Using Insect Larvae and Their Microbiota for Plastic Degradation
Simple Summary
Abstract
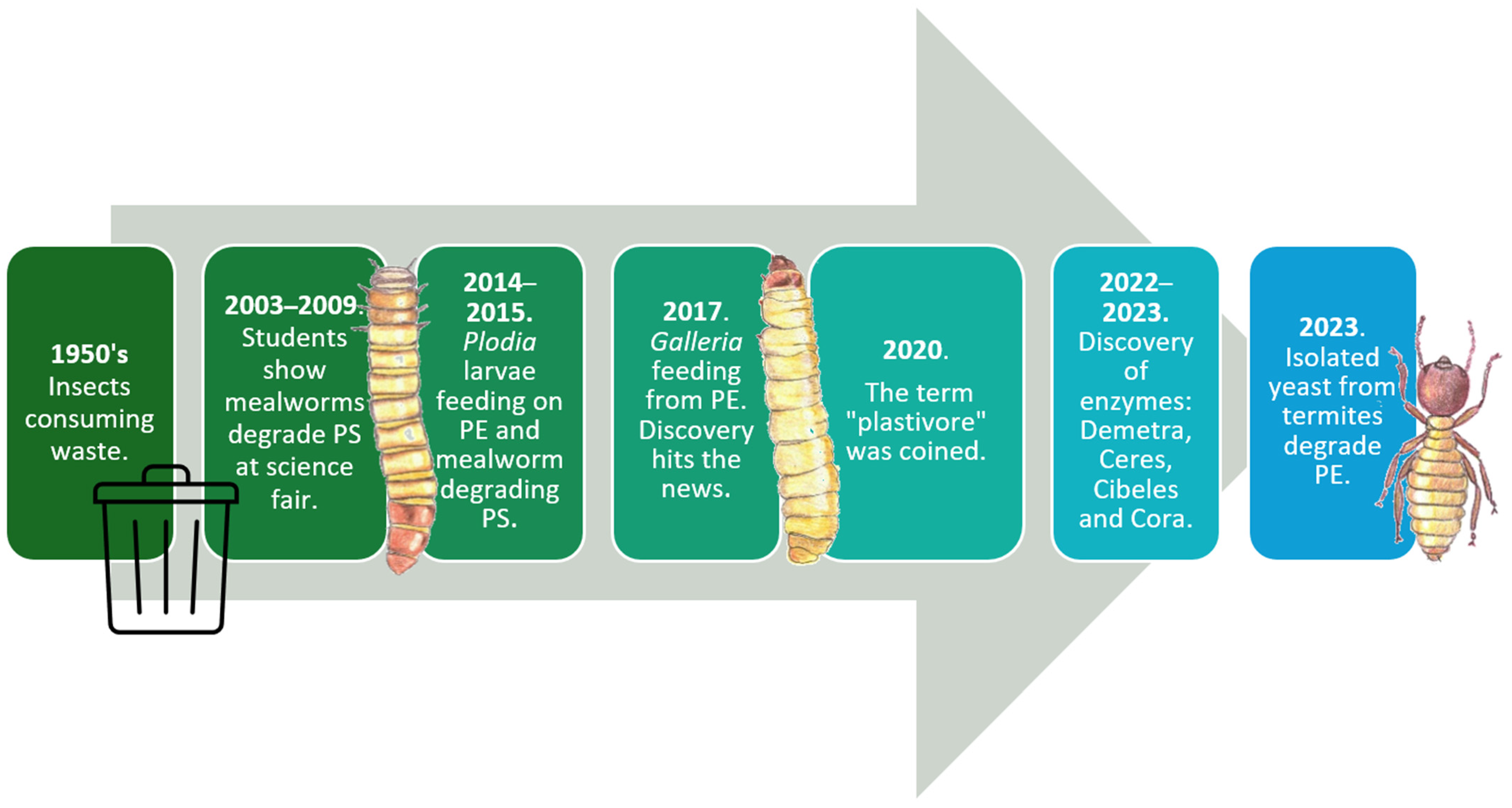
1. Introduction
2. Plastic Pollution
3. Degradation of Plastics—A General Perspective
Microbial Degradation of Plastics
4. Insect Plastic Degradation—Order: Lepidoptera (Butterflies and Moths)
The Waxworm Galleria mellonella (Fabricius, 1798) [Lepidoptera: Pyralidae] Degrades Plastic

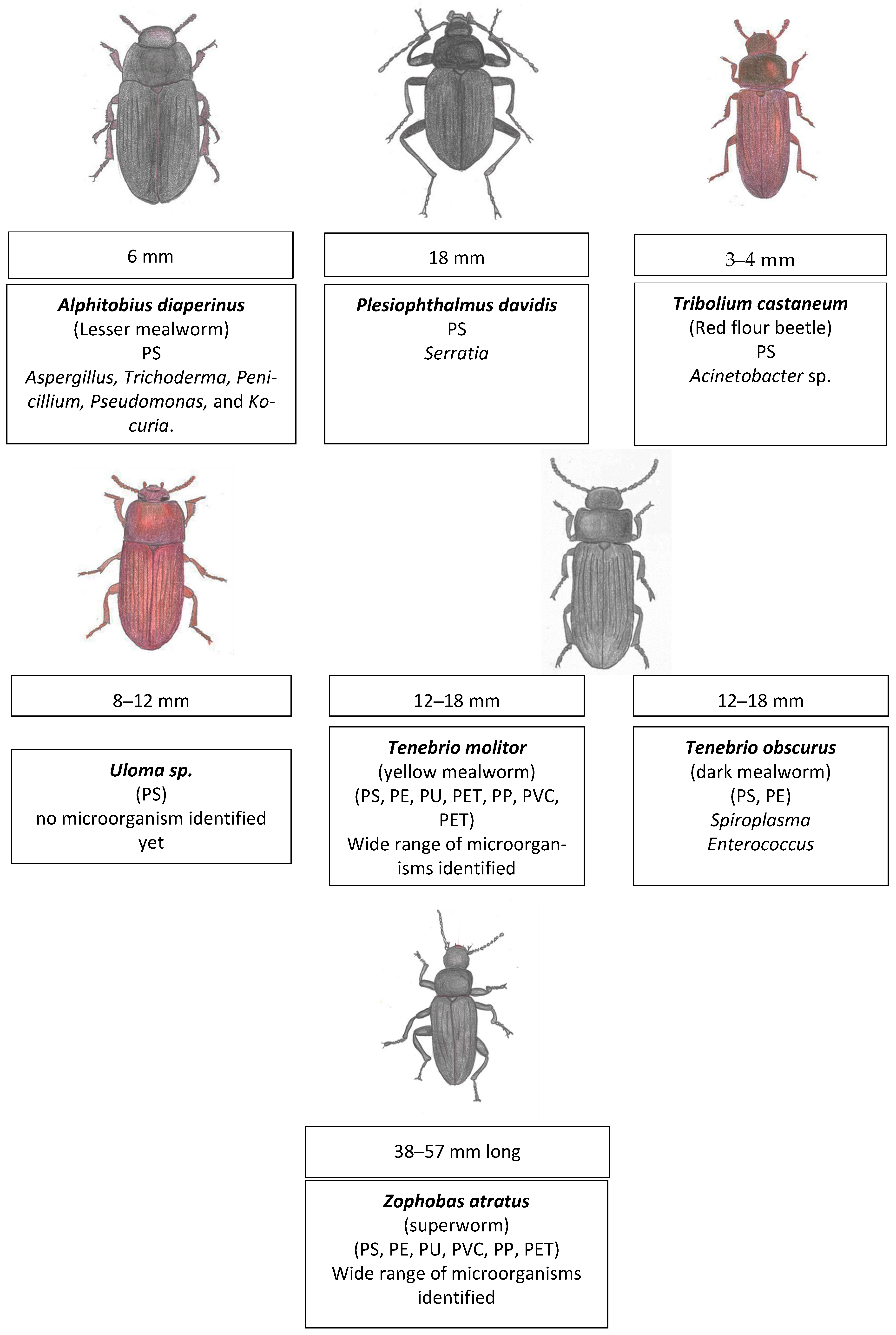
5. Insect Plastic Degradation—Order: Coleoptera (Beetles and Weevils) [111]
5.1. The Yellow Mealworm Tenebrio molitor (Linnaeus, 1758) [Coleoptera: Tenebrionidae] Degrades Plastic
5.2. The Superworm Zophobas atratus (Fabricius, 1776) [Coleoptera: Tenebrionidae] Degrades Plastic
6. Insect Plastic Degradation—Order: Blattodea (Cockroaches and Termites) [18,87,88,171,172]
7. Other Orders from the Class Insecta That Degrade Plastic
8. Analysis of Plastic Degradation After Exposure to Insect Larvae
9. Challenges and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nikiema, J.; Asiedu, Z. A review of the cost and effectiveness of solutions to address plastic pollution. Environ. Sci. Pollut. Res. 2022, 29, 24547–24573. [Google Scholar] [CrossRef]
- MacLeod, M.; Arp, H.P.H.; Tekman, M.B.; Jahnke, A. The global threat from plastic pollution. Science 2021, 373, 61–65. [Google Scholar] [CrossRef]
- Bergeson, A.R.; Bergeson, A.R.; Bergeson, A.R.; Silvera, A.J.; Silvera, A.J.; Silvera, A.J.; Alper, H.S.; Alper, H.S.; Alper, H.S. Bottlenecks in biobased approaches to plastic degradation. Nat. Commun. 2024, 15, 4715. [Google Scholar] [CrossRef] [PubMed]
- Pivato, A.F.; Miranda, G.M.; Prichula, J.; Lima, J.E.; Ligabue, R.A.; Seixas, A.; Trentin, D.S. Hydrocarbon-based plastics: Progress and perspectives on consumption and biodegradation by insect larvae. Chemosphere 2022, 293, 133600. [Google Scholar] [CrossRef] [PubMed]
- Essig, E.O.; Hoskins, W.M.; Linsley, E.G.; Micrelbacher, A.E.; Smith, R.F. A Report on the Penetration of Packaging Materials by Insects. J. Econ. Èntomol. 1943, 36, 822–829. [Google Scholar] [CrossRef]
- Gerhardt, P.D.; Lindgren, D.L. Penetration of packaging films: Film materials used for food packaging tested for resistance to some common stored-product insects. Hilgardia 1954, 8, 3–4. [Google Scholar] [CrossRef]
- Yang, S.-S.; Wu, W.-M.; Bertocchini, F.; Benbow, M.E.; Devipriya, S.P.; Cha, H.J.; Peng, B.-Y.; Ding, M.-Q.; He, L.; Li, M.-X.; et al. Radical innovation breakthroughs of biodegradation of plastics by insects: History, present and future perspectives. Front. Environ. Sci. Eng. 2024, 18, 781–839. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, J.; Wu, W.-M.; Zhao, J.; Yang, J. Complete genome sequence of Bacillus sp. YP1, a polyethylene-degrading bacterium from waxworm’s gut. J. Biotechnol. 2015, 200, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, Y.; Wu, W.-M.; Zhao, J.; Jiang, L. Evidence of Polyethylene Biodegradation by Bacterial Strains from the Guts of Plastic-Eating Waxworms. Environ. Sci. Technol. 2014, 48, 13776–13784. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, J.; Wu, W.-M.; Zhao, J.; Song, Y.; Gao, L.; Yang, R.; Jiang, L. Biodegradation and Mineralization of Polystyrene by Plastic-Eating Mealworms: Part 1. Chemical and Physical Characterization and Isotopic Tests. Environ. Sci. Technol. 2015, 49, 12080–12086. [Google Scholar] [CrossRef]
- Bombelli, P.; Howe, C.J.; Bertocchini, F. Polyethylene bio-degradation by caterpillars of the wax moth Galleria mellonella. Curr. Biol. 2017, 27, R292–R293. [Google Scholar] [CrossRef]
- Arnold, C. This Bug Can Eat Plastic. But Can It Clean Up Our Mess? National Geographic, 2017. Available online: https://www.nationalgeographic.com/science/article/wax-worms-eat-plastic-polyethylene-trash-pollution-cleanup (accessed on 21 January 2025).
- Sanluis-Verdes, A.; Colomer-Vidal, P.; Rodriguez-Ventura, F.; Bello-Villarino, M.; Spinola-Amilibia, M.; Ruiz-Lopez, E.; Illanes-Vicioso, R.; Castroviejo, P.; Cigliano, R.A.; Montoya, M.; et al. Wax worm saliva and the enzymes therein are the key to polyethylene degradation by Galleria mellonella. Nat. Commun. 2022, 13, 5568. [Google Scholar] [CrossRef]
- Spínola-Amilibia, M.; Illanes-Vicioso, R.; Ruiz-López, E.; Colomer-Vidal, P.; Rodriguez-Ventura, F.; Pérez, R.P.; Arias, C.F.; Torroba, T.; Solà, M.; Arias-Palomo, E.; et al. Plastic degradation by insect hexamerins: Near-atomic resolution structures of the polyethylene-degrading proteins from the wax worm saliva. Sci. Adv. 2023, 9, eadi6813. [Google Scholar] [CrossRef]
- Cassone, B.J.; Grove, H.C.; Elebute, O.; Villanueva, S.M.P.; LeMoine, C.M.R. Role of the intestinal microbiome in low-density polyethylene degradation by caterpillar larvae of the greater wax moth, Galleria mellonella. Proc. R. Soc. B Biol. Sci. 2020, 287, 20200112. [Google Scholar] [CrossRef]
- Elsamahy, T.; Sun, J.; Elsilk, S.E.; Ali, S.S. Biodegradation of low-density polyethylene plastic waste by a constructed tri-culture yeast consortium from wood-feeding termite: Degradation mechanism and pathway. J. Hazard. Mater. 2023, 448, 130944. [Google Scholar] [CrossRef]
- Sanchez-Hernandez, J.C. A toxicological perspective of plastic biodegradation by insect larvae. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 248, 109117. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Manap, A.S.A.; Kolobe, S.D.; Monnye, M.; Yudhistira, B.; Fernando, I. Insects for plastic biodegradation—A review. Process. Saf. Environ. Prot. 2024, 186, 833–849. [Google Scholar] [CrossRef]
- Xu, L.; Li, Z.; Wang, L.; Xu, Z.; Zhang, S.; Zhang, Q. Progress in polystyrene biodegradation by insect gut microbiota. World J. Microbiol. Biotechnol. 2024, 40, 143. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Liu, C.; Wang, J.; Jia, P. Recent Advances in Degradation of Polymer Plastics by Insects Inhabiting Microorganisms. Polymers 2023, 15, 1307. [Google Scholar] [CrossRef]
- Goveas, L.C.; Nayak, S.; Kumar, P.S.; Rangasamy, G.; Vidya, S.; Vinayagam, R.; Selvaraj, R.; Vo, D.V.N. Microplastics occurrence, detection and removal with emphasis on insect larvae gut microbiota. Mar. Pollut. Bull. 2023, 188, 114580. [Google Scholar] [CrossRef]
- Elias, H.-G.; Mülhaupt, R. Plastics, General Survey, 1. Definition, Molecular Structure and Properties. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Hoboken, NJ, USA, 2015; pp. 1–70. [Google Scholar]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Mohanan, N.; Montazer, Z.; Sharma, P.K.; Levin, D.B. Microbial and Enzymatic Degradation of Synthetic Plastics. Front. Microbiol. 2020, 11, 580709. [Google Scholar] [CrossRef]
- Telmo, O. Polymers and the Environment. In Polymer Science; Faris, Y., Ed.; IntechOpen: Rijeka, Croatia, 2013; p. Ch. 1. [Google Scholar]
- PlasticsEurope. Plastics—The Facts 2019: An Analysis of European Plastics Production, Demand and Waste Data. Available online: https://plasticseurope.org/wp-content/uploads/2021/10/2019-Plastics-the-facts.pdf (accessed on 28 June 2024).
- Cregut, M.; Bedas, M.; Durand, M.-J.; Thouand, G. New insights into polyurethane biodegradation and realistic prospects for the development of a sustainable waste recycling process. Biotechnol. Adv. 2013, 31, 1634–1647. [Google Scholar] [CrossRef]
- Woo, S.; Song, I.; Cha, H.J. Fast and Facile Biodegradation of Polystyrene by the Gut Microbial Flora of Plesiophthalmus davidis Larvae. Appl. Environ. Microbiol. 2020, 86, e01361-20. [Google Scholar] [CrossRef]
- Kundungal, H.; Synshiang, K.; Devipriya, S.P. Biodegradation of polystyrene wastes by a newly reported honey bee pest Uloma sp. larvae: An insight to the ability of polystyrene-fed larvae to complete its life cycle. Environ. Challenges 2021, 4, 100083. [Google Scholar] [CrossRef]
- Lou, Y.; Ekaterina, P.; Yang, S.-S.; Lu, B.; Liu, B.; Ren, N.; Corvini, P.F.-X.; Xing, D. Biodegradation of Polyethylene and Polystyrene by Greater Wax Moth Larvae (Galleria mellonella L.) and the Effect of Co-diet Supplementation on the Core Gut Microbiome. Environ. Sci. Technol. 2020, 54, 2821–2831. [Google Scholar] [CrossRef]
- Zalasiewicz, J.; Waters, C.N.; Sul, J.A.I.D.; Corcoran, P.L.; Barnosky, A.D.; Cearreta, A.; Edgeworth, M.; Gałuszka, A.; Jeandel, C.; Leinfelder, R.; et al. The geological cycle of plastics and their use as a stratigraphic indicator of the Anthropocene. Anthropocene 2016, 13, 4–17. [Google Scholar] [CrossRef]
- Crutzen, P.J. The “Anthropocene”. In Earth System Science in the Anthropocene; Ehlers, E., Krafft, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 13–18. [Google Scholar]
- Laist, D.W. Impacts of Marine Debris: Entanglement of Marine Life in Marine Debris Including a Comprehensive List of Species with Entanglement and Ingestion Records. In Marine Debris: Sources, Impacts, and Solutions; Coe, J.M., Rogers, D.B., Eds.; Springer: New York, NY, USA, 1997; pp. 99–139. [Google Scholar]
- Zhang, K.; Hamidian, A.H.; Tubić, A.; Zhang, Y.; Fang, J.K.; Wu, C.; Lam, P.K. Understanding plastic degradation and microplastic formation in the environment: A review. Environ. Pollut. 2021, 274, 116554. [Google Scholar] [CrossRef]
- Wu, P.; Huang, J.; Zheng, Y.; Yang, Y.; Zhang, Y.; He, F.; Chen, H.; Quan, G.; Yan, J.; Li, T.; et al. Environmental occurrences, fate, and impacts of microplastics. Ecotoxicol. Environ. Saf. 2019, 184, 109612. [Google Scholar] [CrossRef]
- Vethaak, A.D.; Legler, J. Microplastics and human health. Science 2021, 371, 672–674. [Google Scholar] [CrossRef]
- Zhao, B.; Rehati, P.; Yang, Z.; Cai, Z.; Guo, C.; Li, Y. The potential toxicity of microplastics on human health. Sci. Total Environ. 2023, 912, 168946. [Google Scholar] [CrossRef] [PubMed]
- Zurub, R.E.; Cariaco, Y.; Wade, M.G.; Bainbridge, S.A. Microplastics exposure: Implications for human fertility, pregnancy and child health. Front. Endocrinol. 2024, 14, 1330396. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Manna, C.; Padha, S.; Verma, A.; Sharma, P.; Dhar, A.; Ghosh, A.; Bhattacharya, P. Micro(nano)plastics pollution and human health: How plastics can induce carcinogenesis to humans? Chemosphere 2022, 298, 134267. [Google Scholar] [CrossRef]
- Ziani, K.; Ioniță-Mîndrican, C.-B.; Mititelu, M.; Neacșu, S.M.; Negrei, C.; Moroșan, E.; Drăgănescu, D.; Preda, O.-T. Microplastics: A Real Global Threat for Environment and Food Safety: A State of the Art Review. Nutrients 2023, 15, 617. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xie, E.; Du, Z.; Peng, Z.; Han, Z.; Li, L.; Zhao, R.; Qin, Y.; Xue, M.; Li, F.; et al. Detection of Various Microplastics in Patients Undergoing Cardiac Surgery. Environ. Sci. Technol. 2023, 57, 10911–10918. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Wu, S.; Wei, G. Adverse effects of microplastics and nanoplastics on the reproductive system: A comprehensive review of fertility and potential harmful interactions. Sci. Total Environ. 2023, 903, 166258. [Google Scholar] [CrossRef]
- United Nations Development Programme. Why Aren’t We Recycling More Plastic? United Nations Development Programme. Available online: https://stories.undp.org/why-arent-we-recycling-more-plastic#:~:text=Recycling%20rates%20vary%20by%20location,Some%2012%20percent%20is%20incinerated (accessed on 28 November 2023).
- Yang, Z.; Lü, F.; Zhang, H.; Wang, W.; Shao, L.; Ye, J.; He, P. Is incineration the terminator of plastics and microplastics? J. Hazard. Mater. 2021, 401, 123429. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Yang, H.; Park, S.-A.; Sung, H.K.; Koo, J.M.; Hwang, S.Y.; Jeon, H.; Oh, D.X.; Park, J. Analysis of volatile organic compounds produced during incineration of non-degradable and biodegradable plastics. Chemosphere 2022, 303, 134946. [Google Scholar] [CrossRef] [PubMed]
- Dhali, S.L.; Parida, D.; Kumar, B.; Bala, K. Recent trends in microbial and enzymatic plastic degradation: A solution for plastic pollution predicaments. Biotechnol. Sustain. Mater. 2024, 1, 11. [Google Scholar] [CrossRef]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef] [PubMed]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Fairbrother, A.; Hsueh, H.-C.; Kim, J.H.; Jacobs, D.; Perry, L.; Goodwin, D.; White, C.; Watson, S.; Sung, L.-P. Temperature and light intensity effects on photodegradation of high-density polyethylene. Polym. Degrad. Stab. 2019, 165, 153–160. [Google Scholar] [CrossRef]
- Yousif, E.; Haddad, R. Photodegradation and photostabilization of polymers, especially polystyrene: Review. SpringerPlus 2013, 2, 398. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Hiraga, K.; Takehana, T.; Taniguchi, I.; Yamaji, H.; Maeda, Y.; Toyohara, K.; Miyamoto, K.; Kimura, Y.; Oda, K. A bacterium that degrades and assimilates poly(ethylene terephthalate). Science 2016, 351, 1196–1199. [Google Scholar] [CrossRef]
- Austin, H.P.; Allen, M.D.; Donohoe, B.S.; Rorrer, N.A.; Kearns, F.L.; Silveira, R.L.; Pollard, B.C.; Dominick, G.; Duman, R.; El Omari, K.; et al. Characterization and engineering of a plastic-degrading aromatic polyesterase. Proc. Natl. Acad. Sci. USA 2018, 115, E4350–E4357. [Google Scholar] [CrossRef]
- Danso, D.; Schmeisser, C.; Chow, J.; Zimmermann, W.; Wei, R.; Leggewie, C.; Li, X.; Hazen, T.; Streit, W.R. New Insights into the Function and Global Distribution of Polyethylene Terephthalate (PET)-Degrading Bacteria and Enzymes in Marine and Terrestrial Metagenomes. Appl. Environ. Microbiol. 2018, 84. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, I.E.M.; Wu, P.; Zhao, Y.; Liu, W.; Neumann-Schaal, M.; Pfaff, L.; Barys, J.; Li, Z.; Gao, J.; Han, X.; et al. Molecular and Biochemical Differences of the Tandem and Cold-Adapted PET Hydrolases Ple628 and Ple629, Isolated From a Marine Microbial Consortium. Front. Bioeng. Biotechnol. 2022, 10, 930140. [Google Scholar] [CrossRef] [PubMed]
- Erickson, E.; Gado, J.E.; Avilán, L.; Bratti, F.; Brizendine, R.K.; Cox, P.A.; Gill, R.; Graham, R.; Kim, D.-J.; König, G.; et al. Sourcing thermotolerant poly(ethylene terephthalate) hydrolase scaffolds from natural diversity. Nat. Commun. 2022, 13, 7850. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Yan, W.; Cao, Z.; Ding, M.; Yuan, Y. Current Advances in the Biodegradation and Bioconversion of Polyethylene Terephthalate. Microorganisms 2021, 10, 39. [Google Scholar] [CrossRef]
- Hollis, J.M.; Lovas, F.J.; Jewell, P.R.; Coudert, L.H. Interstellar Antifreeze: Ethylene Glycol. Astrophys. J. 2002, 571, L59–L62. [Google Scholar] [CrossRef]
- Esfe, M.H.; Saedodin, S.; Mahian, O.; Wongwises, S. Efficiency of ferromagnetic nanoparticles suspended in ethylene glycol for applications in energy devices: Effects of particle size, temperature, and concentration. Int. Commun. Heat Mass Transf. 2014, 58, 138–146. [Google Scholar] [CrossRef]
- Westover, C.C.; Long, T.E. Envisioning a BHET Economy: Adding Value to PET Waste. Sustain. Chem. 2023, 4, 363–393. [Google Scholar] [CrossRef]
- Qiu, L.; Yin, X.; Liu, T.; Zhang, H.; Chen, G.; Wu, S. Biodegradation of bis(2-hydroxyethyl) terephthalate by a newly isolated Enterobacter sp. HY1 and characterization of its esterase properties. J. Basic Microbiol. 2020, 60, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Gambarini, V.; Pantos, O.; Kingsbury, J.M.; Weaver, L.; Handley, K.M.; Lear, G. Phylogenetic Distribution of Plastic-Degrading Microorganisms. mSystems 2021, 6. [Google Scholar] [CrossRef]
- Ekanayaka, A.H.; Tibpromma, S.; Dai, D.; Xu, R.; Suwannarach, N.; Stephenson, S.L.; Dao, C.; Karunarathna, S.C. A Review of the Fungi That Degrade Plastic. J. Fungi 2022, 8, 772. [Google Scholar] [CrossRef] [PubMed]
- Montazer, Z.; Najafi, M.B.H.; Levin, D.B. Microbial degradation of low-density polyethylene and synthesis of polyhydroxyalkanoate polymers. Can. J. Microbiol. 2019, 65, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Montazer, Z.; Najafi, M.B.H.; Levin, D.B. In vitro degradation of low-density polyethylene by new bacteria from larvae of the greater wax moth, Galleria mellonella. Can. J. Microbiol. 2021, 67, 249–258. [Google Scholar] [CrossRef]
- O’Leary, N.D.; O’Connor, K.E.; Ward, P.; Goff, M.; Dobson, A.D.W. Genetic Characterization of Accumulation of Polyhydroxyalkanoate from Styrene in Pseudomonas putida CA-3. Appl. Environ. Microbiol. 2005, 71, 4380–4387. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, P.; Zheng, Y.; Li, Q.; Bian, J.; Liang, Q.; Su, T.; Dian, L.; Qi, Q. Unique Raoultella species isolated from petroleum contaminated soil degrades polystyrene and polyethylene. Ecotoxicol. Environ. Saf. 2023, 263, 115232. [Google Scholar] [CrossRef]
- Gambarini, V.; Pantos, O.; Kingsbury, J.M.; Weaver, L.; Handley, K.M.; Lear, G. PlasticDB: A database of microorganisms and proteins linked to plastic biodegradation. Database 2022, 2022. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.; Kaur, A.; Khatri, M.; Singh, G.; Arya, S.K. A review on cutinases enzyme in degradation of microplastics. J. Environ. Manag. 2023, 347, 119193. [Google Scholar] [CrossRef] [PubMed]
- Oda, M.; Numoto, N.; Bekker, G.-J.; Kamiya, N.; Kawai, F. Chapter Eight—Cutinases from thermophilic bacteria (actinomycetes): From identification to functional and structural characterization. In Methods Enzymol; Weber, G., Bornscheuer, U.T., Wei, R., Eds.; Academic Press: New York, NY, USA, 2021; Volume 648, pp. 159–185. [Google Scholar]
- Mamtimin, T.; Ouyang, X.; Wu, W.-M.; Zhou, T.; Hou, X.; Khan, A.; Liu, P.; Zhao, Y.-L.; Tang, H.; Criddle, C.S.; et al. Novel Feruloyl Esterase for the Degradation of Polyethylene Terephthalate (PET) Screened from the Gut Microbiome of Plastic-Degrading Mealworms (Tenebrio Molitor Larvae). Environ. Sci. Technol. 2024, 58, 17717–17731. [Google Scholar] [CrossRef]
- Zrimec, J.; Kokina, M.; Jonasson, S.; Zorrilla, F.; Zelezniak, A. Plastic-Degrading Potential across the Global Microbiome Correlates with Recent Pollution Trends. mBio 2021, 12, e0215521. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.; Schrader, H.; Profe, J.; Dresler, K.; Deckwer, W. Enzymatic Degradation of Poly(ethylene terephthalate): Rapid Hydrolyse using a Hydrolase from T. fusca. Macromol. Rapid Commun. 2005, 26, 1400–1405. [Google Scholar] [CrossRef]
- Son, H.F.; Cho, I.J.; Joo, S.; Seo, H.; Sagong, H.-Y.; Choi, S.Y.; Lee, S.Y.; Kim, K.-J. Rational Protein Engineering of Thermo-Stable PETase from Ideonella sakaiensis for Highly Efficient PET Degradation. ACS Catal. 2019, 9, 3519–3526. [Google Scholar] [CrossRef]
- Bell, E.L.; Smithson, R.; Kilbride, S.; Foster, J.; Hardy, F.J.; Ramachandran, S.; Tedstone, A.A.; Haigh, S.J.; Garforth, A.A.; Day, P.J.R.; et al. Directed evolution of an efficient and thermostable PET depolymerase. Nat. Catal. 2022, 5, 673–681. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, Y.; Liu, X.; Dong, S.; Tian, Y.; Qiao, Y.; Mitra, R.; Han, J.; Li, C.; Han, X.; et al. Computational Redesign of a PETase for Plastic Biodegradation under Ambient Condition by the GRAPE Strategy. ACS Catal. 2021, 11, 1340–1350. [Google Scholar] [CrossRef]
- Lu, H.; Diaz, D.J.; Czarnecki, N.J.; Zhu, C.; Kim, W.; Shroff, R.; Acosta, D.J.; Alexander, B.R.; Cole, H.O.; Zhang, Y.; et al. Machine learning-aided engineering of hydrolases for PET depolymerization. Nature 2022, 604, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Di Rocco, G.; Taunt, H.N.; Berto, M.; Jackson, H.O.; Piccinini, D.; Carletti, A.; Scurani, G.; Braidi, N.; Purton, S. A PETase enzyme synthesised in the chloroplast of the microalga Chlamydomonas reinhardtii is active against post-consumer plastics. Sci. Rep. 2023, 13, 10028. [Google Scholar] [CrossRef]
- Mallet, J. Taxonomy of Lepidoptera: The Scale of the Problem. University College. Available online: https://www.ucl.ac.uk/taxome/lepnos.html (accessed on 25 July 2024).
- Powell, J.A. Chapter 151—Lepidoptera: Moths, Butterflies. In Encyclopedia of Insects, 2nd ed.; Resh, V.H., Cardé, R.T., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 559–587. [Google Scholar]
- Kundungal, H.; Gangarapu, M.; Sarangapani, S.; Patchaiyappan, A.; Devipriya, S.P. Efficient biodegradation of polyethylene (HDPE) waste by the plastic-eating lesser waxworm (Achroia grisella). Environ. Sci. Pollut. Res. 2019, 26, 18509–18519. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Elsamahy, T.; Zhu, D.; Sun, J. Biodegradability of polyethylene by efficient bacteria from the guts of plastic-eating waxworms and investigation of its degradation mechanism. J. Hazard. Mater. 2022, 443, 130287. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Fu, R.; Long, T.; Fan, B.; Guo, C.; Li, L.; Zhang, J.; Zhang, G. Biodegradation of polyethylene by Meyerozyma guilliermondii and Serratia marcescens isolated from the gut of waxworms (larvae of Plodia interpunctella). Sci. Total Environ. 2022, 853, 158604. [Google Scholar] [CrossRef] [PubMed]
- Kesti, S.S.; Thimmappa, S.C. First report on biodegradation of low density polyethylene by rice moth larvae, Corcyra cephalonica (stainton). Holist. Approach Environ. 2019, 9, 79–83. [Google Scholar] [CrossRef]
- Zhang, Z.; Peng, H.; Yang, D.; Zhang, G.; Zhang, J.; Ju, F. Polyvinyl chloride degradation by a bacterium isolated from the gut of insect larvae. Nat. Commun. 2022, 13, 5360. [Google Scholar] [CrossRef] [PubMed]
- Kwadha, C.A.; Ong’amo, G.O.; Ndegwa, P.N.; Raina, S.K.; Fombong, A.T. The Biology and Control of the Greater Wax Moth, Galleria mellonella. Insects 2017, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Kundungal, H.; Amal, R.; Devipriya, S.P. Nature’s Solution to Degrade Long-Chain Hydrocarbons: A Life Cycle Study of Beeswax and Plastic-Eating Insect Larvae. J. Polym. Environ. 2024, 33, 483–496. [Google Scholar] [CrossRef]
- Kong, H.G.; Kim, H.H.; Chung, J.-H.; Jun, J.; Lee, S.; Kim, H.-M.; Jeon, S.; Park, S.G.; Bhak, J.; Ryu, C.-M. The Galleria mellonella Hologenome Supports Microbiota-Independent Metabolism of Long-Chain Hydrocarbon Beeswax. Cell Rep. 2019, 26, 2451–2464.e5. [Google Scholar] [CrossRef] [PubMed]
- Mikulak, E.; Gliniewicz, A.; Przygodzka, M.; Solecka, J. Galleria mellonella L. as model organism used in biomedical and other studies. Przegląd Epidemiol. Epidemiol. Rev. 2018, 72, 57–73. Available online: https://www.przeglepidemiol.pzh.gov.pl/Galleria-mellonella-L-as-model-organism-used-in-biomedical-and-other-studies,180812,0,2.html (accessed on 21 January 2025).
- Asai, M.; Li, Y.; Newton, S.M.; Robertson, B.D.; Langford, P.R. Galleria mellonella–intracellular bacteria pathogen infection models: The ins and outs. FEMS Microbiol. Rev. 2023, 47. [Google Scholar] [CrossRef]
- Peydaei, A.; Bagheri, H.; Gurevich, L.; de Jonge, N.; Nielsen, J.L. Mastication of polyolefins alters the microbial composition in Galleria mellonella. Environ. Pollut. 2021, 280, 116877. [Google Scholar] [CrossRef]
- Shah, R.; Nguyen, T.V.; Marcora, A.; Ruffell, A.; Hulthen, A.; Pham, K.; Wijffels, G.; Paull, C.; Beale, D.J. Exposure to polylactic acid induces oxidative stress and reduces the ceramide levels in larvae of greater wax moth (Galleria mellonella). Environ. Res. 2022, 220, 115137. [Google Scholar] [CrossRef]
- Réjasse, A.; Waeytens, J.; Deniset-Besseau, A.; Crapart, N.; Nielsen-Leroux, C.; Sandt, C. Plastic biodegradation: Do Galleria mellonella Larvae Bioassimilate Polyethylene? A Spectral Histology Approach Using Isotopic Labeling and Infrared Microspectroscopy. Environ. Sci. Technol. 2021, 56, 525–534. [Google Scholar] [CrossRef]
- Cassone, B.J.; Grove, H.C.; Kurchaba, N.; Geronimo, P.; LeMoine, C.M. Fat on plastic: Metabolic consequences of an LDPE diet in the fat body of the greater wax moth larvae (Galleria mellonella). J. Hazard. Mater. 2021, 425, 127862. [Google Scholar] [CrossRef]
- Barrionuevo, J.M.R.; Martín, E.; Cardona, A.G.; Malizia, A.; Chalup, A.; de Cristóbal, R.E.; Garzia, A.C.M. Consumption of low-density polyethylene, polypropylene, and polystyrene materials by larvae of the greater wax moth, Galleria mellonella L. (Lepidoptera, Pyralidae), impacts on their ontogeny. Environ. Sci. Pollut. Res. 2022, 29, 68132–68142. [Google Scholar] [CrossRef]
- Kundungal, H.; Gangarapu, M.; Sarangapani, S.; Patchaiyappan, A.; Devipriya, S.P. Role of pretreatment and evidence for the enhanced biodegradation and mineralization of low-density polyethylene films by greater waxworm. Environ. Technol. 2019, 42, 717–730. [Google Scholar] [CrossRef]
- Peydaei, A.; Bagheri, H.; Gurevich, L.; de Jonge, N.; Nielsen, J.L. Impact of polyethylene on salivary glands proteome in Galleria melonella. Comp. Biochem. Physiol. Part D Genom. Proteom. 2020, 34, 100678. [Google Scholar] [CrossRef]
- Stepnov, A.A.; Lopez-Tavera, E.; Klauer, R.; Lincoln, C.L.; Chowreddy, R.R.; Beckham, G.T.; Eijsink, V.G.H.; Solomon, K.; Blenner, M.; Vaaje-Kolstad, G. Revisiting the activity of two poly(vinyl chloride)- and polyethylene-degrading enzymes. Nat. Commun. 2024, 15, 8501. [Google Scholar] [CrossRef]
- Ren, L.; Men, L.; Zhang, Z.; Guan, F.; Tian, J.; Wang, B.; Wang, J.; Zhang, Y.; Zhang, W. Biodegradation of Polyethylene by Enterobacter sp. D1 from the Guts of Wax Moth Galleria mellonella. Int. J. Environ. Res. Public Heal. 2019, 16, 1941. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gao, D.; Li, Q.; Zhao, Y.; Li, L.; Lin, H.; Bi, Q.; Zhao, Y. Biodegradation of polyethylene microplastic particles by the fungus Aspergillus flavus from the guts of wax moth Galleria mellonella. Sci. Total Environ. 2020, 704, 135931. [Google Scholar] [CrossRef]
- Di Napoli, M.; Silvestri, B.; Castagliuolo, G.; Carpentieri, A.; Luciani, G.; Di Maro, A.; Sorbo, S.; Pezzella, A.; Zanfardino, A.; Varcamonti, M. High density polyethylene (HDPE) biodegradation by the fungus Cladosporium halotolerans. FEMS Microbiol. Ecol. 2022, 99. [Google Scholar] [CrossRef]
- Nyamjav, I.; Jang, Y.; Park, N.; Lee, Y.E.; Lee, S. Physicochemical and Structural Evidence that Bacillus cereus Isolated from the Gut of Waxworms (Galleria mellonella Larvae) Biodegrades Polypropylene Efficiently In Vitro. J. Polym. Environ. 2023, 31, 4274–4287. [Google Scholar] [CrossRef]
- Jiang, S.; Su, T.; Zhao, J.; Wang, Z. Isolation, Identification, and Characterization of Polystyrene-Degrading Bacteria From the Gut of Galleria mellonella (Lepidoptera: Pyralidae) Larvae. Front. Bioeng. Biotechnol. 2021, 9. [Google Scholar] [CrossRef]
- LeMoine, C.M.; Grove, H.C.; Smith, C.M.; Cassone, B.J. A Very Hungry Caterpillar: Polyethylene Metabolism and Lipid Homeostasis in Larvae of the Greater Wax Moth (Galleria mellonella). Environ. Sci. Technol. 2020, 54, 14706–14715. [Google Scholar] [CrossRef]
- Zhong, Z.; Nong, W.; Xie, Y.; Hui, J.H.L.; Chu, L.M. Long-term effect of plastic feeding on growth and transcriptomic response of mealworms (Tenebrio molitor L.). Chemosphere 2022, 287, 132063. [Google Scholar] [CrossRef]
- Young, R.; Ahmed, K.A.; Court, L.; Castro-Vargas, C.; Marcora, A.; Boctor, J.; Paull, C.; Wijffels, G.; Rane, R.; Edwards, O.; et al. Improved reference quality genome sequence of the plastic-degrading greater wax moth, Galleria mellonella. G3 Genes|Genomes|Genetics 2024, 14, jkae070. [Google Scholar] [CrossRef]
- Venegas, S.; Alarcón, C.; Araya, J.; Gatica, M.; Morin, V.; Tarifeño-Saldivia, E.; Uribe, E. Biodegradation of Polystyrene by Galleria mellonella: Identification of Potential Enzymes Involved in the Degradative Pathway. Int. J. Mol. Sci. 2024, 25, 1576. [Google Scholar] [CrossRef]
- Wang, S.; Shi, W.; Huang, Z.; Zhou, N.; Xie, Y.; Tang, Y.; Hu, F.; Liu, G.; Zheng, H. Complete digestion/biodegradation of polystyrene microplastics by greater wax moth (Galleria mellonella) larvae: Direct in vivo evidence, gut microbiota independence, and potential metabolic pathways. J. Hazard. Mater. 2021, 423, 127213. [Google Scholar] [CrossRef]
- O’Connor, K.; Buckley, C.M.; Hartmans, S.; Dobson, A.D. Possible regulatory role for nonaromatic carbon sources in styrene degradation by Pseudomonas putida CA-3. Appl. Environ. Microbiol. 1995, 61, 544–548. [Google Scholar] [CrossRef]
- Noël, G.; Serteyn, L.; Sare, A.R.; Massart, S.; Delvigne, F.; Francis, F. Co-diet supplementation of low density polyethylene and honeybee wax did not influence the core gut bacteria and associated enzymes of Galleria mellonella larvae (Lepidoptera: Pyralidae). Int. Microbiol. 2022, 26, 397–409. [Google Scholar] [CrossRef]
- Gressitt, J.L. Coleopteran. Available online: https://www.britannica.com/animal/beetle (accessed on 20 June 2024).
- Jiang, J.; Xu, H.; Cao, X.; Liang, Y.; Mo, A.; Cao, X.; Liu, Y.; Benbow, M.E.; Criddle, C.S.; Wu, W.-M.; et al. Soil-dwelling grub larvae of Protaetia brevitarsis biodegrade polystyrene: Responses of gut microbiome and host metabolism. Sci. Total Environ. 2024, 934, 173399. [Google Scholar] [CrossRef] [PubMed]
- Cucini, C.; Leo, C.; Vitale, M.; Frati, F.; Carapelli, A.; Nardi, F. Bacterial and fungal diversity in the gut of polystyrene-fed Alphitobius diaperinus (Insecta: Coleoptera). Anim. Gene 2020, 17–18, 200109. [Google Scholar] [CrossRef]
- Wang, Z.; Xin, X.; Shi, X.; Zhang, Y. A polystyrene-degrading Acinetobacter bacterium isolated from the larvae of Tribolium castaneum. Sci. Total Environ. 2020, 726, 138564. [Google Scholar] [CrossRef]
- McConnell, M.W.; Judge, K.A. Body size and lifespan are condition dependent in the mealworm beetle, Tenebrio molitor, but not sexually selected traits. Behav. Ecol. Sociobiol. 2018, 72, 32. [Google Scholar] [CrossRef]
- Jin, L.; Feng, P.; Cheng, Z.; Wang, D. Effect of biodegrading polyethylene, polystyrene, and polyvinyl chloride on the growth and development of yellow mealworm (Tenebrio molitor) larvae. Environ. Sci. Pollut. Res. 2022, 30, 37118–37126. [Google Scholar] [CrossRef] [PubMed]
- Urbanek, A.K.; Rybak, J.; Wróbel, M.; Leluk, K.; Mirończuk, A.M. A comprehensive assessment of microbiome diversity in Tenebrio molitor fed with polystyrene waste. Environ. Pollut. 2020, 262, 114281. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.-Q.; Yang, S.-S.; Ding, J.; Zhang, Z.-R.; Zhao, Y.-L.; Dai, W.; Sun, H.-J.; Zhao, L.; Xing, D.; Ren, N.; et al. Gut Microbiome Associating with Carbon and Nitrogen Metabolism during Biodegradation of Polyethene in Tenebrio larvae with Crop Residues as Co-Diets. Environ. Sci. Technol. 2023, 57, 3031–3041. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-S.; Ding, M.-Q.; Zhang, Z.-R.; Ding, J.; Bai, S.-W.; Cao, G.-L.; Zhao, L.; Pang, J.-W.; Xing, D.-F.; Ren, N.-Q.; et al. Confirmation of biodegradation of low-density polyethylene in dark- versus yellow- mealworms (larvae of Tenebrio obscurus versus Tenebrio molitor) via. gut microbe-independent depolymerization. Sci. Total Environ. 2021, 789, 147915. [Google Scholar] [CrossRef]
- Zaman, I.; Turjya, R.R.; Shakil, S.; Al Shahariar, M.; Emu, R.R.H.; Ahmed, A.; Hossain, M.M. Biodegradation of polyethylene and polystyrene by Zophobas atratus larvae from Bangladeshi source and isolation of two plastic-degrading gut bacteria. Environ. Pollut. 2024, 345, 123446. [Google Scholar] [CrossRef]
- Hong, J.; Han, T.; Kim, Y.Y. Mealworm (Tenebrio molitor Larvae) as an Alternative Protein Source for Monogastric Animal: A Review. Animals 2020, 10, 2068. [Google Scholar] [CrossRef]
- Shafique, L.; Abdel-Latif, H.M.R.; Hassan, F.-U.; Alagawany, M.; Naiel, M.A.E.; Dawood, M.A.O.; Yilmaz, S.; Liu, Q. The Feasibility of Using Yellow Mealworms (Tenebrio molitor): Towards a Sustainable Aquafeed Industry. Animals 2021, 11, 811. [Google Scholar] [CrossRef] [PubMed]
- Ravzanaadii, N.; Kim, S.-H.; Choi, W.-H.; Hong, S.-J.; Kim, N.-J. Nutritional Value of Mealworm, Tenebrio molitor as Food Source. Int. J. Ind. Èntomol. 2012, 25, 93–98. [Google Scholar] [CrossRef]
- Zielińska, E.; Zieliński, D.; Jakubczyk, A.; Karaś, M.; Pankiewicz, U.; Flasz, B.; Dziewięcka, M.; Lewicki, S. The impact of polystyrene consumption by edible insects Tenebrio molitor and Zophobas morio on their nutritional value, cytotoxicity, and oxidative stress parameters. Food Chem. 2020, 345, 128846. [Google Scholar] [CrossRef]
- Jo, Y.H.; Lee, J.H.; Patnaik, B.B.; Keshavarz, M.; Lee, Y.S.; Han, Y.S. Autophagy in Tenebrio molitor Immunity: Conserved Antimicrobial Functions in Insect Defenses. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Machona, O.; Chidzwondo, F.; Mangoyi, R. Tenebrio molitor: Possible source of polystyrene-degrading bacteria. BMC Biotechnol. 2022, 22, 2. [Google Scholar] [CrossRef] [PubMed]
- Brandon, A.M.; Gao, S.-H.; Tian, R.; Ning, D.; Yang, S.-S.; Zhou, J.; Wu, W.-M.; Criddle, C.S. Biodegradation of Polyethylene and Plastic Mixtures in Mealworms (Larvae of Tenebrio molitor) and Effects on the Gut Microbiome. Environ. Sci. Technol. 2018, 52, 6526–6533. [Google Scholar] [CrossRef]
- Brandon, A.M.; Garcia, A.M.; Khlystov, N.A.; Wu, W.-M.; Criddle, C.S. Enhanced Bioavailability and Microbial Biodegradation of Polystyrene in an Enrichment Derived from the Gut Microbiome of Tenebrio molitor (Mealworm Larvae). Environ. Sci. Technol. 2021, 55, 2027–2036. [Google Scholar] [CrossRef]
- Peng, B.-Y.; Su, Y.; Chen, Z.; Chen, J.; Zhou, X.; Benbow, M.E.; Criddle, C.S.; Wu, W.-M.; Zhang, Y. Biodegradation of Polystyrene by Dark (Tenebrio obscurus) and Yellow (Tenebrio molitor) Mealworms (Coleoptera: Tenebrionidae). Environ. Sci. Technol. 2019, 53, 5256–5265. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, J.; Wu, W.-M.; Zhao, J.; Song, Y.; Gao, L.; Yang, R.; Jiang, L. Biodegradation and Mineralization of Polystyrene by Plastic-Eating Mealworms: Part 2. Role of Gut Microorganisms. Environ. Sci. Technol. 2015, 49, 12087–12093. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-S.; Brandon, A.M.; Flanagan, J.C.A.; Yang, J.; Ning, D.; Cai, S.-Y.; Fan, H.-Q.; Wang, Z.-Y.; Ren, J.; Benbow, E.; et al. Biodegradation of polystyrene wastes in yellow mealworms (larvae of Tenebrio molitor Linnaeus): Factors affecting biodegradation rates and the ability of polystyrene-fed larvae to complete their life cycle. Chemosphere 2018, 191, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Su, T.; Zhao, J.; Wang, Z. Biodegradation of Polystyrene by Tenebrio molitor, Galleria mellonella, and Zophobas atratus Larvae and Comparison of Their Degradation Effects. Polymers 2021, 13, 3539. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Gao, J.; Liu, Y.; Zhuang, G.; Peng, X.; Wu, W.-M.; Zhuang, X. Biodegradation of expanded polystyrene and low-density polyethylene foams in larvae of Tenebrio molitor Linnaeus (Coleoptera: Tenebrionidae): Broad versus limited extent depolymerization and microbe-dependence versus independence. Chemosphere 2020, 262, 127818. [Google Scholar] [CrossRef]
- He, L.; Yang, S.-S.; Ding, J.; He, Z.-L.; Pang, J.-W.; Xing, D.-F.; Zhao, L.; Zheng, H.-S.; Ren, N.-Q.; Wu, W.-M. Responses of gut microbiomes to commercial polyester polymer biodegradation in Tenebrio molitor Larvae. J. Hazard. Mater. 2023, 457, 131759. [Google Scholar] [CrossRef] [PubMed]
- Mamtimin, T.; Han, H.; Khan, A.; Feng, P.; Zhang, Q.; Ma, X.; Fang, Y.; Liu, P.; Kulshrestha, S.; Shigaki, T.; et al. Gut microbiome of mealworms (Tenebrio molitor Larvae) show similar responses to polystyrene and corn straw diets. Microbiome 2023, 11, 98. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, L.; Li, X.; Wang, J.; Jin, G. Nitrogen Fixation and Diazotrophic Community in Plastic-Eating Mealworms Tenebrio molitor L. Microb. Ecol. 2022, 85, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Tsochatzis, E.; Berggreen, I.E.; Tedeschi, F.; Ntrallou, K.; Gika, H.; Corredig, M. Gut Microbiome and Degradation Product Formation during Biodegradation of Expanded Polystyrene by Mealworm Larvae under Different Feeding Strategies. Molecules 2021, 26, 7568. [Google Scholar] [CrossRef]
- Gan, S.K.-E.; Phua, S.-X.; Yeo, J.Y.; Heng, Z.S.-L.; Xing, Z. Method for Zero-Waste Circular Economy Using Worms for Plastic Agriculture: Augmenting Polystyrene Consumption and Plant Growth. Methods Protoc. 2021, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Li, Y.; Lu, B.; Liu, Q.; Yang, S.-S.; Liu, B.; Ren, N.; Wu, W.-M.; Xing, D. Response of the yellow mealworm (Tenebrio molitor) gut microbiome to diet shifts during polystyrene and polyethylene biodegradation. J. Hazard. Mater. 2021, 416, 126222. [Google Scholar] [CrossRef]
- Peng, B.-Y.; Xiao, S.; Sun, Y.; Liu, Y.; Chen, J.; Zhou, X.; Wu, W.-M.; Zhang, Y. Unveiling Fragmentation of Plastic Particles during Biodegradation of Polystyrene and Polyethylene Foams in Mealworms: Highly Sensitive Detection and Digestive Modeling Prediction. Environ. Sci. Technol. 2023, 57, 15099–15111. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xian, Z.-N.; Yue, W.; Yin, C.-F.; Zhou, N.-Y. Degradation of polyvinyl chloride by a bacterial consortium enriched from the gut of Tenebrio molitor larvae. Chemosphere 2023, 318, 137944. [Google Scholar] [CrossRef]
- Xian, Z.-N.; Yin, C.-F.; Zheng, L.; Zhou, N.-Y.; Xu, Y. Biodegradation of additive-free polypropylene by bacterial consortia enriched from the ocean and from the gut of Tenebrio molitor larvae. Sci. Total Environ. 2023, 892, 164721. [Google Scholar] [CrossRef] [PubMed]
- Leicht, A.; Masuda, H. Ingestion of Nylon 11 Polymers by the Mealworm (Tenebrio molitor) Beetle and Subsequent Enrichment of Monomer-Metabolizing Bacteria in Fecal Microbiome. Front. Biosci. 2023, 15, 11. [Google Scholar] [CrossRef]
- Leicht, A.; Gatz-Schrupp, J.; Masuda, H. Discovery of Nylon 11 ingestion by mealworm (Tenebrio molitor) larvae and detection of monomer-degrading bacteria in gut microbiota. AIMS Microbiol. 2022, 8, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.; Sun, H.; Wang, Y.; Han, X.; Yu, J.; Zhao, X.; Liu, B. Differences in ingestion and biodegradation of the melamine formaldehyde plastic by yellow mealworms Tenebrio molitor and superworms Zophobas atratus, and the prediction of functional gut microbes. Chemosphere 2024, 352, 141499. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.-Y.; Sun, Y.; Li, P.; Yu, S.; Xu, Y.; Chen, J.; Zhou, X.; Wu, W.-M.; Zhang, Y. Biodegradation of polyvinyl chloride, polystyrene, and polylactic acid microplastics in Tenebrio molitor larvae: Physiological responses. J. Environ. Manag. 2023, 345, 118818. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, J.; Xu, B.; Xu, A.; Cao, S.; Wei, R.; Zhou, J.; Jiang, M.; Dong, W. Biodegradation of polyether-polyurethane foam in yellow mealworms (Tenebrio molitor) and effects on the gut microbiome. Chemosphere 2022, 304, 135263. [Google Scholar] [CrossRef] [PubMed]
- Orts, J.M.; Parrado, J.; Pascual, J.A.; Orts, A.; Cuartero, J.; Tejada, M.; Ros, M. Polyurethane Foam Residue Biodegradation through the Tenebrio molitor Digestive Tract: Microbial Communities and Enzymatic Activity. Polymers 2022, 15, 204. [Google Scholar] [CrossRef]
- Guo, B.; Yin, J.; Hao, W.; Jiao, M. Polyurethane foam induces epigenetic modification of mitochondrial DNA during different metamorphic stages of Tenebrio molitor. Ecotoxicol. Environ. Saf. 2019, 183, 109461. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, C.; Zhao, X.; Weng, Y.; Nan, X.; Han, X.; Li, C.; Liu, B. Ingestion and biodegradation of disposable surgical masks by yellow mealworms Tenebrio molitor larvae: Differences in mask layers and effects on the larval gut microbiome. Sci. Total Environ. 2023, 904, 166808. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-S.; Ding, M.-Q.; Ren, X.-R.; Zhang, Z.-R.; Li, M.-X.; Zhang, L.-L.; Pang, J.-W.; Chen, C.-X.; Zhao, L.; Xing, D.-F.; et al. Impacts of physical-chemical property of polyethylene on depolymerization and biodegradation in yellow and dark mealworms with high purity microplastics. Sci. Total Environ. 2022, 828, 154458. [Google Scholar] [CrossRef] [PubMed]
- Akash, K.; Parthasarathi, R.; Elango, R.; Bragadeeswaran, S. Characterization of Priestia megaterium S1, a polymer degrading gut microbe isolated from the gut of Tenebrio molitor larvae fed on Styrofoam. Arch. Microbiol. 2023, 206, 48. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-W.; Kim, M.; Kim, S.-Y.; Bae, J.; Kim, T.-J. Biodegradation of polystyrene by intestinal symbiotic bacteria isolated from mealworms, the larvae of Tenebrio molitor. Heliyon 2023, 9, e17352. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-G.; Kwak, M.-J.; Kim, Y. Polystyrene microplastics biodegradation by gut bacterial Enterobacter hormaechei from mealworms under anaerobic conditions: Anaerobic oxidation and depolymerization. J. Hazard. Mater. 2023, 459. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Tao, H.; Wong, M.H. Feeding and metabolism effects of three common microplastics on Tenebrio molitor L. Environ. Geochem. Health 2018, 41, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.-Y.; Sun, Y.; Xiao, S.; Chen, J.; Zhou, X.; Wu, W.-M.; Zhang, Y. Influence of Polymer Size on Polystyrene Biodegradation in Mealworms (Tenebrio molitor): Responses of Depolymerization Pattern, Gut Microbiome, and Metabolome to Polymers with Low to Ultrahigh Molecular Weight. Environ. Sci. Technol. 2022, 56, 17310–17320. [Google Scholar] [CrossRef]
- Yang, S.-S.; Wu, W.-M.; Brandon, A.M.; Fan, H.-Q.; Receveur, J.P.; Li, Y.; Wang, Z.-Y.; Fan, R.; McClellan, R.L.; Gao, S.-H.; et al. Ubiquity of polystyrene digestion and biodegradation within yellow mealworms, larvae of Tenebrio molitor Linnaeus (Coleoptera: Tenebrionidae). Chemosphere 2018, 212, 262–271. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, L.; Li, X.; Wang, J.; Wang, H.; Chen, C.; Guo, H.; Han, T.; Zhou, A.; Zhao, X. Different plastics ingestion preferences and efficiencies of superworm (Fab.) and yellow mealworm (Tenebrio molitor Linn.) associated with distinct gut microbiome changes. Sci. Total Environ. 2022, 837, 155719. [Google Scholar] [CrossRef]
- Cheng, X.; Xia, M.; Yang, Y. Biodegradation of vulcanized rubber by a gut bacterium from plastic-eating mealworms. J. Hazard. Mater. 2023, 448, 130940. [Google Scholar] [CrossRef]
- I Rumbos, C.; Athanassiou, C.G. The Superworm, Zophobas morio (Coleoptera: Tenebrionidae): A ‘Sleeping Giant’ in Nutrient Sources. J. Insect Sci. 2021, 21. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Xia, M. Biodegradation and mineralization of polystyrene by plastic-eating superworms Zophobas atratus. Sci. Total Environ. 2020, 708, 135233. [Google Scholar] [CrossRef]
- Lu, B.; Lou, Y.; Wang, J.; Liu, Q.; Yang, S.-S.; Ren, N.; Wu, W.-M.; Xing, D. Understanding the Ecological Robustness and Adaptability of the Gut Microbiome in Plastic-Degrading Superworms (Zophobas atratus) in Response to Microplastics and Antibiotics. Environ. Sci. Technol. 2024, 58, 12028–12041. [Google Scholar] [CrossRef] [PubMed]
- Nyamjav, I.; Jang, Y.; Lee, Y.E.; Lee, S. Biodegradation of polyvinyl chloride by Citrobacter koseri isolated from superworms (Zophobas atratus larvae). Front. Microbiol. 2023, 14. [Google Scholar] [CrossRef]
- Liu, Y.-N.; Bairoliya, S.; Zaiden, N.; Cao, B. Establishment of plastic-associated microbial community from superworm gut microbiome. Environ. Int. 2023, 183, 108349. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Wang, Y.; Guo, H.; Yang, Y.; Qi, N.; Zhao, X.; Gao, S.; Zhou, A. Biodegradation of foam plastics by Zophobas atratus larvae (Coleoptera: Tenebrionidae) associated with changes of gut digestive enzymes activities and microbiome. Chemosphere 2021, 282, 131006. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Han, X.; Sun, H.; Wang, J.; Wang, Y.; Zhao, X. Effects of polymerization types on plastics ingestion and biodegradation by Zophobas atratus larvae, and successions of both gut bacterial and fungal microbiomes. Environ. Res. 2024, 251, 118677. [Google Scholar] [CrossRef]
- Jung, H.; Shin, G.; Park, S.B.; Jegal, J.; Park, S.-A.; Park, J.; Oh, D.X.; Kim, H.J. Circular waste management: Superworms as a sustainable solution for biodegradable plastic degradation and resource recovery. Waste Manag. 2023, 171, 568–579. [Google Scholar] [CrossRef]
- Kim, H.R.; Lee, H.M.; Yu, H.C.; Jeon, E.; Lee, S.; Li, J.; Kim, D.-H. Biodegradation of Polystyrene by Pseudomonas sp. Isolated from the Gut of Superworms (Larvae of Zophobas atratus). Environ. Sci. Technol. 2020, 54, 6987–6996. [Google Scholar] [CrossRef] [PubMed]
- Arunrattiyakorn, P.; Ponprateep, S.; Kaennonsang, N.; Charapok, Y.; Punphuet, Y.; Krajangsang, S.; Tangteerawatana, P.; Limtrakul, A. Biodegradation of polystyrene by three bacterial strains isolated from the gut of Superworms (Zophobas atratus larvae). J. Appl. Microbiol. 2022, 132, 2823–2831. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Y.; Xing, R.; Rensing, C.; Lü, J.; Chen, M.; Zhong, S.; Zhou, S. Reactive Oxygen Species Triggered Oxidative Degradation of Polystyrene in the Gut of Superworms (Zophobas atratus Larvae). Environ. Sci. Technol. 2023, 57, 7867–7874. [Google Scholar] [CrossRef] [PubMed]
- Inward, D.; Beccaloni, G.; Eggleton, P. Death of an order: A comprehensive molecular phylogenetic study confirms that termites are eusocial cockroaches. Biol. Lett. 2007, 3, 331–335. [Google Scholar] [CrossRef]
- Sangiorgio, P.; Verardi, A.; Dimatteo, S.; Spagnoletta, A.; Moliterni, S.; Errico, S. Tenebrio molitor in the circular economy: A novel approach for plastic valorisation and PHA biological recovery. Environ. Sci. Pollut. Res. 2021, 28, 52689–52701. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-X.; Yang, S.-S.; Ding, J.; Ding, M.-Q.; He, L.; Xing, D.-F.; Criddle, C.S.; Benbow, M.E.; Ren, N.-Q.; Wu, W.-M. Cockroach Blaptica dubia biodegrades polystyrene plastics: Insights for superior ability, microbiome and host genes. J. Hazard. Mater. 2024, 479, 135756. [Google Scholar] [CrossRef] [PubMed]
- Kalleshwaraswamy, C.M.; Shanbhag, R.R.; Sundararaj, R. Wood Degradation by Termites: Ecology, Economics and Protection. In Science of Wood Degradation and Its Protection; Sundararaj, R., Ed.; Springer: Singapore, 2022; pp. 147–170. [Google Scholar]
- Côté, W.A. Chemical Composition of Wood. In Principles of Wood Science and Technology: I Solid Wood; Kollmann, F.F.P., Côté, W.A., Eds.; Springer: Berlin/Heidelberg, Germany, 1968; pp. 55–78. [Google Scholar]
- Al-Tohamy, R.; Ali, S.S.; Zhang, M.; Elsamahy, T.; Abdelkarim, E.A.; Jiao, H.; Sun, S.; Sun, J. Environmental and Human Health Impact of Disposable Face Masks During the COVID-19 Pandemic: Wood-Feeding Termites as a Model for Plastic Biodegradation. Appl. Biochem. Biotechnol. 2022, 195, 2093–2113. [Google Scholar] [CrossRef]
- López-Naranjo, E.J.; Alzate-Gaviria, L.M.; Hernández-Zárate, G.; Reyes-Trujeque, J.; Cupul-Manzano, C.V.; Cruz-Estrada, R.H. Effect of biological degradation by termites on the flexural properties of pinewood residue/recycled high-density polyethylene composites. J. Appl. Polym. Sci. 2012, 128, 2595–2603. [Google Scholar] [CrossRef]
- Ali, S.S.; Al-Tohamy, R.; Sun, J.; Wu, J.; Huizi, L. Screening and construction of a novel microbial consortium SSA-6 enriched from the gut symbionts of wood-feeding termite, Coptotermes formosanus and its biomass-based biorefineries. Fuel 2018, 236, 1128–1145. [Google Scholar] [CrossRef]
- Guzmán, L.F.; Tirado, B.; Cruz-Cárdenas, C.I.; Rojas-Anaya, E.; Aragón-Magadán, M.A. De Novo Transcriptome Assembly of Cedar (Cedrela odorata L.) and Differential Gene Expression Involved in Herbivore Resistance. Curr. Issues Mol. Biol. 2024, 46, 8794–8806. [Google Scholar] [CrossRef] [PubMed]
- Baranchikov, Y.; Mozolevskaya, E.; Yurchenko, G.; Kenis, M. Occurrence of the emerald ash borer, Agrilus planipennis in Russia and its potential impact on European forestry. EPPO Bull. 2008, 38, 233–238. [Google Scholar] [CrossRef]
- Mayfield, A.E., III; MacKenzie, M.; Cannon, P.G.; Oak, S.W.; Horn, S.; Hwang, J.; Kendra, P.E. Suitability of California bay laurel and other species as hosts for the non-native redbay ambrosia beetle and granulate ambrosia beetle. Agric. For. Èntomol. 2013, 15, 227–235. [Google Scholar] [CrossRef]
- Meng, P.S.; Hoover, K.; Keena, M.A. Asian Longhorned Beetle (Coleoptera: Cerambycidae), an Introduced Pest of Maple and Other Hardwood Trees in North America and Europe. J. Integr. Pest Manag. 2015, 6. [Google Scholar] [CrossRef]
- Linnakoski, R.; Forbes, K.M. Pathogens—The Hidden Face of Forest Invasions by Wood-Boring Insect Pests. Front. Plant Sci. 2019, 10, 90. [Google Scholar] [CrossRef] [PubMed]
- Boctor, J.; Pandey, G.; Xu, W.; Murphy, D.V.; Hoyle, F.C. Nature’s Plastic Predators: A Comprehensive and Bibliometric Review of Plastivore Insects. Polymers 2024, 16, 1671. [Google Scholar] [CrossRef] [PubMed]
- Milum, V.G. Vitula edmandsii as a Pest of Honeybee Combs. J. Econ. Èntomol. 1953, 46, 710–711. [Google Scholar] [CrossRef][Green Version]
- Wu, W.-M.; Criddle, C.S. Chapter Five—Characterization of biodegradation of plastics in insect larvae. In Methods in Enzymology; Weber, G., Bornscheuer, U.T., Wei, R., Eds.; Academic Press: Cambridge, MA, USA, 2021; Volume 648, pp. 95–120. [Google Scholar]
- Obrador-Viel, T.; Zadjelovic, V.; Nogales, B.; Bosch, R.; Christie-Oleza, J.A. Assessing microbial plastic degradation requires robust methods. Microb. Biotechnol. 2024, 17, e14457. [Google Scholar] [CrossRef]
- Douki, T.; Bard, V.; Boulée, M.; Carrière, M. Extensive HPLC tandem mass spectrometry characterization of soluble degradation products of biodegradable nanoplastics under environmentally relevant temperature and irradiation conditions. Environ. Sci. Nano 2024. [Google Scholar] [CrossRef]
- Picó, Y.; Barceló, D. Pyrolysis gas chromatography-mass spectrometry in environmental analysis: Focus on organic matter and microplastics. TrAC Trends Anal. Chem. 2020, 130, 115964. [Google Scholar] [CrossRef]
- Pereira, M.F.; Rossi, C.C. Overview of rearing and testing conditions and a guide for optimizing Galleria mellonella breeding and use in the laboratory for scientific purposes. APMIS 2020, 128, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Shen, Y.; Li, X.; Liu, X.; Qian, G.; Zhou, J. Feeding preference of insect larvae to waste electrical and electronic equipment plastics. Sci. Total Environ. 2021, 807, 151037. [Google Scholar] [CrossRef]
- Guberman, R. The Complete Plastics Recycling Process. Available online: https://www.rts.com/blog/the-complete-plastics-recycling-process-rts/ (accessed on 12 September 2024).
- Billen, P.; Khalifa, L.; Van Gerven, F.; Tavernier, S.; Spatari, S. Technological application potential of polyethylene and polystyrene biodegradation by macro-organisms such as mealworms and wax moth larvae. Sci. Total Environ. 2020, 735, 139521. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J. Current Plastic Recycling Prices. Available online: https://blog.recycleduklimited.com/current-plastic-recycling-prices (accessed on 12 September 2024).
- Gicole, S.; Dimitriou, A.; Klasios, N.; Tseng, M. Partial consumption of medical face masks by a common beetle species. Biol. Lett. 2024, 20, 20240380. [Google Scholar] [CrossRef] [PubMed]
- Manzano-Agugliaro, F.; Sanchez-Muros, M.; Barroso, F.; Martínez-Sánchez, A.; Rojo, S.; Pérez-Bañón, C. Insects for biodiesel production. Renew. Sustain. Energy Rev. 2012, 16, 3744–3753. [Google Scholar] [CrossRef]
- Siow, H.S.; Sudesh, K.; Ganesan, S. Insect oil to fuel: Optimizing biodiesel production from mealworm (Tenebrio molitor) oil using response surface methodology. Fuel 2024, 371, 132099. [Google Scholar] [CrossRef]
- Ilijin, L.; Nikolić, M.V.; Vasiljević, Z.Z.; Todorović, D.; Mrdaković, M.; Vlahović, M.; Matić, D.; Tadić, N.B.; Perić-Mataruga, V. Sourcing chitin from exoskeleton of Tenebrio molitor fed with polystyrene or plastic kitchen wrap. Int. J. Biol. Macromol. 2024, 268, 131731. [Google Scholar] [CrossRef]
- Hirano, S. Chitin Biotechnology Applications. In Biotechnology Annual Review; El-Gewely, M.R., Ed.; Elsevier: Amsterdam, The Netherlands, 1996; Volume 2, pp. 237–258. [Google Scholar]
- Carbon Emissions and Plastic Waste. QM Recylced Energy. Available online: https://www.qmre.ltd/ (accessed on 16 September 2024).
- Finnveden, G.; Hauschild, M.Z.; Ekvall, T.; Guinée, J.B.; Heijungs, R.; Hellweg, S.; Koehler, A.; Pennington, D.; Suh, S. Recent developments in Life Cycle Assessment. J. Environ. Manag. 2009, 91, 1–21. [Google Scholar] [CrossRef]
- Drugmand, J.-C.; Schneider, Y.-J.; Agathos, S.N. Insect cells as factories for biomanufacturing. Biotechnol. Adv. 2012, 30, 1140–1157. [Google Scholar] [CrossRef]
- Buchholz, K.; Collins, J. The roots—A short history of industrial microbiology and biotechnology. Appl. Microbiol. Biotechnol. 2013, 97, 3747–3762. [Google Scholar] [CrossRef]
- Sadler, J.C.; Wallace, S. Microbial synthesis of vanillin from waste poly(ethylene terephthalate). Green Chem. 2021, 23, 4665–4672. [Google Scholar] [CrossRef]
- Banerjee, G.; Chattopadhyay, P. Vanillin biotechnology: The perspectives and future. J. Sci. Food Agric. 2018, 99, 499–506. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vital-Vilchis, I.; Karunakaran, E. Using Insect Larvae and Their Microbiota for Plastic Degradation. Insects 2025, 16, 165. https://doi.org/10.3390/insects16020165
Vital-Vilchis I, Karunakaran E. Using Insect Larvae and Their Microbiota for Plastic Degradation. Insects. 2025; 16(2):165. https://doi.org/10.3390/insects16020165
Chicago/Turabian StyleVital-Vilchis, Isabel, and Esther Karunakaran. 2025. "Using Insect Larvae and Their Microbiota for Plastic Degradation" Insects 16, no. 2: 165. https://doi.org/10.3390/insects16020165
APA StyleVital-Vilchis, I., & Karunakaran, E. (2025). Using Insect Larvae and Their Microbiota for Plastic Degradation. Insects, 16(2), 165. https://doi.org/10.3390/insects16020165







