Azadirachtin-Mediated Responses in the Maize Weevil, Sitophilus zeamais (Coleoptera: Curculionidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Azadirachtin
2.3. Concentration–Mortality Bioassay
2.4. Survival Analysis
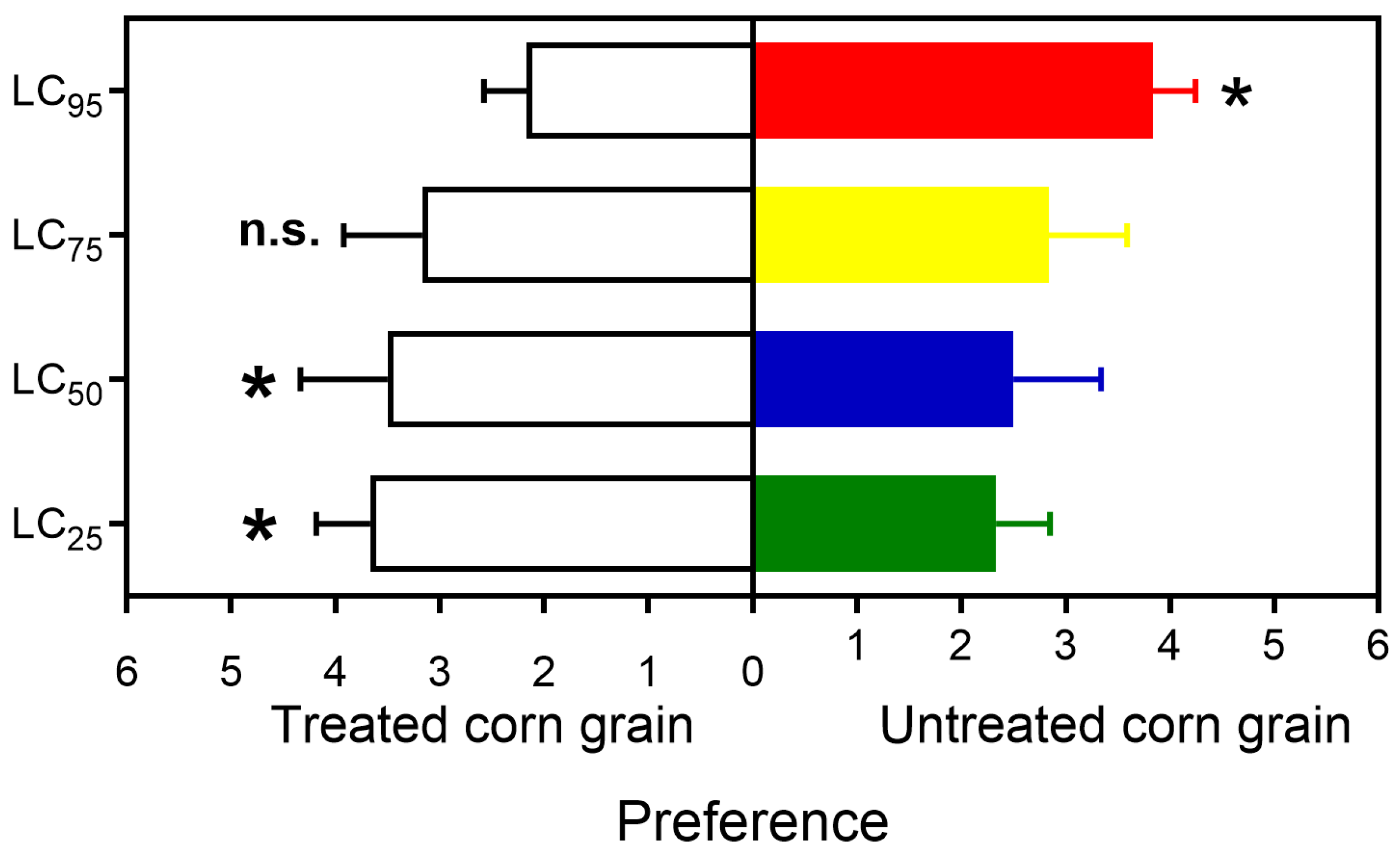
2.5. Adult Food Preference
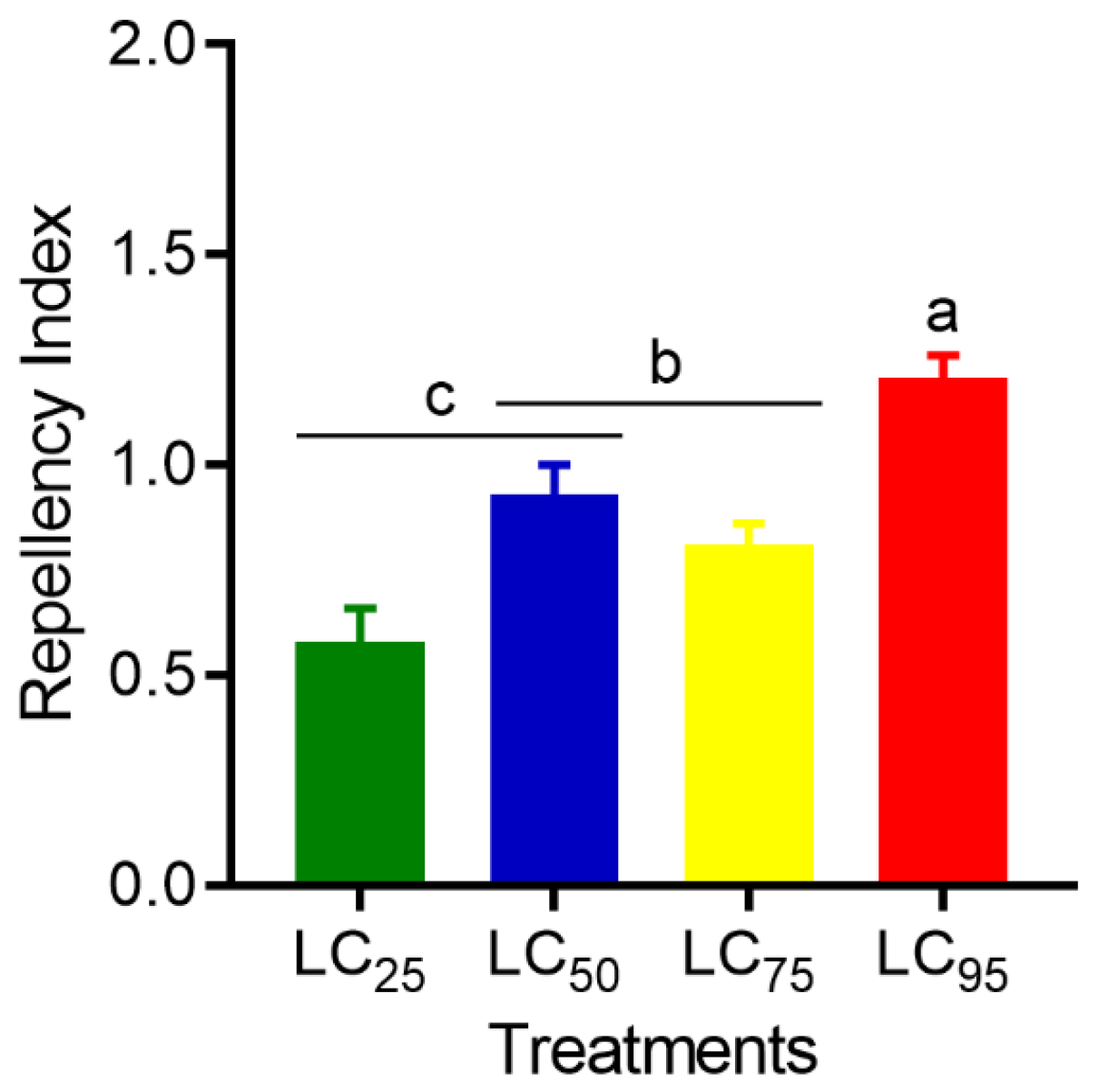
2.6. Repellency
2.7. Statistics
3. Results
3.1. Concentration–Mortality Bioassay
3.2. Survival Analysis
3.3. Adult Food Preference
3.4. Repellency
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daloğlu, I.; Nassauer, J.I.; Riolo, R.L.; Scavia, D. Development of a farmer typology of agricultural conservation behavior in the American corn belt. Agric. Syst. 2014, 129, 93–102. [Google Scholar] [CrossRef]
- Carrera, E.J.; Cejudo-Bastante, M.J.; Hurtado, N.; Heredia, F.J.; González-Miret, M.L. Revalorization of Colombian purple corn Zea mays L. by-products using two-step column chromatography. Food Res. Int. 2023, 169, 112931. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, V.; Paraginski, R.T.; Ferreira, C.D. Grain storage systems and effects of moisture, temperature and time on grain quality—A review. J. Stored Prod. Res. 2021, 91, 101770. [Google Scholar] [CrossRef]
- Yigezu, Y.A.; Alexander, C.E.; Preckel, P.V.; Maier, D.E.; Mason, L.J.; Woloshuk, C.; Lawrence, J.; Moog, D.J. Economics of integrated insect management in stored corn. J. Econ. Entomol. 2010, 103, 1896–1908. [Google Scholar] [CrossRef]
- Jones, T.K.L.; Medina, R.F. Corn stunt disease: An ideal insect–microbial–plant pathosystem for comprehensive studies of vector-borne plant diseases of corn. Plants 2020, 9, 747. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Kavallieratos, N.G.; Campbell, J.F. Competition of three species of Sitophilus on rice and maize. PLoS ONE 2017, 12, e0173377. [Google Scholar] [CrossRef]
- Zhou, Y.; Hui, Y.B.; Feng, L.F.; Zhou, T.; Wang, Q. A method for reconstructing the internal morphological structure of wheat kernels upon Sitophilus zeamais infestation. J. Stored Prod. Res. 2020, 88, 101676. [Google Scholar] [CrossRef]
- Nwosu, L.C. Impact of age on the biological activities of Sitophilus zeamais (Coleoptera: Curculionidae) adults on stored maize: Implications for food security and pest management. J. Econ. Entomol. 2018, 111, 2454–2460. [Google Scholar] [CrossRef]
- Arthur, F.H.; Yue, B.; Wilde, G.E. Susceptibility of stored-product beetles on wheat and maize treated with thiamethoxam: Effects of concentration, exposure interval, and temperature. J. Stored Prod. Res. 2004, 40, 527–546. [Google Scholar] [CrossRef]
- Rumbos, C.I.; Dutton, A.C.; Athanassiou, C.G. Insecticidal efficacy of two pirimiphos-methyl formulations for the control of three stored-product beetle species: Effect of commodity. Crop Prot. 2016, 80, 94–100. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Papagregoriou, A.S.; Buchelos, C.T. Insecticidal and residual effect of three pyrethroids against Sitophilus oryzae (L.) (Coleoptera: Curculionidae) on stored wheat. J. Stored Prod. Res. 2004, 40, 289–297. [Google Scholar] [CrossRef]
- Attia, M.A.; Wahba, T.F.; Shaarawy, N.; Moustafa, F.I.; Guedes, R.N.C.; Dewer, Y. Stored grain pest prevalence and insecticide resistance in Egyptian populations of the red flour beetle Tribolium castaneum (Herbst) and the rice weevil Sitophilus oryzae (L.). J. Stored Prod. Res. 2020, 87, 101611. [Google Scholar] [CrossRef]
- Boyer, S.; Zhang, H.; Lempérière, G. A review of control methods and resistance mechanisms in stored-product insects. Bull. Entomol. Res. 2012, 102, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Brügger, B.P.; Plata-Rueda, A.; Wilcken, C.F.; Souza, L.S.A.D.; Serrão, J.E.; Carvalho, A.G.; Zanuncio, J.C.; Martínez, L.C. Exposure to lemongrass essential oil and its components causes behavior and respiratory disturbs in Anticarsia gemmatalis. Int. J. Pest Manag. 2024, 70, 82–90. [Google Scholar] [CrossRef]
- Farder-Gomes, C.F.; Saravanan, M.; Martinez, L.C.; Plata-Rueda, A.; Zanuncio, J.C.; Serrão, J.E. Azadirachtin-based biopesticide affects the respiration and digestion in Anticarsia gemmatalis caterpillars. Toxin Rev. 2022, 41, 466–475. [Google Scholar] [CrossRef]
- Plata-Rueda, A.; Zanuncio, J.C.; Serrão, J.E.; Martínez, L.C. Origanum vulgare essential oil against Tenebrio molitor (Coleoptera: Tenebrionidae): Composition, insecticidal activity, and behavioral response. Plants 2021, 10, 2513. [Google Scholar] [CrossRef]
- Herrera, J.M.; Zunino, M.P.; Dambolena, J.S.; Pizzolitto, R.P.; Gañan, N.A.; Lucini, E.I.; Zygadlo, J.A. Terpene ketones as natural insecticides against Sitophilus zeamais. Ind. Crops Prod. 2015, 70, 435–442. [Google Scholar] [CrossRef]
- Brahmachari, G. Neem—An omnipotent plant: A retrospection. Chembiochem 2004, 5, 408–421. [Google Scholar] [CrossRef]
- Boeke, S.J.; Boersma, M.G.; Alink, G.M.; van Loon, J.J.; van Huis, A.; Dicke, M.; Rietjens, I.M. Safety evaluation of neem (Azadirachta indica) derived pesticides. J. Ethnopharmacol. 2004, 94, 25–41. [Google Scholar] [CrossRef]
- Kilani-Morakchi, S.; Morakchi-Goudjil, H.; Sifi, K. Azadirachtin-based insecticide: Overview, risk assessments, and future directions. Front. Agron. 2021, 3, 676208. [Google Scholar] [CrossRef]
- Shu, B.; Zhang, J.; Cui, G.; Sun, R.; Yi, X.; Zhong, G. Azadirachtin affects the growth of Spodoptera litura Fabricius by inducing apoptosis in larval midgut. Front. Physiol. 2018, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Zanuncio, J.C.; Mourão, S.A.; Martínez, L.C.; Wilcken, C.F.; Ramalho, F.S.; Plata-Rueda, A.; Soares, M.A.; Serrão, J.E. Toxic effects of the neem oil (Azadirachta indica) formulation on the stink bug predator, Podisus nigrispinus (Heteroptera: Pentatomidae). Sci. Rep. 2016, 6, 30261. [Google Scholar] [CrossRef] [PubMed]
- Oulhaci, C.M.; Denis, B.; Kilani-Morakchi, S.; Sandoz, J.C.; Kaiser, L.; Joly, D.; Aribi, N. Azadirachtin effects on mating success, gametic abnormalities and progeny survival in Drosophila melanogaster (Diptera). Pest Manag. Sci. 2018, 74, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Kraiss, H.; Cullen, E.M. Insect growth regulator effects of azadirachtin and neem oil on survivorship, development and fecundity of Aphis glycines (Homoptera: Aphididae) and its predator, Harmonia axyridis (Coleoptera: Coccinellidae). Pest Manag. Sci. 2008, 64, 660–668. [Google Scholar] [CrossRef]
- Amaral, K.D.; Martínez, L.C.; Lima, M.A.P.; Serrão, J.E.; Della Lucia, T.M.C. Azadirachtin impairs egg production in Atta sexdens leaf-cutting ant queens. Environ. Pollut. 2018, 243, 809–814. [Google Scholar] [CrossRef]
- Qin, D.; Zhang, P.; Zhou, Y.; Liu, B.; Xiao, C.; Chen, W.; Zhang, Z. Antifeeding effects of azadirachtin on the fifth instar Spodoptera litura larvae and the analysis of azadirachtin on target sensilla around mouthparts. Arch. Insect Biochem. Physiol. 2020, 103, e21646. [Google Scholar] [CrossRef]
- Cherry, R.; Nuessly, G. Repellency of the biopesticide, azadirachtin, to wireworms (Coleoptera: Elateridae). Fla. Entomol. 2010, 93, 52–55. [Google Scholar] [CrossRef]
- Martínez, L.C.; Plata-Rueda, A.; Zanuncio, J.C.; Serrao, J.E. Bioactivity of six plant extracts on adults of Demotispa neivai (Coleoptera: Chrysomelidae). J. Insect Sci. 2015, 15, 34. [Google Scholar] [CrossRef]
- Qiao, J.; Zou, X.; Lai, D.; Yan, Y.; Wang, Q.; Li, W.; Deng, S.; Xu, H.; Gu, H. Azadirachtin blocks the calcium channel and modulates the cholinergic miniature synaptic current in the central nervous system of Drosophila. Pest Manag. Sci. 2014, 70, 1041–1047. [Google Scholar] [CrossRef]
- Darwish, Y.A.; Omar, Y.M.; Hassan, R.E.; Mahmoud, M.A. Repellent effects of certain plant essential oil, plant extracts and inorganic salts to granary weevil, Sitophilus granarius (L.). Arch. Phytopathol. Plant Prot. 2013, 46, 1949–1957. [Google Scholar] [CrossRef]
- Franz, A.R.; Knaak, N.; Fiuza, L.M. Toxic effects of essential plant oils in adult Sitophilus oryzae (Linnaeus) (Coleoptera, Curculionidae). Rev. Bras. Entomol. 2011, 55, 116–120. [Google Scholar] [CrossRef]
- Vieira Ribeiro, A.; Almeida Luz, C.E.; Schetino Bastos, C.; Teichmann Krieger, Y.S.; Henriques da Silva, N.; Brandão da Silva, W. Toxicity of botanical and synthetic formulations to the maize weevil, Sitophilus zeamais (Coleoptera: Curculionidae). Rev. Colomb. Entomol. 2017, 43, 167–172. [Google Scholar] [CrossRef]
- Islam, M.S.; Talukder, F.A. Toxic and residual effects of Azadirachta indica, Tagetes erecta and Cynodon dactylon seed extracts and leaf powders towards Tribolium castaneum. J. Plant Dis. Prot. 2005, 112, 594–601. [Google Scholar] [CrossRef]
- da Costa, J.T.; Forim, M.R.; Costa, E.S.; de Souza, J.R.; Mondego, J.M.; Boica, A.L. Effects of different formulations of neem oil-based products on control Zabrotes subfasciatus (Boheman, 1833) (Coleoptera: Bruchidae) on beans. J. Stored Prod. Res. 2014, 56, 49–53. [Google Scholar] [CrossRef]
- Nathan, S.S.; Choi, M.Y.; Seo, H.Y.; Paik, C.H.; Kalaivani, K.; Kim, J.D. Effect of azadirachtin on acetylcholinesterase (AChE) activity and histology of the brown planthopper Nilaparvata lugens (Stal). Ecotox. Environ. Saf. 2008, 70, 244–250. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, T.; Sun, Z.; Li, H.; Qi, X.; Zhong, G.; Yi, X. Azadirachtin acting as a hazardous compound to induce multiple detrimental effects in Drosophila melanogaster. J. Hazard. Mater. 2018, 359, 338–347. [Google Scholar] [CrossRef]
- Jõgar, K.; Kuusik, A.; Metspalu, L.; Hiiesaar, K.; Grishakova, M.; Luik, A. Effects of neem EC on gas exchange, tracheal ventilation, and water loss in diapausing pupae of Pieris brassicae. Entomol. Exp. Appl. 2008, 126, 165–173. [Google Scholar] [CrossRef]
- Fan, S.T.; Zheng, Z.J.; Feng, Q.; Zhu, G.H. Azadirachtin induces fat body apoptosis by suppressing caspase-8 in the fall armyworm, Spodoptera frugiperda. J. Agric. Food Chem. 2024, 72, 19323–19332. [Google Scholar] [CrossRef]
- Silva, C.T.; Wanderley-Teixeira, V.; Cruz, G.S.; Cunha, F.M.; Teixeira, Á.A. Immune and nutritional responses of Podisus nigrispinus (Hemiptera: Pentatomidae) nymphs sprayed with azadirachtin. Austral Entomol. 2020, 59, 215–224. [Google Scholar] [CrossRef]
- Martinez, S.S.; Van Emden, H.F. Growth disruption, abnormalities and mortality of Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae) caused by azadirachtin. Neotrop. Entomol. 2001, 30, 113–125. [Google Scholar] [CrossRef]
- Vieira, C.S.; Bisogno, S.; Salvemini, M.; Loza Telleria, E.; Volf, P. Azadirachtin disrupts ecdysone signaling and alters sand fly immunity. Parasit. Vectors 2024, 17, 526. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Potter, M.F.; Haynes, K.F. Behavioral responses of the bed bug to insecticide residues. J. Med. Entomol. 2009, 46, 51–57. [Google Scholar] [CrossRef]
- Favaro, R.; Garrido, P.M.; Bruno, D.; Braglia, C.; Alberoni, D.; Baffoni, L.; Tettamanti, G.; Porrini, M.P.; Gioia, D.D.; Angeli, S. Combined effect of a neonicotinoid insecticide and a fungicide on honeybee gut epithelium and microbiota, adult survival, colony strength and foraging preferences. Sci. Total Environ. 2023, 905, 167277. [Google Scholar] [CrossRef] [PubMed]
- Kamminga, K.L.; Herbert, D.A., Jr.; Kuhar, T.P.; Malone, S.; Doughty, H. Toxicity, feeding preference, and repellency associated with selected organic insecticides against Acrosternum hilare and Euschistus servus (Hemiptera: Pentatomidae). J. Econ. Entomol. 2009, 102, 1915–1921. [Google Scholar] [CrossRef] [PubMed]
- Baumler, R.E.; Potter, D.A. Knockdown, residual, and antifeedant activity of pyrethroids and home landscape bioinsecticides against Japanese beetles (Coleoptera: Scarabaeidae) on linden foliage. J. Econ. Entomol. 2014, 100, 451–458. [Google Scholar] [CrossRef]
- Capinera, J.L.; Froeba, J.G. Behavioral responses of Schistocerca americana (Orthoptera: Acrididae) to azadirex (neem)-treated host plants. J. Econ. Entomol. 2007, 100, 117–122. [Google Scholar] [CrossRef]
- Silva, W.M.; Martínez, L.C.; Plata-Rueda, A.; Serrão, J.E.; Zanuncio, J.C. Exposure to insecticides causes effects on survival, prey consumption, and histological changes in the midgut of the predatory bug, Podisus nigrispinus (Hemiptera: Pentatomidae). Environ. Sci. Pollut. Res. 2021, 28, 57449–57458. [Google Scholar] [CrossRef]
- Batista, C.H.; Plata-Rueda, A.; Zanuncio, J.C.; Serrão, J.E.; Martínez, L.C. Indoxacarb effects on non-target predator, Podisus distinctus (Hemiptera: Pentatomidae). Environ. Sci. Pollut. Res. 2022, 29, 29967–29975. [Google Scholar] [CrossRef]
- Wang, K.Y.; Zhang, Y.; Wang, H.Y.; Xia, X.M.; Liu, T.X. Influence of three diets on susceptibility of selected insecticides and activities of detoxification esterases of Helicoverpa assulta (Lepidoptera: Noctuidae). Pestic. Biochem. Physiol. 2010, 96, 51–55. [Google Scholar] [CrossRef]
- Zou, C.; Lv, C.; Wang, Y.; Cao, C.; Zhang, G. Larvicidal activity and insecticidal mechanism of Chelidonium majus on Lymantria dispar. Pestic. Biochem. Physiol. 2017, 142, 123–132. [Google Scholar] [CrossRef]
- Akbar, R.; Faheem, B.; Aziz, T.; Ali, A.; Ullah, A.; Khan, I.A.; Sun, J. Evaluating the efficacy of plant extracts in managing the bruchid beetle, Callosobruchus maculatus (Coleoptera: Bruchidae). Insects 2024, 15, 691. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Athanassiou, C.G.; Saitanis, C.J.; Kontodimas, D.C.; Roussos, A.N.; Tsoutsa, M.S.; Anastassopoulou, U.A. Effect of two azadirachtin formulations against adults of Sitophilus oryzae and Tribolium confusum on different grain commodities. J. Food Prot. 2007, 70, 1627–1632. [Google Scholar] [CrossRef]
- Vinha, G.L.; Plata-Rueda, A.; Soares, M.A.; Zanuncio, J.C.; Serrão, J.E.; Martinez, L.C. Deltamethrin-mediated effects on locomotion, respiration, feeding, and histological changes in the midgut of Spodoptera frugiperda caterpillars. Insects 2021, 12, 483. [Google Scholar] [CrossRef] [PubMed]
- Eesiah, S.; Yu, J.; Dingha, B.; Amoah, B.; Mikiashvili, N. Preliminary assessment of repellency and toxicity of essential oils against Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae) on stored organic corn grains. Foods 2022, 11, 2907. [Google Scholar] [CrossRef]
- Plata-Rueda, A.; Fiaz, M.; Brugger, B.P.; Canas, V.; Coelho, R.P.; Zanuncio, J.C.; Martínez, L.C.; Serrão, J.E. Lemongrass essential oil and its components cause effects on survival, locomotion, ingestion, and histological changes of the midgut in Anticarsia gemmatalis caterpillars. Toxin Rev. 2022, 41, 208–217. [Google Scholar] [CrossRef]
- Plata-Rueda, A.; Santos, M.H.D.; Serrão, J.E.; Martínez, L.C. Chemical composition and insecticidal properties of Origanum vulgare (Lamiaceae) essential oil against the stored product beetle, Sitophilus granarius. Agronomy 2022, 12, 2204. [Google Scholar] [CrossRef]
- Annaz, H.; Gonzalez-Coloma, A.; Moullamri, M.; Ajaha, A.; Laglaoui, A.; Bouayad, N.; Rharrabe, K. Chemical profiling and bioactivities of common commercial essential oils against larvae and adults of Tribolium castaneum: Insecticidal, behavioral, and biochemical effects. Ind. Crops Prod. 2024, 222, 120082. [Google Scholar] [CrossRef]
- de Menezes, C.H.M.; Plata-Rueda, A.; Zanuncio, J.C.; Serrão, J.E.; Martínez, L.C. Exposure to insecticides cause mortality, respiratory disturbs, and antifeeding effects in Anticarsia gemmatalis. Phytoparasitica 2024, 52, 15. [Google Scholar] [CrossRef]


| Developmental Stage | No. of Insects | Lethal Concentration | Estimate Value (ppm) | Confidence Interval to 95% (ppm) | Slope ± SE | χ2 (p-Value) |
|---|---|---|---|---|---|---|
| Larva | 90 | LC25 | 0.35 | 0.22–0.54 | 1.710 ± 0.13 | 1.82 (0.10) |
| 90 | LC50 | 3.36 | 2.26–5.13 | |||
| 90 | LC75 | 31.7 | 19.1–58.1 | |||
| 90 | LC95 | 80.3 | 36.3–175 | |||
| Pupa | 90 | LC25 | 7.97 | 5.93–10.1 | 2.392 ± 0.16 | 1.34 (0.24) |
| 90 | LC50 | 23.0 | 18.8–27.9 | |||
| 90 | LC75 | 66.7 | 54.3–84.1 | |||
| 90 | LC95 | 307. | 222–462 | |||
| Adult | 90 | LC25 | 18.6 | 15.5–22.2 | 3.146 ± 0.20 | 1.26 (0.27) |
| 90 | LC50 | 37.7 | 32.3–43.8 | |||
| 90 | LC75 | 76.1 | 64.6–91.5 | |||
| 90 | LC95 | 209. | 165–277 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintero, H.; Quintero Cortes, J.; Plata-Rueda, A.; Martínez, L.C. Azadirachtin-Mediated Responses in the Maize Weevil, Sitophilus zeamais (Coleoptera: Curculionidae). Insects 2025, 16, 294. https://doi.org/10.3390/insects16030294
Quintero H, Quintero Cortes J, Plata-Rueda A, Martínez LC. Azadirachtin-Mediated Responses in the Maize Weevil, Sitophilus zeamais (Coleoptera: Curculionidae). Insects. 2025; 16(3):294. https://doi.org/10.3390/insects16030294
Chicago/Turabian StyleQuintero, Herlinda, Johana Quintero Cortes, Angelica Plata-Rueda, and Luis Carlos Martínez. 2025. "Azadirachtin-Mediated Responses in the Maize Weevil, Sitophilus zeamais (Coleoptera: Curculionidae)" Insects 16, no. 3: 294. https://doi.org/10.3390/insects16030294
APA StyleQuintero, H., Quintero Cortes, J., Plata-Rueda, A., & Martínez, L. C. (2025). Azadirachtin-Mediated Responses in the Maize Weevil, Sitophilus zeamais (Coleoptera: Curculionidae). Insects, 16(3), 294. https://doi.org/10.3390/insects16030294








