The Effect of Elevation Gradient on Distribution and Body Size of Carabid Beetles in the Changbaishan Nature Reserve in Northeast Asia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
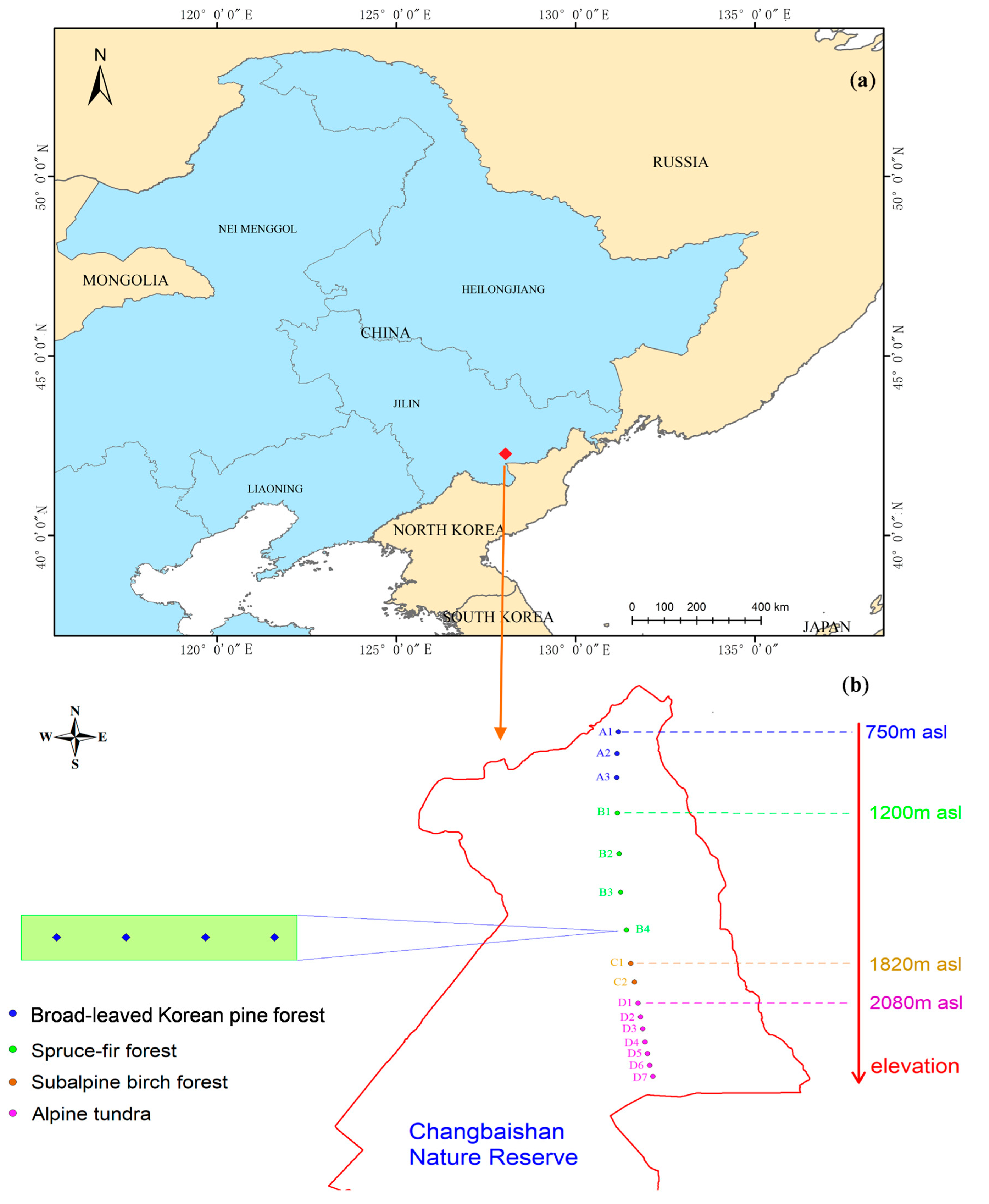
2.1. Study Area
2.2. Experimental Design and Sampling of Carabid Beetles
2.3. Statistical Analyses
3. Results
3.1. Richness and Abundance at Different Elevations
3.2. Abundance of Species in Different Elevations
3.3. Community Composition
3.4. Indicator Species
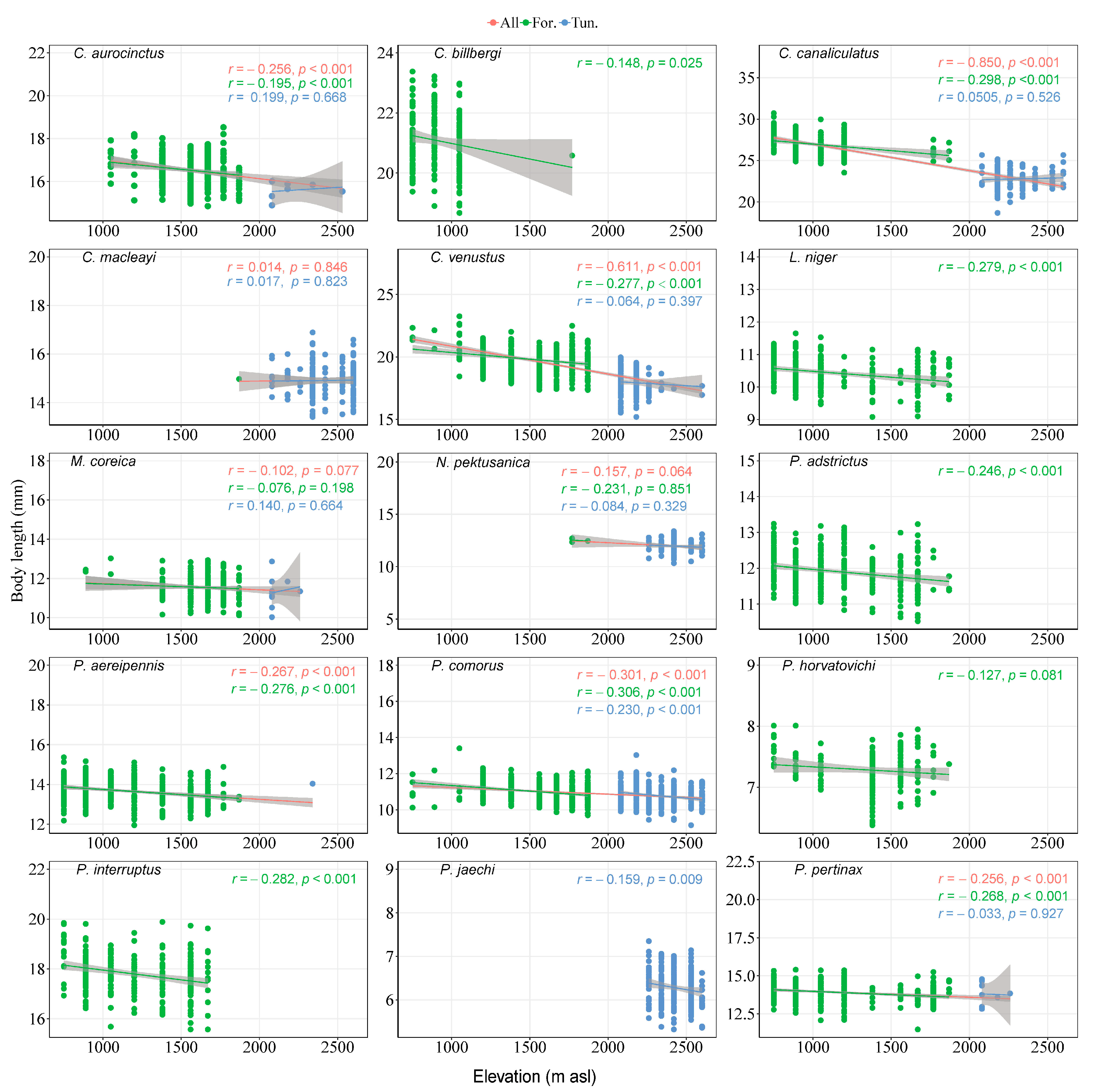
3.5. Body Size of Species
4. Discussion
4.1. Diversity of Species and Elevation
4.2. High-Elevation Species in Alpine Tundra
4.3. Body Size of Species and Elevation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lomolino, M.V. Elevation gradients of species-density: Historical and prospective views. Glob. Ecol. Biogeogr. 2001, 10, 3–13. [Google Scholar] [CrossRef]
- Whittaker, R.J.; Willis, K.J.; Field, R. Scale and species richness: Towards a general, hierarchical theory of species diversity. J. Biogeogr. 2011, 28, 453–470. [Google Scholar] [CrossRef]
- Baudino, F.; Werenkraut, V.; Ruggiero, A. Rapid recovery of the beetle richness-elevation relationship and its environmental correlates after a major volcanic event in northwestern patagonia, argentina. Insect Conserv. Diver. 2020, 13, 404–418. [Google Scholar] [CrossRef]
- Moreira, X.; Abdala-Roberts, L.; Parra-Tabla, V.; Mooney, K.A. Latitudinal variation in herbivory: Influences of climatic drivers, herbivore identity and natural enemies. Oikos 2015, 124, 1444–1452. [Google Scholar] [CrossRef]
- García-López, A.; Micó, E.; Galante, E. From lowlands to highlands: Searching for elevational patterns of species richness and distribution of scarab beetles in Costa Rica. Divers. Distrib. 2011, 18, 543–553. [Google Scholar] [CrossRef]
- Beck, J.; McCain, C.M.; Axmacher, J.C.; Ashton, L.A.; Bärtschi, F.; Brehm, G.; Choi, S.; Cizek, O.; Colwell, R.K.; Fiedler, K. Elevational species richness gradients in a hyperdiverse insect taxon: A global meta-study on geometrid moths. Global Ecol. Biogeogr. 2017, 26, 412–424. [Google Scholar] [CrossRef]
- Colwell, R.K.; Brehm, G.; Cardelus, C.L.; Gilman, A.C.; Longino, J.T. Global warming, elevational range shifts, and lowland biotic attrition in the wet tropics. Science 2008, 322, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Leingärtner, A.; Krauss, J.; Steffan-Ewenter, I. Elevation and experimental snowmelt manipulation affect emergence phenology and abundance of soil-hibernating arthropods. Ecol. Entomol. 2014, 39, 412–418. [Google Scholar] [CrossRef]
- Harvey, J.A.; Tougeron, K.; Gols, R.; Heinen, R.; Abarca, M.; Abram, P.K.; Basset, Y.; Berg, M.; Boggs, C.; Brodeur, J.; et al. Scientists’ warning on climate change and insects. Ecol. Monogr. 2023, 93, e1553. [Google Scholar] [CrossRef]
- Williams, K.K.; Mcmillin, J.D.; Degomez, T.E.; Clancy, K.M.; Miller, A. Influence of elevation on bark beetle (Coleoptera: Curculionidae, Scolytinae) community structure and flight periodicity in ponderosa pine forests of arizona. Environ. Entomol. 2008, 37, 94–109. [Google Scholar] [CrossRef]
- Körner, C. The use of ‘altitude’in ecological research. Trends Ecol. Evol. 2007, 22, 569–574. [Google Scholar] [CrossRef] [PubMed]
- McCain, C.M.; Grytnes, J.A. Elevational gradients in species richness. In Encyclopedia of Life Sciences; John Wiley & Sons, Ltd.: Chichester, UK, 2010. [Google Scholar] [CrossRef]
- Shah, A.A.; Dillon, M.E.; Hotaling, S.; Woods, H.A. High elevation insect communities face shifting ecological and evolutionary landscapes. Curr. Opin. Insect Sci. 2020, 41, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Konvicka, M.; Maradova, M.; Benes, J.; Fric, Z.; Kepka, P. Uphill shifts in distribution of butterflies in the Czech Republic: Effects of changing climate detected on a regional scale. Global Change Biol. 2003, 12, 403–410. [Google Scholar] [CrossRef]
- Franco, A.M.A.; Hill, J.K.; Kitschke, C.; Collingham, Y.C.; Roy, D.B.; Fox, R.; Huntley, B.; Thomas, C.D. Impacts of climate warming and habitat loss on extinctions at species’ low-latitude range boundaries. Glob. Change Biol. 2006, 12, 1545–1553. [Google Scholar] [CrossRef]
- Wilson, R.J.; Gutiérrez, D.; Gutiérrez, J.; Monserrat, V.J. An elevational shift in butterfly species richness and composition accompanying recent climate change. Glob. Change Biol. 2007, 13, 1873–1887. [Google Scholar] [CrossRef]
- Hodkinson, I.D. Terrestrial insects along elevation gradients: Species and community responses to altitude. Biol. Rev. 2005, 80, 489–513. [Google Scholar] [CrossRef]
- Pizzolotto, R.; Gobbi, M.; Brandmayr, P. Changes in ground beetle assemblages above and below the treeline of the Dolomites after almost 30 years (1980/2009). Ecol. Evol. 2014, 4, 1284–1294. [Google Scholar] [CrossRef]
- Eyre, M.D.; Rushton, S.P.; Luff, M.L.; Telfer, M.G. Investigating the relationships between the distribution of British ground beetle species (Coleoptera, Carabidae) and temperature, precipitation and altitude. J. Biogeogr. 2005, 32, 973–983. [Google Scholar] [CrossRef]
- Voigt, W.; Perner, J.; Davis, A.J.; Eggers, T.; Schumacher, J.; Bährmann, R.; Fabian, B.; Heinrich, W.; Kohler, G.; Lichter, D.; et al. Trophic levels are differentially sensitive to climate. Ecology 2003, 84, 2444–2453. [Google Scholar] [CrossRef]
- Liu, S.; Dong, S.; Liu, R.; Meng, Q.; Li, Y.; Zhao, H.; Jin, Y. The distribution patterns and temporal dynamics of carabid beetles (Coleoptera: Carabidae) in the forests of Jiaohe, Jilin Province, China. J. For. Res. 2022, 33, 333–342. [Google Scholar] [CrossRef]
- Gobbi, M.; Armanini, M.; Boscolo, T.; Chirichella, R.; Lencioni, V.; Ornaghi, S.; Mustoni, A. Habitat and landform types drive the distribution of Carabid beetles at high altitudes. Diversity 2021, 13, 142. [Google Scholar] [CrossRef]
- Beckers, N.; Hein, N.; Anneser, A.; Vanselow, K.A.; Löffler, J. Differences in mobility and dispersal capacity determine body size clines in two common alpine-tundra arthropods. Insects 2020, 11, 74. [Google Scholar] [CrossRef]
- Maveety, S.A.; Browne, R.A.; Erwin, T.L. Carabid beetle diversity and community composition as related to altitude and seasonality in Andean forests. Stud. Neotrop Fauna Environ. 2013, 48, 165–174. [Google Scholar] [CrossRef]
- Homburg, K.; Drees, C.; Boutaud, E.; Nolte, D.; Schuett, W.; Zumstein, P.; Ruschkowski, E.; Assmann, T. Where have all the beetles gone? Long–term study reveals carabid species decline in a nature reserve in Northern Germany. Insect Conserv. Diver. 1999, 12, 268–277. [Google Scholar] [CrossRef]
- Moret, P. Altitudinal distribution, diversity and endemicity of Carabidae (Coleoptera) in the páramos of Ecuadorian Andes. Ann. Société Entomol. Fr. 2009, 45, 500–510. [Google Scholar] [CrossRef]
- Maveety, S.A.; Browne, R.A.; Erwin, T.L. Carabidae diversity along an altitudinal gradient in a Peruvian cloud forest (Coleoptera). ZooKeys 2011, 147, 651–666. [Google Scholar] [CrossRef] [PubMed]
- Staunton, K.M.; Nakamura, A.; Burwell, C.J.; Robson, S.K.A.; Williams, S.E. Elevational distribution of flightless ground beetles in the tropical rainforests of North-Eastern Australia. PLoS ONE 2016, 11, e0155826. [Google Scholar] [CrossRef]
- Pizzolotto, R.; Albertini, A.; Gobbi, M.; Brandmayr, P. Habitat diversity analysis along an altitudinal sequence of alpine habitats: The carabid beetle assemblages as a study model. Period Biol. 2016, 118, 241–254. [Google Scholar] [CrossRef]
- Wolda, H. Altitude, habitat and tropical insect diversity. Biol. J. Linn. Soc. 1987, 30, 313–323. [Google Scholar] [CrossRef]
- Homburg, K.; Schuldt, A.; Drees, C.; Assmann, T. Broad-scale geographic patterns in body size and hind wing development of western Palaearctic carabid beetles (Coleoptera: Carabidae). Ecography 2013, 36, 166–177. [Google Scholar] [CrossRef]
- Nolte, D.; Boutaud, E.; Kotze, D.J.; Schuldt, A.; Assmann, T. Habitat specialization, distribution range size and body size drive extinction risk in carabid beetles. Biodivers. Conserv. 2019, 28, 1267–1283. [Google Scholar] [CrossRef]
- Zou, Y.; Sang, W.; Bai, F.; Brennan, E.; Diekman, M.; Liu, Y.; Li, L.; Alice, M.; Shi, H.; Sui, Z.; et al. Large-scale α-diversity patterns in plants and ground beetles (Coleoptera: Carabidae) indicate a high biodiversity conservation value of China’s restored temperate forest landscapes. Divers. Distrib. 2019, 25, 1613–1624. [Google Scholar] [CrossRef]
- Tantowijoyo, W.; Hoffmann, A.A. Variation in morphological characters of two invasive leafminers, Liriomyza huidobrensis and L. sativae, across a tropical elevation gradient. J. Insect Sci. 2011, 11, 69. [Google Scholar] [CrossRef]
- Mähn, L.A.; Hof, C.; Brandl, R.; Pinkert, S. Beyond latitude: Temperature, productivity and thermal niche conservatism drive global body size variation in Odonata. Glob. Ecol. Biogeogr. 2023, 32, 656–667. [Google Scholar] [CrossRef]
- Chown, S.L.; Klok, C.J. Altitudinal body size clines: Latitudinal effects associated with changing seasonality. Ecography 2003, 26, 445–455. [Google Scholar] [CrossRef]
- Bidau, C.J.; Martí, D.A. Clinal variation of body size in Dichroplus pratensis (Orthoptera: Acrididae): Inversion of Bergmann’s and Rensch’s rules. Ann. Entomol. Soc. Am. 2007, 100, 850–860. [Google Scholar] [CrossRef]
- Smith, R.J.; Hines, A.; Richmond, S.; Merrick, M.; Drew, A.; Fargo, R. Altitudinal variation in body size and population density of Nicrophorus investigator (Coleoptera: Silphidae). Environ. Entomol. 2000, 29, 290–298. [Google Scholar] [CrossRef]
- Malo, J.E.; Baonza, J. Are there predictable clines in plant-pollinator interactions along altitudinal gradients? The example of Cytisus scoparius (L.) Link in the Sierra de Guadarrama (Central Spain). Divers. Distrib. 2022, 8, 365–371. [Google Scholar] [CrossRef]
- Atkinson, D. Temperature and organism size—A biological law for ectotherms? Advances Ecol. Res. 1994, 25, 1–58. [Google Scholar]
- Eweleit, L.; Reinhold, K. Body size and elevation: Do Bergmann’s and Rensch’s rule apply in the polytypic bushcricket Poecilimon veluchianus? Ecol. Entomol. 2014, 39, 133–136. [Google Scholar] [CrossRef]
- Kovacs, J.L.; Goodisman, M.A.D. Environmental and genetic influences on queen and worker body size in the social wasp Vespula maculifrons. Insectes Soc. 2010, 57, 53–65. [Google Scholar] [CrossRef]
- Teder, T.; Esperk, T.; Remmel, T.; Sang, A.; Tammaru, T. Counterintuitive size patterns in bivoltine moths: Late-season larvae grow larger despite lower food quality. Oecologia 2010, 162, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Kubota, U.; Loyola, R.D.; Almeida, A.M.; Carvalho, D.A.; Lewinsohn, M. Body size and host range co-determine the altitudinal distribution of Neotropical tephritid flies. Glob. Ecol. Biogeogr. 2007, 16, 632–639. [Google Scholar] [CrossRef]
- Shelomi, M. Where are we now? Bergmann’s rule sensu lato in insects. Am. Nat. 2012, 180, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.A.; Hallas, R.J.; Dean, J.A.; Schiffer, M. Low potential for climatic stress adaptation in a rainforest Drosophila species. Science 2003, 301, 100–102. [Google Scholar] [CrossRef]
- Kumar, A.; O’Donnell, S. Elevation and forest clearing effects on foraging differ between surface—And subterranean—Foraging army ants (Formicidae: Ecitoninae). J. Anim. Ecol. 2009, 78, 91–97. [Google Scholar] [CrossRef]
- Moya-Raygoza, G.; Larsen, K.J.; Nault, A. Geographic and seasonal variation in size and color of adult Corn Leafhoppers (Hemiptera: Cicadellidae) from Mexico. Environ. Entomol. 2005, 34, 1388–1394. [Google Scholar] [CrossRef]
- Ottesen, P.S. Niche segregation of terrestrial alpine beetles (Coleoptera) in relation to environmental gradients and phenology. J. Biogeogr. 1996, 23, 353–369. [Google Scholar] [CrossRef]
- Sota, T. Altitudinal variation in life cycles of carabid beetles: Life-cycle strategy and colonization in alpine zones. Arct. Alp. Res. 1996, 28, 441. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Takami, Y.; Okuzaki, Y.; Sota, T. Genetic differences and phenotypic plasticity in body size between high- and low-altitude populations of the ground beetle Carabus tosanus. J. Evolution Biol. 2012, 25, 1835–1842. [Google Scholar] [CrossRef]
- Okuzaki, Y.; Sota, T. Factors related to altitudinal body size variation in the Earthworm-Eating ground beetle Carabus japonicus. Zool. Sci. 2017, 34, 229–234. [Google Scholar] [CrossRef]
- Van Dijk, T.S. On the relationship between food, reproduction and survival of two carabid beetles: Calathus melanocephalus and Pterostichus versicolor. Ecol. Entomol. 1994, 19, 263–270. [Google Scholar] [CrossRef]
- Ernsting, G.; Isaaks, J.A.; Berg, M.P. Life cycle and food availability indices in Notiophilus biguttatus (Coleoptera, Carabidae). Ecol. Entomol. 1994, 19, 263–270. [Google Scholar] [CrossRef]
- Baranovská, E.; Tajovský, K.; Knapp, M. Changes in the body size of carabid beetles along elevational gradients: A multispecies study of between- and within-population variation. Environ. Entomol. 1999, 48, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Adedoja, O.; Kehinde, T.; Samways, M.J. Asynchrony among insect pollinator groups and flowering plants with elevation. Sci. Rep. 2020, 10, 13268. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Sang, W.G.; Zhou, H.C.; Huang, L.Y.; Axmacher, J.C. Altitudinal diversity patterns of ground beetles (Coleoptera: Carabidae) in the forests of Changbai Mountain, Northeast China. Insect Conserv. Diver. 2014, 7, 161–171. [Google Scholar] [CrossRef]
- Li, J.D.; Wu, B.H.; Sheng, L.X. Jilin Vegetation; Jilin Sci. Technology Press: Changchun, China, 2001; pp. 393–396. [Google Scholar]
- de Arruda, F.V.; Camarota, F.; Silva, R.R.; Izzo, T.J.; Bergamini, L.L.; Almeida, R.P.S. The potential of arboreal pitfall traps for sampling nontargeted bee and wasp pollinators. Entomol. Exp. Appl. 2022, 170, 902–913. [Google Scholar] [CrossRef]
- Wu, J.; Pan, H.; Yang, S.Z.; Niu, X.L. Tree species and elevation influence the assemblage composition of saproxylic beetles in subtropical forest of east China. Forest Ecol. Manag. 2013, 292, 29–38. [Google Scholar] [CrossRef]
- McCune, B.; Mefford, M.J. PC–ORD. Multivariate Analysis of Ecological Data. Version 6; MjM Software: Gleneden Beach, OR, USA, 2011. [Google Scholar]
- Fattorini, S.; Ulrich, W. Drivers of species richness in European Tenebrionidae (Coleoptera). Acta Oecol. 2012, 43, 22–28. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.; Zhou, M.; Ai, D.; Wang, G.; Wang, Y.; Chu, C.; Lundholm, J.T. The effect of environmental heterogeneity on species richness depends on community position along the environmental gradient. Sci. Rep. 2015, 5, 15723. [Google Scholar] [CrossRef]
- Chamberlain, D.; Gobbi, M.; Negro, M.; Caprio, E.; Palestrini, C.; Pedrotti, L.; Brandmayr, P.; Pizzolotto, R.; Rolando, A. Trait-modulated decline of carabid beetle occurrence along elevational gradients across the European Alps. J. Biogeogr. 2020, 47, 1030–1040. [Google Scholar] [CrossRef]
- Yu, X.D.; Lü, L.; Luo, T.H.; Zhou, H.Z. Elevational gradient in species richness pattern of epigaeic beetles and underlying mechanisms at east slope of Balang Mountain in Southwestern China. PLoS ONE 2013, 8, e69177. [Google Scholar] [CrossRef] [PubMed]
- Tremmel, M.; Müller, C. Insect personality depends on environmental conditions. Behav. Ecol. 2013, 24, 386–392. [Google Scholar] [CrossRef]
- Péré, C.; Jactel, H.; Kenis, M. Response of insect parasitism to elevation depends on host and parasitoid life-history strategies. Biol. Lett. 2013, 9, 20130028. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.J.; Sinclair, F.H.; Burwell, C.J. Food web structure changes with elevation but not rainforest stratum. Ecography 2015, 38, 792–802. [Google Scholar] [CrossRef]
- Musthafa, M.M.; Abdullah, F.; Sánchez-Reyes, U.J. Comparative study of spatial patterns and ecological niches of beetles in two Malaysian mountains elevation gradients. J. Insect Conserv. 2018, 22, 757–769. [Google Scholar] [CrossRef]
- Bertuzzo, E.; Carrara, F.; Mari, L.; Altermatt, F.; Rodriguez-Iturbe, I.; Rinaldo, A. Geomorphic controls on elevational gradients of species richness. Proc. Natl. Acad. Sci. USA 2016, 113, 1737–1742. [Google Scholar] [CrossRef]
- Shuai, L.Y.; Ren, C.L.; Yan, W.B.; Song, Y.L.; Zeng, Z.G. Different elevational patterns of rodent species richness between the southern and northern slopes of a mountain. Sci. Rep. 2017, 7, 8743. [Google Scholar] [CrossRef]
- Bommarco, R. Reproduction and energy reserves of a predatory carabid beetle relative to agroecosystem complexity. Ecol. Appl. 1998, 8, 846–853. [Google Scholar] [CrossRef]
- Nowrouzi, S.; Andersen, A.N.; Macfadyen, S.; Staunton, K.M.; VanDerWal, J.; Robson, S.K.A. Ant Diversity and Distribution along Elevation Gradients in the Australian Wet Tropics: The Importance of Seasonal Moisture Stability. PLoS ONE 2016, 11, e0153420. [Google Scholar] [CrossRef]
- Motta, L.; Ruggiero, A.; De Mendoza, G.; Massaferro, J. The species richness-elevation relationship: Global patterns of variation in chironomid richness in mountain lakes. Insect Conserv. Diver. 2019, 12, 339–350. [Google Scholar] [CrossRef]
- Zou, Y.; Sang, W.G.; Hausmann, A.; Axmacher, J.C. High phylogenetic diversity is preserved in species-poor high-elevation temperate moth assemblages. Sci. Rep. 2016, 6, 23045. [Google Scholar] [CrossRef] [PubMed]
- Strathdee, A.T.; Bale, J.S. Life on the edge: Insect ecology in arctic environments. Annu. Rev. Entomol. 1998, 43, 85–106. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.D.; Meng, X.; Shang, J.Y.; Yang, M.Y.; Meng, Q.F.; Gao, W.T.; Wang, G.R. Composition and temporal dynamics of insect community in the tundra zone in Changbai Mountains, Northeast China. Acta Entomol. Sin. 2019, 62, 233–240. [Google Scholar] [CrossRef]
- Advani, N.K.; Parmesan, C.; Singer, M.C. Takeoff temperatures in Melitaea cinxia butterflies from latitudinal and elevational range limits: A potential adaptation to solar irradiance. Ecol. Entomol. 2019, 44, 389–396. [Google Scholar] [CrossRef]
- Nève, G.; Després, L. Cold adaptation across the elevation gradient in an alpine butterfly species complex. Ecol. Entomol. 2020, 45, 997–1003. [Google Scholar] [CrossRef]
- Greenslade, P.M.J. Habitat and altitude distribution of Carabidae (Coleoptera) in Argyll. Scotl. Ecol. Entomol. 1968, 120, 39–54. [Google Scholar] [CrossRef]
- Butterfield, J. Carabid life-cycle strategies and climate change: A study on an altitude transect. Ecol. Entomol. 1996, 21, 9–16. [Google Scholar] [CrossRef]
- Hardy, P.B.; Kinder, P.M.; Sparks, T.H.; Dennis, R. Elevation and habitats: The potential of sites at different altitudes to provide refuges for phytophagous insects during climatic fluctuations. J. Insect Conserv. 2010, 14, 297–303. [Google Scholar] [CrossRef]
- Pérez-valencia, L.I.; Moya-raygoza, G. Body size variation of Diaphorina citri (Hemiptera: Psyllidae) through an elevation gradient. Ann. Entomol. Soc. Am. 2015, 5, 800–806. [Google Scholar] [CrossRef]
- Hill, J.K.; Hodkinson, I.D. Effects of temperature on phenological synchrony and altitudinal distribution of jumping plant-lice (Hemiptera: Psylloidea) on dwarf willow (Salix lapponum) in Norway. Ecol. Entomol. 1995, 20, 237–244. [Google Scholar] [CrossRef]
- Sukhodolskaya, R.; Saveliev, A. Body size variation of ground beetles (Coleoptera: Carabidae) in latitudinal gradient. Period. Biol. 2016, 118, 273–280. [Google Scholar] [CrossRef]
- Zettel, J. Alpine Collembola–adaptations and strategies for survival in harsh environments. Zoology 2000, 102, 73–89. [Google Scholar]
- Blanckenhorn, W.U.; Demont, M. Bergmann and converse bergmann latitudinal clines in arthropods: Two ends of a continuum? Integr. Comp. Biol. 2004, 44, 413–424. [Google Scholar] [CrossRef] [PubMed]




| Adjacent Elevation/Vegetation Type | R2 | p-Value | Adjacent Elevation/Vegetation Type | R2 | p-Value |
|---|---|---|---|---|---|
| A1 vs. A2 | 0.539 | 0.029 * | D3 vs. D4 | 0.268 | 0.124 |
| A2 vs. A3 | 0.244 | 0.035 * | D4 vs. D5 | 0.538 | 0.038 * |
| A3 vs. B1 | 0.796 | 0.027 * | D5 vs. D6 | 0.654 | 0.028 * |
| B1 vs. B2 | 0.724 | 0.039 * | D6 vs. D7 | 0.744 | 0.033 * |
| B2 vs. B3 | 0.295 | 0.118 | A vs. B | 0.591 | <0.001 * |
| B3 vs. B4 | 0.190 | 0.271 | A vs. C | 0.711 | <0.001 * |
| B4 vs. C1 | 0.571 | 0.024 * | A vs. D | 0.436 | <0.001 * |
| C1 vs. C2 | 0.718 | 0.024 * | B vs. C | 0.303 | <0.001 * |
| C2 vs. D1 | 0.699 | 0.020 * | B vs. D | 0.236 | <0.001 * |
| D1 vs. D2 | 0.403 | 0.029 * | C vs. D | 0.159 | <0.001 * |
| D2 vs. D3 | 0.794 | 0.027 * |
| Species | Elevation Gradient | Vegetation Type | ||||
|---|---|---|---|---|---|---|
| Elevation (m) | IndVal | p-Value | Vegetation Type | IndVal | p-Value | |
| Carabus granulatus Linnaeus | 750 | 76.9 | <0.001 | A | 66.7 | <0.001 |
| Leistus niger Gebler | 750 | 59.8 | <0.001 | A | 86.7 | <0.001 |
| Pristosia proxima Morawitz | 750 | 54.3 | <0.010 | A | 64.3 | <0.010 |
| Pterostichus pertinax (Tschitscherine) | 750 | 43.2 | <0.001 | A | 85.5 | <0.001 |
| Pterostichus nigrita (Paykull) | 750 | 68.2 | <0.010 | – | – | – |
| Carabus billbergi Mannerheim | 890 | 47.6 | <0.010 | A | 99.8 | <0.001 |
| Carabus wulffiusi Morawitz | 890 | 52.5 | <0.010 | A | 96.8 | <0.001 |
| Carabus fraterculus Reitter | 1050 | 34.1 | <0.010 | A | 96.3 | <0.001 |
| Carabus seishinensis Lapouge | 1050 | 49.5 | <0.001 | A | 58.2 | <0.001 |
| Pterostichus adstrictus Eschscholtz | – | – | – | A | 80.7 | <0.001 |
| Pterostichus aereipennis (Solsky) | – | – | – | A | 58.2 | <0.001 |
| Pterostichus eobius (Tschitscherine) | – | – | – | A | 56.1 | <0.001 |
| Pterostichus gibbicollis (Mostschulsky) | – | – | – | A | 55.7 | <0.001 |
| Pterostichus interruptus (Dejean) | – | – | – | A | 55.7 | <0.001 |
| Carabus canaliculatus Adams | 1200 | 41.9 | <0.001 | – | – | – |
| Pterostichus tuberculiger (Tschitscherine) | 1200 | 88.5 | <0.001 | – | – | – |
| Cychrus morawitzi koltzei Roeschke | 1380 | 55.2 | <0.001 | B | 52.4 | <0.001 |
| Notiophilus aquaticus (Linnaeus) | 1380 | 48.1 | <0.010 | B | 34.4 | <0.050 |
| Pristosia vigil Tschistcherine | 1380 | 45.9 | <0.010 | B | 60.6 | <0.001 |
| Pterostichus horvatovichi Kirschenhofer | 1380 | 40.6 | <0.001 | B | 50.1 | <0.010 |
| Leistus janae Farkac et Plutenko | 1670 | 61.0 | <0.010 | B | 37.4 | <0.010 |
| Peiyuia sp. | 1670 | 41.4 | <0.001 | B | 42.4 | <0.010 |
| Carabus aurocinctus Motschulsky | – | – | – | B | 80.6 | <0.001 |
| Carabus venustus Morawitz | – | – | – | B | 58.2 | <0.001 |
| Morphodactyla coreica (Jedlicka) | 1770 | 35.1 | <0.010 | C | 55.5 | <0.001 |
| Pterostichus microps Heyden | 1870 | 51.6 | <0.001 | C | 80.4 | <0.001 |
| Xestagonum elytroplanum Morvan | 1870 | 93.1 | <0.001 | C | 85.4 | <0.001 |
| Miscodera arctica Paykull | 2340 | 38.5 | <0.050 | D | 42.9 | <0.010 |
| Nebria pektusanica Horratovich | 2420 | 83.8 | <0.001 | D | 62.5 | <0.001 |
| Pterostichus jaechi Kirschenhofer | 2530 | 65.0 | <0.001 | D | 71.4 | <0.001 |
| Carabus macleayi Dejean | 2600 | 45.4 | <0.001 | D | 91.6 | <0.001 |
| Pterostichus comorus Jedlicka | – | – | – | D | 39.1 | <0.050 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Tong, J.; Xu, M.; Meng, Q.; Shi, Y.; Zhao, H.; Li, Y. The Effect of Elevation Gradient on Distribution and Body Size of Carabid Beetles in the Changbaishan Nature Reserve in Northeast Asia. Insects 2024, 15, 688. https://doi.org/10.3390/insects15090688
Liu S, Tong J, Xu M, Meng Q, Shi Y, Zhao H, Li Y. The Effect of Elevation Gradient on Distribution and Body Size of Carabid Beetles in the Changbaishan Nature Reserve in Northeast Asia. Insects. 2024; 15(9):688. https://doi.org/10.3390/insects15090688
Chicago/Turabian StyleLiu, Shengdong, Jiaqi Tong, Mingfeng Xu, Qingfan Meng, Ying Shi, Hongrui Zhao, and Yan Li. 2024. "The Effect of Elevation Gradient on Distribution and Body Size of Carabid Beetles in the Changbaishan Nature Reserve in Northeast Asia" Insects 15, no. 9: 688. https://doi.org/10.3390/insects15090688




