Mitigating the Threat of Invasive Mosquito Species Expansion: A Comprehensive Entomological Surveillance Study on Kastellorizo, a Remote Greek Island
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. KAP (Knowledge, Attitude, Practices) Survey
2.3. Mosquito Collection
2.3.1. Oviposition Traps
2.3.2. Adult Traps
2.3.3. Human Landing Catches
2.4. Molecular Procedure
DNA Extraction and Polymerase Chain Reaction (PCR) Amplification
3. Results
3.1. Questionnaires
3.2. Mosquito Collections
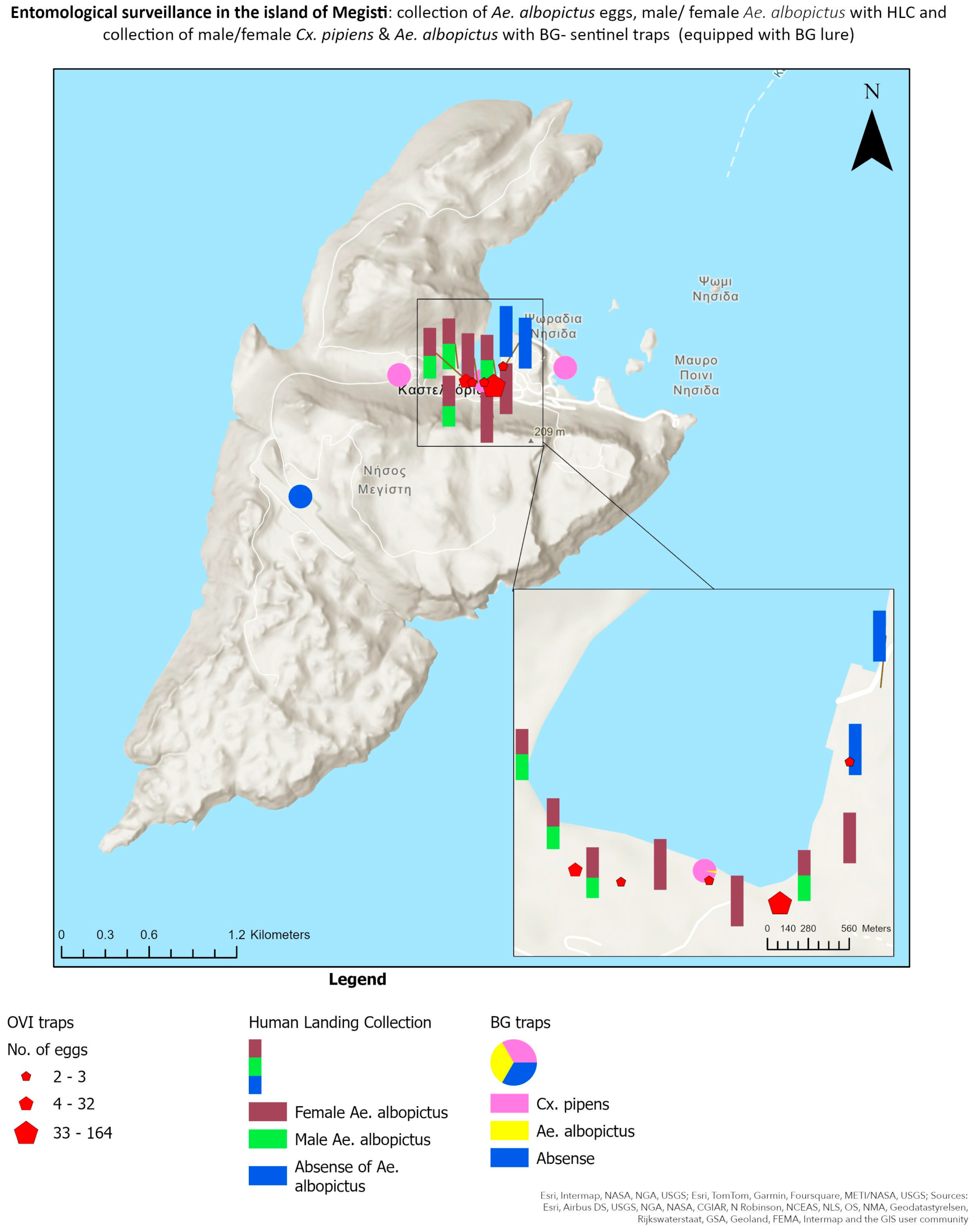

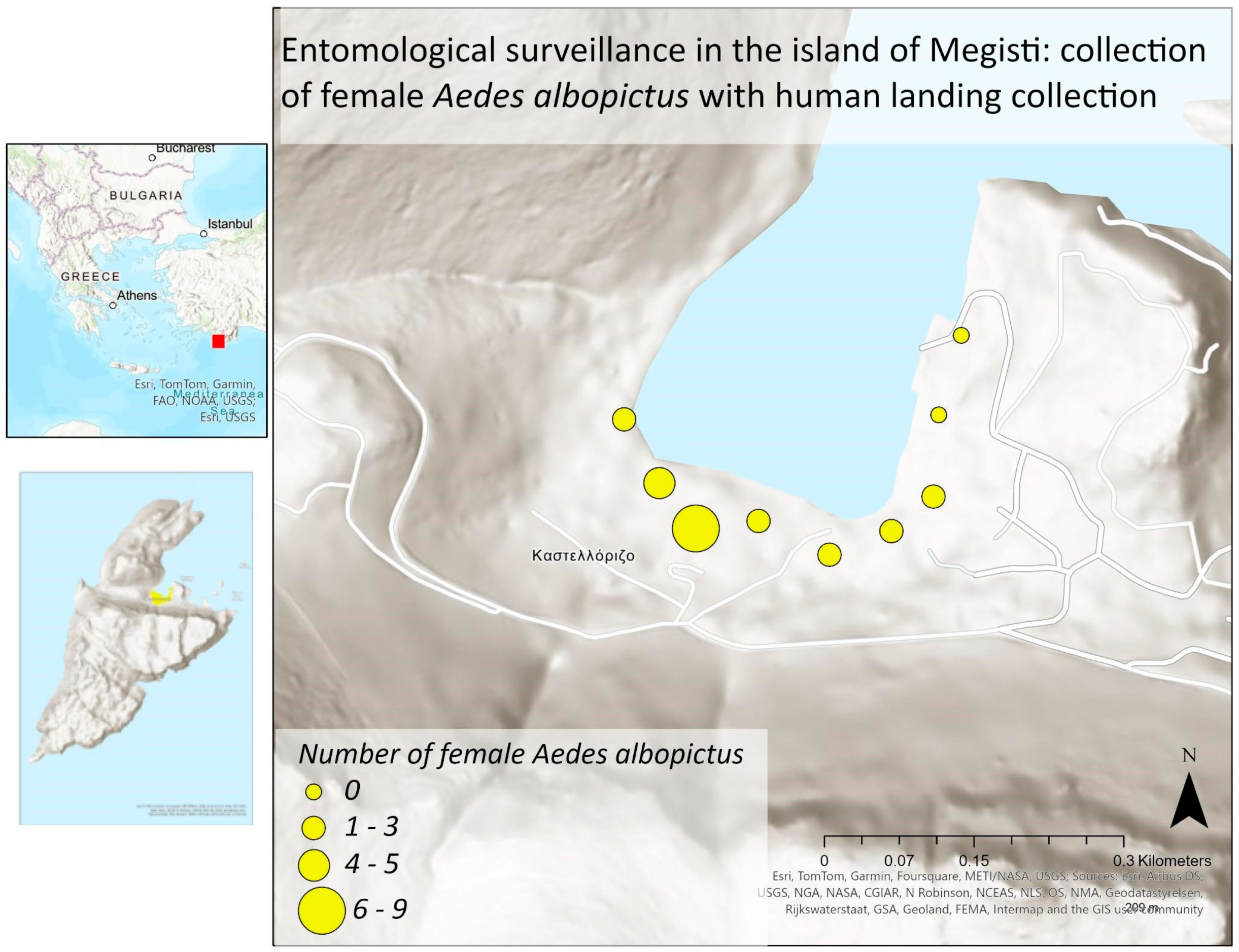
3.3. Molecular Procedures
4. Discussion
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Global Vector Control Response 2017–2030. Available online: https://www.who.int/publications-detail-redirect/9789241512978 (accessed on 2 April 2023).
- Semenza, J.C.; Menne, B. Climate change and infectious diseases in Europe. Lancet Infect. Dis. 2009, 9, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Fauci, A.S.; Morens, D.M. Zika Virus in the Americas. Yet Another Arbovirus Threat. N. Engl. J. Med. 2016, 374, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.U.G.; Reiner, R.C.; Brady, O.J.; Messina, J.P.; Gilbert, M.; Pigott, D.M.; Yi, D.; Johnson, K.; Earl, L.; Marczak, L.B.; et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 2019, 4, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Messina, J.P.; Brady, O.J.; Golding, N.; Kraemer, M.U.G.; Wint, G.R.W.; Ray, S.E.; Pigott, D.M.; Shearer, F.M.; Johnson, K.; Earl, L.; et al. The current and future global distribution and population at risk of dengue. Nat. Microbiol. 2019, 4, 1508–1515. [Google Scholar] [CrossRef]
- Sukhralia, S.; Verma, M.; Gopirajan, S.; Dhanaraj, P.S.; Lal, R.; Mehla, N.; Kant, C.R. From dengue to Zika: The wide spread of mosquito-borne arboviruses. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 3–14. [Google Scholar] [CrossRef]
- Benedict, M.Q.; Levine, R.S.; Hawley, W.A.; Lounibos, L.P. Spread of the tiger: Global risk of invasion by the mosquito Aedes albopictus. Vector Borne Zoonotic Dis. 2007, 7, 76–85. [Google Scholar] [CrossRef]
- Kamgang, B.; Wilson-Bahun, T.A.; Irving, H.; Kusimo, M.O.; Lenga, A.; Wondji, C.S. Geographical distribution of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) and genetic diversity of invading population of Ae. albopictus in the Republic of the Congo. Wellcome Open Res. 2018, 3, 79. [Google Scholar] [CrossRef]
- Medlock, J.; Gandy, S.; Johnston, C.; Handfield, Z.; Neely, K.; Vaux, A. Snap-shot assessment of adult mosquito (Diptera: Culicidae) densities on the Turks and Caicos Islands, February 2022. JEMCA 2022, 41, 1–8. [Google Scholar] [CrossRef]
- ECDC. Aedes aegypti—Factsheet for Experts [Internet]. 2023. Available online: https://www.ecdc.europa.eu/en/disease-vectors/facts/mosquito-factsheets/aedes-aegypti (accessed on 25 September 2023).
- ECDC. Aedes albopictus—Factsheet for Experts [Internet]. 2016. Available online: https://www.ecdc.europa.eu/en/disease-vectors/facts/mosquito-factsheets/aedes-albopictus (accessed on 25 September 2023).
- Brady, O.J.; Godfray, H.C.J.; Tatem, A.J.; Gething, P.W.; Cohen, J.M.; McKenzie, F.E.; Perkins, T.A.; Reiner, R.C., Jr.; Tusting, L.S.; Sinka, M.E.; et al. Vectorial capacity and vector control: Reconsidering sensitivity to parameters for malaria elimination. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 107–117. [Google Scholar] [CrossRef]
- Hu, W.; Clements, A.; Williams, G.; Tong, S. Dengue fever and El Nino/Southern Oscillation in Queensland, Australia: A time series predictive model. Occup. Environ. Med. 2010, 67, 307–311. [Google Scholar] [CrossRef]
- Barrera, R.; Amador, M.; Clark, G.G. Use of the pupal survey technique for measuring Aedes aegypti (Diptera: Culicidae) productivity in Puerto Rico. Am. J. Trop. Med. Hyg. 2006, 74, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Farajollahi, A.; Kesavaraju, B.; Price, D.C.; Williams, G.M.; Healy, S.P.; Gaugler, R.; Nelder, M.P. Field efficacy of BG-Sentinel and industry-standard traps for Aedes albopictus (Diptera: Culicidae) and West Nile virus surveillance. J. Med. Entomol. 2009, 46, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Reiskind, M.H.; Lounibos, L.P. Effects of intraspecific larval competition on adult longevity in the mosquitoes Aedes aegypti and Aedes albopictus. Med. Vet. Entomol. 2009, 23, 62–68. [Google Scholar] [CrossRef]
- Dowling, Z.; Armbruster, P.; LaDeau, S.L.; DeCotiis, M.; Mottley, J.; Leisnham, P.T. Linking mosquito infestation to resident socioeconomic status, knowledge, and source reduction practices in suburban Washington, DC. Ecohealth 2013, 10, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Morse, W.; Izenour, K.; McKenzie, B.; Lessard, S.; Zohdy, S. Perceptions and practices of mosquito-borne diseases in Alabama—Is concern where it should be? BMC Public Health 2019, 19, 987. [Google Scholar] [CrossRef] [PubMed]
- Stefopoulou, A.; Balatsos, G.; Petraki, A.; LaDeau, S.L.; Papachristos, D.; Michaelakis, A. Reducing Aedes albopictus breeding sites through education: A study in urban area. PLoS ONE 2018, 13, e0202451. [Google Scholar] [CrossRef]
- Giatropoulos, A.; Michaelakis, A.; Pontikakos, C. Records of Aedes albopictus and Aedes cretinus (Diptera: Culicidae) in Greece from 2009 to 2011. HPPJ 2012, 5, 49–56. [Google Scholar]
- Medlock, J.M.; Hansford, K.M.; Versteirt, V.; Cull, B.; Kampen, H.; Fontenille, D.; Hendrickx, G.; Zeller, H.; Van Bortel, W.; Schaffner, F. An entomological review of invasive mosquitoes in Europe. Bull. Entomol. Res. 2015, 105, 637–663. [Google Scholar] [CrossRef]
- Badieritakis, Ε.; Papachristos, D.; Latinopoulos, D.; Stefopoulou, A.; Kolimenakis, A.; Bithas, K.; Patsoula, Ε.; Beleri, S.; Maselou, D.; Balatsos, G.; et al. Aedes albopictus (Skuse, 1895) (Diptera: Culicidae) in Greece: 13 years of living with the Asian tiger mosquito. Parasitol. Res. 2018, 117, 453–460. [Google Scholar] [CrossRef]
- Akiner, M.M.; Demirci, B.; Babuadze, G.; Robert, V.; Schaffner, F. Spread of the Invasive Mosquitoes Aedes aegypti and Aedes albopictus in the Black Sea Region Increases Risk of Chikungunya, Dengue, and Zika Outbreaks in Europe. PLoS Negl. Trop. Dis. 2016, 10, e0004664. [Google Scholar]
- Fotakis, E.A.; Mavridis, K.; Kampouraki, A.; Balaska, S.; Tanti, F.; Vlachos, G.; Gewehr, S.; Mourelatos, S.; Papadakis, A.; Kavalou, M.; et al. Mosquito population structure, pathogen surveillance and insecticide resistance monitoring in urban regions of Crete, Greece. PLoS Negl. Trop. Dis. 2022, 16, e0010186. [Google Scholar] [CrossRef] [PubMed]
- ELSTAT. Hellenic Statistical Authority. 2018. Available online: www.elstat.gr (accessed on 25 September 2023).
- Climatology, HNMS, Hellenic National Meteorological Service [Internet]. Available online: http://emy.gr/emy/en/climatology/climatology? (accessed on 9 September 2024).
- Kolimenakis, A.; Tsesmelis, D.; Richardson, C.; Balatsos, G.; Milonas, P.G.; Stefopoulou, A.; Horstick, O.; Yakob, L.; Papachristos, D.P.; Michaelakis, A. Knowledge, Attitudes and Perception of Mosquito Control in Different Citizenship Regimes within and Surrounding the Malakasa Open Accommodation Refugee Camp in Athens, Greece. Int. J. Environ. Res. Public Health 2022, 19, 16900. [Google Scholar] [CrossRef] [PubMed]
- Stefopoulou, A.; LaDeau, S.L.; Syrigou, N.; Balatsos, G.; Karras, V.; Lytra, I.; Boukouvala, E.; Papachristos, D.P.; Milonas, P.G.; Kapranas, A.; et al. Knowledge, Attitude, and Practices Survey in Greece before the Implementation of Sterile Insect Technique against Aedes albopictus. Insects 2021, 12, 212. [Google Scholar] [CrossRef] [PubMed]
- Bisia, M.; Papadopoulos, P.; Filis, S.; Beleri, S.; Tegos, N.; Lamprou, G.K.; Balatsos, G.; Papachristos, D.; Michaelakis, A.; Patsoula, E. Field Evaluation of Commonly Used Adult Mosquito Traps in Greece. Vector Borne Zoonotic Dis. 2023, 23, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Beleri, S.; Balatsos, G.; Karras, V.; Tegos, N.; Sereti, F.; Rachiotis, G.; Hadjichristodoulou, C.; Papadopoulos, N.; Papachristos, D.; Michaelakis, A.; et al. Seasonal Phenological Patterns and Flavivirus Vectorial Capacity of Medically Important Mosquito Species in a Wetland and an Urban Area of Attica, Greece. Trop. Med. Infect. Dis. 2021, 6, 176. [Google Scholar] [CrossRef]
- EU Health Gateways [Internet]. Recommendations for Standar Operating Procedures (SOPs) Development for Vector (Mosquito) Surveillance and Control Activities at Ports and Airports 2022. Available online: https://www.healthygateways.eu/Portals/0/plcdocs/D9_2_EU_HG_SOPs.pdf?ver=2023-02-01-181716-890 (accessed on 25 September 2023).
- WHO [Internet]. Entomological Surveillance for Aedes spp. in the Context of Zika Virus. 2016. Available online: https://www.who.int/publications/i/item/WHO-ZIKV-VC-16.2 (accessed on 25 September 2023).
- Bellini, R.; Michaelakis, A.; Petrić, D.; Schaffner, F.; Alten, B.; Angelini, P.; Aranda, C.; Becker, N.; Carrieri, M.; Di Luca, M.; et al. Practical management plan for invasive mosquito species in Europe: I. Asian tiger mosquito (Aedes albopictus). Travel Med. Infect. Dis. 2020, 35, 101691. [Google Scholar] [CrossRef]
- BG-Sweetscent Mosquito Attractant for Mosquito Traps [Internet]. Available online: https://eu-shop.biogents.com/en/mosquito-trap-accessories/mosquito-attractant-for-mosquito-traps-1x-bg-sweetscent (accessed on 9 September 2024).
- Velo, E.; Balestrino, F.; Kadriaj, P.; Carvalho, D.O.; Dicko, A.; Bellini, R.; Puggioli, A.; Petrić, D.; Michaelakis, A.; Schaffner, F.; et al. A Mark-Release-Recapture Study to EstimateField Performance of Imported Radio-Sterilized Male Aedes albopictus in Albania. Front. Bioeng. Biotechnol. 2022, 10, 833698. [Google Scholar] [CrossRef]
- Becker, N.; Petric, D.; Zgomba, M.; Boase, C.; Madon, M.; Dahl, C.; Kaiser, A. Mosquitoes and Their Control; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Samanidou-Voyadjoglou, A.; Harbach, R.E. Keys to the adult female mosquitoes (Culicidae) of Greece. Eur. Mosq. Bull. 2001, 10, 13–20. [Google Scholar]
- Patsoula, E.; Samanidou-Voyadjoglou, A.; Spanakos, G.; Kremastinou, J.; Nasioulas, G.; Vakalis, N.C. Molecular characterization of the Anopheles maculipennis complex during surveillance for the 2004 Olympic Games in Athens. Med. Vet. Entomol. 2007, 21, 36–43. [Google Scholar] [CrossRef]
- Patsoula, E.; Samanidou-Voyadjoglou, A.; Spanakos, G.; Kremastinou, J.; Nasioulas, G.; Vakalis, N.C. Molecular and morphological characterization of Aedes albopictus in northwestern Greece and differentiation from Aedes cretinus and Aedes aegypti. J. Med. Entomol. 2006, 43, 40–54. [Google Scholar] [CrossRef]
- Darsie, R.F.J.; Samanidou-Voyadjoglou, A. Keys for the identification of the mosquitoes of Greece. J. Am. Mosq. Control Assoc. 1997, 13, 247–254. [Google Scholar] [PubMed]
- Samanidou-Voyadjoglou, A.; Patsoula, E.; Spanakos, G.; Vakalis, N. Confirmation of Aedes albopictus (Skuse) (Diptera: Culicidae) in Greece. Eur. Mosq. Bull. 2005, 19, 10–11. [Google Scholar]
- European Centre for Disease Prevention and Control. Guidelines for the Surveillance of Native Mosquitoes in Europe; ECDC: Stockholm, Sweden, 2014. [Google Scholar]
- Panagopoulou, A.; Tegos, N.; Beleri, S.; Mpimpa, A.; Balatsos, G.; Michaelakis, A.; Hadjichristodoulou, C.; Patsoula, E. Molecular detection of Usutu virus in pools of Culex pipiens mosquitoes in Greece. Acta Trop. 2024, 258, 107330. [Google Scholar] [CrossRef] [PubMed]
- Simonin, Y. Circulation of West Nile Virus and Usutu Virus in Europe: Overview and Challenges. Viruses 2024, 16, 599. [Google Scholar] [CrossRef]
- Balatsos, G.; Beleri, S.; Tegos, N.; Bisia, M.; Karras, V.; Zavitsanou, E.; Papachristos, D.P.; Papadopoulos, N.T.; Michaelakis, A.; Patsoula, A. Overwintering West Nile virus in active Culex pipiens mosquito populations in Greece. Parasites Vectors 2024, 17, 286. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Guidelines for the Surveillance of Invasive Mosquitoes in Europe; ECDC: Stockholm, Sweden, 2012. [Google Scholar]
- Giatropoulos, A.; Balatsos, G.; Lytra, I.; Karras, V.; Koliopoulos, G.; Papachristos, D.; Michaelakis, A. Records of Aedes (Stegomyia) cretinus in Greece before and after the invasion of Aedes (Stegomyia) albopictus (Diptera: Culicidae). EJE 2024, 121, 199–205. [Google Scholar] [CrossRef]
- Giatropoulos, A.; Papachristos, D.; Michaelakis, A.; Kapranas, A.; Emmanouel, N. Laboratory study on larval competition between two related mosquito species: Aedes (Stegomyia) albopictus and Aedes (Stegomyia) cretinus. Acta Trop. 2022, 230, 106389. [Google Scholar] [CrossRef]
- Swan, T.; Russell, T.L.; Staunton, K.M.; Field, M.A.; Ritchie, S.A.; Burkot, T.R. A literature review of dispersal pathways of Aedes albopictus across different spatial scales: Implications for vector surveillance. Parasites Vectors 2022, 15, 303. [Google Scholar] [CrossRef]
- Roiz, D.; Pontifes, P.A.; Jourdain, F.; Diagne, C.; Leroy, B.; Vaissière, A.C.; Tolsá-García, M.J.; Salles, J.M.; Simard, F.; Courchamp, F. The rising global economic costs of invasive Aedes mosquitoes and Aedes-borne diseases. Sci. Total Environ. 2024, 933, 173054. [Google Scholar] [CrossRef]
- Vasquez, M.I.; Notarides, G.; Meletiou, S.; Patsoula, E.; Kavran, M.; Michaelakis, A.; Bellini, R.; Toumazi, T.; Bouyer, J.; Petrić, D. Two invasions at once: Update on the introduction of the invasive species Aedes aegypti and Aedes albopictus in Cyprus—A call for action in Europe. Parasite 2023, 30, 41. [Google Scholar] [CrossRef]
- Kraemer, M.U.G.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.N.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Van Bortel, W.; et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. eLife 2015, 4, e08347. [Google Scholar] [CrossRef] [PubMed]
- Schaffner, F.; Bellini, R.; Petrić, D.; Scholte, E.J.; Zeller, H.; Marrama Rakotoarivony, L. Development of guidelines for the surveillance of invasive mosquitoes in Europe. Parasites Vectors 2013, 6, 209. [Google Scholar] [CrossRef] [PubMed]
- Rezza, G.; Nicoletti, L.; Angelini, R.; Romi, R.; Finarelli, A.C.; Panning, M.; Cordioli, P.; Fortuna, C.; Boros, S.; Magurano, F.; et al. Infection with chikungunya virus in Italy: An outbreak in a temperate region. Lancet 2007, 370, 1840–1846. [Google Scholar] [CrossRef] [PubMed]
- Jeannin, C.; Perrin, Y.; Cornelie, S.; Gloria-Soria, A.; Gauchet, J.D.; Robert, V. An alien in Marseille: Investigations on a single Aedes aegypti mosquito likely introduced by a merchant ship from tropical Africa to Europe. Parasite 2022, 29, 42. [Google Scholar] [CrossRef]
- Wilder-Smith, A.; Quam, M.; Sessions, O.; Rocklov, J.; Liu-Helmersson, J.; Franco, L.; Khan, K. The 2012 dengue outbreak in Madeira: Exploring the origins. Eurosurveillance 2014, 19, 20718. [Google Scholar] [CrossRef]
- Vontas, J.; Kioulos, E.; Pavlidi, N.; Morou, E.; della Torre, A.; Ranson, H. Insecticide resistance in the major dengue vectors Aedes albopictus and Aedes aegypti. Pestic Biochem. Phys. 2012, 104, 126–131. [Google Scholar] [CrossRef]
- Pichler, V.; Caputo, B.; Valadas, V.; Micocci, M.; Horvath, C.; Virgillito, C.; Akiner, M.; Balatsos, G.; Bender, C.; Besnard, G.; et al. Geographic distribution of the V1016G knockdown resistance mutation in Aedes albopictus: A warning bell for Europe. Parasites Vectors 2022, 15, 280. [Google Scholar] [CrossRef]
- Pichler, V.; Bellini, R.; Veronesi, R.; Arnoldi, D.; Rizzoli, A.; Lia, R.P.; Otranto, D.; Montarsi, F.; Carlin, S.; Ballardini, M.; et al. First evidence of resistance to pyrethroid insecticides in Italian Aedes albopictus populations 26 years after invasion. Pest Manag. Sci. 2018, 74, 1319–1327. [Google Scholar] [CrossRef]
- Beebe, N.W.; Ambrose, L.; Hill, L.A.; Davis, J.B.; Hapgood, G.; Cooper, R.D.; Russell, R.C.; Ritchie, S.A.; Reimer, L.J.; Lobo, N.F.; et al. Tracing the tiger: Population genetics provides valuable insights into the Aedes (Stegomyia) albopictus invasion of the Australasian Region. PLoS Negl. Trop. Dis. 2013, 7, e2361. [Google Scholar] [CrossRef]
- Stoddard, S.T.; Morrison, A.C.; Vazquez-Prokopec, G.M.; Soldan, V.P.; Kochel, T.J.; Kitron, U.; Elder, J.P.; Scott, T.W. The Role of Human Movement in the Transmission of Vector-Borne Pathogens. PLoS Negl. Trop. Dis. 2009, 3, e481. [Google Scholar] [CrossRef]
- Bartlett-Healy, K.; Unlu, I.; Obenauer, P.; Hughes, T.; Healy, S.; Crepeau, T.; Farajollahi, A.; Kesavaraju, B.; Fonseca, D.; Schoeler, G.; et al. Larval mosquito habitat utilization and community dynamics of Aedes albopictus and Aedes japonicus (Diptera: Culicidae). J. Med. Entomol. 2012, 49, 813–824. [Google Scholar] [CrossRef]
- Roiz, D.; Wilson, A.L.; Scott, T.W.; Fonseca, D.M.; Jourdain, F.; Müller, P.; Velayudhan, R.; Corbel, V. Integrated Aedes management for the control of Aedes-borne diseases. PLoS Negl. Trop. Dis. 2018, 12, e0006845. [Google Scholar] [CrossRef] [PubMed]
- Andersson, N.; Nava-Aguilera, E.; Arosteguí, J.; Morales-Perez, A.; Suazo-Laguna, H.; Legorreta-Soberanis, J.; Hernandez-Alvarez, C.; Fernandez-Salas, I.; Paredes-Solís, S.; Balmaseda, A.; et al. Evidence based community mobilization for dengue prevention in Nicaragua and Mexico (Camino Verde, the Green Way): Cluster randomized controlled trial. BMJ 2015, 351, h3267. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.P.; AbuBakar, S.; Chinna, K. Community Knowledge, Health Beliefs, Practices and Experiences Related to Dengue Fever and Its Association with IgG Seropositivity. PLoS Negl. Trop. Diss. 2014, 8, e2789. [Google Scholar] [CrossRef] [PubMed]
- Južnič-Zonta, Ž.; Sanpera-Calbet, I.; Eritja, R.; Palmer, J.R.B.; Escobar, A.; Garriga, J.; Oltra, A.; Richter-Boix, A.; Schaffner, F.; Della Torre, A.; et al. Mosquito alert: Leveraging citizen science to create a GBIF mosquito occurrence dataset. Gigabyte 2022. [Google Scholar]
- Eritja, R.; Ruiz-Arrondo, I.; Delacour-Estrella, S.; Schaffner, F.; Álvarez-Chachero, J.; Bengoa, M.; Puig, M.Á.; Melero-Alcíbar, R.; Oltra, A.; Bartumeus, F. First detection of Aedes japonicus in Spain: An unexpected finding triggered by citizen science. Parasites Vectors 2019, 12, 53. [Google Scholar] [CrossRef]
- Mosquito Alert ITALIA 2020−2022: Citizen Engagement, Achievements and Criticisms [Internet]. Available online: https://iris.uniroma1.it/handle/11573/1698000 (accessed on 9 September 2024).
| Demographic Information | Number of Responses (%) |
|---|---|
| Sex | |
| Woman | 20 (65) |
| Man | 11 (35) |
| Type of settlement | |
| Permanent residence | 29 (94) |
| Holiday residence | 2 (6) |
| Ovitraps | Lat | Long | Installation Day | Collection Day | No. of Eggs |
|---|---|---|---|---|---|
| K1 | 36.149806° | 29.592298° | 12 July 2022 | 26 July 2022 | 2 |
| K2 | 36.149044° | 29.589423° | 12 July 2022 | 26 July 2022 | 32 |
| K3 | 36.148920° | 29.589885° | 12 July 2022 | 26 July 2022 | 3 |
| K4 | 36.148889° | 29.590789° | 12 July 2022 | 26 July 2022 | 2 |
| K5 | 36.148671° | 29.591501° | 12 July 2022 | 26 July 2022 | 164 |
| Adult Traps | Location | Lat | Long | Installation Day | Collection Day | Cx. pipiens | Ae. albopictus | Ae. cretinus | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | ||||||
| BG1 | Airport | 36.142480° | 29.576363° | 25 July 2022 | 27 July 2022 | 0 | 1 | 0 | 0 | 0 | 0 |
| BG2 | Public infrastructure | 36.149609° | 29.584378° | 25 July 2022 | 27 July 2022 | 41 | 2 | 0 | 0 | 0 | 0 |
| BG3 | Mandraki | 36.149468° | 29.597007° | 25 July 2022 | 27 July 2022 | 4 | 0 | 0 | 0 | 2 | 1 |
| BG4 | Square (Lazarakis) | 36.148968° | 29.590747° | 25 July 2022 | 27 July 2022 | 28 | 3 | 1 | 0 | 0 | 0 |
| HLC | Lat | Long | Collection Day | Cx. pipiens | Ae. albopictus | ||
|---|---|---|---|---|---|---|---|
| ♀ | ♂ | ♀ | ♂ | ||||
| K_HLC1 | 36.149822° | 29.588870° | 25 July 2022 | 0 | 0 | 1 | 1 |
| K_HLC2 | 36.149230° | 29.589221° | 25 July 2022 | 1 | 2 | 2 | 3 |
| K_HLC3 | 36.148804° | 29.589596° | 25 July 2022 | 0 | 0 | 0 | 0 |
| K_HLC4 | 36.148840° | 29.590294° | 25 July 2022 | 0 | 0 | 0 | 0 |
| K_HLC5 | 36.148499° | 29.591061° | 25 July 2022 | 0 | 0 | 0 | 0 |
| K_HLC6 | 36.148679° | 29.591763° | 25 July 2022 | 0 | 0 | 0 | 0 |
| K_HLC7 | 36.148969° | 29.592251° | 25 July 2022 | 0 | 0 | 0 | 0 |
| K_HLC8 | 36.149701° | 29.592360° | 25 July 2022 | 0 | 0 | 0 | 0 |
| K_HLC9 | 36.150406° | 29.592660° | 25 July 2022 | 0 | 0 | 0 | 0 |
| K_HLC1 | 36.149822° | 29.588870° | 26 July 2022 | 0 | 0 | 0 | 0 |
| K_HLC2 | 36.149230° | 29.589221° | 26 July 2022 | 0 | 0 | 3 | 1 |
| K_HLC3 | 36.148804° | 29.589596° | 26 July 2022 | 0 | 0 | 9 | 6 |
| K_HLC4 | 36.148840° | 29.590294° | 26 July 2022 | 0 | 0 | 3 | 0 |
| K_HLC5 | 36.148499° | 29.591061° | 26 July 2022 | 0 | 0 | 2 | 0 |
| K_HLC6 | 36.148679° | 29.591763° | 26 July 2022 | 0 | 0 | 1 | 1 |
| K_HLC7 | 36.148969° | 29.592251° | 26 July 2022 | 0 | 0 | 2 | 0 |
| K_HLC8 | 36.149701° | 29.592360° | 26 July 2022 | 0 | 0 | 0 | 0 |
| K_HLC9 | 36.150406° | 29.592660° | 26 July 2022 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bisia, M.; Balatsos, G.; Beleri, S.; Tegos, N.; Zavitsanou, E.; LaDeau, S.L.; Sotiroudas, V.; Patsoula, E.; Michaelakis, A. Mitigating the Threat of Invasive Mosquito Species Expansion: A Comprehensive Entomological Surveillance Study on Kastellorizo, a Remote Greek Island. Insects 2024, 15, 724. https://doi.org/10.3390/insects15090724
Bisia M, Balatsos G, Beleri S, Tegos N, Zavitsanou E, LaDeau SL, Sotiroudas V, Patsoula E, Michaelakis A. Mitigating the Threat of Invasive Mosquito Species Expansion: A Comprehensive Entomological Surveillance Study on Kastellorizo, a Remote Greek Island. Insects. 2024; 15(9):724. https://doi.org/10.3390/insects15090724
Chicago/Turabian StyleBisia, Marina, Georgios Balatsos, Stavroula Beleri, Nikolaos Tegos, Evangelia Zavitsanou, Shannon L. LaDeau, Vasilis Sotiroudas, Eleni Patsoula, and Antonios Michaelakis. 2024. "Mitigating the Threat of Invasive Mosquito Species Expansion: A Comprehensive Entomological Surveillance Study on Kastellorizo, a Remote Greek Island" Insects 15, no. 9: 724. https://doi.org/10.3390/insects15090724
APA StyleBisia, M., Balatsos, G., Beleri, S., Tegos, N., Zavitsanou, E., LaDeau, S. L., Sotiroudas, V., Patsoula, E., & Michaelakis, A. (2024). Mitigating the Threat of Invasive Mosquito Species Expansion: A Comprehensive Entomological Surveillance Study on Kastellorizo, a Remote Greek Island. Insects, 15(9), 724. https://doi.org/10.3390/insects15090724







