Stimulatory Functions of Male Genitalia in Tipula (Triplicitipula) colei Alexander and Tipula (Lunatipula) translucida Doane (Diptera: Tipulidae) and Implications for Theories of Genital Evolution
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Genital Morphology and Behavior of T. (T.) colei
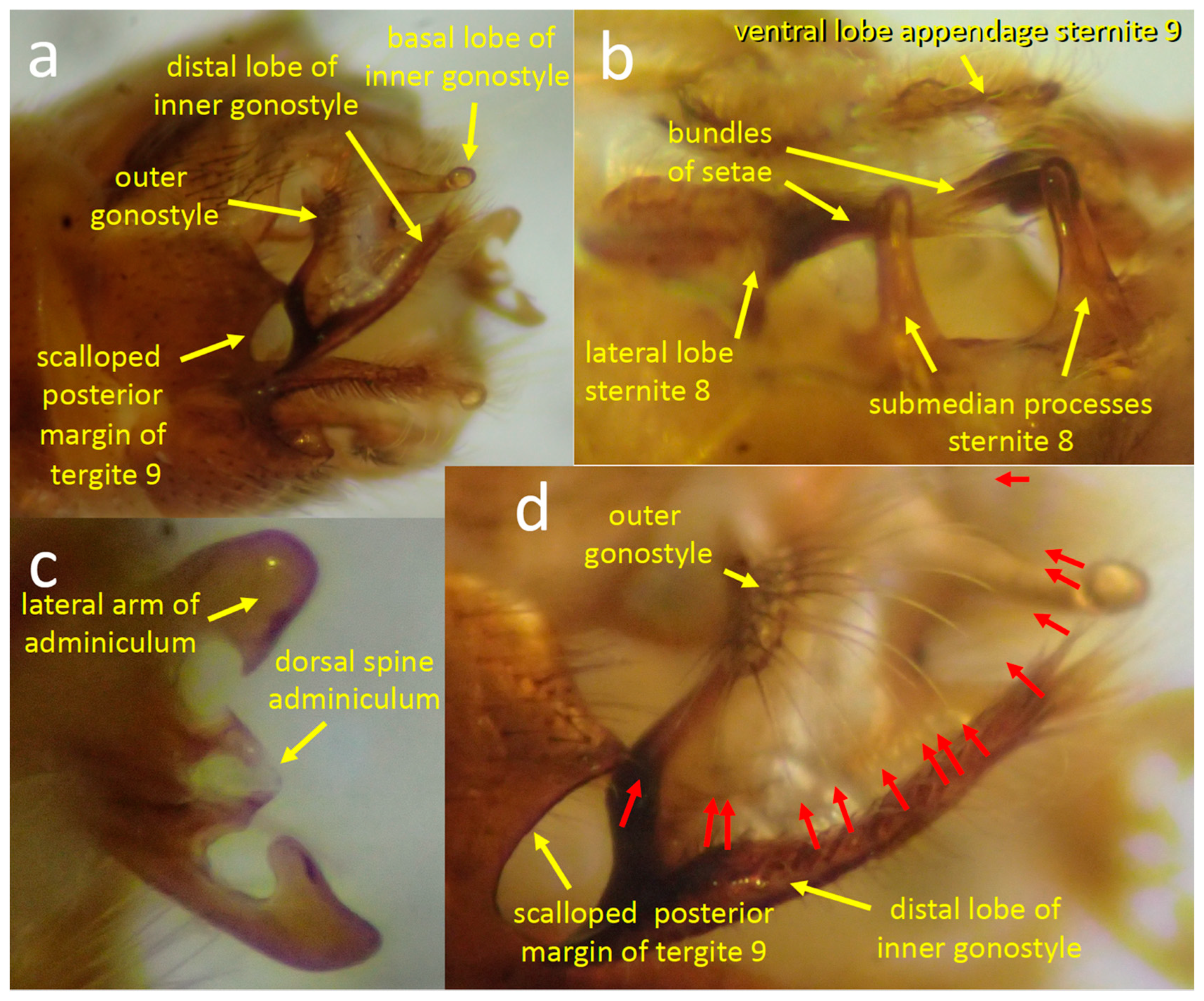
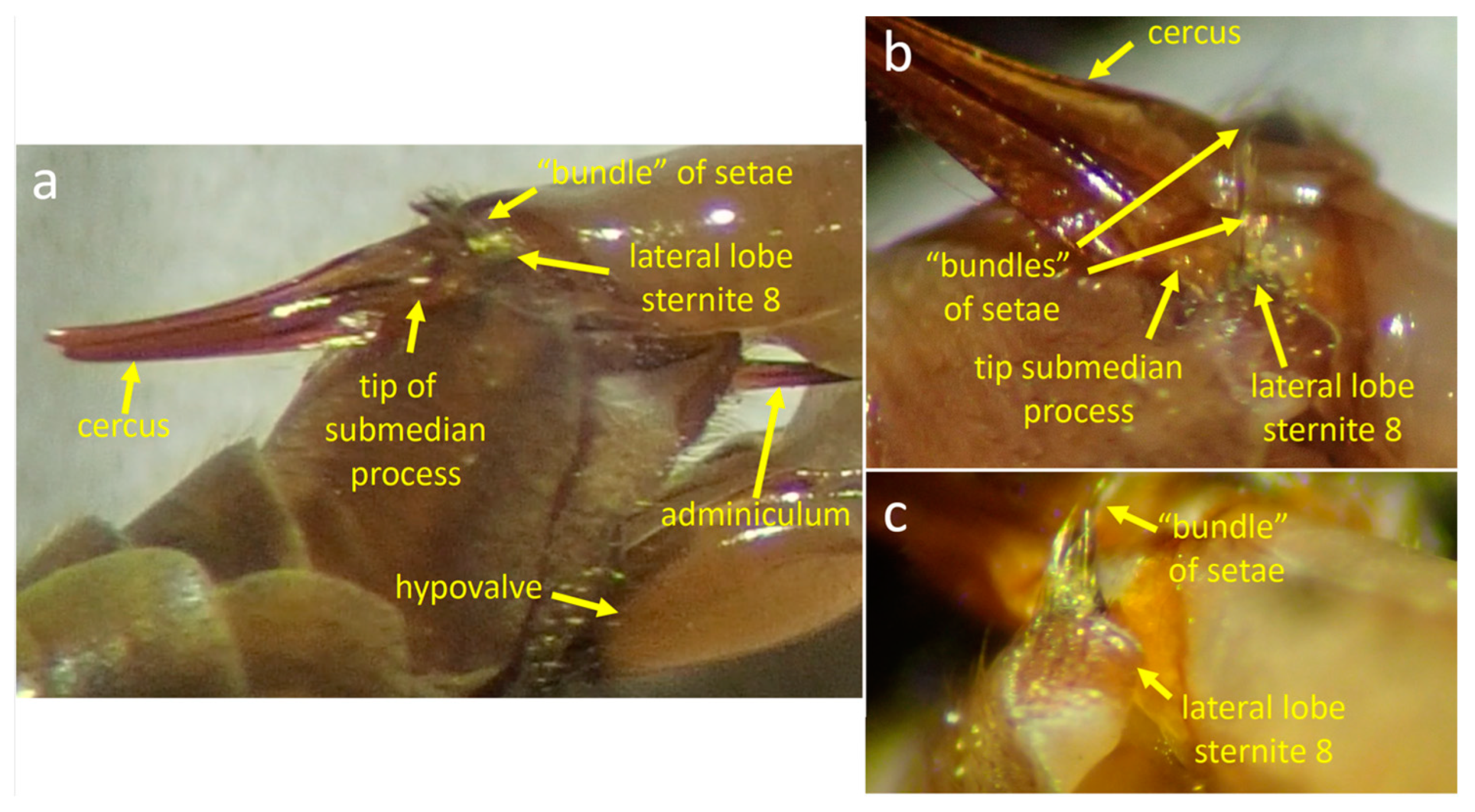
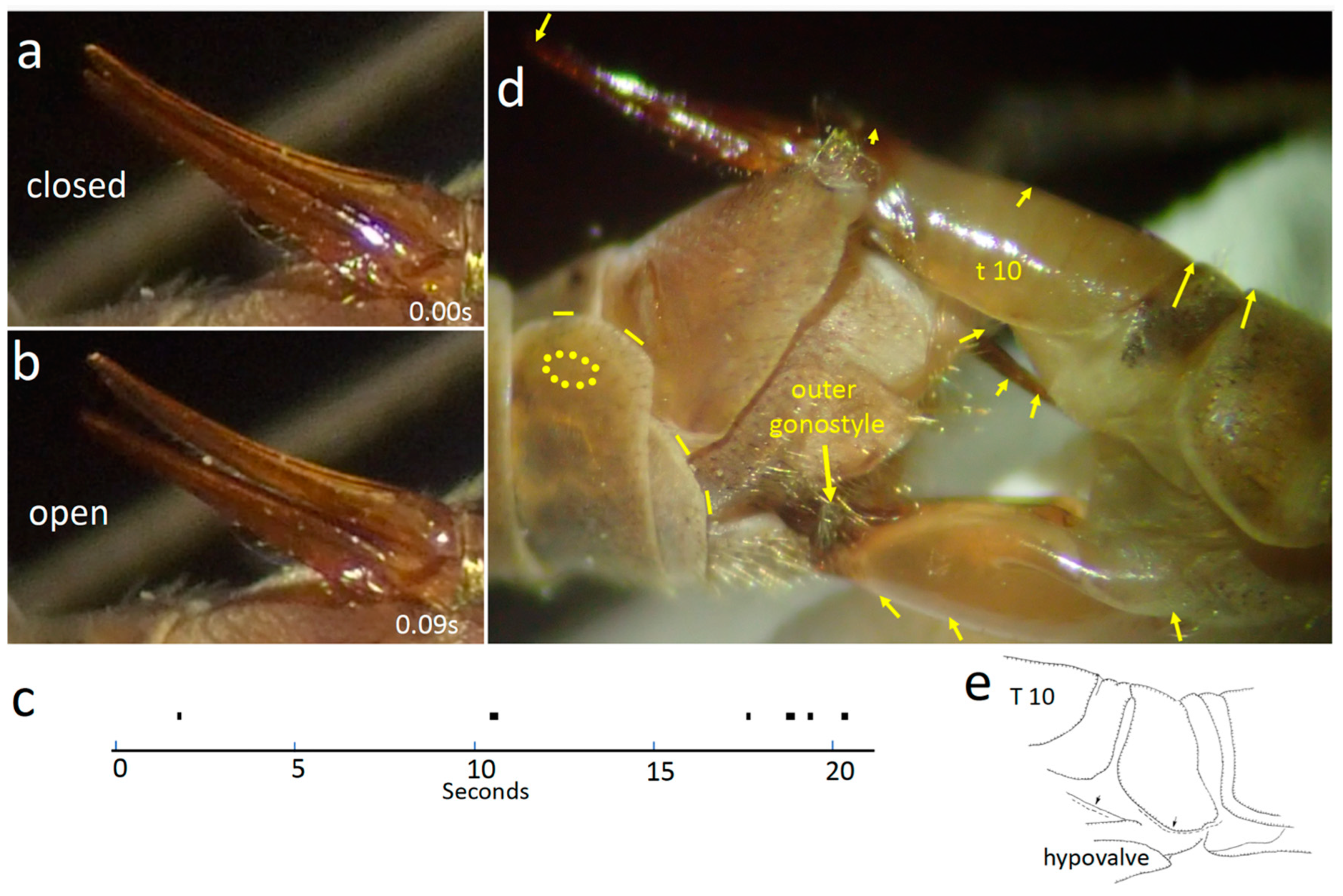
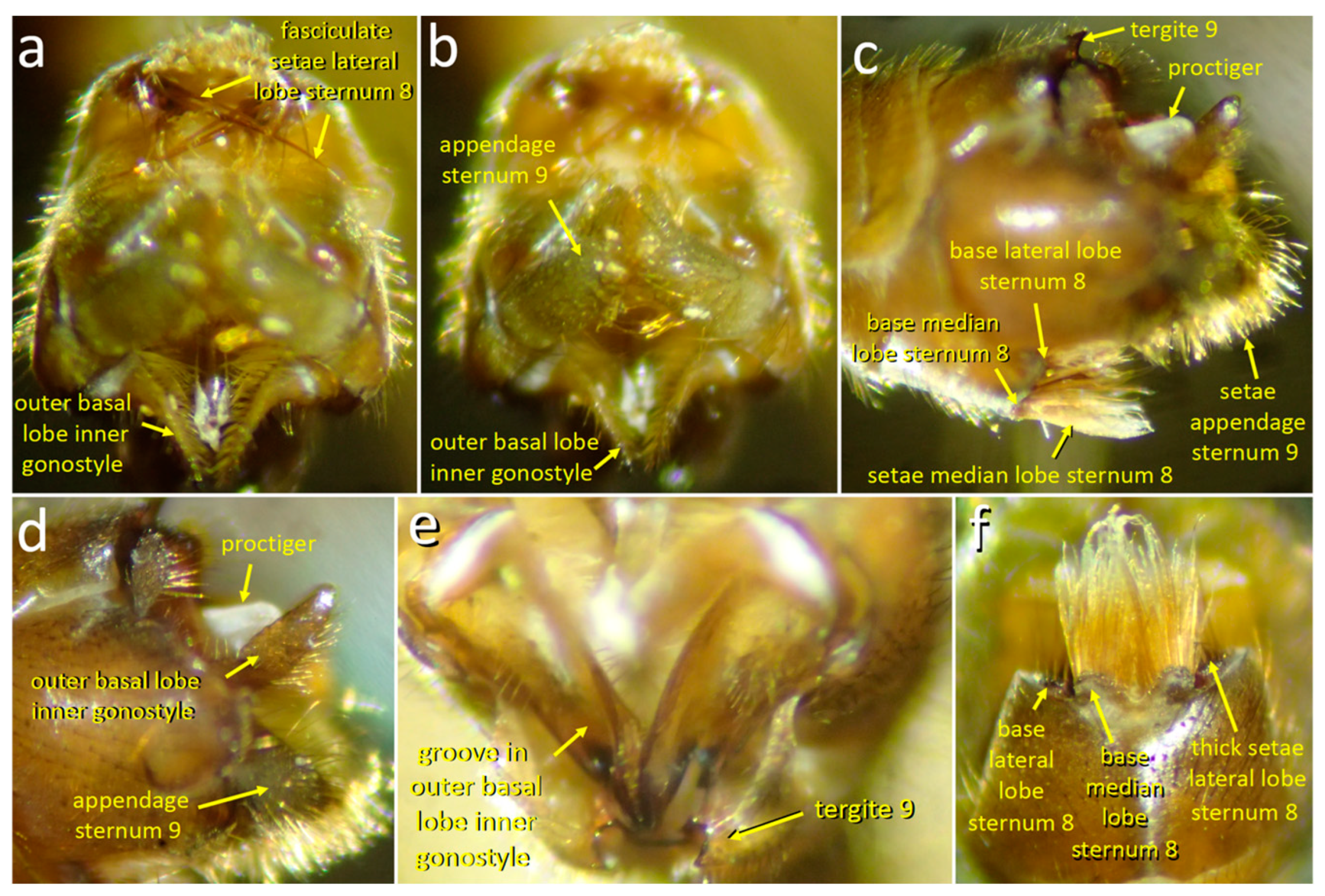
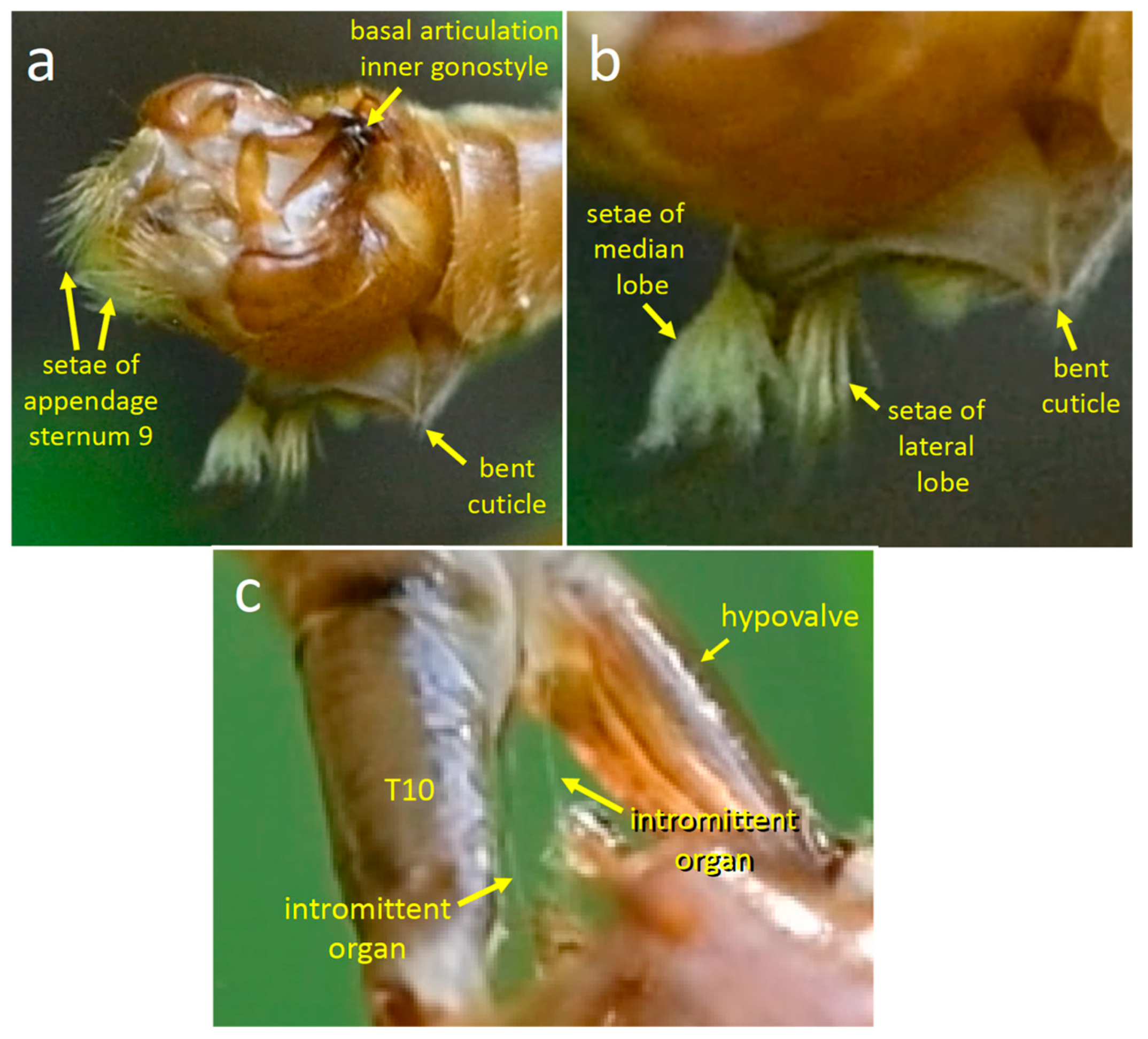
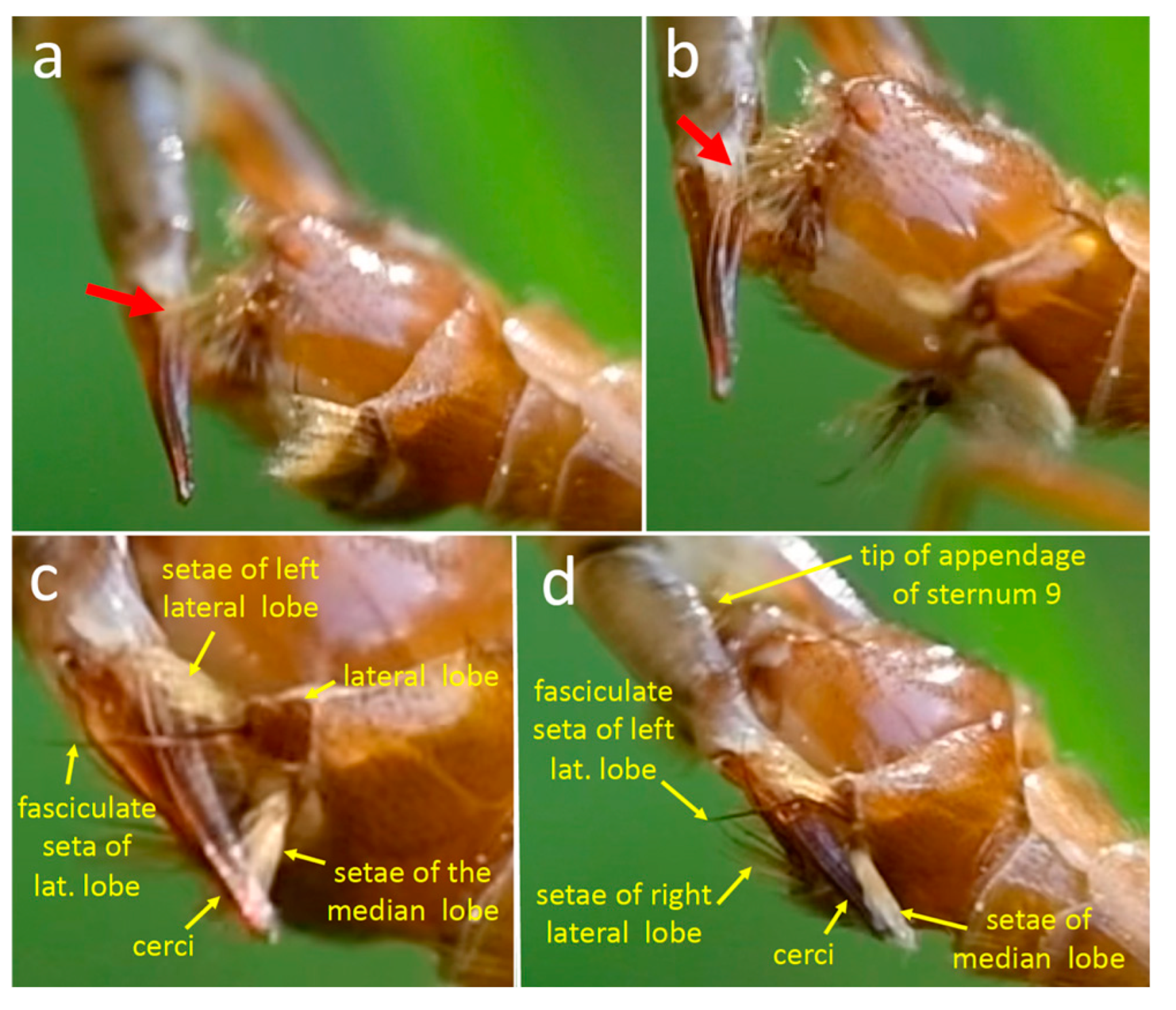
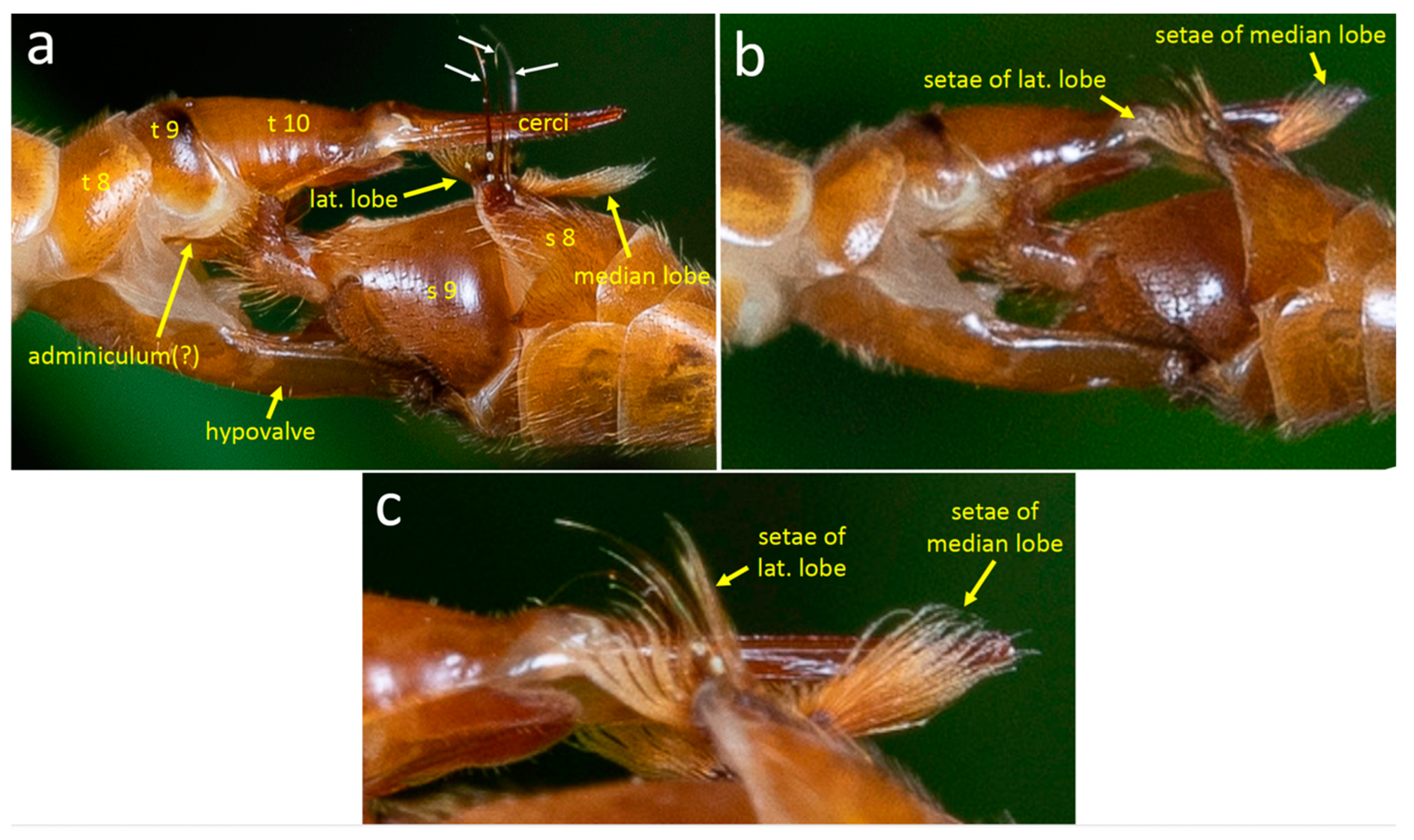
3.1.1. Male Morphology
3.1.2. Female Morphology
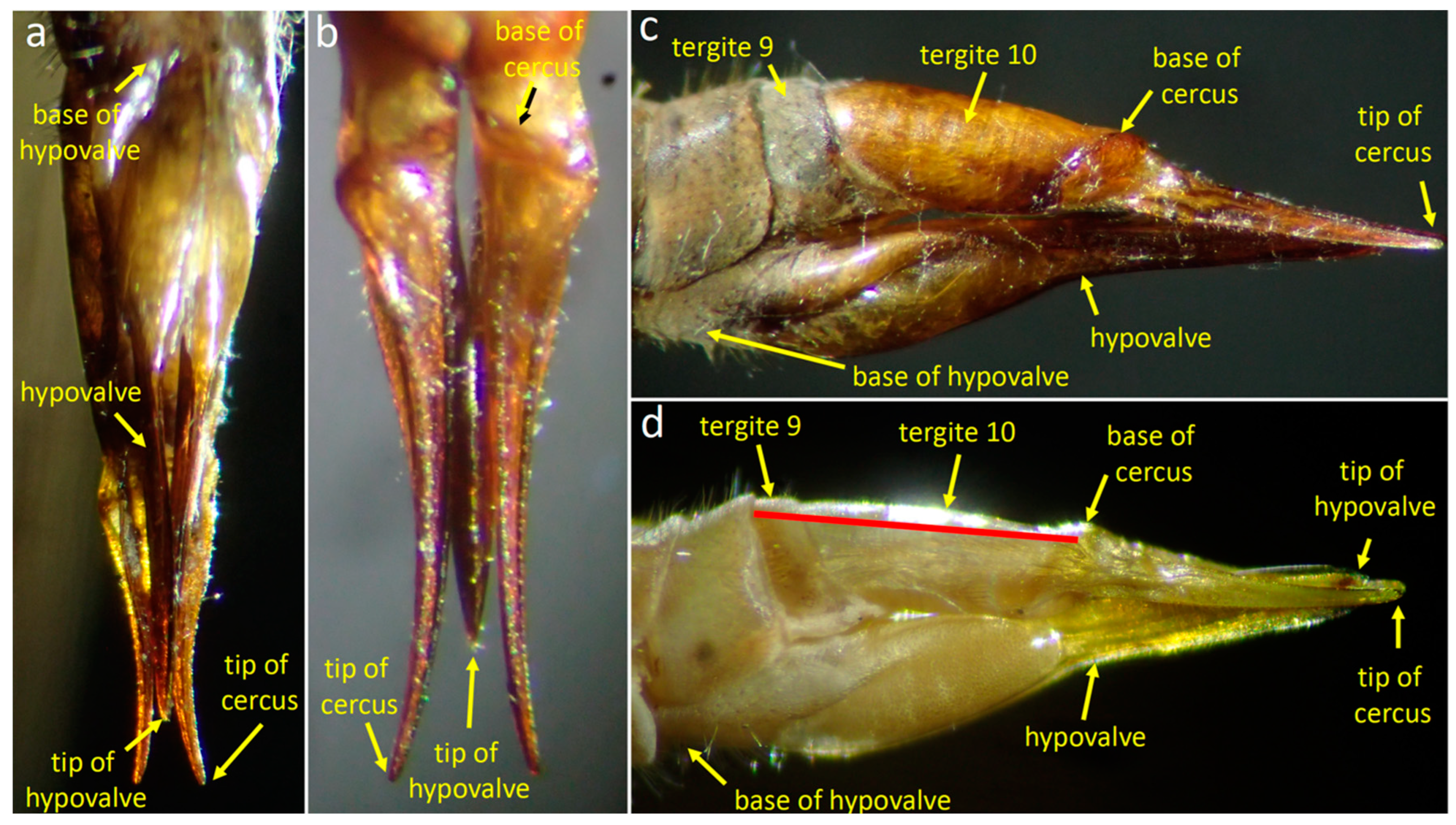
3.1.3. Positions of the Genitalia during Copulation
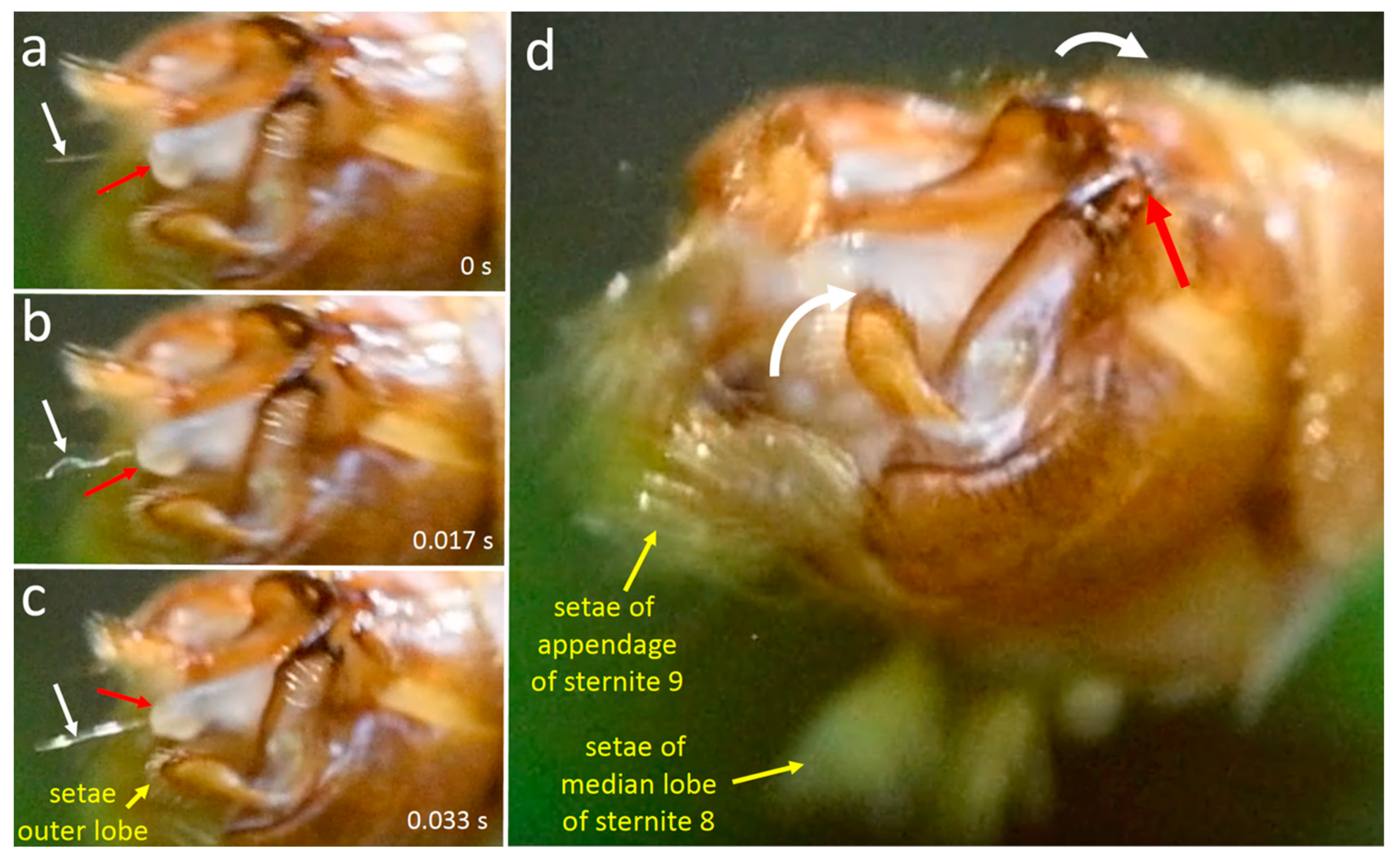
3.1.4. Movements of Male Structures That Contacted the Female during Copulation
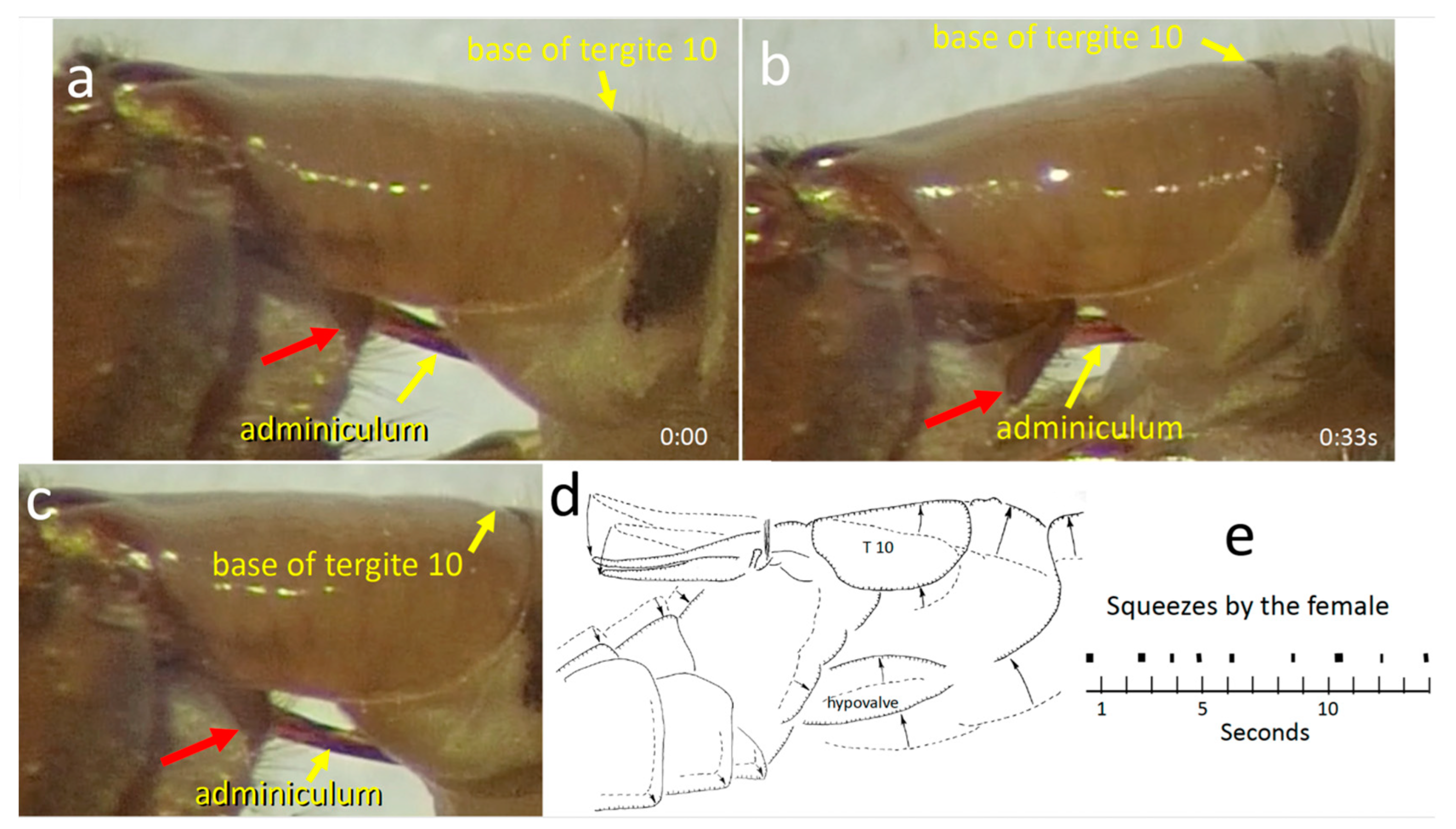
3.1.5. Movements of Female Structures
3.2. Genital Morphology and Behavior of T. (L.) translucida
3.2.1. Female Morphology and Behavior
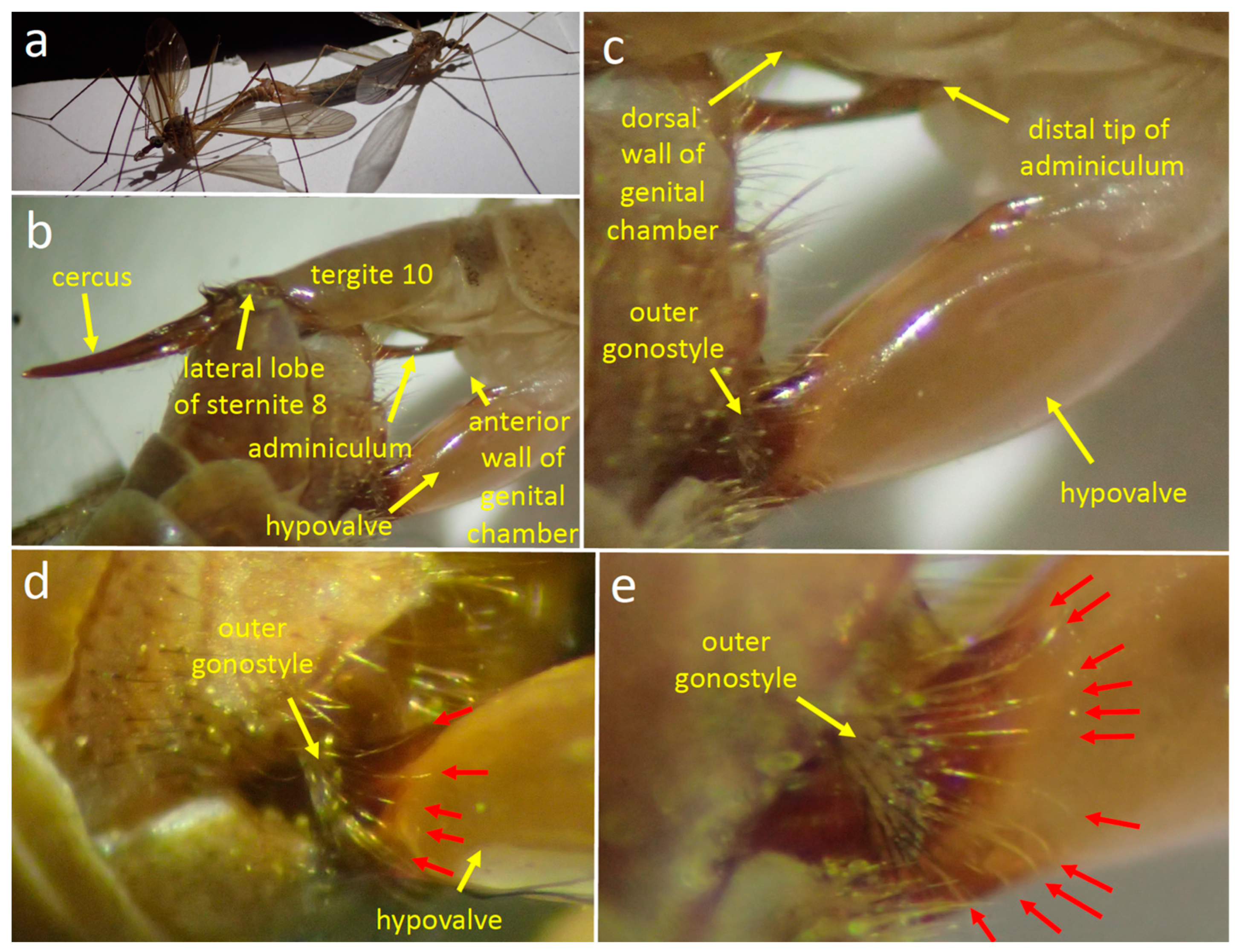
3.2.2. Male Morphology and Behavior during Copulation
3.2.3. Morphology
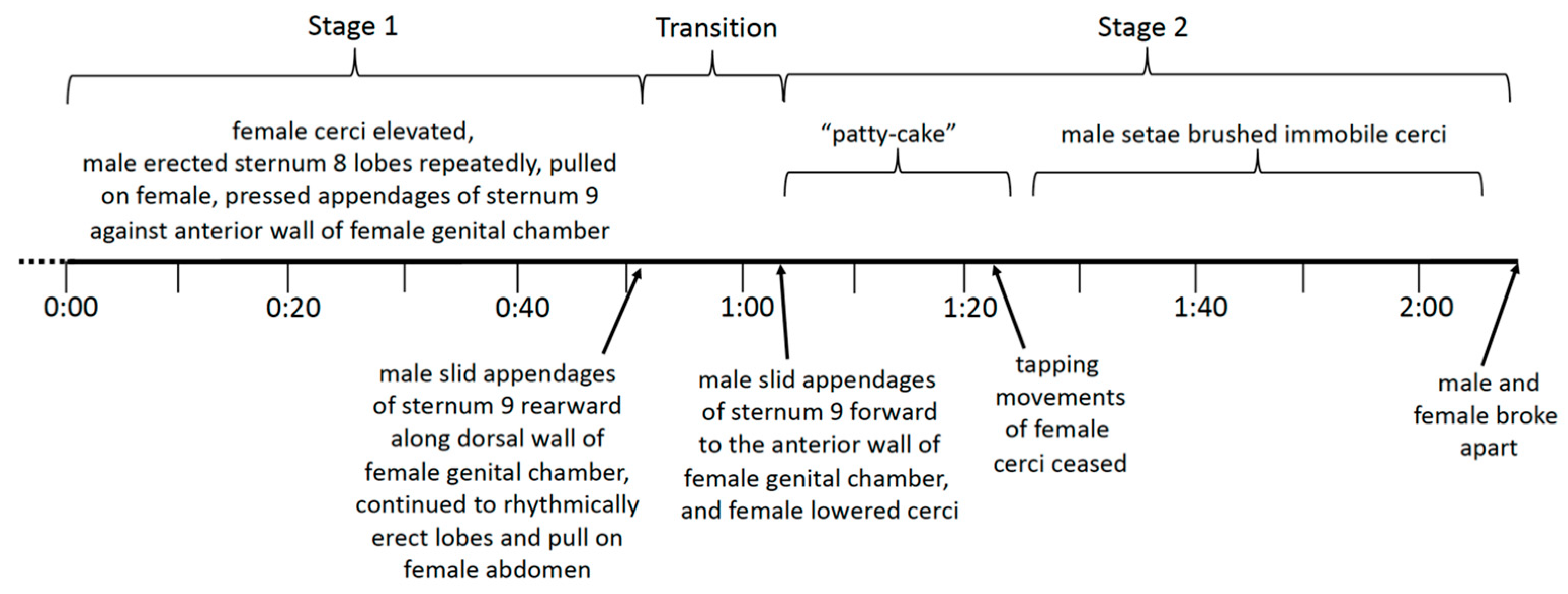
3.2.4. Stage 1
3.2.5. The Transition to Stage 2
3.2.6. Stage 2
3.2.7. Male Genital Behavior Following Copulation
4. Discussion
4.1. Apparent Stimulation Functions for Male Genitalia
4.1.1. T. (T.) colei
4.1.2. T. (L.) translucida
4.2. Phylogenetic Considerations
4.3. Alternative Hypotheses Regarding Function
4.3.1. Sperm Competition
4.3.2. Guide the Female
4.3.3. Apply Chemical Cues
4.3.4. Other Functions That Are Ruled Out
4.4. Limitations of This Study
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eberhard, W.G. Sexual Selection and Animal Genitalia; Harvard University Press: Cambridge, MA, USA, 1985. [Google Scholar]
- Eberhard, W.G. Evolution of genitalia: Theories, evidence, and new directions. Genetica 2010, 138, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Eberhard, W.G. Cryptic female choice and other types of post-copulatory sexual selection. In Cryptic Female Choice in Arthropods; Pereti, A., Aisenberg, A., Eds.; Springer: New York, NY, USA, 2015; pp. 1–26. [Google Scholar]
- Van Haran, M.M.; Rönn, J.L.; Schulthuizen, M.; Arnquist, G. Postmating sexual selection and the enigmatic jawed genitalia of Callosobruchus subinnotatus. Biol. Open 2017, 6, 1008–1012. [Google Scholar] [CrossRef]
- Arnqvist, G.; Rowe, L. Sexual Conflict; Princeton University Press: Princeton, NJ, USA, 2005. [Google Scholar]
- Cocks, O.T.M.; Eady, P.E. Microsurgical manipulation reveals pre-copulatory function of key genital sclerites. J. Exp. Biol. 2018, 221, jeb173427. [Google Scholar] [CrossRef] [PubMed]
- Simmons, L.W. Sexual selection and genital evolution. Austral Entomol. 2014, 53, 1–17. [Google Scholar] [CrossRef]
- Masley, J.P. 170 years of “lock and key”: Genital morphology and reproductive isolation. Int. J. Evol. Evol. Biol. Biol. 2012, 2012, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.M.; Porter, A.H. The lock-and-key hypothesis: Evolutionary and biosystematics interpretation of insect genitalia. Annu. Rev. Entomol. 1989, 34, 231–245. [Google Scholar] [CrossRef]
- Arnqvist, G. Conparative evidence for the 4volution of genitalia by sexual selection. Nature 1998, 393, 784–786. [Google Scholar] [CrossRef]
- Ah-King, M.; Barron, A.; Heerberstein, M.E. Genital evolution: Why are females still understudied? PLoS Biol. 2014, 12, e1001851.45. [Google Scholar] [CrossRef]
- Sloan, N.S.; Simmons, L.W. The evolution of female genitalia. J. Evol. Biol. 2019, 2019, 1–18. [Google Scholar] [CrossRef]
- Williams, G. The Pony Fish’s Glow: And Other Clues to Plan and Purpose in Nature; Basic Books: New York, NY, USA, 1998. [Google Scholar]
- Eberhard, W.G. Species isolation, genital mechanics, and the evolution of species-specific genitalia in three species of Macrodactylus beetles (Coleoptera, Scarabeidae, Melolonthinae). Evolution 1992, 46, 1774–1783. [Google Scholar]
- Eberhard, W.G.; Kariko, S.J. Copulation behavior inside and outside the beetle Macrohaltica jamaicensis (Coleoptera, Chrysomelidae). J. Ethol. 1996, 14, 59–72. [Google Scholar] [CrossRef]
- Crudgington, H.S.; Siva-Jothy, M.T. Genital damage, kicking and early death: The battle of the sexes takes a sinister turn in the bean weevil. Nature 2000, 407, 855–856. [Google Scholar] [CrossRef] [PubMed]
- Flowers, R.W.; Eberhard, W.G. Fitting together: Copulatory linking in some Neotropical Chrysomeloidea. Rev. Biol. Tropical. 2004, 54, 829–842. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Werner, M.; Simmons, L.W. The evolution of male genitalia: Functional integration of genital sclerites in the dung beetle Onthophagus taurus. Biol. J. Linnaean Soc. 2008, 93, 257–266. [Google Scholar] [CrossRef]
- Oosterbroek, P. Catalogue of the Craneflies of the World (Diptera, Tipuloidea: Pediciidae, Limoniidae, Cylindrotomidae, Tipulidae). 2024. Available online: https://ccw.naturalis.nl/index.php (accessed on 3 August 2024).
- Pritchard, G. Biology of Tipulidae. Annu. Rev. Entomol. 1983, 28, 1–22. [Google Scholar] [CrossRef]
- Tangelder, I.R.M. Phylogeny of the Nephrotoma dorsalis species-group (Diptera, Tipulidae), mainly based on genital characters. Beaufortia 1985, 35, 135–174. [Google Scholar]
- Wiegmann, B.; Yeates, D. Phylogeny of Diptera. In Manual of Afrotropical Diptera. Volume 1. Introductory Chapters and Keys to Diptera Families. Suricata 4; Kirk-Spriggs, A.H., Sinclair, B.J., Eds.; South African National Biodiversity Institute: Pretoria, South Africa, 2017; pp. 253–265. [Google Scholar]
- Coe, R.L. Diptera Family Tipulidae. In Handbooks for the Identification of British Insects. Diptera 2. Nematocera: Families Tipulidae to Chironomidae; Royal Entomological Society: London, England, 1950; pp. 1–66. [Google Scholar]
- Alexander, C.P. New subgenera and species of crane-flies from California (Diptera: Tipulidae). Pac. Insects 1965, 7, 333–386. [Google Scholar]
- Hemmingsen, A.M. On the copulation in Phyllolabis hemmingseni Peder Nielsen and Phyllolabis mannheimsiana Peder Nielsen (Limoniinae, Tipulidae, Diptera). Bonn. Zool. Beitr. 1970, 21, 137–144. [Google Scholar]
- Hemmingsen, A.M. Copulatory adaptations of male hypopygium to female tergal ovipository valves (cerci) in some South American crane-flies (Tipulidae). Vidensk. Meddelelser Fra Dan. Naturhistorisk Foren. 1970, 133, 159–178. [Google Scholar]
- Gelhaus, J.K. Systematics and biogeography of the desert crane fly subgenus Tipula (Eremotipula) Alexander (Diptera: Tipulidae). Mem. Am. Entomol. Soc. 2005, 46, 1–242. [Google Scholar]
- Mederos, J.; Pollet, M.; Oosterbroek, P. The crane flies of Martinique, with the description of four new species (Diptera, Tipuloidea). Diversity 2023, 15, 204. [Google Scholar] [CrossRef]
- Sellke, K. Biologische und morphologische Studien an schädlichen Wiesenschnaken (Tipulidae, Dipt.). Z. Für Wiss. Zool. 1936, 148, 465–555. [Google Scholar]
- Neumann, H. Der Bau und die Funktion der mannlichen Genitalapparate von Trichocera annulata Meig. und Tipula paludosa Meig. (Dipt. Nematocera). Dtsch. Entomol. Z. 1958, 5, 235–298. [Google Scholar]
- Hemmingsen, A.M. The function of the peculiar procresses of the 8th sternite in the males of Tipula (Oreomyza) stægeri Peder Nielsen and Tipula (Oreomyza) signata Stæger. Vidensk Meddelelser Fra Dan. Naturhistorisk Foren. 1954, 116, 411–418. [Google Scholar]
- Hemmingsen, A.M. Copulatory adaptations of male hypopygium to female tergal ovipository valves (cerci) in certain crane-flies (Tipulidae). Vidensk. Meddelelser Fra Dan. Naturhistorisk Foren. 1962, 124, 135–163. [Google Scholar]
- Jackson, D.M.; Campbell, R.L. Biology of the European crane fly, Tipula paludosa Meigen in western Washington (Tipulidae; Diptera). Coll. Agric. Res. Cent. Wash. State Univ. Tech. Bull. 1975, 81, 1–23. [Google Scholar]
- Desai, A.S.; Khamkafr, A.G.; Salthe, T.V. Ecology and ethology of crane fly Tipula paludosa Meigen (Tipulidae: Diptera) from Kolhapur region, India. Biolife 2015, 3, 21–25. [Google Scholar]
- Eberhard, W.G.; Gelhaus, J.K. Genitalic stridulation during copulation in a species of crane fly, Tipula (Bellardina) sp. (Diptera: Tipulidae). Rev. Biol. Trop. 2009, 57 (Suppl. S1), 251–256. [Google Scholar]
- Frommer, S.I. Gross morphological studies of the reproductive system in representative North Americn crane flies (Diptera: Tipulidae). Univ. Kans. Sci. Bull. 1963, 44, 535–626. [Google Scholar]
- Hemmingsen, A.M. The oviposition of some crane-fly species (Tipulidae) from different types of localities. Meddelelser Fra Dan. Naturhistorisk Foren. 1952, 114, 365–430. [Google Scholar]
- Hemmingsen, A.M.; Norrevang, A. Cinematographic and other studies of ovipository mechanisms in crane-flies. Vidensk. Meddelelser Fra Dan. Naturhistorisk Foren. 1966, 129, 261–274. [Google Scholar]
- Benda, J. Neural adaptation. Curr. Biol. 2021, 31, R110–R116. [Google Scholar] [CrossRef] [PubMed]
- Rankin, C.H.; Abrams, T.; Barry, R.J.; Bhatnagar, S.; Clayton, D.F.; Colombo, J.; Coppola, G.; Geyer, M.A.; Glanzman, D.L.; Marsland, S.; et al. Habituation revisited: An updated and revised description of the behavioral characteristics of habituation. Neurobiol. Learn. Mem. 2009, 92, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Eberhard, W.G. Female Control: Sexual Selection by Cryptic Female Choice; Princeton University Press: Princeton, NJ, USA, 1996. [Google Scholar]
- Hosken, D.J.; Stockley. Sexual selection and genital evolution. Trends Ecol. Evol. 2004, 19, 87–93. [Google Scholar] [CrossRef]
- Arnqvist, G. Cryptic female choice. In The Evolution of Insect Mating Systems; Shuker, D.M., Simmons, L.W., Eds.; Oxford University Press: New York, NY, USA, 2014; pp. 204–220. [Google Scholar]
- Rodriguez, R.L. Mating is a give-and-take of influence and communication between the sexes. In Cryptic Female Choice in Arthropods; Peretti, A., Aisenberg, Eds.; Springer: New York, NY, USA, 2015; pp. 479–496. [Google Scholar]
- Alexander, C.P. Notes on the tropical American species of the genus Tipula Linnaeus (Tipulidae, Diptera). Part IV. Rev. Entomol. 1951, 22, 265–314. [Google Scholar]
- Eberhard, W.G. Evidence for widespread courtship during copulation in 131 species of insects and spiders, and implications for cryptic female choice. Evolution 1994, 48, 711–733. [Google Scholar] [CrossRef]
- Eberhard, W.G.; Rodriguez, R.L.; Huber, B.A.; Speck, B.; Miller, H. Sexual selection and static allometry: The importance of function. Q. Rev. Biol. 2018, 93, 207–250. [Google Scholar] [CrossRef]
- Stich, H.F. An experimental analysis of the courtship pattern of Tipula oleracea (Diptera). Can. J. Zool. 1963, 41, 99–109. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eberhard, W.G.; Gelhaus, J.K. Stimulatory Functions of Male Genitalia in Tipula (Triplicitipula) colei Alexander and Tipula (Lunatipula) translucida Doane (Diptera: Tipulidae) and Implications for Theories of Genital Evolution. Insects 2024, 15, 680. https://doi.org/10.3390/insects15090680
Eberhard WG, Gelhaus JK. Stimulatory Functions of Male Genitalia in Tipula (Triplicitipula) colei Alexander and Tipula (Lunatipula) translucida Doane (Diptera: Tipulidae) and Implications for Theories of Genital Evolution. Insects. 2024; 15(9):680. https://doi.org/10.3390/insects15090680
Chicago/Turabian StyleEberhard, William G., and Jon K. Gelhaus. 2024. "Stimulatory Functions of Male Genitalia in Tipula (Triplicitipula) colei Alexander and Tipula (Lunatipula) translucida Doane (Diptera: Tipulidae) and Implications for Theories of Genital Evolution" Insects 15, no. 9: 680. https://doi.org/10.3390/insects15090680
APA StyleEberhard, W. G., & Gelhaus, J. K. (2024). Stimulatory Functions of Male Genitalia in Tipula (Triplicitipula) colei Alexander and Tipula (Lunatipula) translucida Doane (Diptera: Tipulidae) and Implications for Theories of Genital Evolution. Insects, 15(9), 680. https://doi.org/10.3390/insects15090680






