Chemical Profiling, Sensory Qualities, and Bioactivities of Essential Oils Obtained from Aloysia citrodora and Bursera graveolens Ecuadorian Plants Against the Mosquito Aedes albopictus (Skuse) (Diptera: Culicidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Botanical Sample Collection and Preservation
2.2. Extraction Method of Botanical Essential Oils
2.3. GC-MS Profiling of A. citrodora and B. graveolens
2.4. Sensory Assessment
2.5. Rearing of Ae. Albopictus Mosquitoes
2.6. Larvicidal Effect
2.7. Oviposition Deterrence Assays
2.8. Protection Efficacy and Protection Time to Humans
2.9. Statistical Analysis
3. Results
3.1. EOs Compositions
3.2. EOs Smell Characterization
3.3. EOs Oviposition Deterrence
3.4. EOs Larvicidal Effect
3.5. EOs Repellent Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| CHIKV | Chikungunya Virus |
| CPT | complete protection time |
| GC-MS | gas chromatography–mass spectrophotometry |
| DAFE | Department of Agricultural, Food, and Environment |
| DENV | Dengue Virus |
| ECDC | European Centre for Disease Prevention and Control |
| EOs | essential oils |
| EU | Europe |
| WNV | West Nile virus |
| OAI | oviposition activity index |
| CI | confidence interval |
| ER | effective repellency |
| HD | hydrodistillation |
| LC50 | lethal concentration values for 50% |
| LC90 | lethal concentration values for 90% |
| PE | protective efficacy |
| PT | protection time |
| RD50 | repellent dosage values for 50% |
| RD90 | repellent dosage values for 90% |
| RI | retention indices |
| RH | relative humidity |
| RMP | relative median potency |
References
- Lamy, K.; Tran, A.; Portafaix, T.; Leroux, M.; Baldet, T. Impact of regional climate change on the mosquito vector Aedes albopictus in a tropical island environment: La Réunion. Sci. Total Environ. 2023, 875, 162484. [Google Scholar] [CrossRef]
- Paz, S. Climate change impacts on vector-borne diseases in Europe: Risks, predictions and actions. Lancet Reg. Health Eur. 2020, 1, 10001. [Google Scholar] [CrossRef]
- Increasing Risk of Mosquito-Borne Diseases in EU/EEA Following Spread of Aedes Species. Available online: https://www.ecdc.europa.eu/en/news-events/increasing-risk-mosquito-borne-diseases-eueea-following-spread-aedes-species (accessed on 22 June 2024).
- Aedes albopictus—Factsheet for Experts. Available online: https://www.ecdc.europa.eu/en/disease-vectors/facts/mosquito-factsheets/aedes-albopictus (accessed on 22 June 2024).
- Muhammad, N.A.F.; Kassim, N.F.A.; Majid, A.H.A.; Rahman, A.A.; Dieng, H.; Avicor, S.W. Biting rhythm and demographic attributes of Aedes albopictus (Skuse) females from different urbanized settings in Penang Island, Malaysia under uncontrolled laboratory conditions. PLoS ONE 2020, 15, e0241688. [Google Scholar] [CrossRef] [PubMed]
- Fikrig, K.; Harrington, L.C. Understanding and interpreting mosquito blood feeding studies: The case of Aedes albopictus. Trends Parasitol. 2021, 37, 959–975. [Google Scholar] [CrossRef]
- Rund, S.S.C.; Labb, L.F.; Benefiel, O.M.; Duffield, G.E. Artificial light at night increases Aedes aegypti mosquito biting behavior with implications for arboviral disease transmission. Am. J. Trop. Med. Hyg. 2020, 103, 2450–2452. [Google Scholar] [CrossRef]
- Valerio, L.; Marini, F.; Bongiorno, G.; Facchinelli, L.; Pombi, M.; Caputo, B.; Maroli, M.; Della Torre, A. Host-feeding patterns of Aedes albopictus (Diptera: Culicidae) in urban and rural contexts within Rome province, Italy. Vector-Borne Zoonot 2009, 10, 291–294. [Google Scholar] [CrossRef]
- Achee, N.L.; Grieco, J.P.; Vatandoost, H.; Seixas, G.; Pinto, J.; Ching-Ng, L.; Martins, A.J.; Juntarajumnong, W.; Corbel, V.; Gouagna, C.; et al. Alternative strategies for mosquito-borne arbovirus control. PLoS Negl. Trop. D 2019, 13, e0006822. [Google Scholar] [CrossRef]
- Xue, R.; Barnard, D.R.; Ali, A. Laboratory and field evaluation of insect repellents as larvicides against the mosquitoes Aedes albopictus and Anopheles albimanus. Med. Vet. Entomol. 2001, 15, 374–380. [Google Scholar] [CrossRef]
- Paupy, C.; Delatte, H.; Bagny, L.; Corbel, V.; Fontenille, D. Aedes albopictus, an arbovirus vector: From the darkness to the light. Microbes Infect. 2009, 11, 1177–1185. [Google Scholar] [CrossRef]
- Mapossa, A.B.; Focke, W.W.; Tewo, R.K.; Androsch, R.; Kruger, T. Mosquito-repellent controlled-release formulations for fighting infectious diseases. Malaria J. 2021, 20, 165. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.N.; Goswami, R.; Pal, A. The insect repellents: A silent environmental chemical toxicant to the health. Environ. Toxicol. Phar. 2017, 50, 91–102. [Google Scholar] [CrossRef]
- Pavela, R. Essential oils for the development of eco-friendly mosquito larvicides: A review. Ind. Crop Prod. 2015, 76, 174–187. [Google Scholar] [CrossRef]
- Vaičiulytė, V.; Ložienė, K.; Taraškevičius, R.; Butkienė, R. Variation of essential oil composition of Thymus pulegioides in relation to soil chemistry. Ind. Crop Prod. 2016, 95, 422–433. [Google Scholar] [CrossRef]
- Prakash, B.; Kedia, A.; Mishra, P.K.; Dubey, N. Plant essential oils as food preservatives to control moulds, mycotoxin contamination and oxidative deterioration of agri-food commodities—Potentials and challenges. Food Control 2014, 47, 381–391. [Google Scholar] [CrossRef]
- Armijos, C.; Ramírez, J.; Salinas, M.; Vidari, G.; Suárez, A. Pharmacology and phytochemistry of Ecuadorian medicinal plants: An update and perspectives. Pharmaceuticals 2021, 14, 1145. [Google Scholar] [CrossRef] [PubMed]
- Bahramsoltani, R.; Rostamiasrabadi, P.; Shahpiri, Z.; Marques, A.M.; Rahimi, R.; Farzaei, M.H. Aloysia citrodora Paláu (Lemon verbena): A review of phytochemistry and pharmacology. J. Ethnopharmacol. 2018, 222, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Comelli, N.C.; Diez, P.A.; Rodríguez, M.R.; Denett, G.O.; López, T.E.; Bracamonte, D.M.; Ortiz, E.V.; Sampietro, D.A.; Duchowicz, P.R. Excito-repellent and pesticide-likeness properties of essential oils on Carpophilus dimidiatus (Fabricius) (Nitidulidae) and Oryzaephilus mercator (L.) (Silvanidae). J. Chem. Inf. Model. 2023, 64, 2467–2487. [Google Scholar] [CrossRef] [PubMed]
- Jumbo, L.O.V.; Corrêa, M.J.M.; Gomes, J.M.; Armijos, M.J.G.; Valarezo, E.; Mantilla-Afanador, J.G.; Machado, F.P.; Rocha, L.; Aguiar, R.W.; Oliveira, E.E. Potential of Bursera graveolens essential oil for controlling bean weevil infestations: Toxicity, repellence, and action targets. Ind. Crop Prod. 2022, 178, 114611. [Google Scholar] [CrossRef]
- Farina, P.; Venturi, F.; Ascrizzi, R.; Flamini, G.; Ortega, R.D.C.; Echeverría, M.C.; Ortega, S.; Zinnai, A.; Bedini, S.; Conti, B. Andean plants essential oils: A scented alternative to synthetic insecticides for the control of blowflies. Insects 2021, 12, 894. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Standards and Technology. NIST/EPA/NIH Mass Spectral Library, NIST Standard Reference Database Number 69; The NIST Mass Spectrometry Data Center: Gaithersburg, MD, USA, 2014.
- Adams, R.P. Identification of Essential Oil Components by Gas. Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; p. 804. [Google Scholar]
- Müller, P.; Engeler, L.; Vavassori, L.; Suter, T.; Guidi, V.; Gschwind, M.; Tonolla, M.; Flacio, E. Surveillance of invasive Aedes mosquitoes along Swiss traffic axes reveals different dispersal modes for Aedes albopictus and Ae. japonicus. PLoS Negl. Trop. Dis. 2020, 14, e0008705. [Google Scholar] [CrossRef]
- Bedini, S.; Flamini, G.; Ascrizzi, R.; Venturi, F.; Ferroni, G.; Bader, A.; Girardi, J.; Conti, B. Essential oils sensory quality and their bioactivity against the mosquito Aedes albopictus. Sci. Rep. 2018, 8, 17857. [Google Scholar] [CrossRef] [PubMed]
- Najar, B.; Pistelli, L.; Venturi, F.; Ferroni, G.; Giovanelli, S.; Cervelli, C.; Bedini, S.; Conti, B. Salvia spp. essential oils against the Arboviruses vector Aedes albopictus (Diptera: Culicidae): Bioactivity, composition, and sensorial profile—Stage 1. Biology 2020, 9, 206. [Google Scholar] [CrossRef]
- Guidelines for Laboratory and Field Testing of Mosquito Larvicides. Available online: https://iris.who.int/bitstream/handle/10665/69101/WHO_CDS_WHOPES_GCDPP_2005.13.pdf?sequence=1 (accessed on 25 January 2025).
- Abbott, W.S. A Method of Computing the Effectiveness of an Insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Reuss, F.; Kreß, A.; Braun, M.; Magdeburg, A.; Pfenninger, M.; Müller, R.; Mehring, M. Knowledge on exotic mosquitoes in Germany, and public acceptance and effectiveness of Bti and two self-prepared insecticides against Aedes japonicus japonicus. Sci. Rep. 2020, 10, 18901. [Google Scholar] [CrossRef] [PubMed]
- Conti, B.; Canale, A.; Bertoli, A.; Gozzini, F.; Pistelli, L. Essential oil composition and larvicidal activity of six Mediterranean aromatic plants against the mosquito Aedes albopictus (Diptera: Culicidae). Parasitol. Res. 2010, 107, 1455–1461. [Google Scholar] [CrossRef]
- Control of Neglected Tropical Diseases (NTD). Available online: https://www.who.int/publications/i/item/WHO-HTM-NTD-WHOPES-2009.4 (accessed on 25 June 2024).
- The Good Scents Company—Flavor, Fragrance, Food and Cosmetics Ingredients information. Available online: https://www.thegoodscentscompany.com/index.html (accessed on 8 July 2024).
- Hung, N.H.; Satyal, P.; Hieu, H.V.; Chuong, N.T.H.; Dai, D.N.; Huong, L.T.; Tai, T.A.; Setzer, W.N. Mosquito larvicidal activity of the essential oils of Erechtites species growing wild in Vietnam. Insects 2019, 10, 47. [Google Scholar] [CrossRef]
- Demirak, M.Ş.Ş.; Canpolat, E. Plant-based bioinsecticides for mosquito control: Impact on insecticide resistance and disease transmission. Insects 2022, 13, 162. [Google Scholar] [CrossRef]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent activity of essential oils: A review. Bioresour. Technol. 2009, 101, 372–378. [Google Scholar] [CrossRef]
- Monzote, L.; Hill, G.M.; Cuellar, A.; Scull, R.; Setzer, W.N. Chemical composition and anti-proliferative properties of Bursera graveolens essential eil. Nat. Prod. Commun. 2012, 7, 1934578X1200701130. [Google Scholar] [CrossRef]
- Fon-Fay, F.M.; Pino, J.A.; Hernández, I.; Rodeiro, I.; Fernández, M.D. Chemical composition and antioxidant activity of Bursera graveolens (Kunth) Trianaet Planch essential oil from Manabí, Ecuador. J. Essent. Oil Res. 2019, 31, 211–216. [Google Scholar] [CrossRef]
- Fitsiou, E.; Mitropoulou, G.; Spyridopoulou, K.; Vamvakias, M.; Bardouki, H.; Galanis, A.; Chlichlia, K.; Kourkoutas, Y.; Panayiotidis, M.; Pappa, A. Chemical composition and evaluation of the biological properties of the essential oil of the dietary phytochemical Lippia citriodora. Molecules 2018, 23, 123. [Google Scholar] [CrossRef] [PubMed]
- Argyropoulou, C.; Daferera, D.; Tarantilis, P.A.; Fasseas, C.; Polissiou, M. Chemical composition of the essential oil from leaves of Lippia citriodora H.B.K. (Verbenaceae) at two developmental stages. Biochem. Syst. Ecol. 2007, 35, 831–837. [Google Scholar] [CrossRef]
- Andrade-Ochoa, S.; Sánchez-Aldana, D.; Chacón-Vargas, K.F.; Rivera-Chavira, B.E.; Sánchez-Torres, L.E.; Camacho, A.D.; Nogueda-Torres, B.; Nevárez-Moorillón, G.V. Oviposition deterrent and larvicidal and pupaecidal activity of seven essential oils and their major components against Culex quinquefasciatus Say (Diptera: Culicidae): Synergism–antagonism effects. Insects 2018, 9, 25. [Google Scholar] [CrossRef]
- Giatropoulos, A.; Papachristos, D.P.; Kimbaris, A.; Koliopoulos, G.; Polissiou, M.G.; Emmanouel, N.; Michaelakis, A. Evaluation of bioefficacy of three Citrus essential oils against the dengue vector Aedes albopictus (Diptera: Culicidae) in correlation to their components enantiomeric distribution. Parasitol. Res. 2012, 111, 2253–2263. [Google Scholar] [CrossRef]
- Liu, F.; Chen, L.; Appel, A.G.; Liu, N. Olfactory responses of the antennal trichoid sensilla to chemical repellents in the mosquito, Culex quinquefasciatus. J. Insect Physiol. 2013, 59, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Sun, J.; Dai, J. Dose-dependent behavioral response of the mosquito Aedes albopictus to floral odorous compounds. J. Insect Sci. 2013, 13, 127. [Google Scholar] [CrossRef]
- Spinozzi, E.; Maggi, F.; Bonacucina, G.; Pavela, R.; Boukouvala, M.C.; Kavallieratos, N.G.; Canale, A.; Romano, D.; Desneux, N.; Wilke, A.B.; et al. Apiaceae essential oils and their constituents as insecticides against mosquitoes—A review. Ind. Crop Prod. 2021, 171, 113892. [Google Scholar] [CrossRef]
- Trongtokit, Y.; Rongsriyam, Y.; Komalamisra, N.; Apiwathnasorn, C. Comparative repellency of 38 essential oils against mosquito bites. Phytother. Res. 2005, 19, 303–309. [Google Scholar] [CrossRef]
- Bedini, S.; Flamini, G.; Cosci, F.; Ascrizzi, R.; Benelli, G.; Conti, B. Cannabis sativa and Humulus lupulus essential oils as novel control tools against the invasive mosquito Aedes albopictus and fresh water snail Physella acuta. Ind. Crop Prod. 2016, 85, 318–323. [Google Scholar] [CrossRef]
- Substances Added to Food (Formerly EAFUS). Available online: https://www.hfpappexternal.fda.gov/scripts/fdcc/index.cfm?set=FoodSubstances&id=LEMONVERBENAOILLIPPIACITRIODORA (accessed on 23 January 2025).
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. F 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Luker, H.A.; Salas, K.R.; Esmaeili, D.; Holguin, F.O.; Bendzus-Mendoza, H.; Hansen, I.A. Repellent efficacy of 20 essential oils on Aedes aegypti mosquitoes and Ixodes scapularis ticks in contact-repellency assays. Sci. Rep. 2023, 13, 1705. [Google Scholar] [CrossRef] [PubMed]
- Elechosa, M.A.; Di Leo Lira, P.; Juárez, M.A.; Viturro, C.I.; Heit, C.I.; Molina, A.C.; Martínez, A.J.; López, S.; Molina, A.M.; Van Baren, C.M.; et al. Essential oil chemotypes of Aloysia citrodora (Verbenaceae) in Northwestern Argentina. Biochem. Syst. Ecol. 2017, 74, 19–29. [Google Scholar] [CrossRef]

| Main Odor | A.citrodora | B. graveolens |
|---|---|---|
| Positive Attributes | ||
| Fruity | Lemon, lemongrass | n.d. |
| Floral | Orange blossom | n.d. |
| Balsamic | Mint, menthol | n.d. |
| Spicy | Sweet pastry, candied lemon, candied orange | n.d. |
| Off-flavors | ||
| Chemical | n.d. | Mold, backwater, swamp |
| Pharmaceutical | n.d. | Carbolic acid, paint |
| Essential Oil (EO) | Total No. of Eggs Laid | Average No. of Eggs/Ovitrap | % ER | OAI | ||
|---|---|---|---|---|---|---|
| Control | Treated | Control | Treated | |||
| A. citrodora | 344 | 235 | 68.80 ± 19.02 b | 47.00 ± 13.95 a | 31.97 ± 8.72 b | −0.19 ± 0.06 b |
| B. graveolens | 993 | 363 | 198.60 ± 14.31 a | 72.60 ± 23.16 a | 63.69 ± 10.07 a | −0.47 ± 0.11 a |
| A. citrodora | B. graveolens | |
|---|---|---|
| LC50 a (CI) | 88.543 (79.384–96.302) | 146.528 (138.427–154.942) |
| LC90 b (CI) | 131.446 (121.157–145.647) | 208.464 (191.457–237.813) |
| Slope ± SD | 7.468 ± 0.896 | 8.370 ± 1.075 |
| Intercept ± SD | −14.542 ± 1.833 | −18.129 ± 2.333 |
| χ2 (df) | 0.754 (4) | 2.125 (4) |
| P | 0.944 | 0.713 |
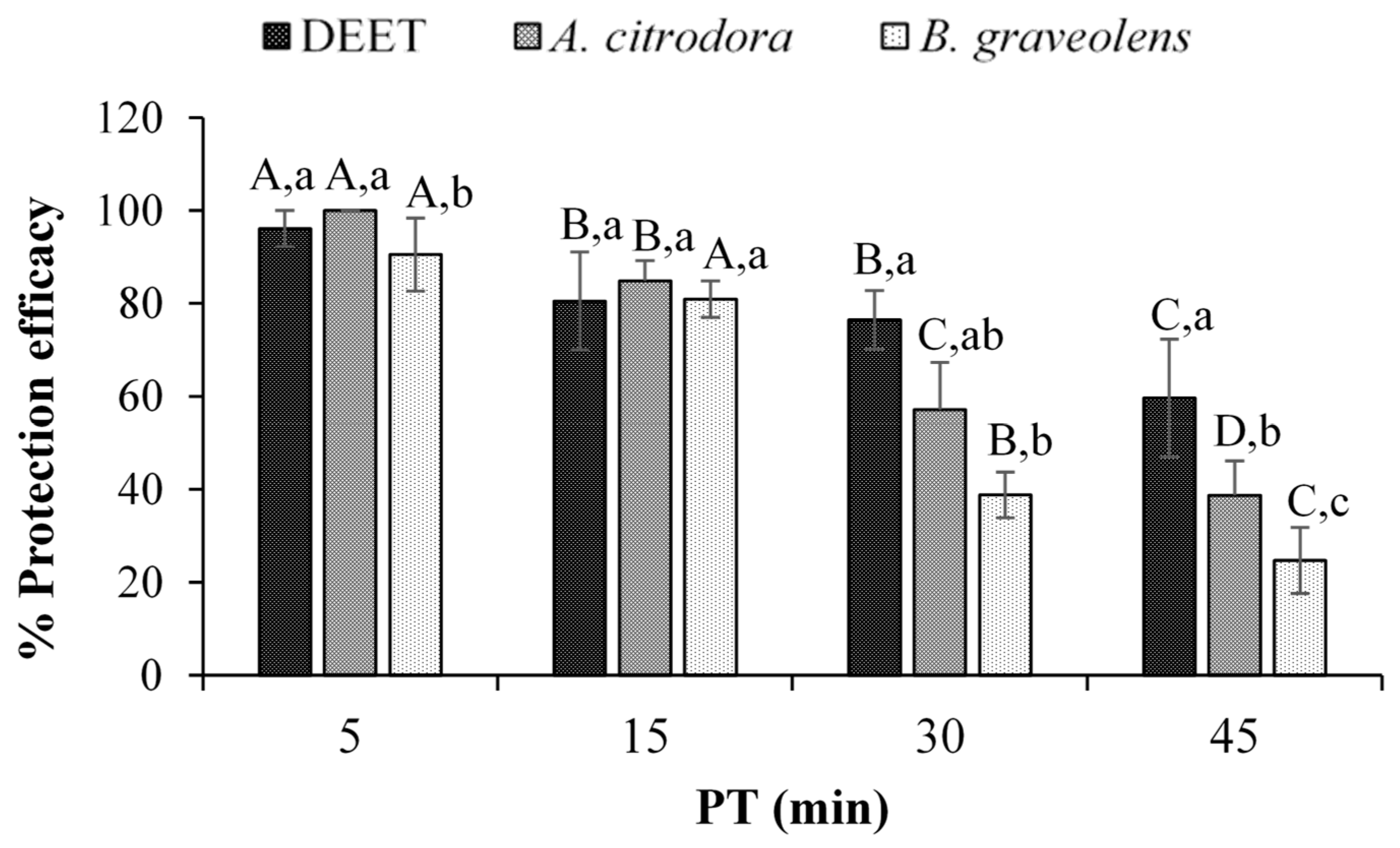
| Essential Oil | Dose (µL of EO cm−2 of Skin) | Protection Efficacy ± SD (%) | ||||
|---|---|---|---|---|---|---|
| Time After the Application of the Repellent (min) | ||||||
| 0 | 5 | 15 | 30 | 45 | ||
| A. citrodora | 0.04 | 100 A,a | 100 A,a | 84.87 ± 4.37 B,cd | 57.13 ± 10.19 C,b | 38.61 ± 7.44 D,c |
| 0.12 | 100 A,a | 100 A,a | 89.32 ± 6.65 B,abc | 68.35 ± 6.94 C,a | 46.92 ± 0.67 D,b | |
| 0.20 | 100 A,a | 100 A,a | 94.83 ± 1.25 B,a | 73.91 ± 1.12 C,a | 68.30 ± 3.80 D,a | |
| B. graveolens | 0.04 | 100 A,a | 90.55 ± 7.85 B,b | 80.89 ± 3.94 B,d | 38.77 ± 4.90 C,c | 24.73 ± 7.10 D,d |
| 0.12 | 100 A,a | 92.54 ± 3.01 B,b | 86.87 ± 5.26 B,bcd | 50.74 ± 3.60 C,b | 40.01 ± 6.37 C,bc | |
| 0.20 | 100 A,a | 100 A,a | 92.00 ± 7.67 B,ab | 72.61 ± 7.67 C,a | 63.98 ± 2.05 C,a | |
| DEET | 0.04 | 100 A,a | 96.15 ± 3.85 A,a | 80.54 ± 10.54 B,d | 76.41 ± 6.34 B,a | 59.64 ± 12.67 C,a |
| A.citrodora | B. graveolens | DEET | |
|---|---|---|---|
| RD50 a (CI) | 0.104 (0.079–0.140) | 0.136 (0.107–0.189) | 0.035 (0.022–0.046) |
| RD90 b (CI) | 1.057 (0.521–5.185) | 1.107 (0.566–4.555) | 0.200 (0.157–0.282) |
| Slope ± SD | 1.273± 0.258 | 1.408 ± 0.265 | 1.683 ± 0.224 |
| Intercept ± SD | 1.251± 0.268 | 1.219 ± 0.271 | 2.457 ± 0.238 |
| χ2 (df) | 2.495 (1) | 3.063 (1) | 0.860 (2) |
| P | 0.114 | 0.080 | 0.651 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parichanon, P.; Ascrizzi, R.; Tani, C.; Echeverria, M.C.; Andrade, S.O.; Paredes, H.; Taglieri, I.; Flamini, G.; Venturi, F.; Conti, B. Chemical Profiling, Sensory Qualities, and Bioactivities of Essential Oils Obtained from Aloysia citrodora and Bursera graveolens Ecuadorian Plants Against the Mosquito Aedes albopictus (Skuse) (Diptera: Culicidae). Insects 2025, 16, 202. https://doi.org/10.3390/insects16020202
Parichanon P, Ascrizzi R, Tani C, Echeverria MC, Andrade SO, Paredes H, Taglieri I, Flamini G, Venturi F, Conti B. Chemical Profiling, Sensory Qualities, and Bioactivities of Essential Oils Obtained from Aloysia citrodora and Bursera graveolens Ecuadorian Plants Against the Mosquito Aedes albopictus (Skuse) (Diptera: Culicidae). Insects. 2025; 16(2):202. https://doi.org/10.3390/insects16020202
Chicago/Turabian StyleParichanon, Prangthip, Roberta Ascrizzi, Camilla Tani, Maria Cristina Echeverria, Sania Ortega Andrade, Hugo Paredes, Isabella Taglieri, Guido Flamini, Francesca Venturi, and Barbara Conti. 2025. "Chemical Profiling, Sensory Qualities, and Bioactivities of Essential Oils Obtained from Aloysia citrodora and Bursera graveolens Ecuadorian Plants Against the Mosquito Aedes albopictus (Skuse) (Diptera: Culicidae)" Insects 16, no. 2: 202. https://doi.org/10.3390/insects16020202
APA StyleParichanon, P., Ascrizzi, R., Tani, C., Echeverria, M. C., Andrade, S. O., Paredes, H., Taglieri, I., Flamini, G., Venturi, F., & Conti, B. (2025). Chemical Profiling, Sensory Qualities, and Bioactivities of Essential Oils Obtained from Aloysia citrodora and Bursera graveolens Ecuadorian Plants Against the Mosquito Aedes albopictus (Skuse) (Diptera: Culicidae). Insects, 16(2), 202. https://doi.org/10.3390/insects16020202











