Chronic Exposure to Field-Level Thiamethoxam Impairs Gut Tissue and Reduces Honeybee (Apis cerana) Survival
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Honeybee Sample Handling and Preparation of Pesticides
2.2. Sugar Solution Consumption and Survival
2.3. Samples for Transcriptome and Metabolome
2.4. Hematoxylin and Eosin (HE) Staining of the Gut Tissue
2.5. RNA-Seq Sequencing and Transcriptomic Analysis of the Head and Gut
2.6. Metabolomic Analysis of the Head
2.7. Verification of DEGs Using Real-Time Quantitative PCRs
3. Results
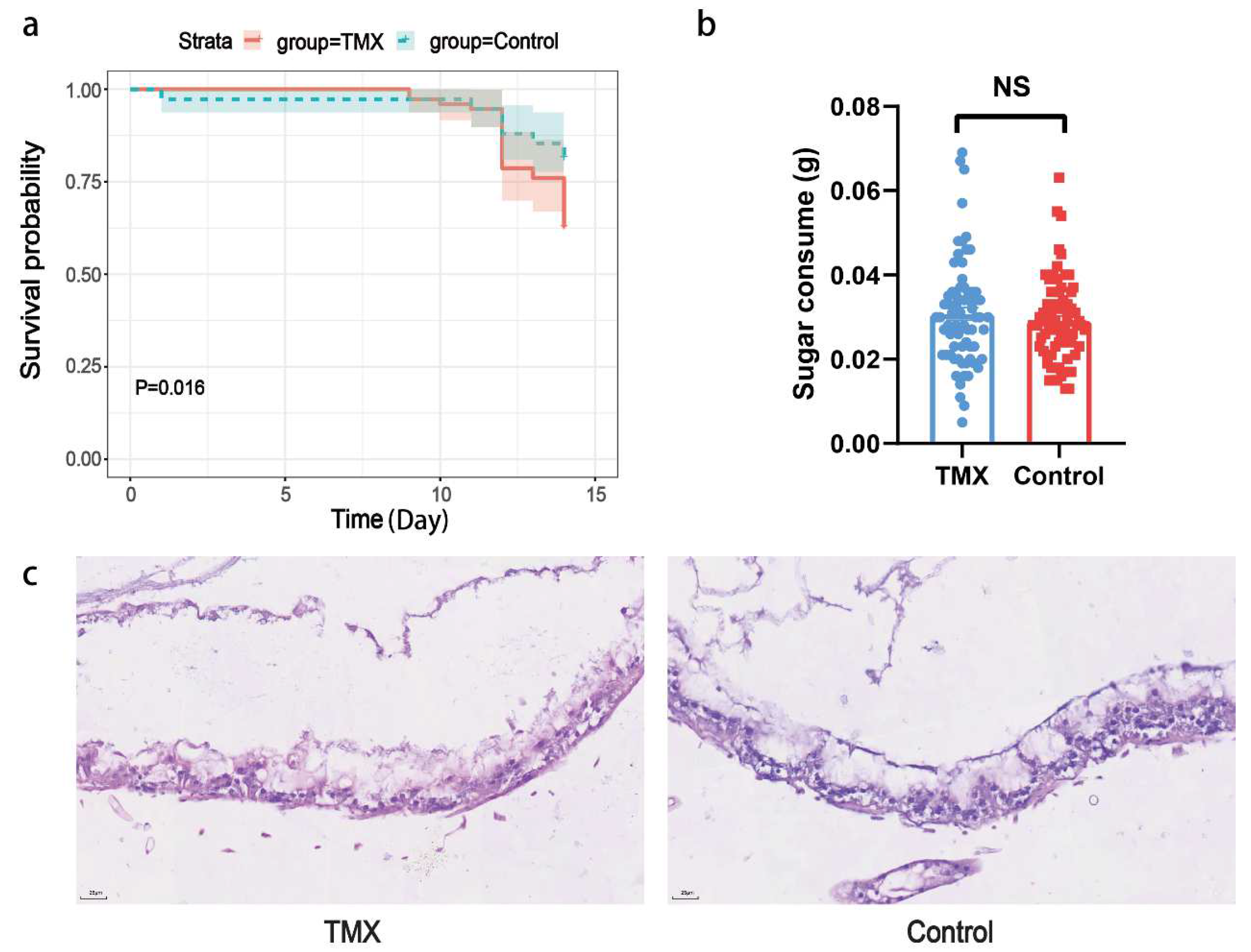
3.1. Honeybee Mortality Rates, Sugar Solution Consumption, and Gut HE Staining
3.2. Gut Tissue Transcriptome Analyses
3.3. Head Transcriptome Analyses
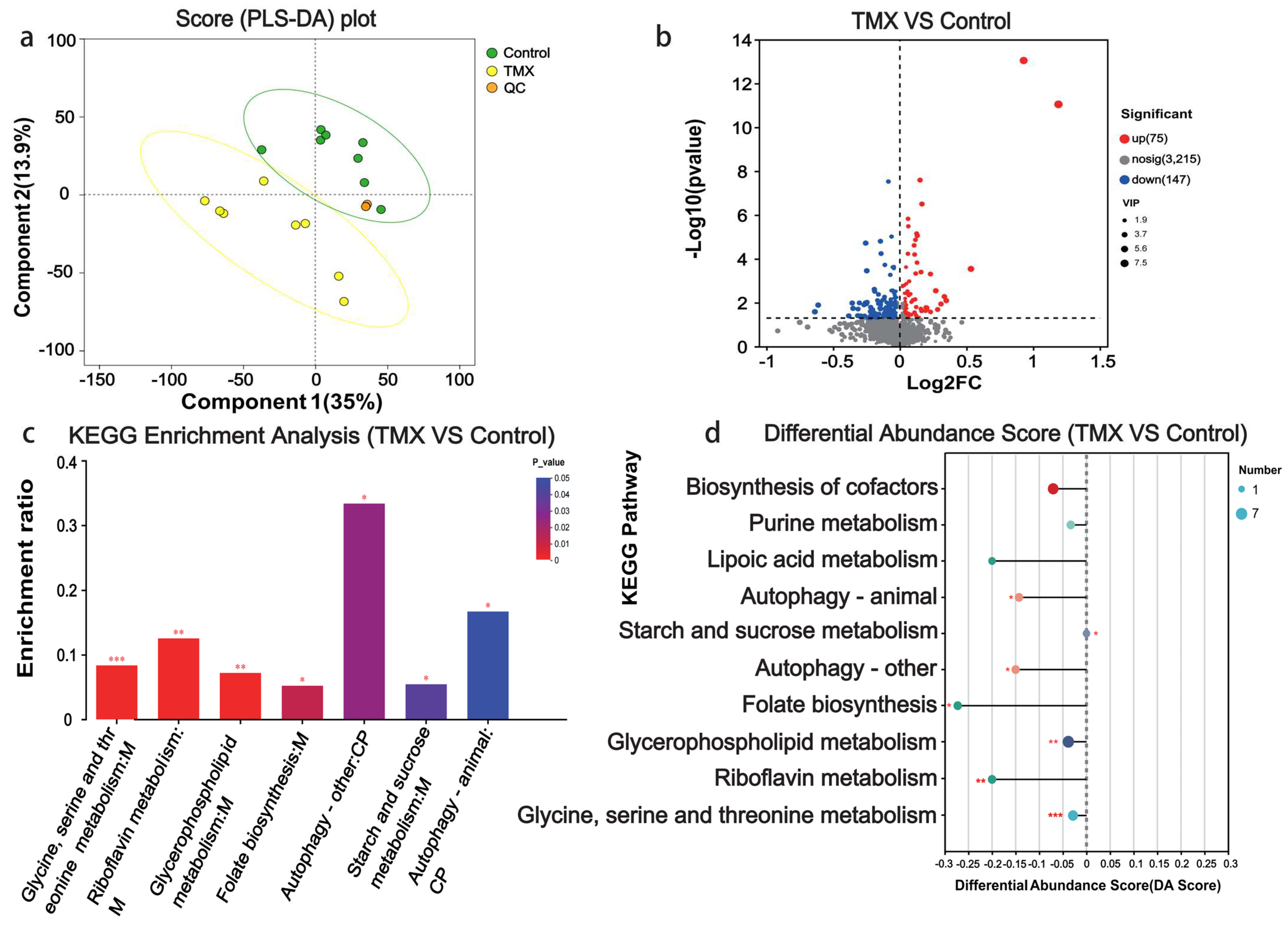
3.4. Head Metabolome Analyses
3.5. Quantitative PCR of Candidate Genes of Gut and Head
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aizen, M.A.; Garibaldi, L.A.; Cunningham, S.A.; Klein, A.M. Long-Term Global Trends in Crop Yield and Production Reveal No Current Pollination Shortage but Increasing Pollinator Dependency. Curr. Biol. 2008, 18, 1572–1575. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Ma, C.; Gustave, W.; Orr, M.C.; Yuan, Z.; Chen, J.; Yang, G.; Niu, Z.; Zhou, Q.; Xia, C.; et al. The impact of heavy metal pollution on wild bee communities in smallholder farmlands. Environ. Res. 2023, 233, 116515. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Ma, C.; Gustave, W.; Orr, M.; Sritongchuay, T.; Yuan, Z.; Wang, M.; Zhang, X.; Zhou, Q.; Huang, Y.; et al. Effects of arsenic and selenium pollution on wild bee communities in the agricultural landscapes. Sci. Total Environ. 2024, 907, 168052. [Google Scholar] [CrossRef] [PubMed]
- Doublet, V.; Labarussias, M.; de Miranda, J.R.; Moritz, R.F.A.; Paxton, R.J. Bees under stress: Sublethal doses of a neonicotinoid pesticide and pathogens interact to elevate honey bee mortality across the life cycle. Environ. Microbiol. 2015, 17, 969–983. [Google Scholar] [CrossRef]
- Goulson, D. De-intensify agriculture. Nature 2015, 521, S58. [Google Scholar]
- Fries, I. Nosema ceranae in European honey bees (Apis mellifera). J. Invertebr. Pathol. 2010, 103, S73–S79. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Z.; Zhuang, M.; Wang, L.; Li, K.; Yao, J.; Yang, H.; Huang, J.; Hao, Y.; Ying, F.; et al. Transcriptome Profiling Reveals a Novel Mechanism of Antiviral Immunity Upon Sacbrood Virus Infection in Honey Bee Larvae (Apis cerana). Front. Microbiol. 2021, 12, 615893. [Google Scholar] [CrossRef]
- Brown, M.J.F.; Dicks, L.V.; Paxton, R.J.; Baldock, K.C.R.; Barron, A.B.; Chauzat, M.P.; Freitas, B.M.; Goulson, D.; Jepsen, S.; Kremen, C.; et al. A horizon scan of future threats and opportunities for pollinators and pollination. PeerJ 2016, 4, e2249. [Google Scholar] [CrossRef]
- Douglas, M.R.; Tooker, J.F. Large-Scale Deployment of Seed Treatments Has Driven Rapid Increase in Use of Neonicotinoid Insecticides and Preemptive Pest Management in US Field Crops. Environ. Sci. Technol. 2015, 49, 5088–5097. [Google Scholar] [CrossRef]
- Simon-Delso, N.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Chagnon, M.; Downs, C.; Furlan, L.; Gibbons, D.W.; Giorio, C.; Girolami, V.; et al. Systemic insecticides (neonicotinoids and fipronil): Trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res. 2015, 22, 5–34. [Google Scholar] [CrossRef]
- Xiao, J.; Xu, X.; Wang, F.; Ma, J.; Liao, M.; Shi, Y.; Fang, Q.; Cao, H. Analysis of exposure to pesticide residues from Traditional Chinese Medicine. J. Hazard. Mater. 2019, 365, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, T. A potential link among biogenic amines-based pesticides, learning and memory, and colony collapse disorder: A unique hypothesis. Neurochem. Int. 2013, 62, 122–136. [Google Scholar] [CrossRef]
- European Food Safety, A.; Adriaanse, P.; Arce, A.; Focks, A.; Ingels, B.; Jolli, D.; Lambin, S.; Rundlof, M.; Sussenbach, D.; Del Aguila, M.; et al. Revised guidance on the risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA J. 2023, 21, e07989. [Google Scholar] [CrossRef]
- Wen, X.; Ma, C.; Sun, M.; Wang, Y.; Xue, X.; Chen, J.; Song, W.; Li-Byarlay, H.; Luo, S. Pesticide residues in the pollen and nectar of oilseed rape (Brassica napus L.) and their potential risks to honey bees. Sci. Total Environ. 2021, 786, 147443. [Google Scholar] [CrossRef]
- Tong, Z.; Duan, J.; Wu, Y.; Liu, Q.; He, Q.; Shi, Y.; Yu, L.; Cao, H. A survey of multiple pesticide residues in pollen and beebread collected in China. Sci. Total Environ. 2018, 640–641, 1578–1586. [Google Scholar] [CrossRef]
- Yue, M.; Luo, S.; Liu, J.; Wu, J. Apis cerana is less sensitive to most neonicotinoids, despite of their smaller body mass. J. Econ. Entomol. 2018, 111, 39–42. [Google Scholar] [CrossRef]
- Mommaerts, V.; Reynders, S.; Boulet, J.; Besard, L.; Sterk, G.; Smagghe, G. Risk assessment for side-effects of neonicotinoids against bumblebees with and without impairing foraging behavior. Ecotoxicology 2010, 19, 207–215. [Google Scholar] [CrossRef]
- Ma, C.; Shi, X.; Chen, S.; Han, J.; Bai, H.; Li, Z.; Li-Byarlay, H.; Bai, L. Combined pesticides in field doses weaken honey bee (Apis cerana F.) flight ability and analyses of transcriptomics and metabolomics. Pestic. Biochem. Physiol. 2024, 201, 105793. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, Y.; Sun, J.; Imran, M.; Yang, H.; Wu, J.; Zou, Y.; Li-Byarlay, H.; Luo, S. Impact of acute oral exposure to thiamethoxam on the homing, flight, learning acquisition and short-term retention of Apis cerana. Pest Manag. Sci. 2019, 75, 2975–2980. [Google Scholar] [CrossRef]
- Brandt, A.; Gorenflo, A.; Siede, R.; Meixner, M.; Büchler, R. The neonicotinoids thiacloprid, imidacloprid, and clothianidin affect the immunocompetence of honey bees (Apis mellifera L.). J. Insect Physiol. 2016, 86, 40–47. [Google Scholar] [CrossRef]
- Dively, G.P.; Embrey, M.S.; Kamel, A.; Hawthorne, D.J.; Pettis, J.S. Assessment of Chronic Sublethal Effects of Imidacloprid on Honey Bee Colony Health. PLoS ONE 2017, 12, e0181297. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Chen, S.; Li, J.; Shi, X.; Zou, Y.; Luo, S. The impact of chronic exposure to field-level thiamethoxam on sunflower visitation and yield for Apis cerana. Apidologie 2023, 55, 5. [Google Scholar] [CrossRef]
- Arena, M.; Sgolastra, F. A meta-analysis comparing the sensitivity of bees to pesticides. Ecotoxicology 2014, 23, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Manjon, C.; Troczka, B.J.; Zaworra, M.; Beadle, K.; Randall, E.; Hertlein, G.; Singh, K.S.; Zimmer, C.T.; Homem, R.A.; Lueke, B.; et al. Unravelling the Molecular Determinants of Bee Sensitivity to Neonicotinoid Insecticides. Curr. Biol. 2018, 28, 1137–1143.e1135. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Luo, Q.H.; Hou, C.S.; Wang, Q.; Dai, P.L.; Gao, J.; Liu, Y.J.; Diao, Q.Y. Sublethal effects of imidacloprid on targeting muscle and ribosomal protein related genes in the honey bee Apis mellifera L. Sci. Rep. 2017, 7, 15943. [Google Scholar] [CrossRef]
- Domingues, C.E.C.; Abdalla, F.C.; Balsamo, P.J.; Pereira, B.V.R.; Hausen, M.A.; Costa, M.J.; Silva-Zacarin, E.C.M. Thiamethoxam and picoxystrobin reduce the survival and overload the hepato-nephrocitic system of the Africanized honeybee. Chemosphere 2017, 186, 994–1005. [Google Scholar] [CrossRef]
- Liu, Y.J.; Qiao, N.H.; Diao, Q.Y.; Jing, Z.; Vukanti, R.; Dai, P.L.; Ge, Y. Thiacloprid exposure perturbs the gut microbiota and reduces the survival status in honeybees. J. Hazard. Mater. 2020, 389, 121818. [Google Scholar] [CrossRef]
- Li-Byarlay, H.; Rittschof, C.C.; Massey, J.H.; Pittendrigh, B.R.; Robinson, G.E. Socially responsive effects of brain oxidative metabolism on aggression. Proc. Natl. Acad. Sci. USA 2014, 111, 12533–12537. [Google Scholar] [CrossRef]
- Matsuda, K.; Ihara, M.; Sattelle, D.B. Neonicotinoid Insecticides: Molecular Targets, Resistance, and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 241–255. [Google Scholar] [CrossRef]
- Nauen, R.; Ebbinghaus-Kintscher, U.; Schmuck, R. Toxicity and nicotinic acetylcholine receptor interaction of imidacloprid and its metabolites in Apis mellifera (Hymenoptera: Apidae). Pest Manag. Sci. 2001, 57, 577–586. [Google Scholar] [CrossRef]
- Chen, Y.R.; Tzeng, D.T.W.; Ting, C.; Hsu, P.S.; Wu, T.H.; Zhong, S.; Yang, E.C. Missing Nurse Bees-Early Transcriptomic Switch From Nurse Bee to Forager Induced by Sublethal Imidacloprid. Front. Genet. 2021, 12, 665927. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Guo, Y.; Wu, Y.Y.; Dai, P.L.; Dai, S.J.; Diao, Q.Y.; Gao, J. Acute and chronic effects of sublethal neonicotinoid thiacloprid to Asian honey bee (Apis cerana cerana). Pestic. Biochem. Physiol. 2023, 194, 105483. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.R.; Tzeng, D.T.W.; Lin, S.S.; Yang, E.C. Sublethal Imidacloprid Administration to Honey Bee Workers is More Lethal to the Queen Larvae. Environ. Toxicol. Chem. 2024, 43, 2232–2242. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, K.; Wu, X.; Zheng, H.; Liao, X. Association of specific gut microbiota with polyethylene microplastics caused gut dysbiosis and increased susceptibility to opportunistic pathogens in honeybees. Sci. Total Environ. 2024, 918, 170642. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kessler, S.; Tiedeken, E.J.; Simcock, K.L.; Derveau, S.; Mitchell, J.; Softley, S.; Stout, J.C.; Wright, G.A. Bees prefer foods containing neonicotinoid pesticides. Nature 2015, 521, 74–76. [Google Scholar] [CrossRef]
- Muth, F.; Gaxiola, R.L.; Leonard, A.S. No evidence for neonicotinoid preferences in the bumblebee Bombus impatiens. R. Soc. Open Sci. 2020, 7, 191883. [Google Scholar] [CrossRef]
- Paris, L.; Peghaire, E.; Mone, A.; Diogon, M.; Debroas, D.; Delbac, F.; El Alaoui, H. Honeybee gut microbiota dysbiosis in pesticide/parasite co-exposures is mainly induced by Nosema ceranae. J. Invertebr. Pathol. 2020, 172, 107348. [Google Scholar] [CrossRef] [PubMed]
- Czerwinski, M.A.; Sadd, B.M. Detrimental interactions of neonicotinoid pesticide exposure and bumblebee immunity. Exp. Zool. Part A Ecol. Integr. Physiol. 2017, 327, 273–283. [Google Scholar] [CrossRef]
- Leng, H.; Simon, A.K.; Horwood, N.J. Blocking glycosphingolipid production alters autophagy in osteoclasts and improves myeloma bone disease. Autophagy 2024, 20, 930–932. [Google Scholar] [CrossRef]
- Stuart, L.M.; Boulais, J.; Charriere, G.M.; Hennessy, E.J.; Brunet, S.; Jutras, I.; Goyette, G.; Rondeau, C.; Letarte, S.; Huang, H.; et al. A systems biology analysis of the Drosophila phagosome. Nature 2007, 445, 95–101. [Google Scholar] [CrossRef]
- Herb, M.; Gluschko, A.; Farid, A.; Kronke, M. When the Phagosome Gets Leaky: Pore-Forming Toxin-Induced Non-Canonical Autophagy (PINCA). Front. Cell. Infect. Microbiol. 2022, 12, 834321. [Google Scholar] [CrossRef]
- Luo, F.; Zhang, C.; Shi, Z.; Mao, T.; Jin, L.H. Notch signaling promotes differentiation, cell death and autophagy in Drosophila hematopoietic system. Insect Biochem. Mol. Biol. 2024, 173, 104176. [Google Scholar] [CrossRef]
- Grotewold, L.; Ruther, U. The Wnt antagonist Dickkopf-1 is regulated by Bmp signaling and c-Jun and modulates programmed cell death. EMBO J. 2002, 21, 966–975. [Google Scholar] [CrossRef]
- Tchantchou, F.; Graves, M.; Rogers, E.; Ortiz, D.; Shea, T.B. N-acteyl cysteine alleviates oxidative damage to central nervous system of ApoE-deficient mice following folate and vitamin E-deficiency. J. Alzheimer’s Dis. 2005, 7, 135–138; discussion 173–180. [Google Scholar] [CrossRef]
- Koenig, M.K.; Perez, M.; Rothenberg, S.; Butler, I.J. Juvenile onset central nervous system folate deficiency and rheumatoid arthritis. J. Child Neurol. 2008, 23, 106–107. [Google Scholar] [CrossRef]
- Selak, I.; Skaper, S.D.; Varon, S. Pyruvate participation in the low molecular weight trophic activity for central nervous system neurons in glia-conditioned media. J. Neurosci. 1985, 5, 23–28. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, P.; Almeida, A.; Bolanos, J.P. Brain energy metabolism in glutamate-receptor activation and excitotoxicity: Role for APC/C-Cdh1 in the balance glycolysis/pentose phosphate pathway. Neurochem. Int. 2013, 62, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Gomeza, J.; Ohno, K.; Betz, H. Glycine transporter isoforms in the mammalian central nervous system: Structures, functions and therapeutic promises. Curr. Opin. Drug Discov. Dev. 2003, 6, 675–682. [Google Scholar]
- Enokido, Y.; Suzuki, E.; Iwasawa, K.; Namekata, K.; Okazawa, H.; Kimura, H. Cystathionine beta-synthase, a key enzyme for homocysteine metabolism, is preferentially expressed in the radial glia/astrocyte lineage of developing mouse CNS. FASEB J. 2005, 19, 1854–1856. [Google Scholar] [CrossRef] [PubMed]
- Naghashpour, M.; Jafarirad, S.; Amani, R.; Sarkaki, A.; Saedisomeolia, A. Update on riboflavin and multiple sclerosis: A systematic review. Iran. J. Basic Med. Sci. 2017, 20, 958–966. [Google Scholar] [CrossRef]
- Liu, X.; Wei, Q.; Yang, X.; Wang, X.; Zhang, J.; Xu, R.; Zhang, H.; Wang, S.; Wan, X.; Jiang, L.; et al. Lipidomics Reveals Dysregulated Glycerophospholipid Metabolism in the Corpus Striatum of Mice Treated with Cefepime. ACS Chem. Neurosci. 2021, 12, 4449–4464. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Ma, C.; Zhao, W.; Kuang, H.; Tian, Y.; Zhang, H.; Xue, Y.; Li-Byarlay, H.; Dong, K.; Gong, X. Chronic Exposure to Field-Level Thiamethoxam Impairs Gut Tissue and Reduces Honeybee (Apis cerana) Survival. Insects 2025, 16, 372. https://doi.org/10.3390/insects16040372
Guo Y, Ma C, Zhao W, Kuang H, Tian Y, Zhang H, Xue Y, Li-Byarlay H, Dong K, Gong X. Chronic Exposure to Field-Level Thiamethoxam Impairs Gut Tissue and Reduces Honeybee (Apis cerana) Survival. Insects. 2025; 16(4):372. https://doi.org/10.3390/insects16040372
Chicago/Turabian StyleGuo, Yulong, Changsheng Ma, Wenzheng Zhao, Haiou Kuang, Yakai Tian, Haoyuan Zhang, Yunfei Xue, Hongmei Li-Byarlay, Kun Dong, and Xueyang Gong. 2025. "Chronic Exposure to Field-Level Thiamethoxam Impairs Gut Tissue and Reduces Honeybee (Apis cerana) Survival" Insects 16, no. 4: 372. https://doi.org/10.3390/insects16040372
APA StyleGuo, Y., Ma, C., Zhao, W., Kuang, H., Tian, Y., Zhang, H., Xue, Y., Li-Byarlay, H., Dong, K., & Gong, X. (2025). Chronic Exposure to Field-Level Thiamethoxam Impairs Gut Tissue and Reduces Honeybee (Apis cerana) Survival. Insects, 16(4), 372. https://doi.org/10.3390/insects16040372






