Effects of Dietary Zinc Chloride and Zinc Sulfate on Life History Performance and Hemolymph Metabolism of Spodoptera litura (Lepidoptera: Noctuidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Artificial Diet Content and Preparation
2.2. Insects Source and Rearing
2.3. Life Table Analysis
2.4. Extraction and Pretreatment of Metabolite
2.5. UHPLC-MS/MS Analysis
3. Results
3.1. Effects of Dietary ZnCl2 on the Development and Survival of S. litura
3.2. Effects of Dietary ZnCl2 on Longevity and Fecundity of S. litura Adults
3.3. Effects of Dietary ZnCl2 on the Population Parameters of S. litura
3.4. Effect of Dietary ZnSO4 on Life History Performances
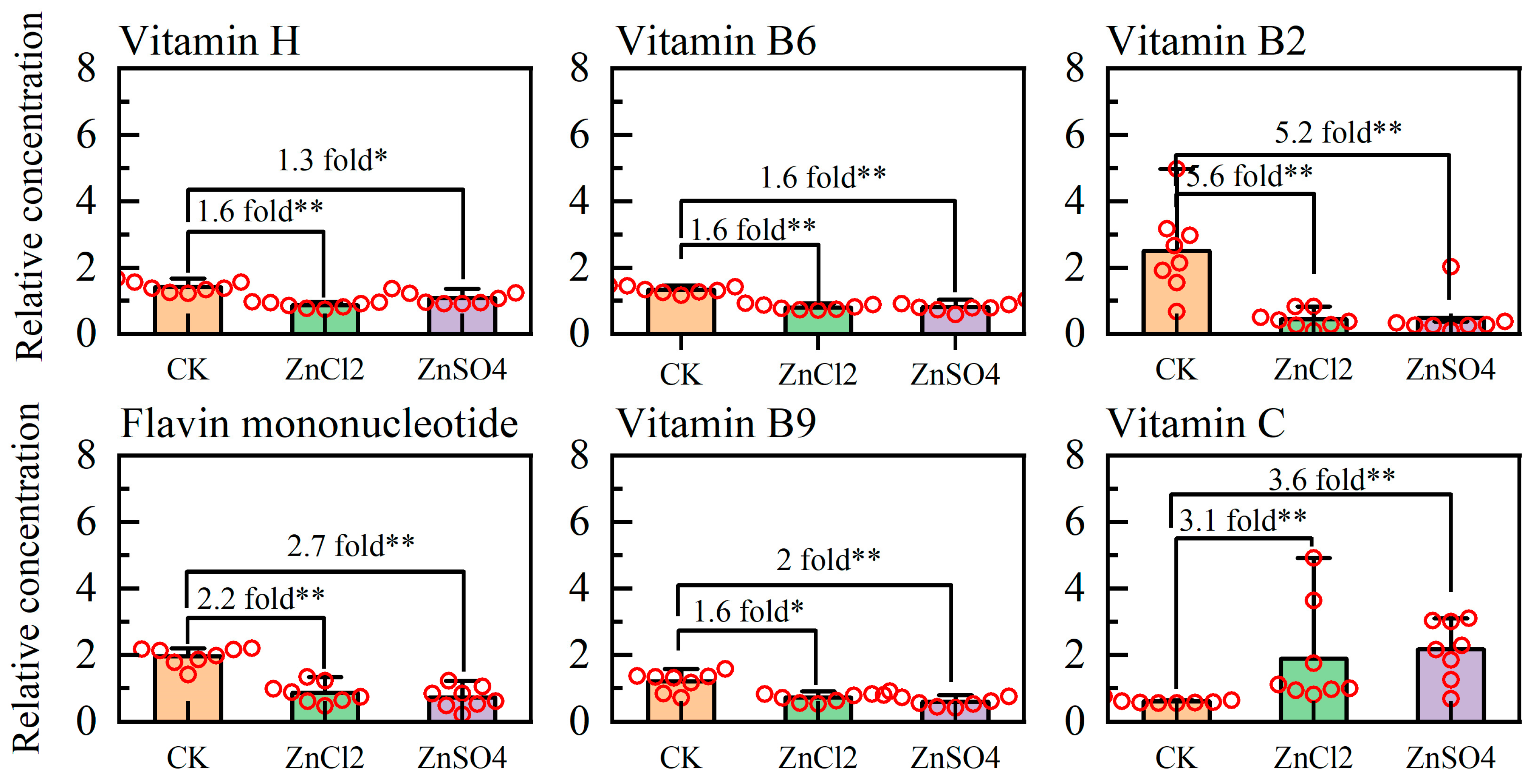
3.5. Metabolic Profiles of S. litura Fed Diets with Different Treatments
3.6. Metabolic Pathway of S. litura Fed Diets with Different Treatments
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Augustyniak, M.; Juchimiuk, J.; Przybyłowicz, W.J.; Mesjasz-Przybyłowicz, J.; Babczyńska, A.; Migula, P. Zinc-induced DNA damage and the distribution of metals in the brain of grasshoppers by the comet assay and micro-PIXE. Comp. Biochem. Phys. C 2006, 144, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kim, E.; Gwag, B.; Sohn, S.; Koh, J.Y. Zinc-induced cortical neuronal death with features of apoptosis and necrosis: Mediation by free radicals. Neuroscience 1999, 89, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Zinc coordination environments in proteins determine zinc functions. J. Trace Elem. Med. Biol. 2005, 19, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Sterenborg, I.; Vork, N.A.; Verkade, S.K.; van Gestel, C.A.; van Straalen, N.M. Dietary zinc reduces uptake but not metallothionein binding and elimination of cadmium in the springtail, Orchesella cincta. Environ. Toxicol. Chem. 2003, 22, 1167–1171. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.T.; Zhen, J.; Leng, J.Y.; Cai, L.; Ji, H.I.; Keller, B.B. Zinc as a countermeasure for cadmium toxicity. Acta Pharmacol. Sin. 2021, 42, 340–346. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Gao, J.; Shahzad, M.; Han, Z.; Wang, Z.; Li, J.; Sjölinder, H. Zinc supplementation protects against cadmium accumulation and cytotoxicity in Madin-Darby bovine kidney cells. PLoS ONE 2014, 9, e103427. [Google Scholar] [CrossRef]
- Montalvo, D.; Degryse, F.; Da Silva, R.; Baird, R.; McLaughlin, M. Agronomic effectiveness of zinc sources as micronutrient fertilizer. Adv. Agron. 2016, 139, 215–267. [Google Scholar]
- De Lange, C.; Pluske, J.; Gong, J.; Nyachoti, C. Strategic use of feed ingredients and feed additives to stimulate gut health and development in young pigs. Livest. Sci. 2010, 134, 124–134. [Google Scholar] [CrossRef]
- Shu, Y.; Zhang, G.; Wang, J. Response of the common cutworm Spodoptera litura to zinc stress: Zn accumulation, metallothionein and cell ultrastructure of the midgut. Sci. Total Environ. 2012, 438, 210–217. [Google Scholar] [CrossRef]
- Zirpel, L.; Parks, T.N. Zinc inhibition of group I mGluR-mediated calcium homeostasis in auditory neurons. JARO-J. Assoc. Res. Otolaryngol. 2001, 2, 180. [Google Scholar] [CrossRef]
- Garay, E.; Schuth, N.; Barbanente, A.; Tejeda-Guzmán, C.; Vitone, D.; Osorio, B.; Clark, A.H.; Nachtegaal, M.; Haumann, M.; Dau, H. Tryptophan regulates Drosophila zinc stores. Proc. Natl. Acad. Sci. USA 2022, 119, e2117807119. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xin, Z.Z.; Song, J.; Zhu, X.Y.; Liu, Q.N.; Zhang, D.Z.; Tang, B.P.; Zhou, C.L.; Dai, L.S. Transcriptome analysis reveals potential antioxidant defense mechanisms in Antheraea pernyi in response to zinc stress. J. Agric. Food Chem. 2018, 66, 8132–8141. [Google Scholar] [CrossRef]
- Jiang, D.; Dong, X.W.; Yan, S.C. Heavy metal accumulation/excretion in and food utilization of Lymantria dispar larvae fed with Zn-or Pb-stressed Populus alba berolinensis leaves. Environ. Entomol. 2018, 47, 1329–1336. [Google Scholar] [CrossRef]
- Kafel, A.; Rozpędek, K.; Szulińska, E.; Zawisza-Raszka, A.; Migula, P. The effects of cadmium or zinc multigenerational exposure on metal tolerance of Spodoptera exigua (Lepidoptera: Noctuidae). Environ. Sci. Pollut. Res. 2014, 21, 4705–4715. [Google Scholar] [CrossRef] [PubMed]
- Belon, E.; Boisson, M.; Deportes, I.; Eglin, T.; Feix, I.; Bispo, A.; Galsomies, L.; Leblond, S.; Guellier, C. An inventory of trace elements inputs to French agricultural soils. Sci. Total Environ. 2012, 439, 87–95. [Google Scholar] [CrossRef]
- Hejna, M.; Gottardo, D.; Baldi, A.; Dell’Orto, V.; Cheli, F.; Zaninelli, M.; Rossi, L. Nutritional ecology of heavy metals. Animal 2018, 12, 2156–2170. [Google Scholar] [CrossRef]
- Lidman, J.; Jonsson, M.; Berglund, Å.M. The effect of lead (Pb) and zinc (Zn) contamination on aquatic insect community composition and metamorphosis. Sci. Total Environ. 2020, 734, 139406. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Zou, B.; Li, N.; Li, Z. Heavy metal contamination in soils and food crops around Dabaoshan mine in Guangdong, China: Implication for human health. Environ. Geochem. Health 2009, 31, 707–715. [Google Scholar] [CrossRef]
- Wu, J.; Li, Z.; Xu, Y.; Lou, D. Studies on characteristic of zinc extravagant absorption by vegetables. Chin. J. Soil Sci. 1996, 27, 228–229. [Google Scholar]
- Gul, S.; Naz, A.; Fareed, I.; Khan, A.; Irshad, M. Speciation of heavy metals during co-composting of livestock manure. Pol. J. Chem. Technol. 2015, 17, 19–23. [Google Scholar] [CrossRef]
- Ingelmo, F.; Molina, M.J.; Soriano, M.D.; Gallardo, A.; Lapeña, L. Influence of organic matter transformations on the bioavailability of heavy metals in a sludge based compost. J. Environ. Manag. 2012, 95, S104–S109. [Google Scholar] [CrossRef] [PubMed]
- Jakubus, M.; Dach, J.; Starmans, D. Bioavailability of copper and zinc in pig and cattle slurries. Fresenius Environ. Bull. 2013, 22, 995–1002. [Google Scholar]
- Luo, L.; Ma, Y.; Zhang, S.; Wei, D.; Zhu, Y.-G. An inventory of trace element inputs to agricultural soils in China. J. Environ. Manag. 2009, 90, 2524–2530. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, F.A.; Smith, S.R.; Alloway, B.; Carlton-Smith, C.; Chambers, B. An inventory of heavy metals inputs to agricultural soils in England and Wales. Sci. Total Environ. 2003, 311, 205–219. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.; Yang, M.; Li, W. Content of heavy metals in animal feeds and manures from farms of different scales in northeast China. Int. J Environ. Res. Public Health 2012, 9, 2658–2668. [Google Scholar] [CrossRef] [PubMed]
- Naito, W.; Kamo, M.; Tsushima, K.; Iwasaki, Y. Exposure and risk assessment of zinc in Japanese surface waters. Sci. Total Environ. 2010, 408, 4271–4284. [Google Scholar] [CrossRef]
- EFSA Panel on Additives; Products or Substances used in Animal Feed (FEEDAP). Scientific Opinion on the safety and efficacy of zinc compounds (E6) as feed additives for all animal species (zinc acetate, dihydrate; zinc chloride, anhydrous; zinc oxide; zinc sulfate, heptahydrate; zinc sulfate, monohydrate; zinc chelate of amino acids, hydrate; zinc chelate of glycine, hydrate), based on a dossier submitted by FEFANA asbl. EFSA J. 2015, 13, 4058. [Google Scholar]
- EFSA Panel on Additives; Products or Substances used in Animal Feed (FEEDAP). Scientific opinion on safety and efficacy of zinc compounds (E6) as feed additives for all animal species: Zinc sulfate monohydrate, based on a dossier submitted by helm AG. EFSA J. 2012, 10, 2572. [Google Scholar] [CrossRef]
- Jin, P.; Chen, J.; Zhan, H.; Huang, S.; Wang, J.; Shu, Y. Accumulation and excretion of zinc and their effects on growth and food utilization of Spodoptera litura (Lepidoptera: Noctuidae). Ecotoxicol. Environ. Saf. 2020, 202, 110883. [Google Scholar] [CrossRef]
- Shu, Y.; Gao, Y.; Sun, H.; Zou, Z.; Zhou, Q.; Zhang, G. Effects of zinc exposure on the reproduction of Spodoptera litura Fabricius (Lepidoptera: Noctuidae). Ecotoxicol. Environ. Saf. 2009, 72, 2130–2136. [Google Scholar] [CrossRef]
- Augustyniak, M.; Babczyńska, A.; Augustyniak, M. Oxidative stress in newly-hatched Chorthippus brunneus—The effects of zinc treatment during diapause, depending on the female’s age and its origins. Comp. Biochem. Phys. C 2011, 154, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhafar, Z.M.; Sharaby, A. Effect of zinc sulfate against the red palm weevil Rhynchophorus ferrugineus with reference to their histological changes on the larval midgut and adult reproductive system. J. Agric. Sci. Technol. 2012, 2, 888. [Google Scholar]
- Sawsan, S.M.; Sharaby, A.; Ebadah, I.M.; El-Behery, H. Efficiency of zinc sulfate and some volatile oils on some insect pests of the tomato crop. Glob. Adv. Res. J. Agric. Sci. 2015, 4, 182–187. [Google Scholar]
- Huang, D.; Kong, J.; Seng, Y. Effects of the heavy metal Cu2+ on growth, development, and population dynamics of Spodoptera litura (Lepidoptera: Noctuidae). J. Econ. Entomol. 2012, 105, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wu, C.; Tu, J.; Ling, Y.; Hu, N.; Zhang, Y.; Su, K.; Wang, P. Assessment of cadmium-induced hepatotoxicity and protective effects of zinc against it using an improved cell-based biosensor. Sens. Actuat. A-Phys. 2013, 199, 156–164. [Google Scholar] [CrossRef]
- Yang, Y.; Qi, J.; Wang, Z.; Zhou, Z.; Zhao, C.; Dong, X.; Li, X.; Li, C. Evaluating the effects of Cu2+ on the development and reproduction of Spodoptera litura (Lepidoptera: Noctuidae) based on the age-stage, two-sex life table. J. Insect Sci. 2022, 22, 4. [Google Scholar] [CrossRef]
- Chevrette, M.G.; Carlson, C.M.; Ortega, H.E.; Thomas, C.; Ananiev, G.E.; Barns, K.J.; Book, A.J.; Cagnazzo, J.; Carlos, C.; Flanigan, W. The antimicrobial potential of Streptomyces from insect microbiomes. Nat. Commun. 2019, 10, 516. [Google Scholar] [CrossRef] [PubMed]
- Modoux, M.; Rolhion, N.; Mani, S.; Sokol, H. Tryptophan metabolism as a pharmacological target. Trends Pharmacol. Sci. 2021, 42, 60–73. [Google Scholar] [CrossRef]
- Chen, N.; Du, N.; Wang, W.; Liu, T.; Yuan, Q.; Yang, Y. Real-time monitoring of dynamic microbial Fe (III) respiration metabolism with a living cell-compatible electron-sensing probe. Angew. Chem. Int. Edit. 2022, 61, e202115572. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, J.; Wu, J. Improvement of semi-artificial diet for Spodoptera litura. J. Northwest A&F Univ. 2016, 44, 109–113. [Google Scholar]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX–MSChart: A Computer Program for the Population Projection Based on Age-Stage, Two-Sex Life Table. Available online: http://140.120.197.173/ecology/prod02.htm (accessed on 22 July 2024).
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap; Chapman and Hall/CRC: New York, NY, USA, 1994. [Google Scholar]
- Chi, H.; Fu, J.; You, M. Age-stage, two-sex life table and its application in population ecology and integrated pest management. Acta Entomol. Sin. 2019, 62, 255–262. [Google Scholar]
- Goodman, D. Optimal life histories, optimal notation, and the value of reproductive value. Am. Nat. 1982, 119, 803–823. [Google Scholar] [CrossRef]
- Cheng, S.; Dai, P.; Li, R.; Chen, Z.; Liang, P.; Xie, X.; Zhen, C.; Gao, X. The sulfoximine insecticide sulfoxaflor exposure reduces the survival status and disrupts the intestinal metabolism of the honeybee Apis mellifera. J. Hazard. Mater. 2023, 442, 130109. [Google Scholar] [CrossRef]
- Praharaj, S.; Skalicky, M.; Maitra, S.; Bhadra, P.; Shankar, T.; Brestic, M.; Hejnak, V.; Vachova, P.; Hossain, A. Zinc biofortification in food crops could alleviate the zinc malnutrition in human health. Molecules 2021, 26, 3509. [Google Scholar] [CrossRef]
- Rogalska, J.; Brzóska, M.M.; Roszczenko, A.; Moniuszko-Jakoniuk, J. Enhanced zinc consumption prevents cadmium-induced alterations in lipid metabolism in male rats. Chem-Biol. Interact. 2009, 177, 142–152. [Google Scholar] [CrossRef]
- Wani, M.Y.; Rather, R.; Bashir, M.; Shafi, S.; Rani, S. Effect of zinc on the larval growth and quality cocoon parameters of silkworm (Bombyx mori L.): A review. Int. J. Fauna Biol. Stud. 2018, 5, 31–36. [Google Scholar]
- Shu, Y.; Du, Y.; Wang, J. Molecular characterization and expression patterns of Spodoptera litura heat shock protein 70/90, and their response to zinc stress. Comp. Biochem. Phys. A 2011, 158, 102–110. [Google Scholar] [CrossRef]
- Barata, C.; Lekumberri, I.; Vila-Escalé, M.; Prat, N.; Porte, C. Trace metal concentration, antioxidant enzyme activities and susceptibility to oxidative stress in the tricoptera larvae Hydropsyche exocellata from the Llobregat river basin (NE Spain). Aquat. Toxicol. 2005, 74, 3–19. [Google Scholar] [CrossRef]
- Ferrero, A.; Torreblanca, A.; Garcerá, M.D. Assessment of the effects of orally administered ferrous sulfate on Oncopeltus fasciatus (Heteroptera: Lygaeidae). Environ. Sci. Pollut. Res. 2017, 24, 8551–8561. [Google Scholar] [CrossRef] [PubMed]
- Li, X.D.; Jiang, G.F.; Li, R.; Bai, Y.; Zhang, G.S.; Xu, S.J.; Deng, W.A. Molecular strategies of the pygmy grasshopper Eucriotettix oculatus adapting to long-term heavy metal pollution. Ecotoxicol. Environ. Saf. 2024, 276, 116301. [Google Scholar] [CrossRef] [PubMed]
- Augustyniak, M.; Babczyńska, A.; Kozłowski, M.; Sawczyn, T.; Augustyniak, M. Effects of zinc and female aging on nymphal life history in a grasshopper from polluted sites. J. Insect Physiol. 2008, 54, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Shephard, A.M.; Mitchell, T.S.; Henry, S.B.; Oberhauser, K.S.; Kobiela, M.E.; Snell-Rood, E.C. Assessing zinc tolerance in two butterfly species: Consequences for conservation in polluted environments. Insect Conserv. Diver. 2020, 13, 201–210. [Google Scholar] [CrossRef]
- Vallee, B.L.; Falchuk, K.H. The biochemical basis of zinc physiology. Physiol. Rev. 1993, 73, 79–118. [Google Scholar] [CrossRef]
- Falchuk, K.H.; Montorzi, M. Zinc physiology and biochemistry in oocytes and embryos. In Zinc Biochemistry, Physiology, and Homeostasis; Springer: Berlin/Heidelberg, Germany, 2001; pp. 199–209. [Google Scholar]
- Kavitha, S.; Sivaprasad, S.; Saidulla, B.; Yellamma, K. Effect of zinc chloride and zinc sulfate on the silkworm, Bombyx mori growth tissue proteins and economic parameters of sericulture. Bioscan 2012, 7, 189–195. [Google Scholar]
- Capinera, J.L.; Rodrigues, C.G. Biology and control of the leatherleaf slug Leidyula floridana (Mollusca: Gastropoda: Veronicellidae). Fla. Entomol. 2015, 98, 243–253. [Google Scholar] [CrossRef]
- Kozlowski, J.; JasKulsKa, M.; Kozlowska, M. Evaluation of the effectiveness of iron phosphate and the parasitic nematode Phasmarhabditis hermaphrodita in reducing plant damage caused by the slug Arion vulgaris Moquin-Tandon, 1885. Folia Malacol. 2014, 22, 293–300. [Google Scholar] [CrossRef]
- Rae, R.G.; Robertson, J.F.; Wilson, M.J. Optimization of biological (Phasmarhabditis hermaphrodita) and chemical (iron phosphate and metaldehyde) slug control. Crop Prot. 2009, 28, 765–773. [Google Scholar] [CrossRef]
- Halpern, M.; Gasith, A.; Bresler, V.; Broza, M. The protective nature of Chironomus luridus larval tubes against copper sulfate. J. Insect Sci. 2002, 2, 8. [Google Scholar] [CrossRef]
- Kelkenberg, M.; Odman-Naresh, J.; Muthukrishnan, S.; Merzendorfer, H. Chitin is a necessary component to maintain the barrier function of the peritrophic matrix in the insect midgut. Insect Biochem. Mol. 2015, 56, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.J.; Huang, Y.L.; Yu, H.Z.; Li, N.Y.; Xie, Y.X.; Zhang, Q.; Zeng, X.D.; Hu, H.; Huang, A.J.; Yi, L. Silencing of the chitin synthase gene is lethal to the Asian citrus psyllid, Diaphorina citri. Int. J. Mol. Sci. 2019, 20, 3734. [Google Scholar] [CrossRef] [PubMed]
- Merzendorfer, H.; Zimoch, L. Chitin metabolism in insects: Structure, function and regulation of chitin synthases and chitinases. J. Exp. Biol. 2003, 206, 4393–4412. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Merzendorfer, H.; Zhang, W.; Zhang, J.; Muthukrishnan, S. Biosynthesis, turnover, and functions of chitin in insects. Annu. Rev. Entomol. 2016, 61, 177–196. [Google Scholar] [CrossRef]
- Huang, X.; Tsuji, N.; Miyoshi, T.; Motobu, M.; Islam, M.K.; Alim, M.A.; Fujisaki, K. Characterization of glutamine: Fructose-6-phosphate aminotransferase from the ixodid tick, Haemaphysalis longicornis, and its critical role in host blood feeding. Int. J. Parasitol. 2007, 37, 383–392. [Google Scholar] [CrossRef]
- Kato, N.; Dasgupta, R.; Smartt, C.; Christensen, B. Glucosamine: Fructose-6-phosphate aminotransferase: Gene characterization, chitin biosynthesis and peritrophic matrix formation in Aedes aegypti. Insect Mol. Biol. 2002, 11, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Mueller, C.R.; Fuchs, J.F.; Wessely, V.; Lan, Q.; Christensen, B.M. Regulatory mechanisms of chitin biosynthesis and roles of chitin in peritrophic matrix formation in the midgut of adult Aedes aegypti. Insect Biochem. Mol. 2006, 36, 1–9. [Google Scholar] [CrossRef]
- Arakane, Y.; Baguinon, M.C.; Jasrapuria, S.; Chaudhari, S.; Doyungan, A.; Kramer, K.J.; Muthukrishnan, S.; Beeman, R.W. Both UDP N-acetylglucosamine pyrophosphorylases of Tribolium castaneum are critical for molting, survival and fecundity. Insect Biochem. Mol. 2011, 41, 42–50. [Google Scholar] [CrossRef]
- Bhagath Kumar, P.; Nagendra Pratap, S.; Kasi Viswanath, K.; Sampath Kumar, R.; Dinakara Rao, A. UDP-N-Acetyl glucosamine pyrophosphorylase as novel target for controlling Aedes aegypti–molecular modeling, docking and simulation studies. Int. J. Mosquito Res. 2014, 1, 17–24. [Google Scholar]
- Graack, H.-R.; Cinque, U.; Kress, H. Functional regulation of glutamine: Fructose-6-phosphate aminotransferase 1 (GFAT1) of Drosophila melanogaster in a UDP-N-acetylglucosamine and cAMP-dependent manner. Biochem. J. 2001, 360, 401–412. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, D.; Wang, L.; Li, J.; Wang, W. Glucosamine: Fructose-6-phosphate amidotransferase in the white shrimp Litopenaeus vannamei: Characterization and regulation under alkaline and cadmium stress. Ecotoxicology 2015, 24, 1754–1764. [Google Scholar] [CrossRef] [PubMed]
- Bryskier, A.; Dini, C. Peptidoglycan synthesis inhibitors. In Antimicrobial Agents; Wiley: Hoboken, NJ, USA, 2005; pp. 377–400. [Google Scholar]
- Lagorce, A.; Le Berre-Anton, V.; Aguilar-Uscanga, B.; Martin-Yken, H.; Dagkessamanskaia, A.; François, J. Involvement of GFA1, which encodes glutamine–fructose-6-phosphate amidotransferase, in the activation of the chitin synthesis pathway in response to cell-wall defects in Saccharomyces cerevisiae. Eur. J. Biochem. 2002, 269, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Dubovskiy, I.; Martemyanov, V.; Vorontsova, Y.; Rantala, M.; Gryzanova, E.; Glupov, V. Effect of bacterial infection on antioxidant activity and lipid peroxidation in the midgut of Galleria mellonella L. larvae (Lepidoptera, Pyralidae). Comp. Biochem. Phys. C 2008, 148, 1–5. [Google Scholar] [CrossRef]
- Coskun, M.; Kayis, T.; Gulsu, E.; Alp, E. Effects of selenium and vitamin E on enzymatic, biochemical, and immunological biomarkers in Galleria mellonella L. Sci. Rep. 2020, 10, 9953. [Google Scholar] [CrossRef]
- Farjan, M.; Dmitryjuk, M.; Lipiński, Z.; Biernat-Łopieńska, E.; Żółtowska, K. Supplementation of the honey bee diet with vitamin C: The effect on the antioxidative system of Apis mellifera carnica brood at different stages. J. Apicult. Res. 2012, 51, 263–270. [Google Scholar] [CrossRef]
- Flora, S.; Shrivastava, R.; Mittal, M. Chemistry and pharmacological properties of some natural and synthetic antioxidants for heavy metal toxicity. Curr. Med. Chem. 2013, 20, 4540–4574. [Google Scholar] [CrossRef]
- Pardini, R.S. Toxicity of oxygen from naturally occurring redox-active pro-oxidants. Arch. Insect Biochem. 1995, 29, 101–118. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. Transl. Integr. 1997, 82, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Mahajan, S. Effect of ascorbic acid on longevity and biochemical alterations in Callosobruchus maculatus F. (Coleoptera: Bruchidae). Arch. Gerontol. Geriat. 1994, 18, 149–157. [Google Scholar] [CrossRef]
- El-Demerdash, F.; Yousef, M.; Kedwany, F.; Baghdadi, H. Role of α-tocopherol and β-carotene in ameliorating the fenvalerate-induced changes in oxidative stress, hemato-biochemical parameters, and semen quality of male rats. J. Environ. Sci. Health B 2004, 39, 443–459. [Google Scholar] [CrossRef]
- Sodhi, S.; Sharma, A.; Brar, A.; Brar, R. Effect of α tocopherol and selenium on antioxidant status, lipid peroxidation and hepatopathy induced by malathion in chicks. Pestic. Biochem. Phys. 2008, 90, 82–86. [Google Scholar] [CrossRef]
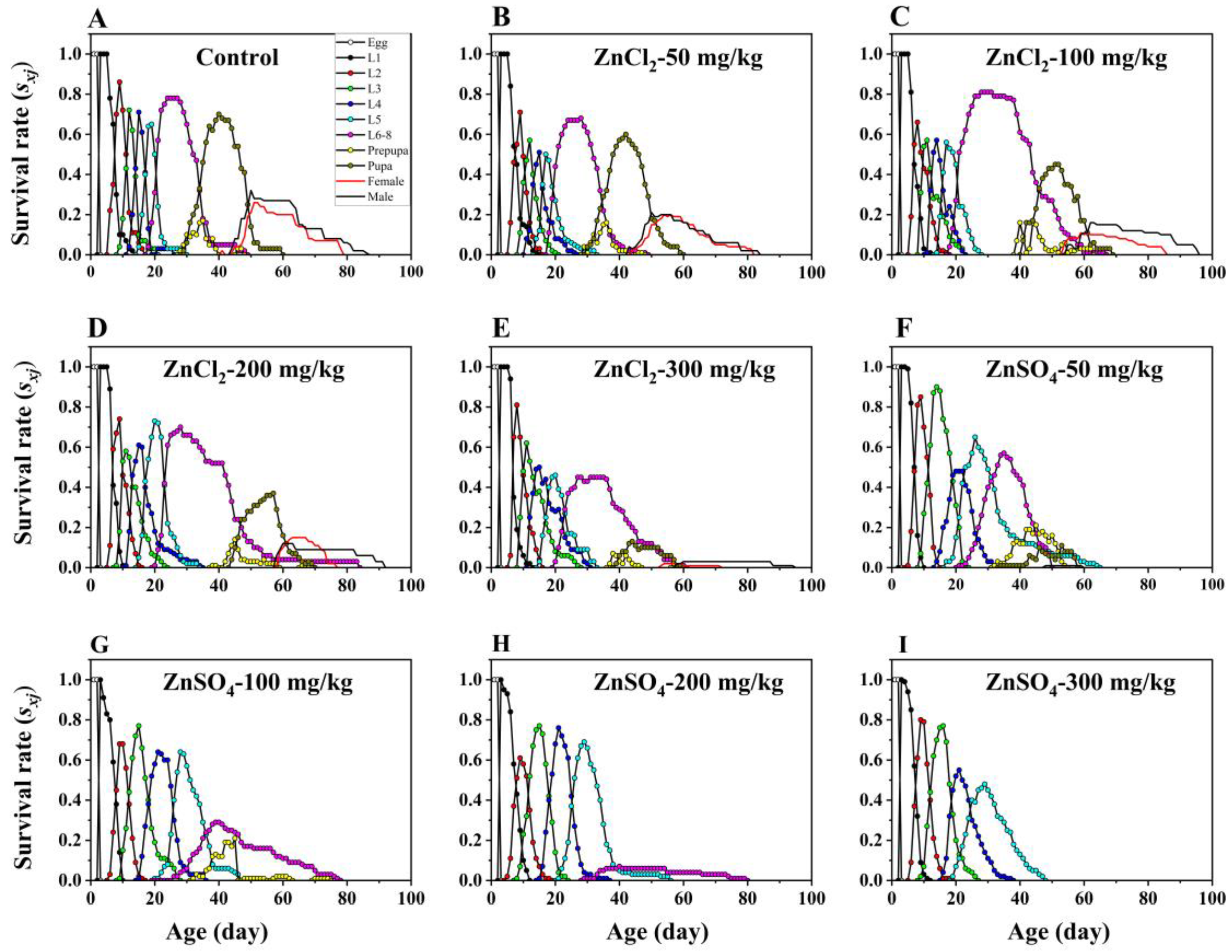
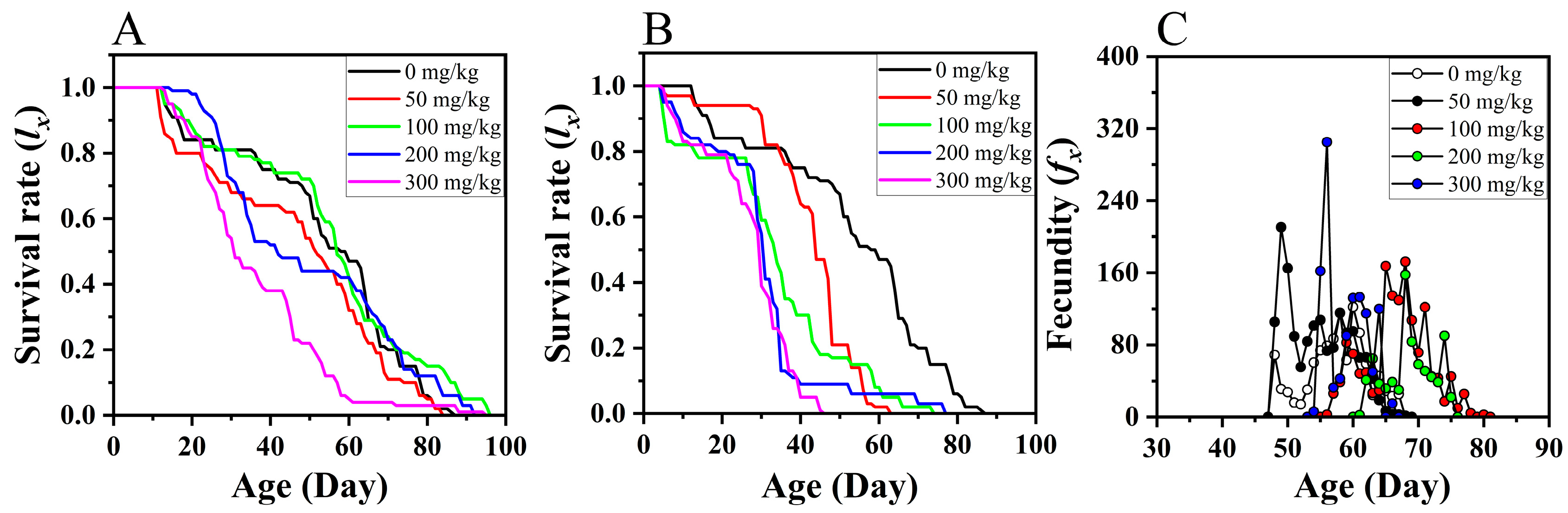
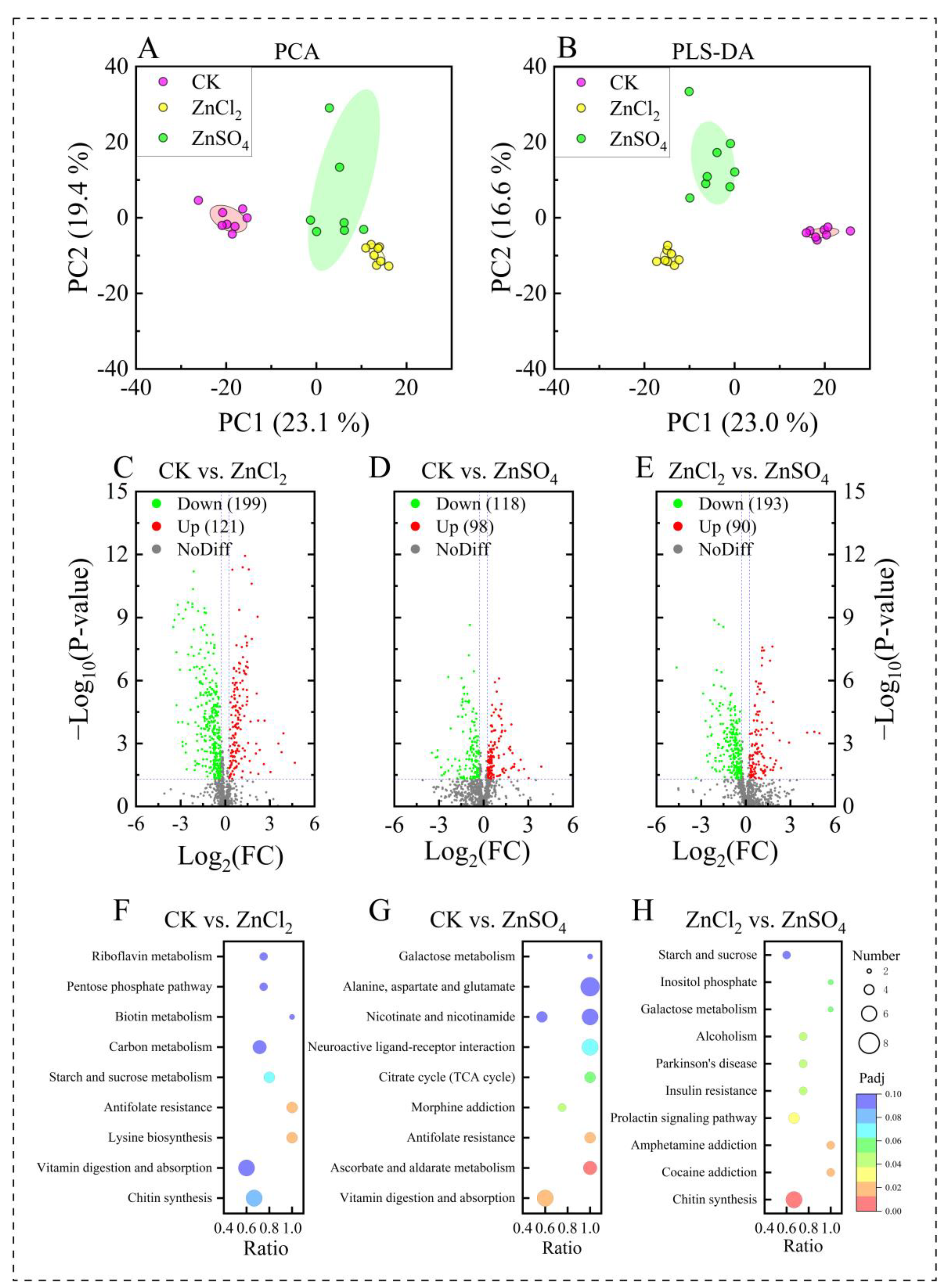
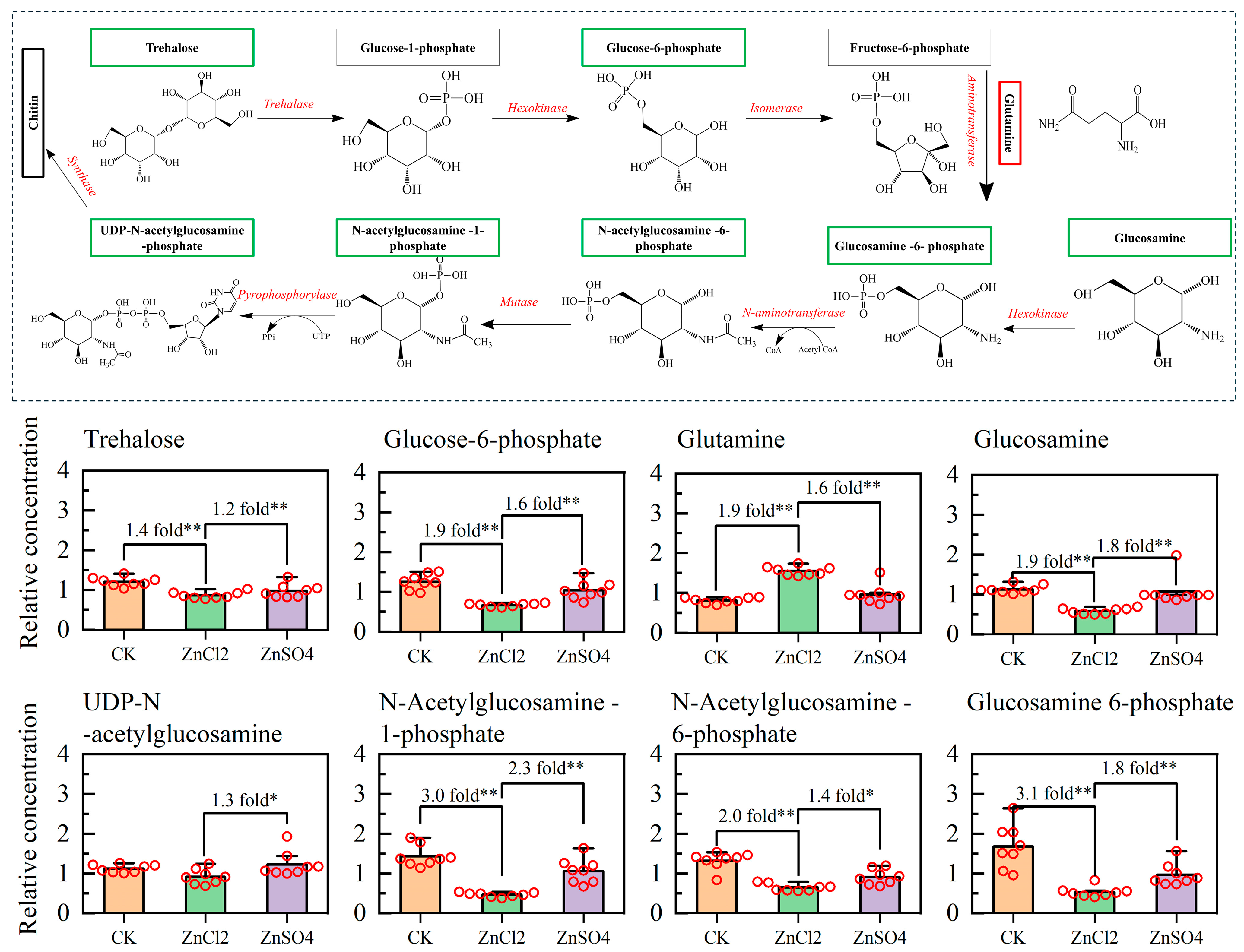
| Life History Parameters | Concentration (mg/kg) | ||||
|---|---|---|---|---|---|
| 0 | 50 | 100 | 200 | 300 | |
| Egg (d) | 3.0 ± 0.00a (100) | 3.0 ± 0.00a (100) | 3.0 ± 0.00a (100) | 3.0 ± 0.00a (100) | 3.0 ± 0.00a (100) |
| L1 (d) | 5.0 ± 0.17a (99) | 4.8 ± 0.15a (88) | 4.8 ± 0.15a (100) | 4.7 ± 0.12a (100) | 4.7 ± 0.14a (100) |
| L2 (d) | 3.9 ± 0.07a (88) | 3.4 ± 0.09bc (83) | 3.2 ± 0.08c (100) | 3.5 ± 0.09b (100) | 3.5 ± 0.13bc (100) |
| L3 (d) | 3.1 ± 0.06d (84) | 3.2 ± 0.10cd (80) | 3.3 ± 0.08c (94) | 3.6 ± 0.10b (100) | 4.2 ± 0.18a (91) |
| L4 (d) | 3.2 ± 0.05d (84) | 3.3 ± 0.09d (77) | 3.5 ± 0.05c (87) | 4.1 ± 0.08b (97) | 4.5 ± 0.12a (84) |
| L5 (d) | 3.4 ± 0.06c (81) | 3.5 ± 0.06c (68) | 4.2 ± 0.06b (83) | 5.4 ± 0.15a (86) | 5.5 ± 0.24a (61) |
| L6–8 (d) | 12.6 ± 0.22d (81) | 14.4 ± 0.34c (66) | 24.4 ± 0.82a (77) | 23.4 ± 0.80a (42) | 19.1 ± 1.17b (19) |
| Prepupa | 1.45 ± 0.07c (81) | 1.51 ± 0.06bc (66) | 1.92 ± 0. 05a (77) | 1.95 ± 0.07a (42) | 2.01 ± 0.16a (19) |
| Pupa (d) | 13.6 ± 0.21a (76) | 13.1 ± 0.13ab (48) | 13.0 ± 0.12b (33) | 13.0 ± 0.14b (27) | 13.4 ± 0.20ab (5) |
| Preadult (d) | 48.5 ± 0.44c (66) | 48.8 ± 0.51c (48) | 57.8 ± 0.54b (33) | 59.4 ± 0.26a (27) | 56.4 ± 0.99b (5) |
| Preadult survival rate (%) | 66.0 ± 4.74a (66) | 47.9 ± 4.99b (48) | 33.0 ± 4.69cd (33) | 27.0 ± 4.43d (27) | 5.4 ± 2.04e (5) |
| Oviposition days (d) | 6.5 ± 0.75b (34) | 7.3 ± 0.85b (24) | 10.7 ± 1.17a (15) | 4.8 ± 1.35b (15) | 6.9 ± 3.04 (2) |
| Female longevity (d) | 14.1 ± 1.69a (34) | 15.8 ± 1.83a (24) | 15.9 ± 2.32a (15) | 12.7 ± 0.92a (15) | 12.5 ± 4.93 (2) |
| Male longevity (d) | 20.9 ± 1.92b (32) | 17.8 ± 2.11b (24) | 23.3 ± 2.58ab (18) | 23.2 ± 3.27ab (12) | 31.5 ± 2.94a (3) |
| Fecundity | 620.4 ± 137.30b (34) | 1022.4 ± 247.13a (24) | 895.3 ± 238.30ab (15) | 592.9 ± 246.71b (15) | 900.7 ± 106.67 (2) |
| Concentration (mg/kg) | Population Parameters | |||
|---|---|---|---|---|
| r (d−1) | λ (d−1) | R0 (Offspring/Individual) | T (d) | |
| 0 | 0.0920 ± 0.0058a | 1.0963 ± 0.0063a | 211.05 ± 54.74a | 57.88 ± 1.30b |
| 50 | 0.0985 ± 0.0065a | 1.1035 ± 0.0071a | 245.02 ± 72.82a | 55.40 ± 1.19b |
| 100 | 0.0728 ± 0.0059bc | 1.0755 ± 0.0064bc | 134.46 ± 47.13a | 66.39 ± 1.43a |
| 200 | 0.0637 ± 0.0094cd | 1.0658 ± 0.0100cd | 88.99 ± 41.63ab | 68.18 ± 1.23a |
| 300 | 0.0494 ± 0.0095d | 1.0507 ± 0.0100d | 21.16 ± 11.52b | 58.99 ± 1.82b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, J.; Xia, Z.; Yang, Y.; Li, C.; Wang, Z. Effects of Dietary Zinc Chloride and Zinc Sulfate on Life History Performance and Hemolymph Metabolism of Spodoptera litura (Lepidoptera: Noctuidae). Insects 2024, 15, 687. https://doi.org/10.3390/insects15090687
Qi J, Xia Z, Yang Y, Li C, Wang Z. Effects of Dietary Zinc Chloride and Zinc Sulfate on Life History Performance and Hemolymph Metabolism of Spodoptera litura (Lepidoptera: Noctuidae). Insects. 2024; 15(9):687. https://doi.org/10.3390/insects15090687
Chicago/Turabian StyleQi, Jingwei, Zhenzhou Xia, Yang Yang, Chuanren Li, and Zailing Wang. 2024. "Effects of Dietary Zinc Chloride and Zinc Sulfate on Life History Performance and Hemolymph Metabolism of Spodoptera litura (Lepidoptera: Noctuidae)" Insects 15, no. 9: 687. https://doi.org/10.3390/insects15090687
APA StyleQi, J., Xia, Z., Yang, Y., Li, C., & Wang, Z. (2024). Effects of Dietary Zinc Chloride and Zinc Sulfate on Life History Performance and Hemolymph Metabolism of Spodoptera litura (Lepidoptera: Noctuidae). Insects, 15(9), 687. https://doi.org/10.3390/insects15090687





