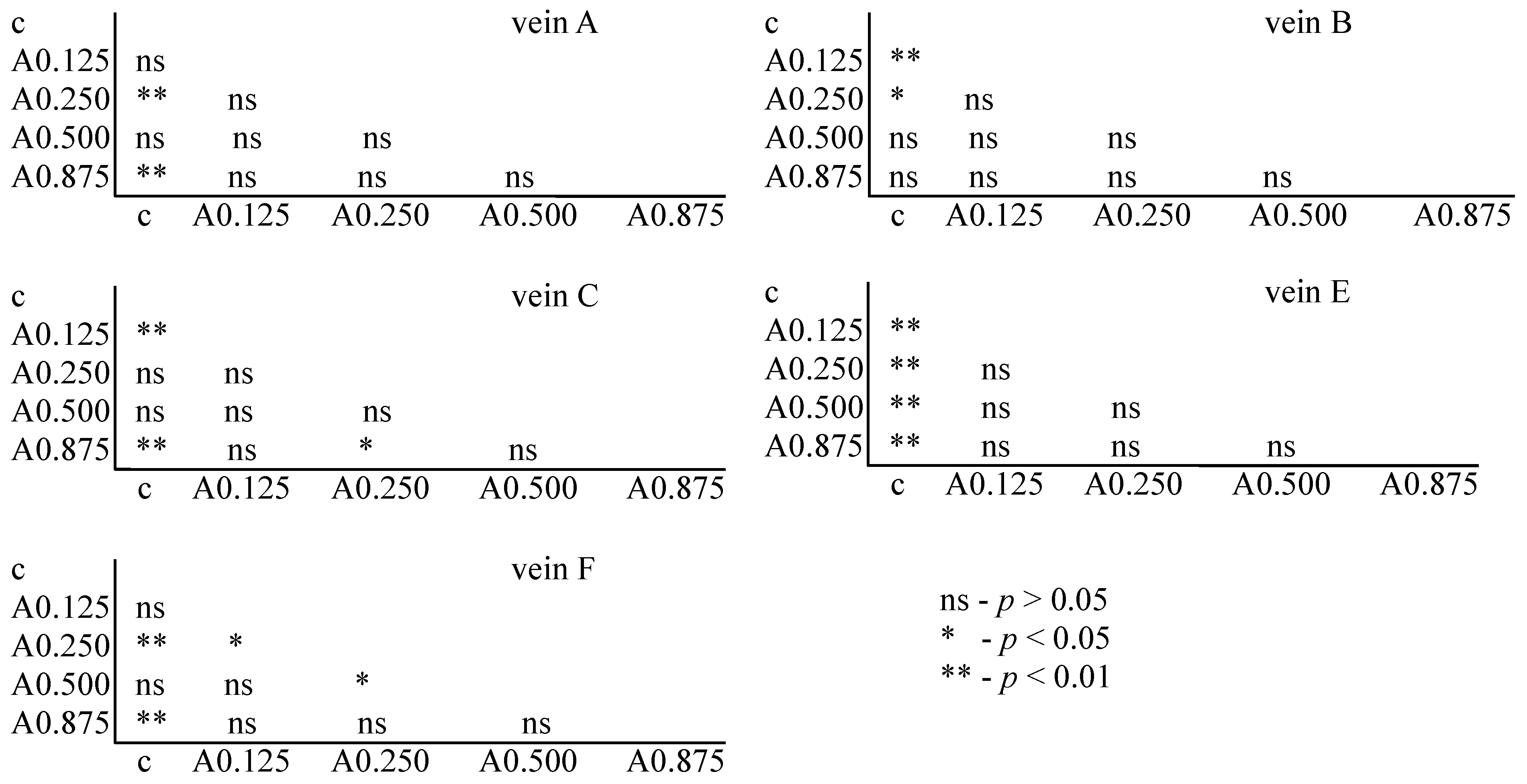Simple Summary
Long-term exposure to low concentrations of insecticides can cause several adverse effects, both on target insects and on other organisms. Low doses can also generate tolerance in surviving offspring. One simple method to detect the consequences of long-term exposure to residual toxins is to measure deviations from ideal body symmetry. Fluctuating asymmetry (FA) is evidence that insects were stressed during development. This study aimed to verify FA in the wing veins of Drosophila suzukii, a fruit pest controlled by the insecticide acetamiprid. To determine whether low doses of insecticide in the diet induce the asymmetry effect, multigenerational insect breeding was carried out on media supplemented with different concentrations of two insecticides. Nicotine was used as a positive control. Even in the first generation, low doses reduced fertility and caused vein asymmetry. This effect persisted in subsequent generations, indicating a lack of tolerance that led to complete insect death after 10 generations. D. suzukii proved extremely sensitive to acetamiprid, and FA is a good index of this sensitivity.
Abstract
Long-term exposure to low concentrations of toxic substances can cause several adverse consequences ranging from molecular to morphological. Sublethal doses may also lead to increased tolerance in the offspring of surviving individuals. One of the consequences of such stress is deviations from the ideal body symmetry during development, reflected by increased levels of fluctuating asymmetry (FA). This research aimed to verify FA in the wing veins of insects belonging to the Drosophilidae family—Drosophila suzukii, a fruit pest controlled by the insecticide acetamiprid, a neonicotinoid. To determine whether FA varied depending on insecticides present in the diet, multigenerational cultures of D. suzukii were carried out on media supplemented with different concentrations (below the LC50) of two insecticides. Nicotine was used as a positive control. Fecundity decreased, the number of insects decreased, and breeding did not continue beyond the tenth generation. However, the FA level at different concentrations was similar, and high FA values were observed even at lower acetamiprid concentrations. We did not see significant changes in FA levels in subsequent generations. D. suzukii proved extremely sensitive to acetamiprid, and FA is a good index of this sensitivity.
1. Introduction
All organisms are constantly exposed to harmful substances present in the environment. The rapid development of agriculture obliges the production of a wide variety of pesticides, raising many questions regarding their safety and potential health and environmental risks. Often, pesticide residues that have been partially decomposed or diluted accumulate in soil, water, and plants in low doses [1,2,3]. In this way, they can directly or indirectly affect vertebrates and invertebrates. The best-known example of the negative impact of such substances on insects is pollinating insects. Bees can absorb pesticides from various sources, including food, direct contact with contaminated surfaces, inhalation, and exposure to water containing dissolved toxins. In most of these cases, insecticide concentrations are low, and their effects range from negligible to lethal [4,5,6,7,8,9,10].
One of the insects exposed to contact with insecticides at low concentrations is the vinegar fly, Drosophila suzukii (Matsumura, 1931), Diptera: Drosophilidae, also called the spotted wing Drosophila (SWD). It is a polyphagous fruit pest from Southeast Asia [11]. This species spread to Europe, Africa and Oceania [12]. This invasive species lays its eggs in intact fruits using its sclerotized ovipositor. Direct damage consists of larvae feeding on the pericarp, and secondary damage is caused by pathogens that penetrate the fruit, causing faster rotting and economic depreciation [13].
This pest is difficult to control, and many factors can expose its populations to sublethal doses of insecticides. One of these insecticides is acetamiprid (ACE (N-(6-chloropyridin-3-yl) methyl)-N′-cyano-N-methylethanimidamide), a neonicotinoid. Neonicotinoids are a class of insecticides that have a strong affinity for insect receptors, so it is believed that they are safe for other organisms [14]. Unfortunately, research shows that they can negatively affect other vertebrates, including mammals [14,15,16]. Neonicotinoids have a structure similar to nicotine; they are highly effective pesticides but are currently banned in Europe and the USA. (In this study, we used nicotine as a positive sample.) Like nicotine, neonicotinoids are agonists of nicotinic acetylcholine receptors in the nervous system. There is considerable concern about the bioavailability of neonicotinoids in the environment and the possible exposure of non-target organisms to insecticide residues, which have been detected at low concentrations in, e.g., pollen, nectar, soil, and water [17,18].
Sublethal effects are important for D. suzukii because its small size, feeding method, short development cycle, and use of different plant species as hosts mean that insecticides have a limited effect. Low doses of harmful substances can negatively affect the organism, especially in cases of long-term exposure [19,20]. This concerns various factors, such as survival, development, reproduction, learning ability, and behavior [4,21,22,23]. One method of determining whether chronic exposure to low concentrations of acetamiprid in food has negative effects is describing biomarkers of environmental stress such as fluctuating asymmetry (FA), a measure of developmental instability. The FA index reflects the degree of deviations between sides regarding the size of a given feature. FA is present when external stressors disrupt developmental processes that normally promote symmetrical growth. This is a non-directional, random, small deviation from perfect symmetry [24,25,26]. Among other insects, the emergence of FA under the influence of various factors has been demonstrated in D. melanogaster exposed to the antibiotic neomycin [27]; in D. ananassae as a result of nutritional stress [28]; and in D. buzzatii and D. koepferae raised on alternative breeding substrates [29]. Thus, we decided to determine to what extent it can be used to study D. suzukii. We aimed to test [i] whether exposing insects to sublethal doses of acetamiprid and nicotine in the diet causes deviations from wing symmetry; [ii] whether FA correlates with increasing concentrations of toxic substances; and [iii] whether there are FA changes in subsequent generations of insects compared with the F1 generation; in other words, can adaptation to these insecticides be observed?
2. Materials and Methods
2.1. SWD Colony and Experimental Design
A D. suzukii colony was established from wild individuals collected in 2019 from infested fruit growing (sweet cherries) near Wrzesnia in central Poland (N 52°18′56.931″ E 17°27′47.136″). The insects were reared on a modified sugar/yeast diet as described previously [30], in standard conditions: 23 ± 2 °C, under a 12:12 light-dark cycle, at 60% humidity. After optimizing rearing conditions, 5 virgin females and males were transferred to vials containing diet supplemented with sublethal toxin doses. After 72 h, the parent insects (P) were removed. The new, first generation of insects (definitely over 100 individuals in each bottle) was partly intended for analysis (30 individuals), and the rest was poured into new bottles with media so that the females would lay eggs and after 3 days poured out and disposed of. The new, second generation (three-day imago) was poured onto fresh diet with the tested substances, and the procedure was analogous until the tenth generation. Five replicates were performed for each concentration in seven independent experiments. Control diets without nicotine (N) and acetamiprid (A) were conducted in parallel. N and A were supplied by Sigma-Aldrich (St. Louis, MO, USA). To assess survival, emerged flies were counted on day 14 after oviposition. To calculate the percentage survival and sublethal doses in transgenerational studies, 50% survival percentage (SP50) was calculated as 50% of the total eggs that developed into adult flies. Dose-response tests were performed for acetamiprid and nicotine. To select insecticide doses for further analysis, acetamiprid was added to the medium at concentrations of 0.125, 0.25, 0.50, 0.875, and 1.0 μL/mL, and nicotine was added at concentrations of 0.05, 0.1, 0.15, 0.2, 0.3, 0.4, and 0.5 mg/mL. From the populations reared on standard medium, 100 Drosophila suzukii eggs were collected using a stereomicroscope and placed on a medium consisting of nicotine, acetamiprid, and standard medium. To assess survival, the number of hatched flies was determined. Three sublethal concentrations of acetamiprid were selected: 0.125, 0.250, and 0.500 µg/mL. We also used 0.875, which is above SP50 doses. Two concentrations of nicotine were used as positive controls (sublethal, 0.1 mg/mL; above SP50 doses, 0.2).
2.2. FA Measurement in the Wing Veins
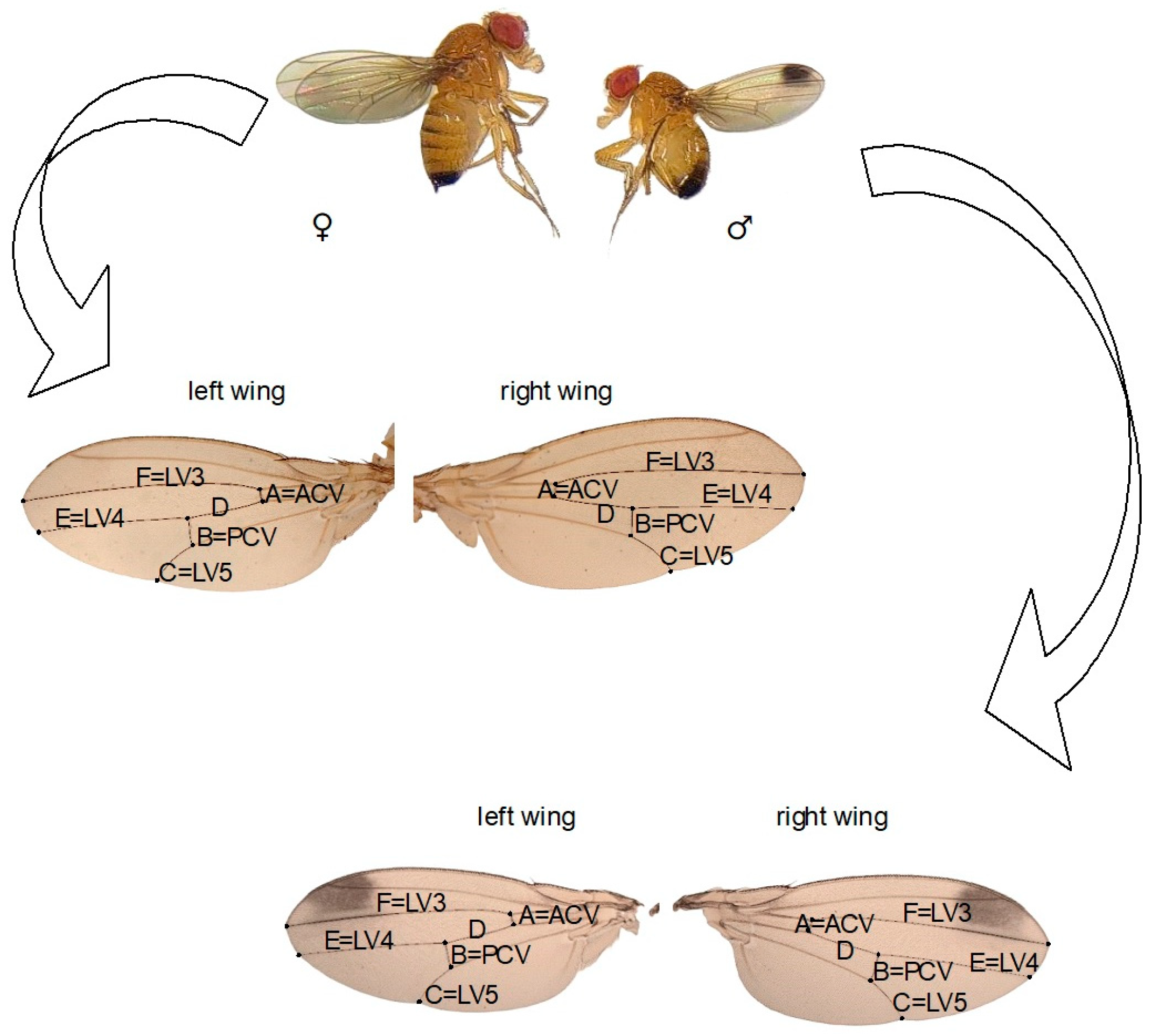
We randomly selected 15 female and 15 male D. suzukii flies (3 days old) reared on a diet with different concentrations of nicotine and acetamiprid from the first (F1), fifth (F5), and tenth (F10) generations. The insects were anesthetized using ether, and their wings were removed, placed on a slide, mounted in Euparal, and covered with a coverslip. Measurements of the veins of the left and right wings were taken under a microscope at 4× magnification using the CellSens software (Olympus Co., Tokyo, Japan). Six veins on the wing, marked A-F (Figure 1), were measured three times to reduce the risk of errors. FA was defined as the absolute difference between the veins of the right and left wings, standardized by the mean.
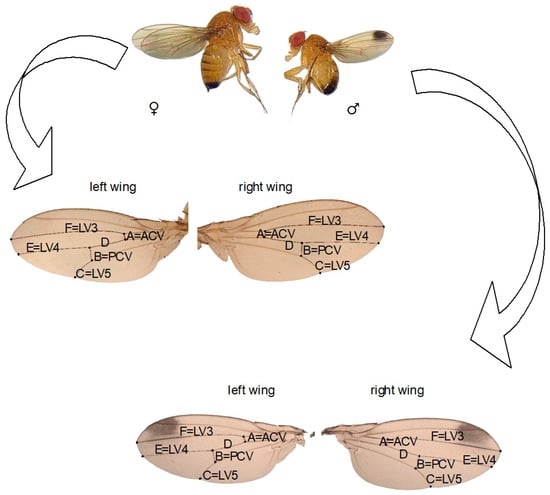
Figure 1.
Drosophila suzukii adult. Right and left wings of a male and a female; from A to F, the measured veins are marked (LV 3,4,5 = longitudinal vein 3,4,5; PCV = posterior cross vein; ACV = anterior cross vein).
2.3. Statistical Analysis
Data were statistically analyzed using StatSoft Statistica (StatSoft Inc., Tulsa, OK, USA). The following statistics were calculated for vein length: arithmetic mean, minimum, maximum, standard deviation, and coefficient of variation. To determine the coefficient of FA, basic statistics, analysis of variance (ANOVA), the Shapiro–Wilk test, the Tukey test to check statistical significance, principal component analysis, and a dendrogram were calculated. Triplicate measurements of vein lengths were performed (90 measurements for each vein, A to F, from each of the six test concentrations plus the control, for generations F1, F5, and F10; F5—without A0.875 µg/mL, F10—without acetamiprid). The Kruskal–Wallis test determined the difference between triplicate wing measurements.
3. Results
The first results indicated that D. suzukii was so sensitive to acetamiprid that it could not be continued to the tenth generation, although this attempt was made three times. In generation F5, no adults survived at a concentration of 0.875. In generation F10, at concentrations of 0.125, 0.250, and 0.500, only two to five adults were recorded, so proper analyses could not be performed.
3.1. Vein Length
The absolute difference in the lengths of both veins yielded similar results, indicating high precision. Since there was no significant difference between replicates (p > 0.05), the data were combined. Acetamiprid and nicotine affected the average length of D. suzukii wing veins in F1. All veins except anterior cross-vein A were the longest in the control samples. In the remaining experiments, adding insecticides reduced vein length. No correlation was observed between the reduced length of the veins and the concentration of the tested toxic substances (Table 1).

Table 1.
Descriptive statistics for five studied veins in Drosophila suzukii wings. Yellow indicates the highest average vein length, and green indicates the lowest.
3.2. Fluctuating Asymmetry
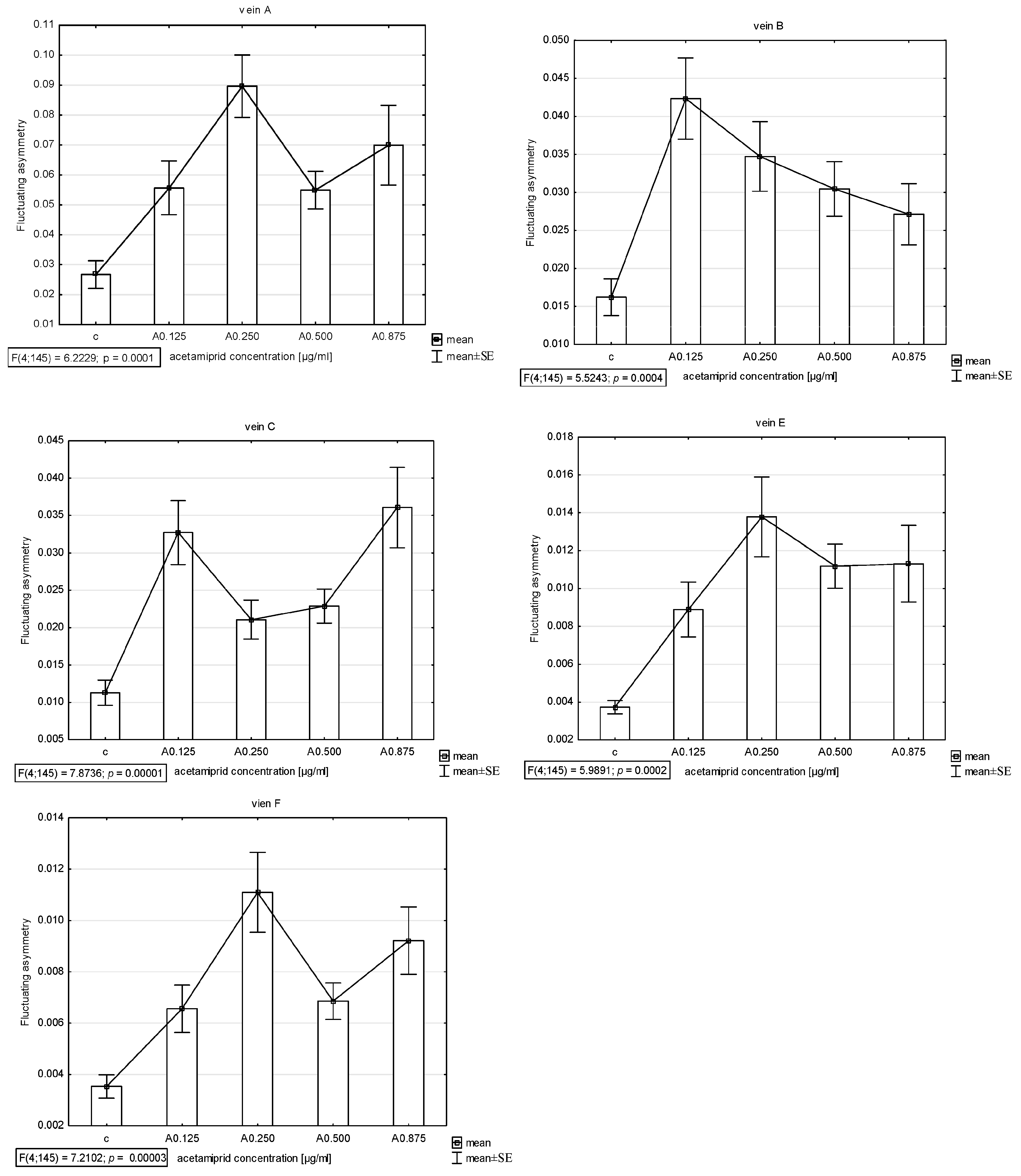
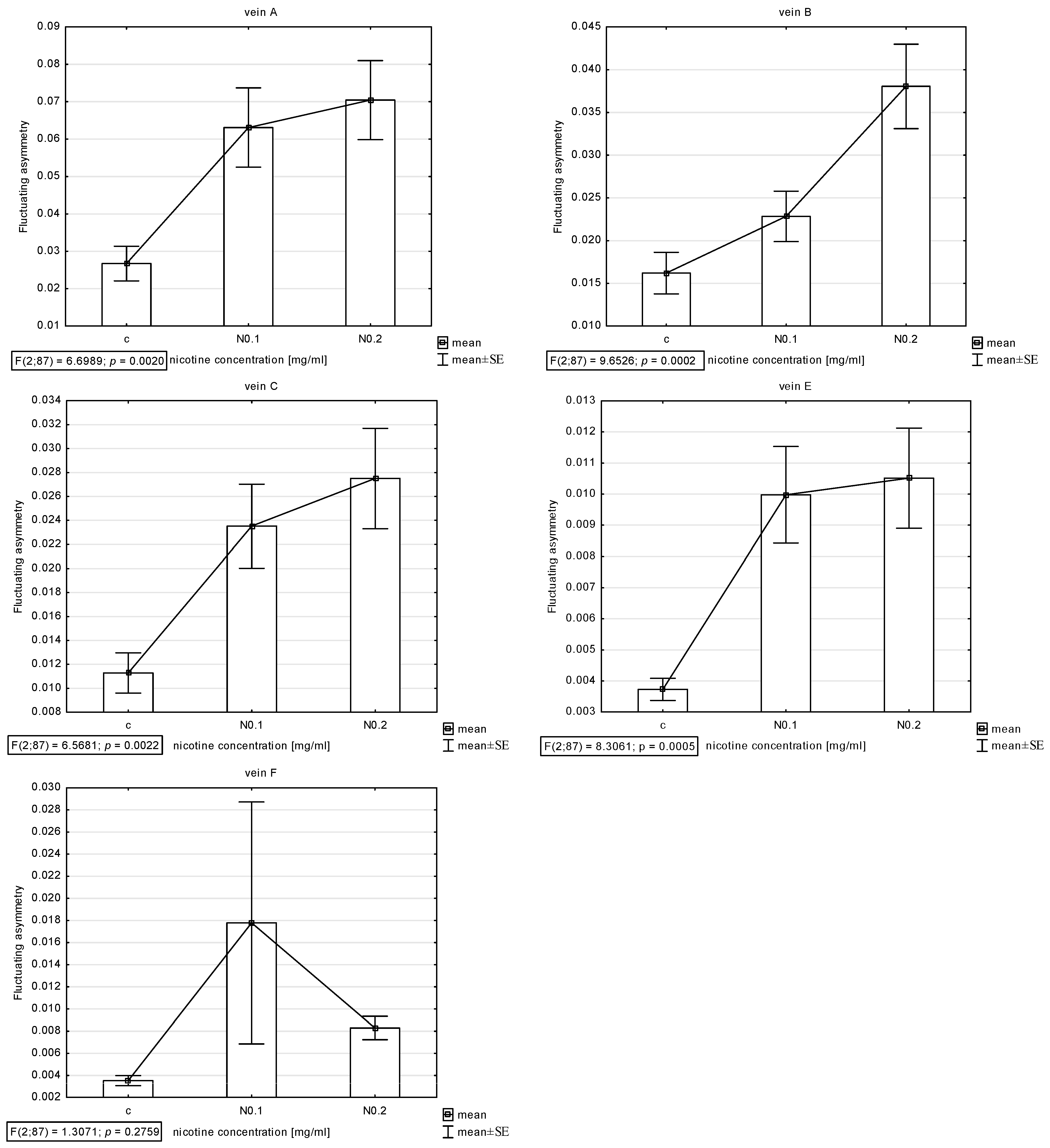
The presence of asymmetry was confirmed via an ANOVA examining the differences in length between longer and shorter veins in pairs in all veins (A, B, C, E, and F) except D, which was excluded from further analyses. The Shapiro–Wilk test showed that the FA index distribution was normal. Thus, the descriptive statistics were calculated. The highest FA for vein A was noted for acetamiprid at a concentration of 0.250 µg/mL and nicotine at a concentration of 0.2 mg/mL; for vein B, the highest FA was for acetamiprid at 0.125 µg/mL and nicotine at 0.2 mg/mL; for vein C, the highest was for acetamiprid at 0.875 µg/mL and nicotine at 0.2 mg/mL; for vein E, the highest was for acetamiprid at 0.250 µg/mL and nicotine at 0.2 mg/mL; for vein F, the highest was for acetamiprid at 0.250 µg/mL and nicotine at 0.1 mg/mL (Table 2; Figure 2 and Figure 3).

Table 2.
Descriptive statistics for fluctuating asymmetry in five examined F1 Drosophila suzukii wing veins. Blue indicates the highest mean FA observed for acetamiprid, and green indicates the highest mean FA observed for nicotine.
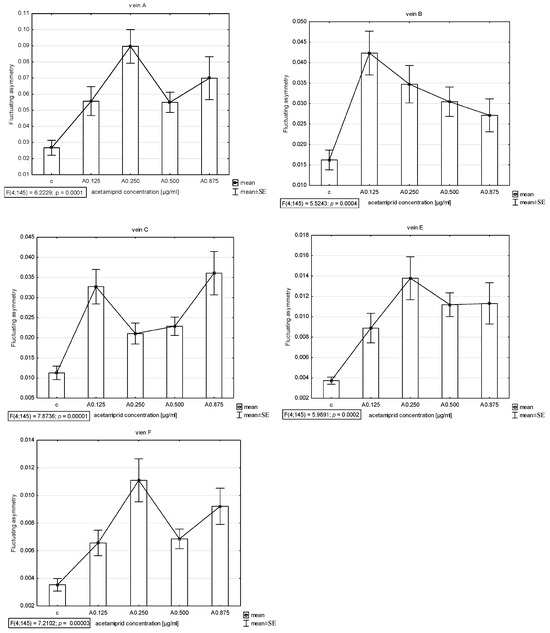
Figure 2.
Graph of mean and standard error (SE) of fluctuating asymmetry for five studied veins (A, B, C, E, F) for four different concentrations of acetamiprid (0.125, 0.250, 0.500, and 0.875 µg/mL) and control. ANOVA (F) and p-value calculated.
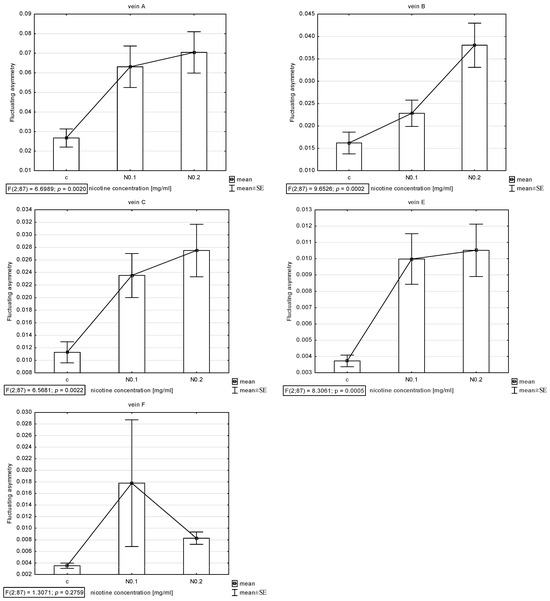
Figure 3.
Graph of mean and standard error (SE) of fluctuating asymmetry for five studied veins (A, B, C, E, F) for two concentrations of nicotine (N: 0.1 and 0.2 mg/mL). ANOVA (F) and p-value calculated.
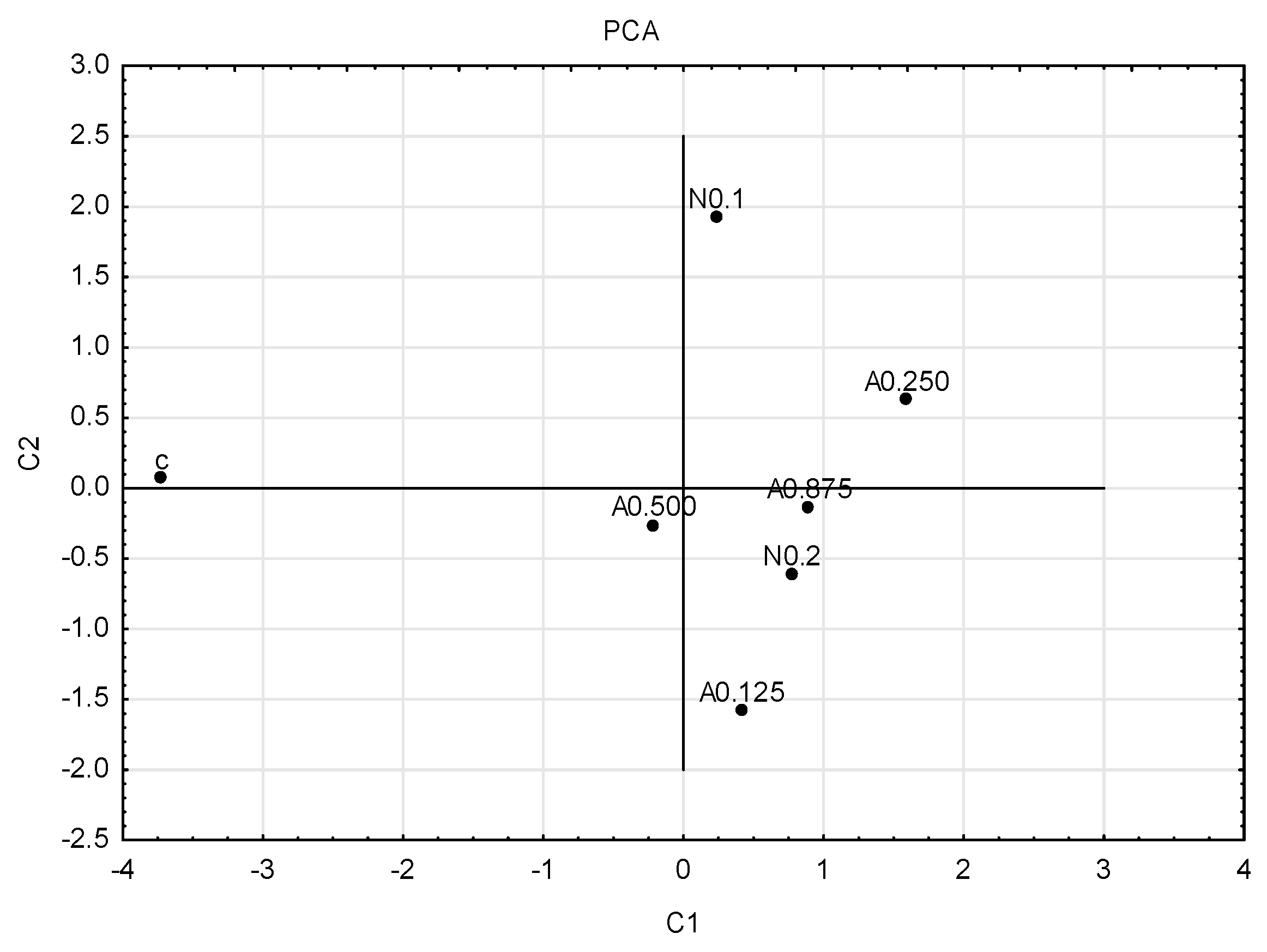
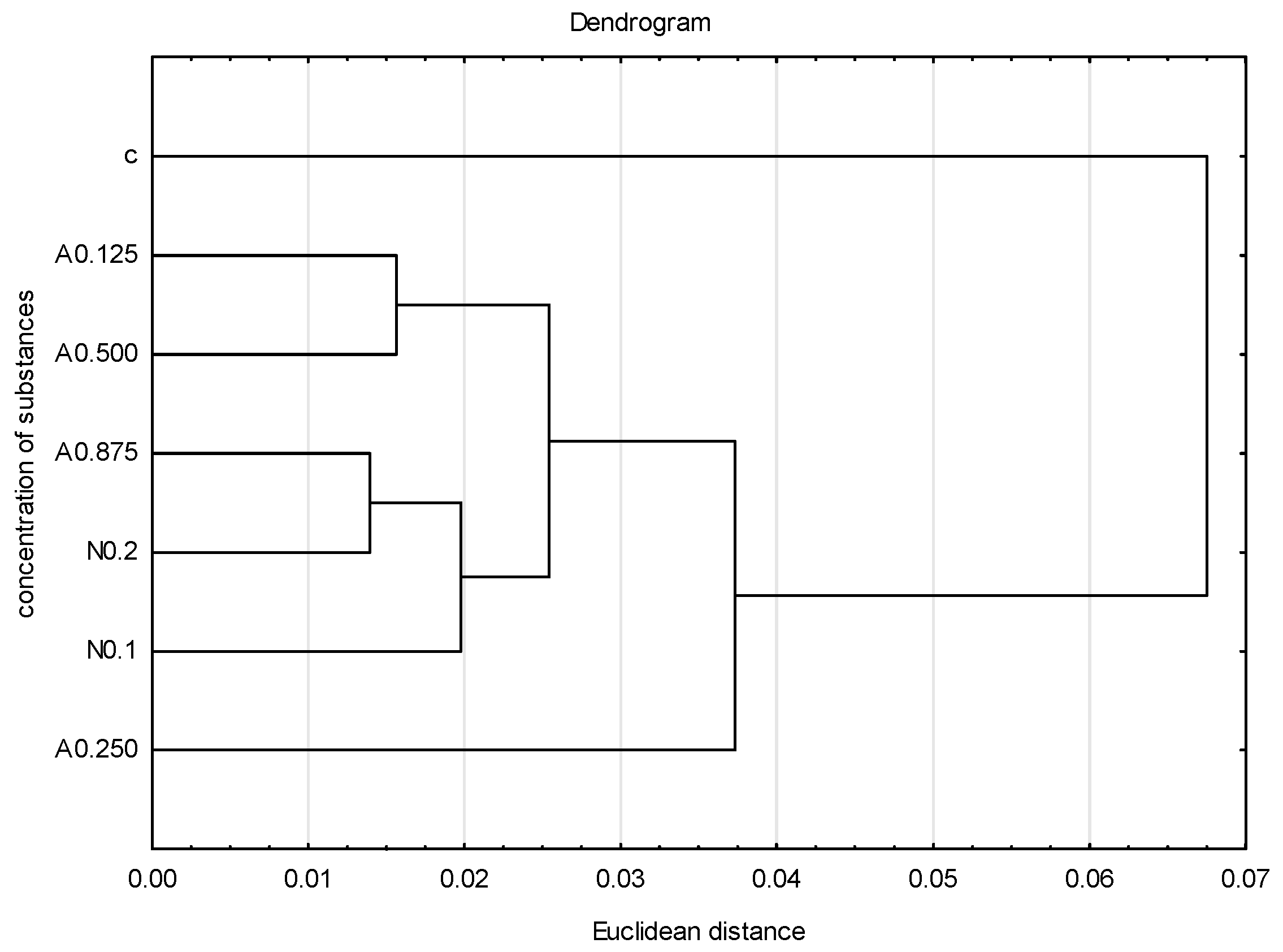
To graphically illustrate the obtained results, principal component analysis (PCA) (Figure 4) and cluster analysis (dendrogram) (Figure 5) were performed. Sublethal concentrations of acetamiprid at 0.125 and 0.500 formed one group, and concentrations of acetamiprid at 0.875 and nicotine at 0.2 (which are above SP50 doses) formed the other. The concentrations of acetamiprid at 0.250 and the sample without toxic substances were the most distinct.
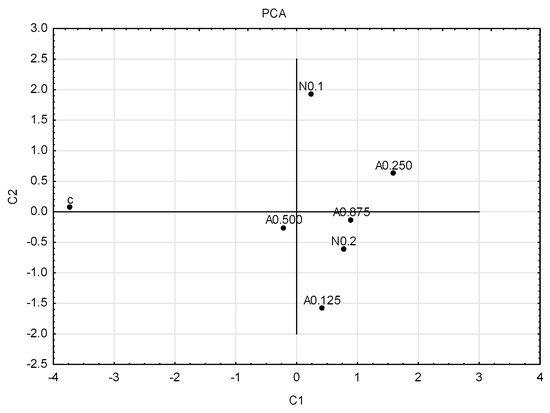
Figure 4.
Graph of principal component analysis (PCA). First generation of insects; for four concentrations of acetamiprid (A = acetamiprid: 0.125, 0.250, 0.500, 0.875 µg/mL), for two concentrations of nicotine (N = nicotine: 0.1 and 0.2 mg/mL), and for the control (c).
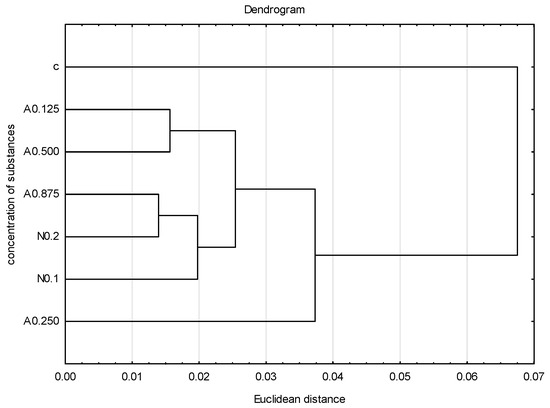
Figure 5.
Dendrogram based on five tested veins (A, B, C, E, F) showing the hierarchical relationships between groups. Groups represent different concentrations of acetamiprid (A = acetamipirid: 0.125, 0.250, 0.500, and 0.875 µg/mL), of nicotine (N = nicotine: 0.1 and 0.2 mg/mL), and the control group (c).
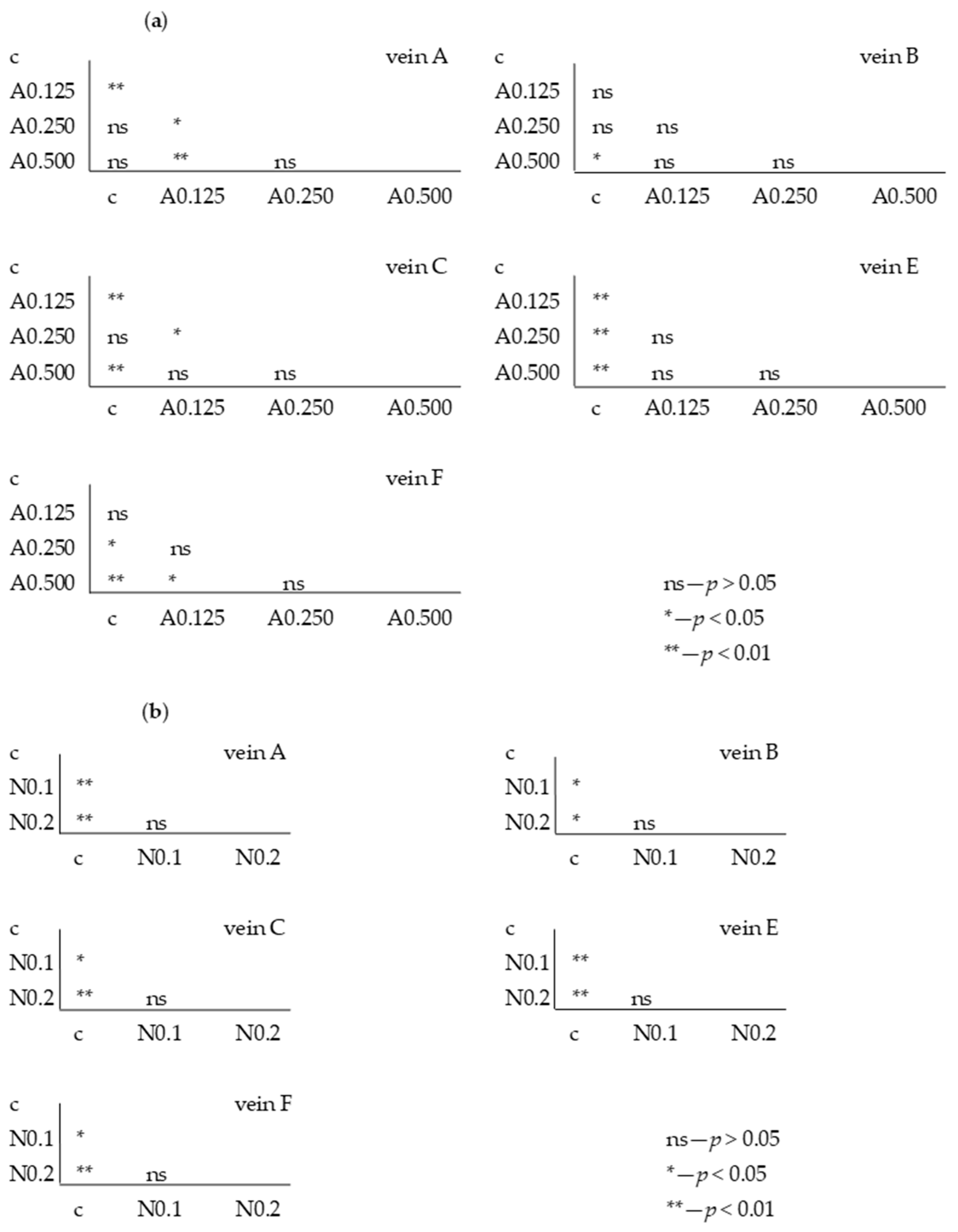
The Tukey test was performed. The test showed that for acetamiprid, significant differences between the control and FA veins were observed: for vein A at concentrations of 0.250 and 0.875 µg/mL; for vein B at concentrations of 0.125 and 0.250 µg/mL; for vein C at concentrations of 0.125 and 0.875 µg/mL; for vein E at all tested concentrations; and for vein F at concentrations of 0.250 and 0.875 µg/mL. In veins C and F, significant differences were also found between the 0.250 and 0.875 µg/mL concentrations and 0.500 and 0.875 µg/mL concentrations, respectively (Figure 6).
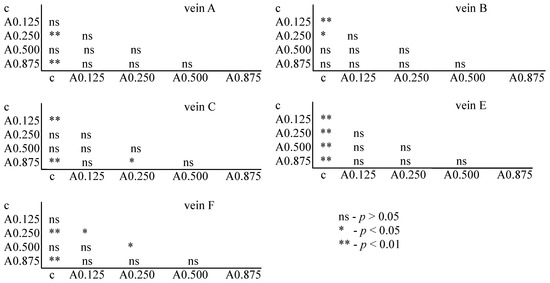
Figure 6.
Tukey test for fluctuating asymmetry between tested acetamiprid concentrations (A = acetamiprid: 0.125, 0.250, 0.500, and 0.875 µg/mL) and control (c) for veins A, B, C, E, and F; p < 0.01 = statistically significant differences, p < 0.005 = statistically significant differences, p > 0.05 = differences statistically insignificant (ns).
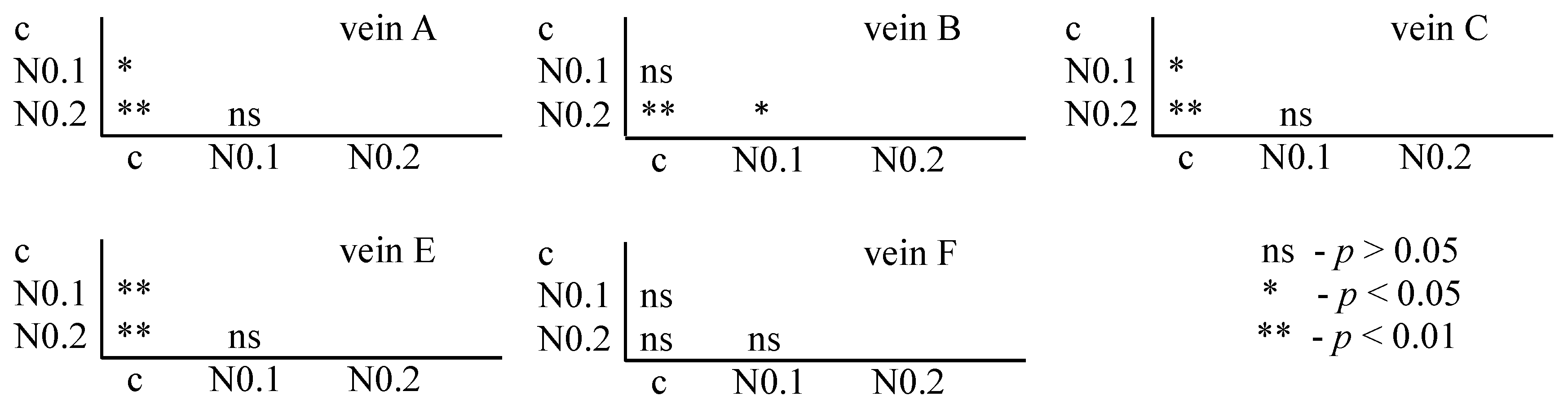
For nicotine, the Tukey test showed significant differences between control and FA veins: vein A at concentrations of 0.1 and 0.2 mg/mL; vein B at a concentration of 0.2 mg/mL; and veins C and E at concentrations of 0.1 and 0.2 mg/mL. In vein B, significant differences were also found between the 0.1 and 0.2 mg/mL concentrations (Figure 7).
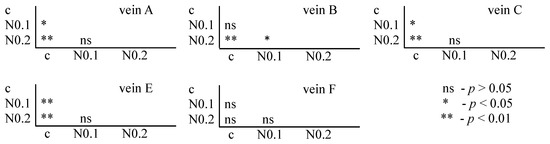
Figure 7.
Tukey test for fluctuating asymmetry between tested nicotine concentrations (N = nicotine: 0.1 and 0.2 mg/mL) and control (c) for veins A, B, C, E, and F; p < 0.01 = statistically significant differences, p < 0.005 = statistically significant differences, p > 0.05 = differences statistically insignificant (ns).
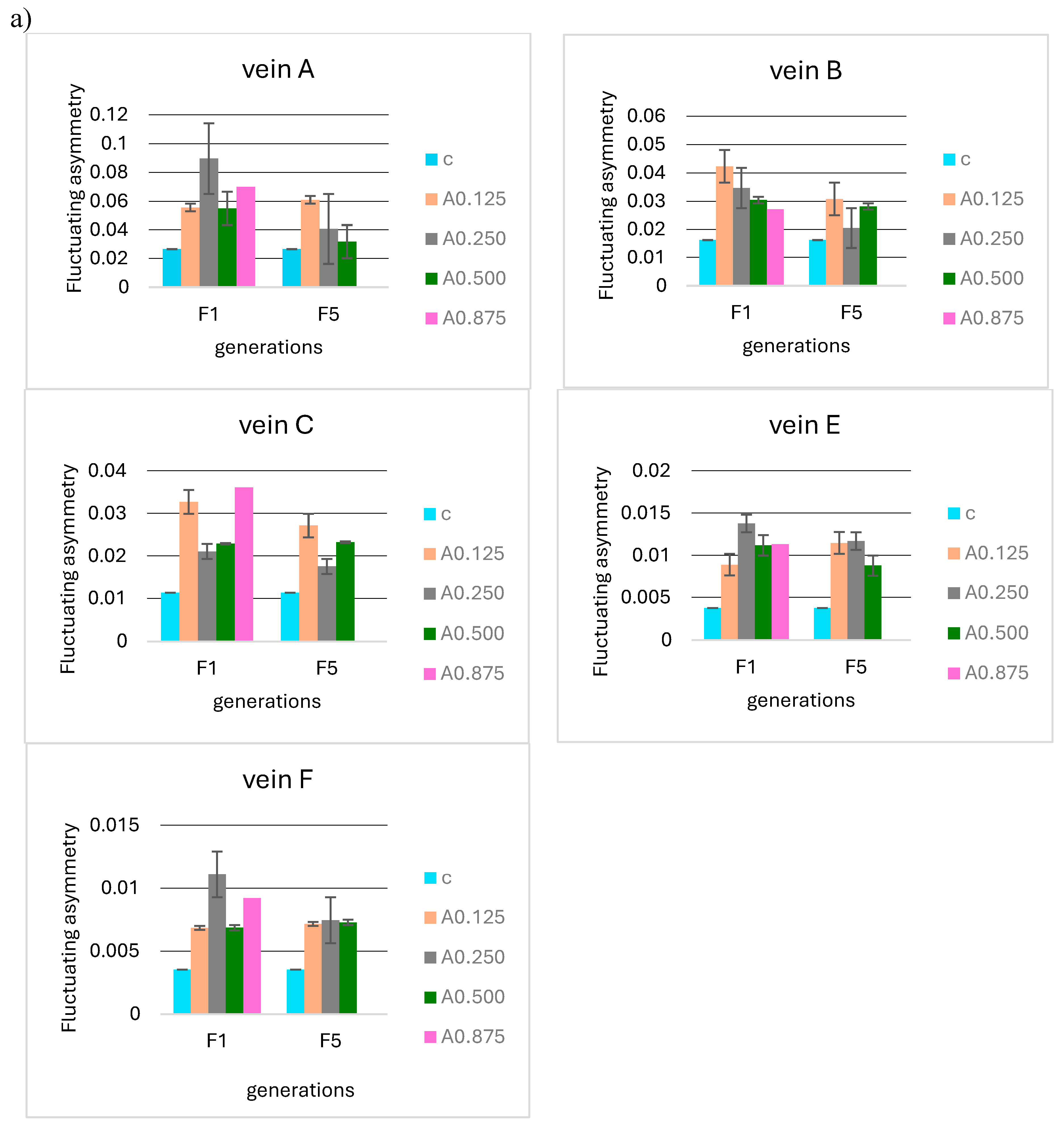
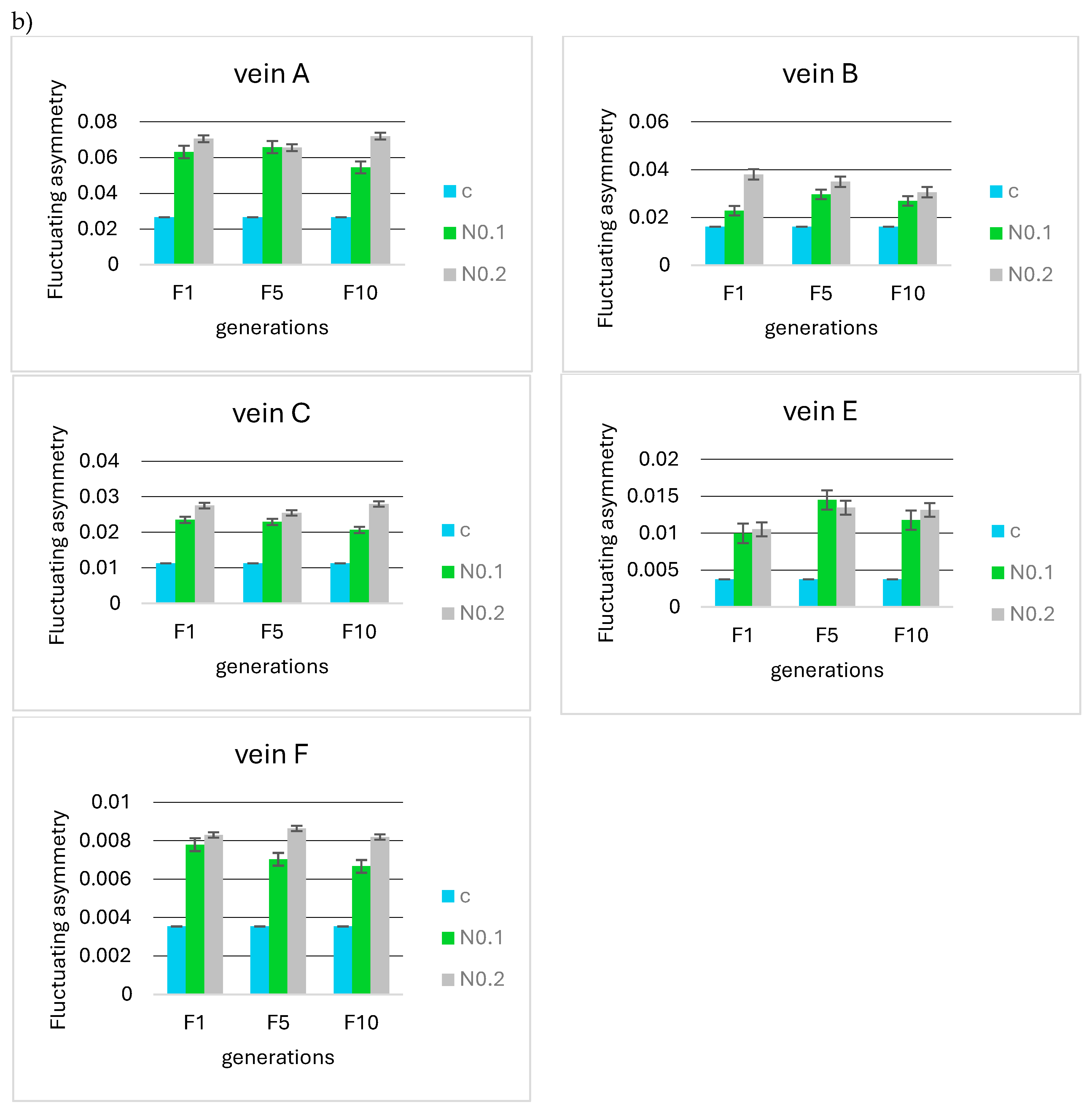
No significant differences were observed when analyzing the F5 and F10 generations in relation to the first generation in terms of the mean FA for 0.125, 0.250, 0.500, and 0.875 µg/mL acetamiprid concentrations (Figure 8a) and 0.1 and 0.2 mg/mL nicotine concentrations (Figure 8b). Fluctuating asymmetry did not increase in subsequent generations and was smaller than in F1 in some cases.
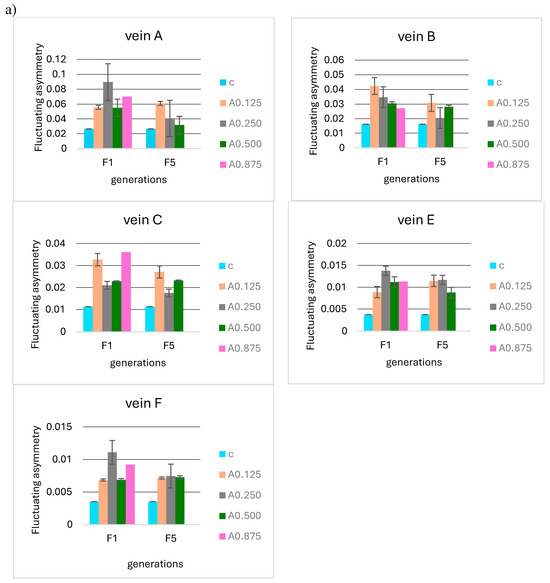
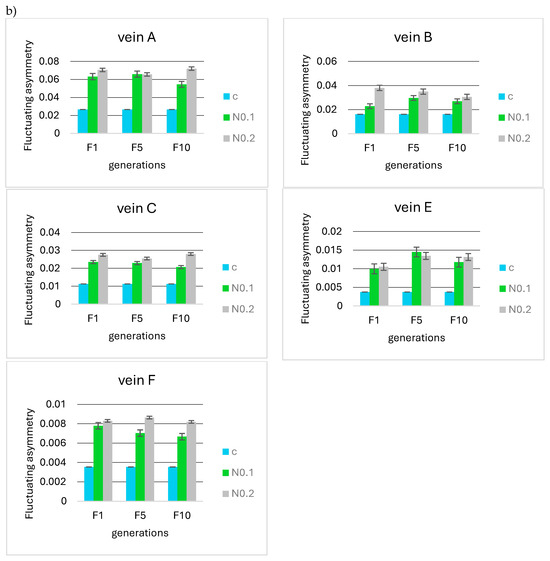
Figure 8.
Mean fluctuating asymmetry of veins A, B, C, E, and F observed in successive insect generations (F1 = first generation, F5 = fifth generation, F10 = tenth generation) for the control (c) and (a) different concentrations of acetamiprid (A0.125, 0.250, 0.500, 0.875 µg/mL) and (b) different concentrations of nicotine (N0.1 and 0.2 mg/mL).
For the fifth generation, the Tukey test was also performed, showing statistically significant differences for individual veins between the control sample in some acetamiprid and nicotine concentrations (Figure 9a); however, these were not significant between F1 and F5.
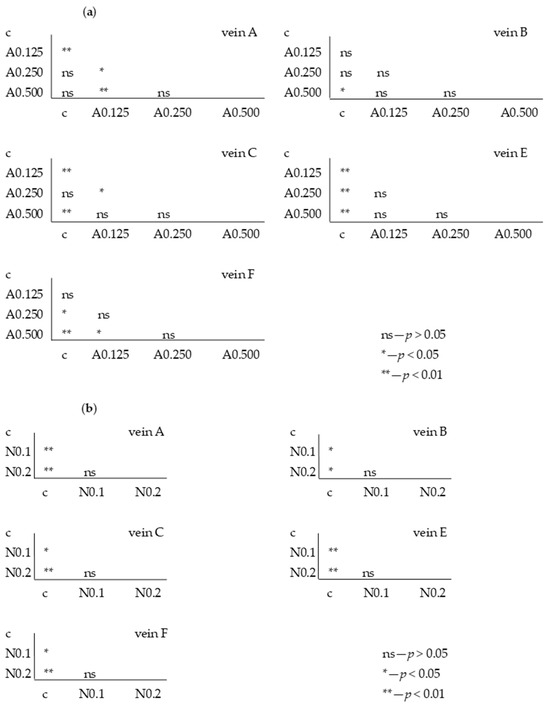
Figure 9.
Tukey test for fluctuating asymmetry between control (c) and tested acetamiprid concentrations (A = acetamiprid: 0.125, 0.250, 0.500, 0.875 µg/mL) (a) and between control (c) and tested nicotine concentrations (N = nicotine: 0.1 and 0.2 mg/mL) (b) for veins A, B, C, E, and F; p < 0.01 = statistically significant differences, p < 0.005 = statistically significant differences, p > 0.05 = differences statistically insignificant (ns); fifth generation.
For nicotine, the Tukey test showed significant differences between the FA of the control and all tested concentrations (Figure 9b), but these were not significant at the intergenerational level.
4. Discussion
Despite the undoubted benefits of reasonable insecticide use, these chemicals can be serious risks to humans or the environment [31]. Neonicotinoids, a new group of insecticides, have become popular in recent years; initially, they were considered a safe alternative to other substances that control pests, but many of them are dangerous and have already been banned. One of the few neonicotinoids currently approved is acetamiprid, a neurotoxin that affects the nervous system of insects and is used to control SWD, an invasive fruit pest. Despite renewed approval from the Commission Implementing Regulation, this substance has been detected in residues in many products, possibly posing previously unrecognized risks [32]. The EFSA recommends updating its residue limits based on new information on the risks involved and continuing research. We decided to use SWD to study the effects of sublethal doses of acetamiprid. Acetamiprid is classified as harmful to SWD parasitoids because it causes rapid mortality and reduced survival time in offspring and does not show parasitism [33]. As a positive control with a documented harmful effect on Drosophila, we used nicotine [23,34]. In earlier reports, we confirmed that in low doses, acetamiprid adversely affects D. suzukii development and reproduction, and we were interested in whether it similarly affects another bioindicator of stress, fluctuating asymmetry.
This small, random deviation from ideal symmetry measures developmental instability, a sensitive biomarker of environmental stress [24,25,26,35]. Higher FA has been proved to be a measure of susceptibility to pesticides in, for example, lizards [36], frogs [37], birds [38], fish [39,40], and invertebrates: Odonata [41], Apis [42], Triatoma [43,44], carabid beetles [45], and Drosophila [46,47].
FA in locomotor features may be particularly important in the fitness context; therefore, we decided to study wings—or more precisely, their veins—to determine whether long-term exposure to various sublethal acetamiprid and nicotine concentrations throughout the developmental period would cause asymmetry. Studies conducted on D. melanogaster have shown FA in wings under the influence of the antibiotic neomycin [27], but not all veins have shown FA, and it has not always been precisely correlated with increased antibiotic concentrations. In our studies, five of the six analyzed veins showed differences between the right and left wings under the influence of sublethal doses of insecticides, classified as FA after statistical analysis. Compared with the control, the differences were statistically significant and correlated with increased insecticide concentrations in most cases. The average wing vein length was smaller in the insects exposed to toxins than in the controls. Similar results have been reported for D. melanogaster by [27,30,48]. Their studies showed that both acetamiprid and nicotine, even in exceedingly small doses, are a strong controlling factor for SWD, disturbing developmental stability. Importantly, we can relate the results of our analyses to other studies on the effects of the same concentrations of acetamiprid and nicotine [23] and link the appearance of FA to locomotor dysfunction occurring under the influence of the tested toxins. This is a significant result. In subsequent generations of SWD, we did not observe major differences in the FA index. The results were similar, possibly indicating that insects do not acquire resistance to this pesticide. The reduced number of flies in subsequent generations indicates high acetamiprid activity even at low doses; with each subsequent generation, the number of insects exposed to insecticides decreased compared with the control culture. Acetamiprid had a greater effect on the decreasing numbers than nicotine used in higher concentrations. This also shows the validity of using FA as an environmental stress indicator. Exposure to low doses of insecticide and their influence on insects have been observed in many different experiments. Among sublethal effects, one can mention modification of antioxidant enzymes, altered neurotransmission, especially inhibited acetylcholinesterase activity, or structural aberrations [49]. Research on nicotine showed that sublethal effects, like reduced vitality or fertility, due to physiological malfunctions and structural malformations, may significantly decrease the fitness of flies [30]. A major problem with the widespread use of insecticides is the emergence of resistance. This enlarges the toxic effects of pesticides on the environment and leads to an increase in the amount of insecticides required to effectively control resistant insects. In the case of acetamiprid, we did not observe the acquisition of resistance expressed in fluctuating asymmetry. On the one hand, this confirms the effectiveness of this pesticide in controlling SWD even when used at low doses over several generations. On the other hand, attention should be paid to the risk of harmful effects of low doses in case of interactions with non-target organisms, including humans. This is especially true since small amounts of residues of this compound can be found not only in the environment but also in food.
5. Conclusions
The conclusions from our research are as follows:
- Sublethal doses of acetamiprid and nicotine disrupt the developmental stability of Drosophila suzukii, which manifests as fluctuating asymmetry in wing veins.
- Exposing insects to these insecticides throughout development reduces wing vein length, consequently reducing wing size.
- It is likely that persistent FA in subsequent SWD generations affects increased mortality—assuming that more disturbed individuals do not develop from eggs or larvae.
- FA can be used to analyze the effects of acetamiprid even at low, sublethal concentrations.
Author Contributions
Conceptualization, A.L.-W. and E.M.C. methodology, A.L.-W.; investigation, A.L.-W. and E.M.C.; resources, A.L.-W.; data curation, A.L.-W.; writing—original draft preparation, A.L.-W.; writing—review and editing, E.M.C.; visualization, A.L.-W.; supervision, E.M.C.; project administration, A.L.-W. and E.M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bonmatin, J.-M.; Giorio, C.; Girolami, V.; Goulson, D.; Kreutzweiser, D.; Krupke, C.; Liess, M.; Long, E.; Marzaro, M.; Mitchell, E.A.D.; et al. Environmental fate and exposure; neonicotinoids and fipronil. Environ. Sci. Pollut. Res. Int. 2015, 22, 35–67. [Google Scholar] [CrossRef] [PubMed]
- Botías, C.; David, A.; Horwood, J.; Abdul-Sada, A.; Nicholls, E.; Hill, E.; Goulson, D. Neonicotinoid Residues in Wildflowers, a Potential Route of Chronic Exposure for Bees. Environ. Sci. Technol. 2015, 49, 12731–12740. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, I.; Imadi, S.; Shazadi, K.; Gul, A.; Hakeem, K. Effects of Pesticides on Environment. In Plant, Soil and Microbes; Hakeem, K., Akhtar, M., Abdullah, S., Eds.; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sub-lethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- Aliouane, Y.; El Hassani, A.K.; Gary, V.; Armengaud, C.; Lambin, M.; Gauthier, M. Subchronic exposure of honeybees to sub-lethal doses of pesticides: Effects on behavior. Environ. Toxcicol. Chem. 2009, 28, 113–122. [Google Scholar] [CrossRef]
- Blacquière, T.; Smagghe, G.; van Gestel, C.A.; Mommaerts, V. Neonicotinoids in bees: A review on concentrations, side-effects, and risk assessment. Ecotoxicology 2012, 21, 973–992. [Google Scholar] [CrossRef] [PubMed]
- van der Sluijs, J.P.; Simon-Delso, N.; Goulson, D.; Maxim, L.; Bonmatin, J.-M.; Belzunces, L.P. Neonicotinoids, bee disorders and the sustainability of pollinator services. Curr. Opin. Environ. Sustain. 2013, 5, 293–305. [Google Scholar] [CrossRef]
- Gregorc, A.; Alburaki, M.; Rinderer, N.; Sampson, B.; Knight, P.R.; Karim, S.; Adamczyk, J. Effects of coumaphos and imidacloprid on honeybee (Hymenoptera: Apidae) lifespan and antioxidant gene regulations in laboratory experiments. Sci. Rep. 2018, 8, 15003. [Google Scholar] [CrossRef]
- Serrão, J.E.; Plata-Rueda, A.; Martínez, L.C.; Zanuncio, J.C. Side-effects of pesticides on non-target insects in agriculture: A mini review. Sci. Nat. 2022, 109, 17. [Google Scholar] [CrossRef]
- Bartling, M.-T.; Brandt, A.; Hollert, H.; Vilcinskas, A. Current Insights into Sublethal Effects of Pesticides on Insects. Int. J. Mol. Sci. 2024, 25, 6007. [Google Scholar] [CrossRef]
- Asplen, M.K.; Anfora, G.; Biondi, A.; Choi, D.; Chu, D.; Daane, K.M.; Gibert, P.; Gutierrez, A.P.; Hoelmer, K.A.; Hutchison, W.D.; et al. Invasion biology of spotted wing Drosophila (Drosophila suzukii): A global perspective and future priorities. J. Pest. Sci. 2015, 88, 469–494. [Google Scholar] [CrossRef]
- Garcia, F.R.M.; Lasa, R.; Funes, C.F.; Buzzetti, K. Drosophila suzukii Management in Latin America: Current Status and Perspectives. J. Econ. Entomol. 2022, 115, 1008–1023. [Google Scholar] [CrossRef]
- Bühlmann, I.; Gossner, M.M. Invasive Drosophila suzukii outnumbers native controphics and causes substantial damage to fruits of forest plants. NeoBiota 2022, 77, 39–77. [Google Scholar] [CrossRef]
- Costas-Ferreira, C.; Faro, L.R.F. Neurotoxic Effects of Neonicotinoids on Mammals: What Is There beyond the Activation of Nicotinic Acetylcholine Receptors?—A Systematic Review. Int. J. Mol. Sci. 2021, 22, 8413. [Google Scholar] [CrossRef] [PubMed]
- Zuščíková, L.; Bažány, D.; Greifová, H.; Knížatová, N.; Kováčik, A.; Lukáč, N.; Jambor, T. Screening of Toxic Effects of Neonicotinoid Insecticides with a Focus on Acetamiprid: A Review. Toxics 2023, 11, 598. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.A.; Lehmler, H.-J.; Kolpin, D.W.; Hladik, M.L.; Vargo, J.D.; Schilling, K.E.; LeFevre, G.H.; Peeples, T.L.; Poch, M.C.; LaDuca, L.E.; et al. A Critical Review on the Potential Impacts of Neonicotinoid Insecticide Use: Current Knowledge of Environmental Fate, Toxicity, and Implications for Human Health. Environ. Sci. Process. Impacts 2020, 22, 1315–1346. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Kaur, P.; Gan, J. A critical review on the accumulation of neonicotinoid insecticides in pollen and nectar. Influencing factors and implications for pollinator exposure. Sci. Total Environ. 2023, 899, 165670. [Google Scholar] [CrossRef]
- Wood, T.J.; Goulson, D. The environmental risks of neonicotinoid pesticides: A review of the evidence post 2013. Environ. Sci. Pollut. Res. Int. 2017, 24, 17285–17328. [Google Scholar] [CrossRef]
- Schöneberg, T.; Lewis, M.T.; Burrack, H.J.; Grieshop, M.; Isaacs, R.; Rendon, D.; Rogers, M.; Rothwell, N.; Sial, A.A.; Walton, V.M.; et al. Cultural Control of Drosophila suzukii in Small Fruit-Current and Pending Tactics in the U.S. Insects 2021, 12, 172. [Google Scholar] [CrossRef] [PubMed]
- Shaw, B.; Brain, P.; Wijnen, H.; Fountain, M.T. Implications of sub-lethal rates of insecticides and daily time of application on Drosophila suzukii lifecycle. Crop Prot. 2019, 121, 182–194. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, Z.; Cui, K.; Zhao, Y.; Han, J.; Liu, F.; Mu, W. Effects of Sub-lethal Concentrations of Cyantraniliprole on the Development, Fecundity and Nutritional Physiology of the Black Cutworm Agrotis ipsilon (Lepidoptera: Noctuidae). PLoS ONE 2016, 11, e0156555. [Google Scholar] [CrossRef]
- Brandt, A.; Gorenflo, A.; Siede, R.; Meiner, M.; Buchler, R. The neonicotinoids thiacloprid, imidacloprid, and clothianidin affect the immunocompetence of honeybees (Apis mellifera L.). J. Insect Physiol. 2016, 86, 4–47. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska-Wosik, A.; Chudzińska, E.; Wojnicka-Półtorak, A. Genotoxic effects of sub-lethal doses of nicotine and acetamiprid in neuroblasts of Drosophila melanogaster and Drosophila suzukii. Ecotoxicol. Environ. Saf. 2024, 280, 116585. [Google Scholar] [CrossRef]
- Graham, J.; Raz, S.; Hel-Or, H.; Eviatar, N. Fluctuating Asymmetry: Methods, Theory, and Applications. Symmetry 2010, 2, 466–540. [Google Scholar] [CrossRef]
- Graham, J.H. Fluctuating Asymmetry and Developmental Instability, a Guide to Best Practice. Symmetry 2021, 13, 9. [Google Scholar] [CrossRef]
- De Coster, G.; Van Dongen, S.; Malaki, P.; Muchane, M.; Alcantara-Exposito, A.; Matheve, H.; Lens, L. Fluctuating Asymmetry and Environmental Stress: Understanding the Role of Trait History. PLoS ONE 2013, 8, e57966. [Google Scholar] [CrossRef] [PubMed]
- Büyükgüzel, K.; Büyükgüzel, E.; Chudzińska, E.; Lewandowska-Wosik, A.; Gaj, R.; Adamski, Z. Drosophila melanogaster Response to Feeding with Neomycin-Based Medium Expressed in Fluctuating Asymmetry. Insects 2020, 11, 378. [Google Scholar] [CrossRef]
- Vishalakshi, C.; Singh, B.N. Effect of environmental stress on fluctuating asymmetry in certain morphological traits in Drosophila ananassae: Nutrition and larval crowding. Can. J. Zool. 2008, 86, 427–437. [Google Scholar] [CrossRef]
- Soto, I.M.; Carreira, V.P.; Soto, E.M.; Hasson, E. Wing morphology and fluctuating asymmetry depend on the host plant in cactophilic Drosophila. J. Evol. Biol. 2008, 21, 598–609. [Google Scholar] [CrossRef]
- Chowański, S.Z.; Chudzińska, E.; Lelario, F.; Ventrella, E.; Marciniak, P.; Miądowicz-Kobielska, M.; Spochacz, M.; Szymczak, M.; Scrano, L.; Bufo, S.A.; et al. Insecticidal properties of Solanum nigrum and Armoracia rusticana extracts on reproduction and development of Drosophila melanogaster. Ecotoxicol. Environ. Saf. 2018, 162, 454–463. [Google Scholar] [CrossRef]
- Ahmad, M.F.; Ahmad, F.A.; Alsayegh, A.A.; Zeyaullah, M.; AlShahrani, A.M.; Muzammil, K.; Saati, A.A.; Wahab, S.; Elbendary, E.Y.; Kambal, N.; et al. Pesticides impacts on human health and the environment with their mechanisms of action and possible countermeasures. Heliyon 2024, 10, e29128. [Google Scholar] [CrossRef]
- Hernandez-Jerez, A.; Coja, T.; Paparella, M.; Price, A.; Henri, J.; Focks, A.; Louisse, J.; Terron, A.; Binaglia, M.; Munoz Guajardo, I.; et al. Statement on the toxicological properties and maximum residue levels of acetamiprid and its metabolites. EFSA J. 2024, 22, e8759. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann Schlesener, D.C.; Wollmann, J.; de Bastos Pazini, J.; Costa Padilha, A.; Grützmacher, A.D.; Mello Garcia, F.R. Insecticide Toxicity to Drosophila suzukii (Diptera: Drosophilidae) parasitoids: Trichopria anastrephae (Hymenoptera: Diapriidae) and Pachycrepoideus vindemmiae (Hymenoptera: Pteromalidae). J. Eco. Entomol. 2019, 112, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Velazquez-Ulloa, N.A. A Drosophila model for developmental nicotine exposure. PLoS ONE 2017, 12, e0177710. [Google Scholar] [CrossRef]
- De Beasley, A.E.; Bonisoli-Alquati, A.; Mousseau, T.A. The use of fluctuating asymmetry as a measure of environmentally induced developmental instability: A meta-analysis. Eco. Indic. 2013, 30, 218–226. [Google Scholar] [CrossRef]
- Simbula, G.; Vignoli, L.; Carretero, M.A.; Kaliontzopoulou, A. Fluctuating asymmetry as biomarker of pesticides exposure in the Italian wall lizards (Podarcis siculus). Zoology 2021, 147, 125928. [Google Scholar] [CrossRef]
- Zhelev, Z.; Mollov, I.; Tsonev, S. Application of Fluctuating Asymmetry Values in Pelophylax ridibundus (Amphibia: Anura: Ranidae) Meristic Traits as a Method for Assessing Environmental Quality of Areas with Different Degrees of Urbanization. Diversity 2023, 15, 118. [Google Scholar] [CrossRef]
- Eeva, T.; Tanhuanpää, S.; Råbergh, C.; Airaksinen, S.; Nikinmaa, M.; Lehikoinen, E. Biomarkers and fluctuating asymmetry as indicators of pollution-induced stress in two hole-nesting passerines. Funct. Ecol. 2000, 14, 235–243. [Google Scholar] [CrossRef]
- Allenbach, D.M. Fluctuating asymmetry and exogenous stress in fishes: A review. Rev. Fish Biol. Fish. 2011, 21, 355–376. [Google Scholar] [CrossRef]
- Seixas, L.B.; Gonzalez Neves dos Santos, A.F.; Neves dos Santos, L. Fluctuating asymmetry: A tool for impact assessment on fish populations in a tropical polluted bay, Brazil. Ecol. Indic. 2016, 71, 522–532. [Google Scholar] [CrossRef]
- Chang, X.; Zhai, B.; Liu, X.; Wang, M. Effects of temperature stress and pesticide exposure on fluctuating asymmetry and mortality of Copera annulata (Selys) (Odonata: Zygoptera) larvae. Ecotoxicol. Environ. Saf. 2007, 67, 120–127. [Google Scholar] [CrossRef]
- Nunes, L.A.; Araújo, E.D.D.; Marchini, L.C. Fluctuating asymmetry in Apis mellifera (Hymenoptera: Apidae) as bioindicator of anthropogenic environments. Rev. Biol. Trop. 2015, 63, 673–682. [Google Scholar] [CrossRef]
- Nattero, J.; Piccinali, R.V.; Gaspe, M.S.; Gürtler, R.E. Fluctuating asymmetry and exposure to pyrethroid insecticides in Triatoma infestans populations in northeastern Argentina. Infect. Genet. Evol. 2019, 74, 103925. [Google Scholar] [CrossRef] [PubMed]
- Vilaseca, C.; Pinto, C.F.; Órdenes-Claveria, R.; Laroze, D.; Méndez, M.A.; Benítez, H.A. Insect Fluctuating Asymmetry: An Example in Bolivian Peridomestic Populations of Triatoma infestans (Klug, 1834) (Hemiptera: Reduviidae). Symmetry 2022, 14, 526. [Google Scholar] [CrossRef]
- Ivanković Tatalović, L.; Anđelić, B.; Jelić, M.; Kos, T.; Benítez, H.A.; Šerić Jelaska, L. Fluctuating Asymmetry as a Method of Assessing Environmental Stress in Two Predatory Carabid Species within Mediterranean Agroecosystems. Symmetry 2020, 12, 1890. [Google Scholar] [CrossRef]
- Floate, K.D.; Fox, A.S. Flies understress: A test of fluctuating asymmetry as a biomonitor of environmental quality. Ecol. Appl. 2000, 10, 1541–1550. [Google Scholar] [CrossRef]
- Antipin, M.I.; Imasheva, A.G. Genetic Variability and Fluctuating Asymmetry of Morphological Traits in Drosophila melanogaster Reared on a Pesticide-Containing Medium. Russ. J. Genet. 2001, 37, 247–252. [Google Scholar] [CrossRef]
- Ventrella, E.; Adamski, Z.; Chudzińska, E.; Miądowicz-Kobielska, M.; Marciniak, P.; Büyükgüzel, E.; Erdem, M.; Falabella, P.; Scrano, L.; Bufo, S.A. Solanum tuberosum and Lycopersicon esculentum leaf extracts and single metabolites affect development and reproduction of Drosophila melanogaster. PLoS ONE 2016, 11, e0155958. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Matviishyn, T.M.; Husak, V.V.; Storey, J.M.; Storey, K.B. Pesticide toxicity: A mechanistic approach. EXCLI J. 2018, 17, 1101–1136. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).