef]
- Xue, D.; Chen, T.; Wu, Y. Stability evaluation of candidate reference genes for real-time qPCR normalization in Rhyzopertha dominica (Coleoptera: Bostrycidae). J. Econ. Entomol. 2024, 117, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.H.; Tang, M.; Li, X.-F.; Zhu, L.; Li, W.; Deng, P.; Zhai, Q.; Wu, G.; Yan, X.H. Evaluation of reference genes for quantitative expression analysis in Mylabris sibirica (Coleoptera, Meloidae). Front. Physiol. 2024, 15, 1345836. [Google Scholar] [CrossRef]

| Gene | Primer Sequences (5′-3′, F/R) | Efficieny (E) (%) | Regression Coefficient (R2) |
|---|---|---|---|
| GAPDH | AGTCGCCGTAAATGATCCCT | 110.68 | 0.9971 |
| CGTCGACTGTGCCATTGAAT | |||
| RPL32 | GCAAAAACCCGTCATATGCT | 110.17 | 0.998 |
| TGATACGCCATGTGCAATTT | |||
| RPL7A | TGCAAATACAATCCGAGCTG | 107.71 | 0.9916 |
| GGCAGGAAGAGCACAAGTTC | |||
| RPS18 | CGTGCTGGAGAATGTTCTGA | 105.82 | 0.9972 |
| GCTGGCTATATTTGCCATCAA | |||
| RPL13a | GGCGTACCTCCACCTTATGA | 106.76 | 0.9978 |
| CAGAATTTGCGACCAGGTTT | |||
| RPL18 | CATTACGCTCACCAACAGGA | 111.19 | 0.998 |
| CTGGAGCAGGACCAAAGTGT | |||
| Actin | TGTCACTGTATGCCTCTGGT | 109.67 | 0.9978 |
| TACCAGCCAAATCCAAACGC | |||
| RPS7 | CGCGAGCTTGAGAAGAAGTT | 109.18 | 0.9994 |
| AGAACGTGGACGCTTCTGTT | |||
| RPS3 | ATCCACTCAGGTGACCCTTG | 114.35 | 0.9956 |
| AACGGCCTCTTAGGTCCAAT | |||
| VATB | GGTCTACCGCACAACGAAAT | 126.92 | 0.9933 |
| ACCTAGCGGTTTCCATGTTG | |||
| EF1A | GCCAGAAGCTGTACCTGGAG | 106.76 | 0.9994 |
| TGTCACCGGCTACATAACCA | |||
| Target gene | |||
| VgR | TGGCGAAGACGAGAAAAACT | - | - |
| TCGTCCGACAAATCGTAACA | |||
| FoxL 2 | CAGCAGCCTATACGCAACAA | - | - |
| AGGAGGCCAATAAGCTGGAT | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, Z.; Zhang, J.; Chen, Q.; Li, H.; Zhang, Z.; Duan, Y.; Jiang, Y.; Li, T.; Miao, J.; Wu, Y. Comprehensive Screening and Validation of Stable Internal Reference Genes for Accurate qRT-PCR Analysis in Holotrichia parallela under Diverse Biological Conditions and Environmental Stresses. Insects 2024, 15, 661. https://doi.org/10.3390/insects15090661
Gong Z, Zhang J, Chen Q, Li H, Zhang Z, Duan Y, Jiang Y, Li T, Miao J, Wu Y. Comprehensive Screening and Validation of Stable Internal Reference Genes for Accurate qRT-PCR Analysis in Holotrichia parallela under Diverse Biological Conditions and Environmental Stresses. Insects. 2024; 15(9):661. https://doi.org/10.3390/insects15090661
Chicago/Turabian StyleGong, Zhongjun, Jing Zhang, Qi Chen, Huiling Li, Ziqi Zhang, Yun Duan, Yueli Jiang, Tong Li, Jin Miao, and Yuqing Wu. 2024. "Comprehensive Screening and Validation of Stable Internal Reference Genes for Accurate qRT-PCR Analysis in Holotrichia parallela under Diverse Biological Conditions and Environmental Stresses" Insects 15, no. 9: 661. https://doi.org/10.3390/insects15090661
APA StyleGong, Z., Zhang, J., Chen, Q., Li, H., Zhang, Z., Duan, Y., Jiang, Y., Li, T., Miao, J., & Wu, Y. (2024). Comprehensive Screening and Validation of Stable Internal Reference Genes for Accurate qRT-PCR Analysis in Holotrichia parallela under Diverse Biological Conditions and Environmental Stresses. Insects, 15(9), 661. https://doi.org/10.3390/insects15090661






