Population Dynamics of Galerucella birmanica and Its Aggregation Behavior in Brasenia schreberi Aquaculture System
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
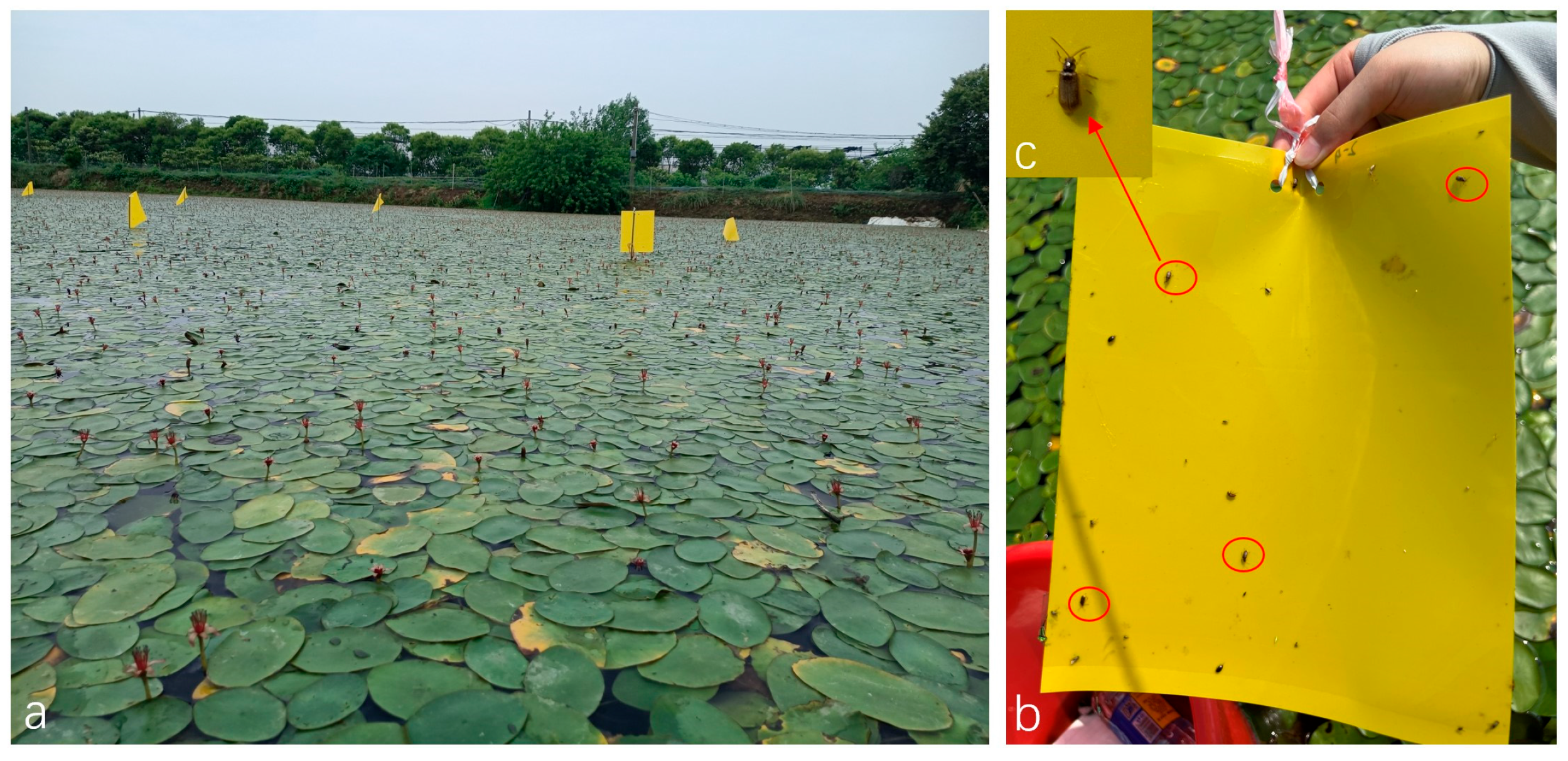
2.1. Study Location
2.2. Monitoring of Galerucella birmanica
2.3. Comparison of Galerucella birmanica Occurrence in Relatively Intact and Severely Chewed Areas
2.4. Two-Choice Tests
2.5. GC–MS Analysis for Volatiles from Intact and Chewed B. schreberi Leaves
2.6. Verification of Volatile Substance Attracting Galerucella birmanica
2.7. Statistical Analysis
3. Results
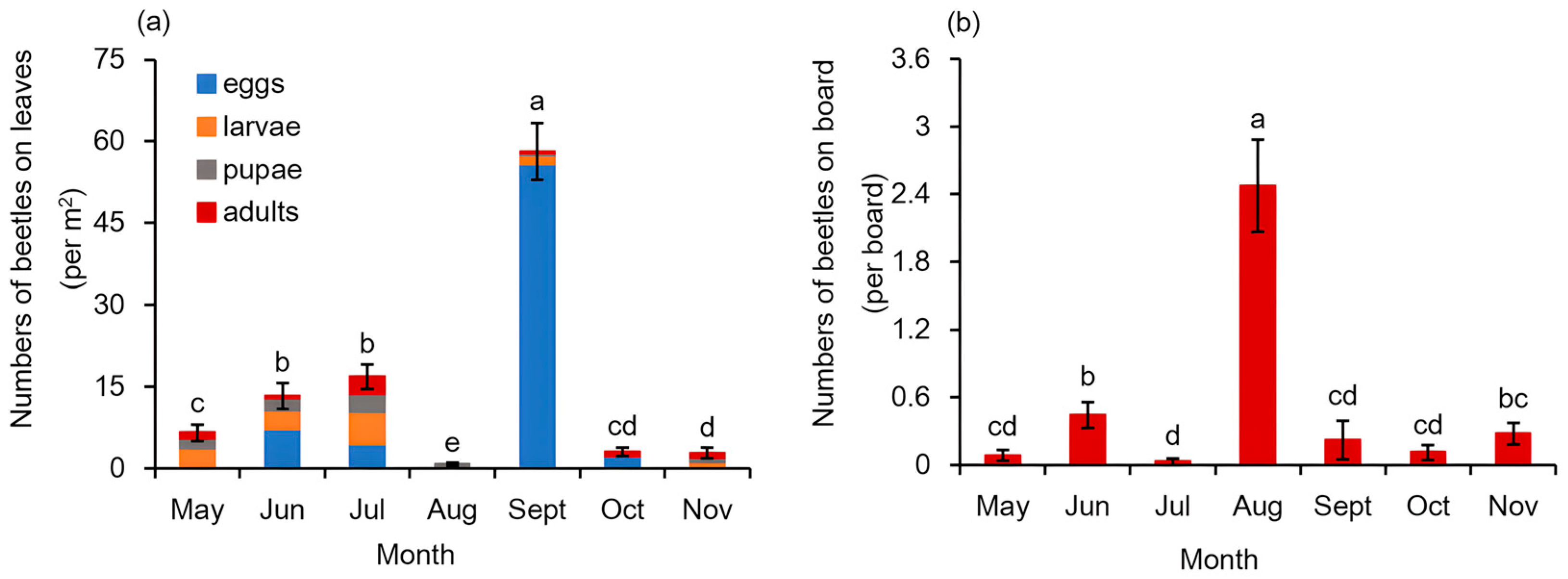
3.1. Monthly Occurrence of Galerucella birmanica in Brasenia schreberi Pond
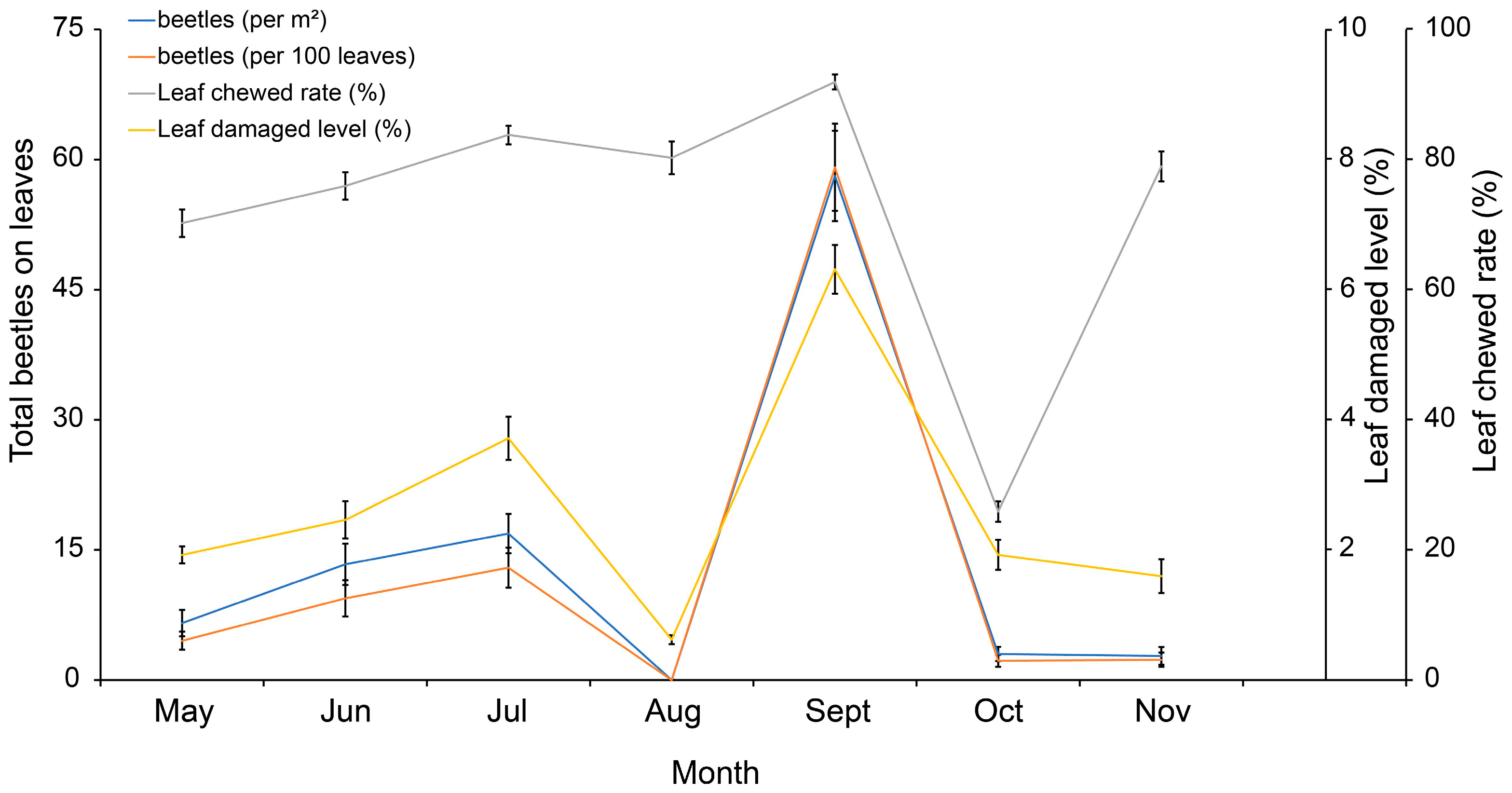
3.2. Leaf Damage Condition of Brasenia schreberi and Its Correlation with Galerucella birmanica Occurrence
3.3. Aggregation of Galerucella birmanica in Brasenia schreberi Pond
3.4. Volatiles Differed Between Intact and Chewed Brasenia schreberi Leaves
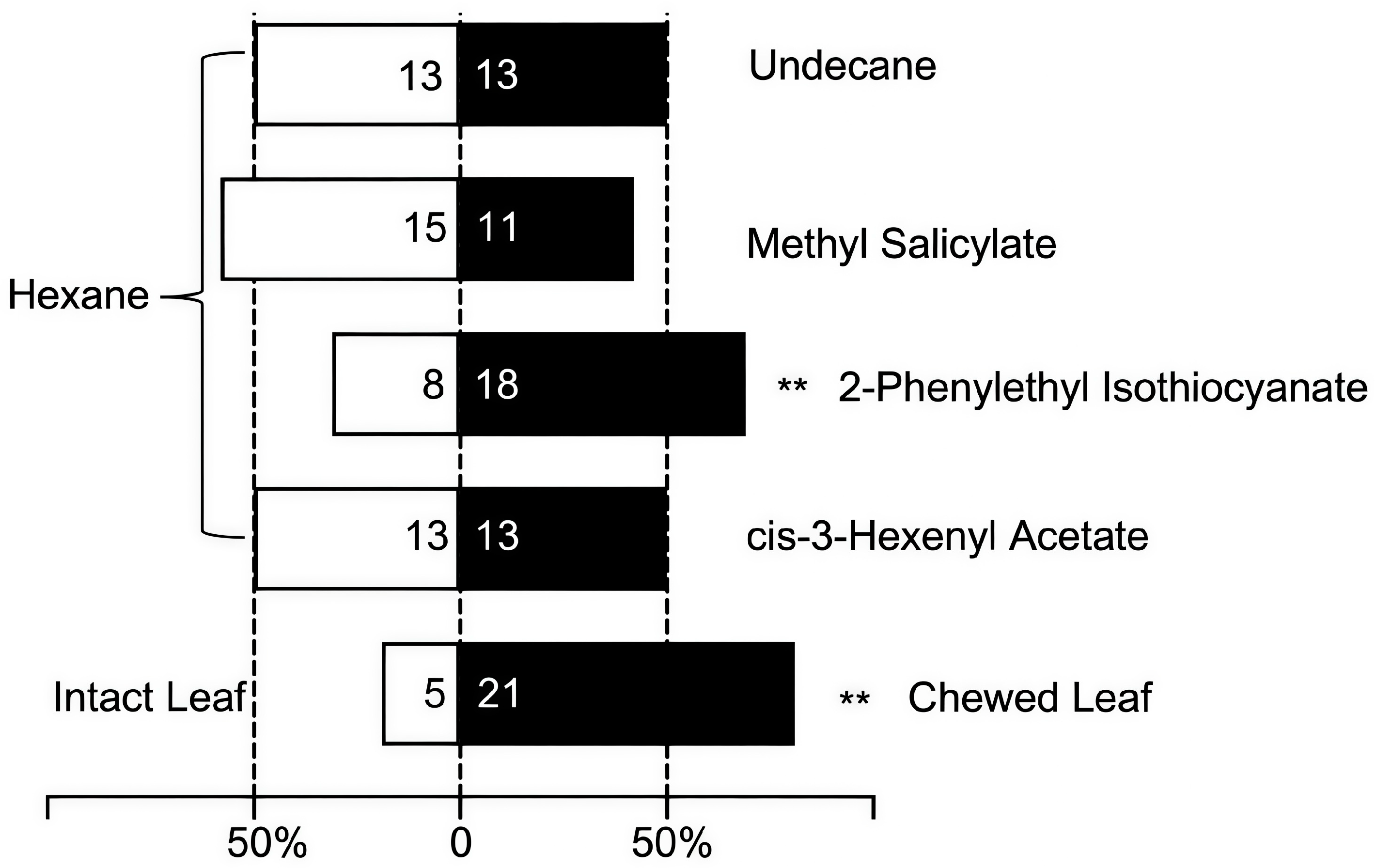
3.5. Two-Choice Tests of Volatiles from Brasenia schreberi by Adult Galerucella birmanica
4. Discussions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, M.L.; Osborn, J.M. Pollen ontogeny in Brasenia (Cabombaceae, Nymphaeales). Am. J. Bot. 2006, 93, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Drzymulska, D. On the history of Brasenia Schreb. in the European Pleistocene. Veg. Hist. Archaeobot. 2017, 27, 527–534. [Google Scholar] [CrossRef]
- Feng, S.; Luan, D.; Ning, K.; Shao, P.; Sun, P. Ultrafiltration isolation, hypoglycemic activity analysis and structural characterization of polysaccharides from Brasenia schreberi. Int. J. Biol. Macromol. 2019, 135, 141–151. [Google Scholar] [CrossRef]
- Zhou, C.; Zhong, Z. Watershield (Brasenia schreberi J. F. Gmel.), from popular vegetable to endangered species. World J. Agric. Soil Sci. 2021, 6, 2020. [Google Scholar] [CrossRef]
- Schneider, E.L.; Carlquist, S. Vessels in Brasenia (Cabombaceae): New perspectives on vessel origin in primary xylem of angiosperms. Am. J. Bot. 1996, 83, 1236–1240. [Google Scholar] [CrossRef]
- Liu, G.; Feng, S.; Yan, J.; Luan, D.; Sun, P.; Shao, P. Antidiabetic potential of polysaccharides from Brasenia schreberi regulating insulin signaling pathway and gut microbiota in type 2 diabetic mice. Curr. Res. Food Sci. 2022, 5, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Yu, X.; Liu, J.; Li, J.; Ai, T.; Yin, C.; Liu, H.; Qin, R. A special polysaccharide hydrogel coated on Brasenia schreberi: Preventive effects against ulcerative colitis via modulation of gut microbiota. Food Funct. 2023, 14, 3564–3575. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, Y.; Fang, Q.; Feng, R.; Zhang, J.; Zhou, W.; Wei, Q. Polysaccharides from Brasenia schreberi with great antioxidant ability and the potential application in yogurt. Molecules 2023, 29, 150. [Google Scholar] [CrossRef]
- Xie, C.; Li, J.; Pan, F.; Fu, J.; Zhou, W.; Lu, S.; Li, P.; Zhou, C. Environmental factors influencing mucilage accumulation of the endangered Brasenia schreberi in China. Sci. Rep. 2018, 8, 17955. [Google Scholar] [CrossRef]
- Lu, Z.; Zhu, J.; Zhu, S.; Chen, Z. Preliminary studies on the beetle (Galerucella birmanica Jacoby)—An insect pest of waterchestnut and watershield. Sci. Agric. Sin. 1984, 5, 73–76. [Google Scholar]
- Ding, J.; Blossey, B.; Du, Y.; Zheng, F. Galerucella birmanica (Coleoptera: Chrysomelidae), a promising potential biological control agent of water chestnut, Trapa natans. Biol. Control 2006, 36, 80–90. [Google Scholar] [CrossRef]
- Simmons, W.; Blossey, B. Host plant phylogeny does not fully explain host choice and feeding preferences of Galerucella birmanica, a promising biological control herbivore of Trapa natans. Biol. Control 2023, 180, 105201. [Google Scholar] [CrossRef]
- Han, Q.; Huang, M.; Li, S.; Zeng, X.; Hu, G.; Tang, C. Occurrence and integrated control measures of Galerucella birmanica Jacoby. J. Anhui Agric. Sci. 2015, 43, 80–82. [Google Scholar] [CrossRef]
- Ding, J.; Wang, Y.; Jin, X. Monitoring populations of Galerucella birmanica (Coleoptera: Chrysomelidae) on Brasenia schreberi and Trapa natans (Lythraceae): Implications for biological control. Biol. Control 2007, 43, 71–77. [Google Scholar] [CrossRef]
- Pemberton, R.W. Natural enemies of Trapa spp. in Northeast Asia and Europe. Biol. Control 1999, 14, 168–180. [Google Scholar]
- Schuman, M.C.; Baldwin, I.T. The layers of plant responses to insect herbivores. Annu. Rev. Entomol. 2016, 61, 373–394. [Google Scholar] [CrossRef] [PubMed]
- Aljbory, Z.; Chen, M.S. Indirect plant defense against insect herbivores: A review. Insect Sci. 2018, 25, 2–23. [Google Scholar] [CrossRef] [PubMed]
- de Bobadilla, M.F.; Vitiello, A.; Erb, M.; Poelman, E.H. Plant defense strategies against attack by multiple herbivores. Trends Plant Sci. 2022, 27, 528–535. [Google Scholar] [CrossRef]
- Pinero, J.C.; Souder, S.K.; Vargas, R.I. Vision-mediated exploitation of a novel host plant by a tephritid fruit fly. PLoS ONE 2017, 12, e0174636. [Google Scholar] [CrossRef]
- Zhang, J.; Bisch-Knaden, S.; Fandino, R.A.; Yan, S.; Obiero, G.F.; Grosse-Wilde, E.; Hansson, B.S.; Knaden, M. The olfactory coreceptor IR8a governs larval feces-mediated competition avoidance in a hawkmoth. Proc. Natl. Acad. Sci. USA 2019, 116, 21828–21833. [Google Scholar] [CrossRef]
- Wang, J.; Wei, J.; Yi, T.; Li, Y.Y.; Xu, T.; Chen, L.; Xu, H. A green leaf volatile, (Z)-3-hexenyl-acetate, mediates differential oviposition by Spodoptera frugiperda on maize and rice. BMC Biol. 2023, 21, 140. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Zhang, S.S.; Niu, B.L.; Ji, D.F.; Liu, X.J.; Li, M.W.; Bai, H.; Palli, S.R.; Wang, C.Z.; Tan, A.J. A determining factor for insect feeding preference in the silkworm, Bombyx mori. PLoS Biol. 2019, 17, e3000162. [Google Scholar] [CrossRef]
- Wang, C.; Li, G.; Miao, C.; Zhao, M.; Wang, B.; Guo, X. Nonanal modulates oviposition preference in female Helicoverpa assulta (Lepidoptera: Noctuidae) via the activation of peripheral neurons. Pest Manag. Sci. 2020, 76, 3159–3167. [Google Scholar] [CrossRef]
- Magalhães, D.M.; Borges, M.; Laumann, R.A.; Blassioli Moraes, M.C. Influence of multiple- and single-species infestations on herbivore-induced cotton volatiles and Anthonomus grandis behaviour. J. Pest Sci. 2018, 91, 1019–1032. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, S. GC-MS analysis of volatile oil content for Brasenia schreberi. In Proceedings of the 2011 International Conference on Electronics and Optoelectronics, Dalian, China, 29–31 July 2011. [Google Scholar] [CrossRef]
- Zhang, J.; Komail Raza, S.A.; Wei, Z.; Keesey, I.W.; Parker, A.L.; Feistel, F.; Chen, J.; Cassau, S.; Fandino, R.A.; Grosse-Wilde, E.; et al. Competing beetles attract egg laying in a hawkmoth. Curr. Biol. 2022, 32, 861–869.e8. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.D.; Liu, Y.J.; Liu, J.H.; Liu, Y. Pheromones emitted by both female and male moths regulate coordination between the sexes for Agriphila aeneociliella (Lepidoptera: Crambidae). Insect Sci. 2023, 30, 1481–1492. [Google Scholar] [CrossRef] [PubMed]
- Ekholm, A.; Faticov, M.; Tack, A.J.M.; Berger, J.; Stone, G.N.; Vesterinen, E.; Roslin, T. Community phenology of insects on oak: Local differentiation along a climatic gradient. Ecosphere 2021, 12, e03785. [Google Scholar] [CrossRef]
- Owens, C.H.; Lee, M.J.; Grim, M.; Schroeder, J.; Young, H.S. Interactions between temperature and predation impact insect emergence in alpine lakes. Ecosphere 2023, 14, e4619. [Google Scholar] [CrossRef]
- Zheng, F.S.; Du, Y.Z.; Wang, Z.J.; Xu, J.J. Effect of temperature on the demography of Galerucella birmanica (Coleoptera: Chrysomelidae). Insect Sci. 2008, 15, 375–380. [Google Scholar] [CrossRef]
- Liu, N.; Gou, W.; Ma, W.; Tang, L.; Hu, G.; Sun, Y. Effects of temperature on the growth, development and reproduction of Diorhabda rybakowi (Coleoptera: Chrysomelidae). Plant Prot. 2023, 49, 220–226. [Google Scholar] [CrossRef]
- Rao, S.; Cossé, A.A.; Zilkowski, B.W.; Bartlelt, R.J. Aggregation pheromone of the cereal leaf beetle: Field evaluation and emission from males in the laboratory. J. Chem. Ecol. 2003, 29, 2165–2175. [Google Scholar] [CrossRef]
- Lacoume, S.; Bressac, C.; Chevrier, C. Sperm production and mating potential of males after a cold shock on pupae of the parasitoid wasp Dinarmus basalis (Hymenoptera: Pteromalidae). J. Insect Physiol. 2007, 53, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chen, Z.; Qu, J.; Zhang, F.; Yin, X.; Xu, Y. Responses of Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) adults to cold acclimation and the related changes of activities of several enzymes in their bodies. Acta Entomol. Sin. 2010, 53, 147–153. [Google Scholar] [CrossRef]
- Qin, J.; Zhang, L.; Cheng, Y.; Luo, L.; Lei, C.; Jiang, X. Age-stage two-sex life table for laboratory populations of oriental armyworm Mythimna separata (Walker) under different temperatures. Acta Phytophylac. Sin. 2017, 44, 729–736. [Google Scholar] [CrossRef]
- Huang, L.H.; Chen, B.; Kang, L. Impact of mild temperature hardening on thermotolerance, fecundity, and Hsp gene expression in Liriomyza huidobrensis. J. Insect Physiol. 2007, 53, 1199–1205. [Google Scholar] [CrossRef]
- Zheng, F.; Zheng, Y.; Lu, Y.; Qiang, C.; Ding, J. Spatial distribution pattern of water chestnut beetle (Galerucella birmanica Jacoby). Chin. J. Eco-Agric. 2006, 14, 171–175. [Google Scholar]
- Chen, Y.; Yan, X. The role of glucosinolates in plant-biotic environment interactions. Acta Ecol. Sin. 2007, 27, 2584–2593. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Erb, M. Tritrophic interactions mediated by herbivore-induced plant volatiles: Mechanisms, ecological relevance, and application potential. Annu. Rev. Entomol. 2018, 63, 433–452. [Google Scholar] [CrossRef]
- Ahuja, I.; Rohloff, J.; Bones, A.M. Defence mechanisms of Brassicaceae: Implications for plant-insect interactions and potential for integrated pest management. A review. Agron. Sustain. Dev. 2010, 30, 311–348. [Google Scholar] [CrossRef]
- Renwick, J.A.A. The chemical world of crucivores: Lures, treats and traps. Entomol. Exp. Appl. 2003, 104, 35–42. [Google Scholar] [CrossRef]
- Agrawal, A.A.; Kurashige, N.S. A role for isothiocyanates in plant resistance against the specialist herbivore Pieris rapae. J. Chem. Ecol. 2003, 29, 1403–1415. [Google Scholar] [CrossRef] [PubMed]
- Bartlet, E.; Blight, M.M.; Hick, A.J.; Williams, I.H. The responses of the cabbage seed weevil (Ceutorhynchus assimilis) to the odor of oilseed rape (Brassica napus) and to some volatile isothiocyanates. Entomol. Exp. Appl. 1993, 68, 295–302. [Google Scholar] [CrossRef]
- Smart, L.E.; Blight, M.M. Response of the pollen beetle, Meligethes aeneus, to traps baited with volatiles from oilseed rape, Brassica napus. J. Chem. Ecol. 2000, 26, 1051–1064. [Google Scholar] [CrossRef]
- Rodrigo, F.; Burgueno, A.P.; Gonzalez, A.; Rossini, C. Better together: Volatile-mediated intraguild effects on the preference of Tuta absoluta and Trialeurodes vaporariorum for tomato plants. J. Chem. Ecol. 2023, 49, 725–741. [Google Scholar] [CrossRef]
- Engelberth, J.; Alborn, H.T.; Schmelz, E.A.; Tumlinson, J.H. Airborne signals prime plants against insect herbivore attack. Proc. Natl. Acad. Sci. USA 2004, 101, 1781–1785. [Google Scholar] [CrossRef]
- Wari, D.; Aboshi, T.; Shinya, T.; Galis, I. Integrated view of plant metabolic defense with particular focus on chewing herbivores. J. Integr. Plant Biol. 2022, 64, 449–475. [Google Scholar] [CrossRef]
- Wang, P.; Lou, Y.-G. Screening and field evaluation of synthetic plant volatiles as attractants for Anagrus nilaparvatae Pang et Wang, an egg parasitoid of rice planthoppers. Chin. J. Appl. Entomol. 2013, 50, 431–440. [Google Scholar] [CrossRef]
- Gong, Q.; Wang, Y.; He, L.; Huang, F.; Zhang, D.; Wang, Y.; Wei, X.; Han, M.; Deng, H.; Luo, L.; et al. Molecular basis of methyl-salicylate-mediated plant airborne defence. Nature 2023, 622, 139–148. [Google Scholar] [CrossRef]
- Yao, C.; Du, L.; Liu, Q.; Hu, X.; Ye, W.; Turlings, T.C.J.; Li, Y. Stemborer-induced rice plant volatiles boost direct and indirect resistance in neighboring plants. New Phytol. 2023, 237, 2375–2387. [Google Scholar] [CrossRef]
- Yang, K.; Gong, X.L.; Li, G.C.; Huang, L.Q.; Ning, C.; Wang, C.Z. A gustatory receptor tuned to the steroid plant hormone brassinolide in Plutella xylostella (Lepidoptera: Plutellidae). eLife 2020, 9, e64114. [Google Scholar] [CrossRef]
- Hao, X.; Wang, S.; Fu, Y.; Liu, Y.; Shen, H.; Jiang, L.; McLamore, E.S.; Shen, Y. The WRKY46-MYC2 module plays a critical role in E-2-hexenal-induced anti-herbivore responses by promoting flavonoid accumulation. Plant Commun. 2024, 5, 100734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Han, W.H.; Zhang, F.B.; Wang, J.X.; Liu, S.S.; Wang, X.W. Attraction of Nicotiana benthamiana to Bemisia tabaci is related to a chemical signal in plant volatile, undecane. Pest Manag. Sci. 2024; in press. [Google Scholar] [CrossRef]
- Shivaramu, S.; Damodaram, K.J.P.; Bhatnagar, A.; Naga, K.C.; Byregowda, V.Y.; Bairwa, A.; Sharma, S.; Singh, R.K.; Singh, B.; Kempraj, V. Influence of Bemisia tabaci-induced plant volatiles on the host-choice behavior of Tuta absoluta. J. Chem. Ecol. 2025, 51, 3. [Google Scholar] [CrossRef] [PubMed]
- Holmes, K.D.; Agrawal, A.A. Induced resistance mitigates the effect of plant neighbors on susceptibility to herbivores. Ecosphere 2021, 12, e03334. [Google Scholar] [CrossRef]
- Foba, C.N.; Shi, J.H.; An, Q.Q.; Liu, L.; Hu, X.J.; Hegab, M.; Liu, H.; Zhao, P.M.; Wang, M.Q. Volatile-mediated tritrophic defense and priming in neighboring maize against Ostrinia furnacalis and Mythimna separata. Pest Manag. Sci. 2023, 79, 105–113. [Google Scholar] [CrossRef]
- Choh, Y.; Ozawa, R.; Takabayashi, J. Do plants use airborne cues to recognize herbivores on their neighbours? Exp. Appl. Acarol. 2013, 59, 263–273. [Google Scholar] [CrossRef]
- Gurr, G.M.; Wratten, S.D.; Landis, D.A.; You, M. Habitat management to suppress pest populations: Progress and prospects. Annu. Rev. Entomol. 2017, 62, 91–109. [Google Scholar] [CrossRef]





| Beetles on Leaves (per m2) | Beetles on Leaves (per One Hundred Leaves) | Beetles on Board | |||||
|---|---|---|---|---|---|---|---|
| Egg | Larva | Pupa | Adult | Total | |||
| Leaf chewed rate | 0.3846 (0.0000) | 0.2416 (0.0001) | 0.2264 (0.0003) | 0.1011 (0.1095) | 0.4533 (0.0000) | 0.4641 (0.000) | 0.1586 (0.0117) |
| Leaf damaged level | 0.6431 (0.0000) | 0.3919 (0.0000) | 0.3509 (0.0000) | 0.2691 (0.0000) | 0.7809 (0.0000) | 0.7783 (0.0000) | −0.3034 (0.0000) |
| No. | Retention Time/Min | Compounds | CAS Number | Contents *b (ng) | t-test *c (p Value) | |
|---|---|---|---|---|---|---|
| Chewed Leaves | Intact Leaves | |||||
| 1 | 3.959 | Toluene | 108-88-3 | 58.39 ± 4.14 | 58.97 ± 4.06 | |
| 2 | 4.499 | Octane *a | 111-65-9 | 200.00 ± 0.00 | 200.00 ± 0.00 | |
| 3 | 4.737 | 3-ethyl-Hexane | 619-99-8 | 366.01 ± 24.49 | 365.64 ± 21.61 | |
| 4 | 5.988 | Ethylbenzene | 100-41-4 | 187.36 ± 26.85 | 179.84 ± 24.95 | |
| 5 | 6.185 | p-Xylene | 106-42-3 | 546.35 ± 55.36 | 543.52 ± 54.18 | |
| 6 | 6.714 | Styrene | 100-42-5 | 289.524 ± 23.44 | 289.64 ± 17.76 | |
| 7 | 7.046 | Heptanal | 111-71-7 | 230.112 ± 15.65 | 230.132 ± 12.64 | |
| 8 | 7.56 | 4-Ethylbenzoic acid | 619-64-7 | 86.316 ± 12.50 | 101.932 ± 6.81 | |
| 9 | 8.559 | Benzaldehyde | 100-52-7 | 665.544 ± 24.83 | 670.554 ± 13.43 | |
| 10 | 8.722 | 1,2,3-trimethyl-Benzene | 526-73-8 | 59.346 ± 3.94 | 58.646 ± 3.41 | |
| 11 | 8.893 | (1S)-(1)-beta-Pinene | 18172-67-3 | 161.38 ± 15.37 | 159.63 ± 16.62 | |
| 12 | 9.272 | 6-methyl-5-Hepten-2-one | 110-93-0 | 276.52 ± 16.61 | 282.864 ± 10.41 | |
| 13 | 9.308 | cis-3-Hexenyl Acetate | 3681-71-8 | 917.33 ± 29.56 | 604.034 ± 23.24 | 0.001 |
| 14 | 9.552 | Octanal | 124-13-0 | 232.19 ± 22.16 | 223.388 ± 19.23 | |
| 15 | 9.822 | Phenol | 108-95-2 | 224.094 ± 17.12 | 226.398 ± 15.62 | |
| 16 | 10.289 | D-Limonene | 138-86-3 | 304.58 ± 21.10 | 300.4 ± 18.07 | |
| 17 | 10.357 | 2-ethyl-1-Hexanol | 104-76-7 | 177.65 ± 9.31 | 176.19 ± 10.34 | |
| 18 | 10.826 | 2-Phenethyl isothiocyanate | 2257-09-2 | 595.37 ± 28.42 | 356.00 ± 13.44 | 0.000 |
| 19 | 11.316 | 3-Carene | 13466-78-9 | 189.00 ± 34.52 | 185.05 ± 30.89 | |
| 20 | 12.126 | 2,6,10-trimethyl-Dodecane | 3891-98-3 | 222.22 ± 8.25 | 217.92 ± 8.77 | |
| 21 | 12.192 | γ-Chlorobutyrophenone | 939-52-6 | 287.82 ± 43.66 | 323.826 ± 22.37 | |
| 22 | 13.306 | (E)-2-Hexenyl benzoate | 76841-70-8 | 157.74 ± 11.90 | 142.974 ± 14.15 | |
| 23 | 13.337 | Undecane | 1120-21-4 | 771.44 ± 34.72 | 1003.28 ± 47.88 | 0.000 |
| 24 | 14.112 | 2-butyl-1-Octanol | 3913-02-8 | 160.01 ± 14.42 | 158.91 ± 10.25 | |
| 25 | 14.261 | Nonanal | 124-19-6 | 175.60 ± 7.44 | 159.80 ± 8.25 | |
| 26 | 15.278 | Camphor | 76-22-2 | 154.43 ± 13.11 | 156.30 ± 11.13 | |
| 27 | 16.212 | Naphthalene | 91-20-3 | 184.13 ± 6.60 | 191.71 ± 7.12 | |
| 28 | 16.502 | Methyl salicylate | 119-36-8 | 1079.84 ± 49.39 | 532.11 ± 18.23 | 0.000 |
| 29 | 16.902 | 2,6,10-trimethyl-Tetradecane | 14905-56-7 | 109.19 ± 22.45 | 113.38 ± 16.37 | |
| 30 | 17.234 | Benzothiazole | 95-16-9 | 171.96 ± 3.68 | 171.95 ± 2.95 | |
| 31 | 18.075 | Stearic acid | 57-11-4 | 142.30 ± 4.94 | 149.25 ± 4.72 | |
| 32 | 18.448 | 2-methyl-Undecane | 97659-99-9 | 318.48 ± 26.72 | 314.55 ± 21.37 | |
| 33 | 18.421 | Tridecane | 629-50-5 | 377.56 ± 21.05 | 387.42 ± 11.08 | |
| 34 | 18.561 | 3-methyl-Tridecane | 6418-41-3 | 107.24 ± 19.29 | 109.31 ± 17.34 | |
| 35 | 18.933 | Oleic acid | 112-80-1 | 372.65 ± 39.87 | 380.96 ± 31.36 | |
| 36 | 19.151 | Tetradecane | 629-59-4 | 200.07 ± 22.43 | 195.71 ± 11.36 | |
| 37 | 20.132 | 2-methyl-1-Hexadecanol | 2490-48-4 | 579.88 ± 105.67 | 557.93 ± 35.53 | |
| 38 | 20.363 | Dimethyl phthalate | 131-11-3 | 211.96 ± 27.77 | 202.21 ± 17.90 | |
| 39 | 21.424 | α-Farnesene | 502-61-4 | 948.01 ± 8.00 | 947.66 ± 4.68 | |
| 40 | 21.569 | Butylated Hydroxytoluene | 128-37-0 | 1608.61 ± 164.07 | 1612.82 ± 131.27 | |
| 41 | 22.316 | (Z)-9-Octadecenoic acid | 8051-88-5 | 1766.76 ± 129.92 | 1743.13 ± 77.81 | |
| 42 | 22.903 | Diethyltoluamide | 134-62-3 | 101.82 ± 7.38 | 98.55 ± 5.20 | |
| 43 | 23.385 | 2-Dodecen-1-ylsuccinic anhydride | 26544-38-7 | 256.41 ± 35.97 | 287.61 ± 29.21 | |
| 44 | 24.215 | Behenic alcohol | 661-19-8 | 512.80 ± 18.80 | 522.41 ± 11.97 | |
| 45 | 24.771 | tert-Hexadecanethiol | 25360-09-2 | 131.28 ± 12.63 | 137.69 ± 8.46 | |
| 46 | 25.134 | Methyl cis-9,10-epoxyoctadecanoate | 2566-91-8 | 336.41 ± 26.73 | 307.52 ± 13.27 | |
| 47 | 26.312 | 2,6-Ditert-butyl-4-ethylphenol | 4130-42-1 | 157.00 ± 10.54 | 158.95 ± 12.13 | |
| 48 | 27.438 | Albocycline | 25129-91-3 | 112.61 ± 14.01 | 123.36 ± 6.15 | |
| 49 | 27.536 | 1-Heptatriacotanol | 105794-58-9 | 150.99 ± 9.91 | 160.01 ± 11.11 | |
| 50 | 28.548 | Obtusilactone | 56799-51-0 | 186.20 ± 31.83 | 189.92 ± 10.50 | |
| 51 | 29.694 | Dibutyl phthalate | 84-74-2 | 725.29 ± 39.45 | 698.03 ± 20.26 | |
| 52 | 32.045 | Heptacosane | 593-49-7 | 472.62 ± 25.03 | 454.58 ± 21.81 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, Y.; Zhou, C. Population Dynamics of Galerucella birmanica and Its Aggregation Behavior in Brasenia schreberi Aquaculture System. Insects 2025, 16, 371. https://doi.org/10.3390/insects16040371
Wang Y, Wang Y, Zhou C. Population Dynamics of Galerucella birmanica and Its Aggregation Behavior in Brasenia schreberi Aquaculture System. Insects. 2025; 16(4):371. https://doi.org/10.3390/insects16040371
Chicago/Turabian StyleWang, Yini, Yahong Wang, and Changfang Zhou. 2025. "Population Dynamics of Galerucella birmanica and Its Aggregation Behavior in Brasenia schreberi Aquaculture System" Insects 16, no. 4: 371. https://doi.org/10.3390/insects16040371
APA StyleWang, Y., Wang, Y., & Zhou, C. (2025). Population Dynamics of Galerucella birmanica and Its Aggregation Behavior in Brasenia schreberi Aquaculture System. Insects, 16(4), 371. https://doi.org/10.3390/insects16040371





