Simple Summary
This review examines the potential of bees, especially honey bees, as bioindicators for environmental particulate matter detection, utilizing their ability to collect particles through electrostatic forces. By drawing comparisons between pollen adhesion and particulate matter adhesion, the study investigates factors such as bee morphology and the physicochemical properties of particles. Key pollutants—including heavy metals, microplastics, nanoplastics, pathogens, pesticides, radionuclides, and volatile organic compounds—are assessed for their potential for electrostatic adhesion. The findings underscore the value of bees in monitoring environmental health and addressing biodiversity challenges, offering innovative methodologies for biomonitoring and ecosystem protection.
Abstract
Bees (Hymenoptera, Anthophila) are widely recognized for their essential ecological roles, including pollination and biodiversity maintenance. Recently, their ability to collect environmental particulate matter through electrostatic forces has been explored for biomonitoring purposes. This review integrates knowledge on electrostatic pollen adhesion with emerging insights into particulate matter adhesion to bees, emphasizing their potential as bioindicators. The mechanisms of electrostatic adhesion, influenced by factors such as the physicochemical properties of particulate matter and bee morphology, are discussed in detail. Additionally, the study evaluates the adhesion efficiency of pollutants, including heavy metals, microplastics, nanoplastics, pathogens, pesticides, radionuclides, and volatile organic compounds. This multidisciplinary approach underscores the role of bees in advancing environmental monitoring methodologies and offers innovative tools for assessing ecosystem health while addressing the drivers of bee decline.
1. Introduction
Bees (Hymenoptera, Anthophila) are a globally distributed and diverse monophyletic taxon with over 20,000 described species [,]. Bees include several families, such as Andrenidae (e.g., mining bees), Apidae (e.g., bumble bees, carpenter bees, honey bees), Halictidae (e.g., sweat bees), Megachilidae (e.g., leafcutter bees, mason bees), among others []. These insects have been extensively studied due to their invaluable ecological roles; in particular, many bee species, especially wild bees [,], support wild plant communities and agriculture productivity through pollination service, thereby ensuring the maintenance of biodiversity and global food security [,].
In recent years, bees have also been investigated for their promising potential in biomonitoring []. Bees are found worldwide [], and given their sensitivity to climatic [] and environmental changes [], coupled with the ease and low cost of sampling, as well as their well-known taxonomy and ecology, they are considered ideal bioindicators []. Through bee biomonitoring, it is possible to assess the qualitative status of the environment, including climate changes, habitat degradation and fragmentation, the degree of biodiversity, pollination efficiency, the presence of pollutants (e.g., heavy metals, microplastics and nanoplastics (MPs/NPs), pesticides, radionuclides, volatile organic compounds (VOCs)), and the spread of pathogens (e.g., bacteria, fungi, viruses) [,,,]. Wild bees are generally used less frequently as bioindicators than honey bees [], particularly in biomonitoring related to climate changes [], habitat degradation and fragmentation [], and the degree of biodiversity [], as they are highly sensitive to environmental changes due to their ecological specialization []. In contrast, honey bees are preferred as bioindicators because they can be economically bred, are abundant and widespread across various habitats, and are easier to recognize, sample, and manage in experimental settings [,,,]. Their use is primarily extended to biomonitoring of pollutants [,,,] and pathogens [,,] dispersed in the environment (particulate matter). Indeed, honey bees travel long distances during foraging and can inadvertently collect particulate matter on their hairy bodies, bringing it back to the hive where it accumulates in honey, pollen, and wax, effectively acting as bioaccumulators [,,,,,]. However, using honey bees for biomonitoring may not fully capture the range of particulate matter exposure experienced by diverse wild bee populations [,]. Indeed, unlike wild bees, honey bees have distinct behavioral and ecological traits, and thus they may not interact with all environmental sources of particulate matter in the same way as wild bees []. For example, honey bees predominantly forage on flowers and do not frequently come into contact with soil or other non-floral surfaces where certain types of particulate matter may accumulate. Instead, wild bees display a wider variety of nesting habits, foraging strategies, and habitat use, often interacting with the soil [,]. Plus, honey bees possess distinct anatomical traits, such as body size, hair density, and leg morphology, that differ from those of many wild bee species, potentially affecting their ability to trap and transport certain types of particulate matter. Additionally, it is not possible to introduce honey bees into areas where they are absent without considering their potential impact on biodiversity []. Therefore, in certain environmental contexts, the use of wild bees may be preferred. For instance, bumblebees have been used to detect heavy metals [], and Megachilidae have been employed to detect pesticides [].
The ability of bees to collect particulate matter on their bodies is, at least in part, the result of its adhesion due to electrostatic forces [,], similarly to the process of pollen adhesion []. Electrostatic forces generally promote pollen adhesion to bees when they visit flowers, facilitating pollination and playing a crucial role in bee ecology [,,,,,,]. In this context, an approach to bee biomonitoring based on electrostatic forces could be an innovative and powerful tool when applied to particulate matter detection. Indeed, a better understanding of how electrostatic forces are involved in bee ecology, and how these forces are influenced by the physicochemical properties of particulate matter and bee morphology, could be valuable for designing more effective experimental protocols and identifying new types of particulate matter detectable via bee biomonitoring. This would enable the collection of more meaningful data on environmental conditions and the factors driving bee decline. To this end, this review will first discuss electrostatic pollen adhesion to bees in detail, as a valid model for understanding electrostatic particulate matter adhesion to bees. Second, this information will be used to describe how and which types of particulate matter may electrostatically adhere to bees, based on their physicochemical properties. However, before proceeding, it is important to clarify that, at least in bumblebees and honey bees, electrostatic forces are involved not only in pollen adhesion but also in other aspects of bee ecology, such as the communication through electroreception (i.e., the perception of external electric fields, a phenomenon common to many animals [,] and the electroreception of flowers [].
2. Search Strategy and Selection Criteria
To ensure a comprehensive and relevant selection of studies, we employed a systematic search strategy across multiple key databases, including Google Scholar, PubMed, ScienceDirect, Scopus, and Web of Science. The search was conducted using specific keywords relevant to the topic, such as “bees particulate matter detection”, “bees biomonitoring”, “bees electrostatic force”, and “bees electrostatic adhesion”. Studies were selected based on predefined inclusion criteria, prioritizing research focused on the biomonitoring of particulate matter using bees and electrostatic force. The selected studies were then ranked according to their relevance to the research question, the rigor of their methodology, and their contribution to the existing body of knowledge. While recognizing the value of other review methodologies, such as meta-analysis or systematic reviews, we opted for a narrative review approach due to the heterogeneity of the included studies. Our aim was to provide a comprehensive overview of the current state of research rather than a statistical synthesis.
3. Electrostatic Pollen Adhesion to Bees
Like other flying insects, bees acquire a positive electric potential during flight []. Although the underlying mechanisms of this phenomenon are not yet fully understood, it is partially attributed to the triboelectric effect [], which refers to the transfer of electric charge between materials that come into contact or slide against each other []. Bees gain a positive electric potential by losing electrons through aerodynamic friction during flight, as the atmosphere is positively charged under clear weather conditions [,]. In contrast, negative charges continuously migrate from the atmosphere to the ground, causing earthed objects, such as flowers, to acquire a negative electric potential [,]. As illustrated in Figure 1, when a bee approaches a flower, the difference in electric potential between the bee and the flower generates two interacting electric fields [,]. This interaction is known as the electrostatic force or Coulomb force, which arises due to the behavior of electric charges: when one charge is positive and the other is negative, they attract each other, whereas like charges (either both negative or both positive) result in a repulsive force between the interacting bodies []. The electrostatic force (F) is governed by the Coulomb law, expressed as:
In this equation, k represents the proportionality constant (k = 8.987 × 109 N × m2/C2), which determines the magnitude of the force in newtons (N); q1 and q2 denote the interacting bodies approximated as point charges in coulombs (C); d2 represents the square of the linear distance between the two bodies in meters (m) [,,,]. Thus, the intensity of electrostatic force depends on the magnitude of the charges and reaches its maximum at the shortest distance between the two bodies. Consequently, at very short distances, the positive charges on the bee induce a strong negative polarization in the flower in the direction of the bee. As a result, pollen, which is also negatively charged, is attracted to the positively charged bee until it adheres electrostatically to the insect [,,,,,,].
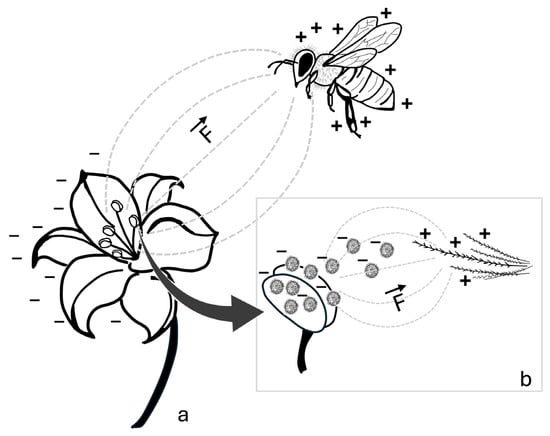
Figure 1.
Schematic representation of the electrostatic interaction between a bee and a flower. (a) The potential difference between the positively charged bee and the negatively charged flower generates an interaction of electric fields (---), resulting in an electrostatic force (F). (b) Enlarged detail showing negatively charged pollen grains detaching from the negatively charged anthers and being electrostatically attracted to the positively charged branched hairs of the bee. The direction and orientation of the electrostatic force are indicated by the vector (→). Symbols: +, positive charge; −, negative charge.
More specifically, electrostatic pollen adhesion is influenced by weather conditions [], gravity [], and, most notably, two key factors: the chemical composition of pollen [] and the morphology of the interacting bodies (i.e., the flower, the pollen, and the bee) [,,]. Understanding these factors is essential for enhancing current knowledge on bee-based biomonitoring on particulate matter, as they may also affect electrostatic particulate matter adhesion to bees. Therefore, the following sections will examine their impact on electrostatic pollen adhesion in detail.
3.1. Pollen Chemical Composition
At the chemical level, the primary contributor to electrostatic pollen adhesion appears to be the chemical composition of sporopollenin, which is the main constituent of exine (i.e., the outer layer of pollen grains []). Experimental studies on synthetic materials derived from sporopollenin have demonstrated that some functional groups contained in this substance, such as amino (-NH2) and carboxyl (-COOH) groups, can ionize, and acquire an electric charge []. Moreover, environmental pH plays a crucial role in this ionization process [,]. Additionally, charged sporopollenin has been observed to electrostatically adsorb various substances, including copper atoms [] and proteins []. Similarly to pollen, different types of particulate matter may exhibit distinct electrostatic behaviors, due to their chemical composition.
3.2. Morphology of the Interacting Bodies
The morphology of the bodies interacting within the “flower-pollen-bee system” influences the distribution of the electric fields around them, and, consequently, the strength of electrostatic force [,], as illustrated in Figure 2. The surface charge and, consequently, the surface charge density are inversely correlated with the radius of surface curvature. As a result, a smaller radius (in the most extreme case, a sharp point) exhibits a higher surface charge density []. This phenomenon is known as the “point discharge” or “point effect”.
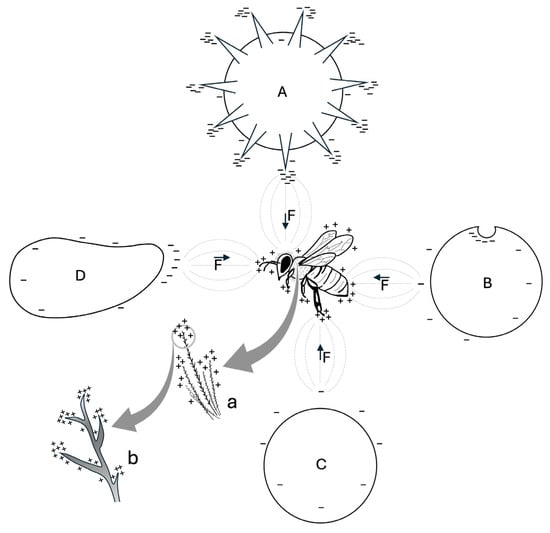
Figure 2.
Effect of morphology on the distribution of positive (+) and negative (−) surface charge density on a bee and particles with different shapes (A, B, C, D). Due to point discharge, the radius of surface curvature determines the accumulation of electric charges on the objects shown. On the bee, positive charges accumulate primarily on the legs, antennae, and branched hairs. On the particles, negative charges are more concentrated on spines, concavities, and other small structures: echinate pollen grain (A); concave particle (B); rounded particle with lower surface charge density (C); elongated particle (D). The interaction between the electric fields (---) of the bee and each particle generates an electrostatic force (F), with direction and orientation indicated by the vector (→). (a) magnification of hairs; (b) magnification in which it is shown the higher concentration of positive charges on the tip of the hair branches.
As a result, on the flower, the charge density increases along contours and sharp components (e.g., anthers, edges of petals, stigma) as well as on small components (e.g., trichomes) [,]. This mechanism favors the transfer of negatively charged pollen from the anthers to the bee, and from the bee to the stigmas, when the insect visits other flowers []. This process may also be crucial for electrostatic particulate matter adhesion.
Similarly, as a result of point discharge, the sculpture of pollen grains—the protuberances, concavities, and grooves on their exine—may enhance the charge density on their surface. For example, it has been proposed that a protruding pollen morphology, such as the echinate sculpture (i.e., covered with external spines), may promote electrostatic pollen adhesion to the stigma and to pollinators [,] (see Figure 2). Additionally, these structures increase the contact area with the substrate, thereby amplifying van der Waals forces [,]. Van der Waals forces are defined as interactions between atoms and molecules, separated by any medium (including air or vacuum), based on their mutual distance. The electrostatic interactions between the shifting electron clouds that comprise molecules and other materials give rise to these nonbonded forces, which have a quantum mechanical origin []. These dispersive forces—comprising Keesom, Debye, and London contributions—are most active at relatively short distances compared to charge–charge interactions (approximately 10 nm at the nanoscale or even farther in materials at the mesoscopic level) []. Van der Waals forces, together with the sculpture of exine and viscous substances (e.g., pollenkitt), are involved in the mechanism of pollen retention/detachment in/from anthers [,,], and promote pollen adhesion to experimental surfaces [] and to the stigma []. Therefore, van der Waals forces may also work in synergy with electrostatic force in pollen adhesion to the body of bees, as both forces are significant for particles with diameter <100 μm []. However, while electrostatic force dominates for particles >50 µm, van der Waals forces influence smaller particles [], and these forces are amplified with particle size reduction []. Thus, the influence of electrostatic force and van der Waals forces on pollen may change with particle size, as each plant species produces pollen grains of varying sizes, ranging from less than 10 µm to over 100 µm []. Moreover, the weight of pollen grains must be considered, as lighter pollen grains are more likely to overcome gravity, and be affected by electrostatic force []. These findings align with the assumptions of Zeghloul et al. (2017) []: the smaller and lighter the particles, the higher the ratio of electrostatic force to gravitational force, making their electrostatic separation easier. Consequently, as with pollen, both the morphology and the size of particles are crucial for electrostatic particulate matter adhesion.
Lastly, bee morphology plays a significant role in electrostatic pollen adhesion [,,,,]. Bees are covered by millions of branched hairs, which are essential for mechanical pollen adhesion and transport [,,,], but are also electrostatically charged [,]. These hairs may enhance electrostatic pollen adhesion through point discharge: since hair branches can be approximated to thin points, they may exhibit a high charge density on their surface. Additionally, the numerosity of these hairs can amplify this phenomenon, particularly in bee species with wide hairy areas (see Figure 2). Furthermore, similar to the cuticle of other insects [], the bee cuticle may possess electrostatic characteristics, potentially contributing to both electrostatic pollen and particulate matter adhesion.
Having outlined the process of electrostatic pollen adhesion and the key factors involved—the chemical composition of pollen and the morphology of the interacting bodies—it is now possible to apply this information to electrostatic particulate matter adhesion to bees. In the following paragraph, particulate matter will be analyzed in terms of physicochemical properties that may promote its electrostatic adhesion to bees and its detection via bee biomonitoring.
4. Analysis of the Physicochemical Properties Involved in Electrostatic Particulate Matter Adhesion to Bees
In this paragraph, the three hypothetical modalities of electrostatic particulate adhesion to bees—direct, indirect, and combined—will be briefly described. Next, the efficiency of electrostatic adhesion of certain types of particulate matter to bees, including heavy metals, MPs/NPs, pathogens, pesticides, radionuclides, and VOCs, will be discussed with reference to the most recent research on their physicochemical properties. As it is not feasible to cover all types of particulate matter, only select examples from each category of particulate matter will be presented.
4.1. Modalities of Electrostatic Particulate Matter Adhesion to Bees
Following the assumptions of Perugini et al. (2011) [], it can be hypothesized that various types of particulate matter may adhere electrostatically to bees through at least three modes: the first is direct electrostatic adhesion, in which particulate matter adheres to the bee’s body due to its electrostatic charge; the second is indirect electrostatic adhesion, wherein particulate matter binds to an intermediary substance—such as pollen [] or other materials—that may adhere to the bee, or that has already adhered to the bee and can further attract particulate matter []; the third is combined electrostatic adhesion, which results from the synergistic effect of the bee’s morphological electrostatic properties and the electrostatic properties of intermediary substances accumulated on its body. These three mechanisms may operate simultaneously, with their relative intensity varying depending on the physicochemical properties of each type of particulate matter, as discussed below.
4.2. Heavy Metals
Heavy metals primarily occur in dispersed ionic form [] or as constituents of other pollutants [,]. They are continuously released from both natural sources and anthropogenic activities—including agriculture, industries, mining, and transportation—becoming hazardous pollutants due to their non-biodegradable nature and their ability to enter food chains, where they exert toxic effects on organisms [,,,]. While some heavy metals are essential for biological functions, they become toxic in excess, whereas others are harmful even at low concentrations [,].
Due to these concerns, environmental pollution by heavy metals has been extensively studied through bee biomonitoring [,,,,,,,,,,,,,,,]. Indeed, atmospheric heavy metals can adhere directly to bees and/or indirectly reach them via contaminated pollen []. In particular, direct adhesion may be partially driven by electrostatic force, as heavy metals are often present as ions, similar to what has been observed in studies on microorganisms. Research suggests that electrostatic interactions contribute to microbial cell walls via functional groups such as carboxyl, amino, hydroxyl, phosphate, thiol [,,]. Similarly, indirect and combined adhesion via pollen attached to bees may be facilitated by electrostatic force. Supporting this hypothesis, sporopollenin has been observed to electrostatically adsorb copper atoms []. Finally, heavy metals may reach bees through electrostatic adhesion of pollutants that contain them, such as MPs/NPs [] and pesticides [].
4.3. Microplastics and Nanoplastics
MPs/NPs originate either from the degradation of various plastic materials or as microcomponents intentionally produced for industrial and cosmetic applications [,]. Owing to their diverse origins, each type of MPs/NPs exhibits unique chemical composition, shape (e.g., fibers, films, fragments), size, and weight, resulting in heterogeneous physicochemical properties [,,,]. These properties contribute to their exceptionally long degradation time [], rendering MPs/NPs nearly ubiquitous in the environment. As a consequence, they pose a serious threat to organisms by infiltrating biological tissues and inducing adverse effects such as toxicity and metabolic disturbances, which remain incompletely understood [,,]. Additionally, MPs/NPs can be transferred through food chains [,,], release toxic additives and heavy metals into the environment [,,], and serve as substrate for biofilms of pathogenic microorganisms [,].
Despite the severity of MPs/NPs pollution, research on their impact in the terrestrial environment remains limited, whereas their presence and effects have been extensively studied in aquatic ecosystems [,,,]. Consequently, studies investigating bees themselves as bioindicators of MPs/NPs are still scarce [,,]. However, bees have been observed to accumulate MPs/NPs on their body [], likely due to electrostatic force, given that MPs/NPs have been detected in the atmosphere [], on flowers [], and, hypothetically, on pollen [,]. Pollen may, therefore, act as an intermediate in indirect and combined electrostatic adhesion of MPs/NPs to bees. While no direct evidence currently confirms electrostatic MPs/NPs adhesion to bees, this hypothesis is supported by studies demonstrating electrostatic adhesion of MPs/NPs to algae and plants [,,,]. Furthermore, Wang et al. (2022) [] described MPs/NPs adhesion to soil as a result of both electrostatic and van der Waals forces, suggesting that these forces may also act in synergy to facilitate the electrostatic adhesion of pollen and particulate matter to bees. Given the heterogeneity of MPs/NPs in terms of chemical composition, size, and shape, the relative contributions of electrostatic force and their interaction with van der Waals forces in MPs/NPs adhesion to bees may vary depending on the specific characteristics of the particles.
For instance, the chemical composition of MPs/NPs may influence their electrostatic adhesion to bees, depending on the type of functional groups present on their surface and/or the presence of additives and their respective functional groups. Indeed, some of these functional groups may ionize and acquire a charge similar to those found in sporopollenin []. Furthermore, the chemical composition of MPs/NPs can determine their charge polarity—either positive or negative—as suggested by the study of Park et al. (2008) [] on various plastic materials. Consequently, this charge polarity may influence the electrostatic adhesion of MPs/NPs to bees. As indicated by Park et al. (2008) [], plastic materials exhibit specific charge tendency in a triboelectric series (i.e., a ranking of materials based on their propensity to gain or lose electrons []). The ranking is as follows, from negative to positive: Polychloroethylene with a high polymerization degree (HPVC)—Suspension Polyvinyl Chloride Resin (SPVC)—Copolymer Polypropylene (COPP)—Homopolypropylene (HOMOPP)—Low Density Polyethylene (LDPE)—High Density Polyethylene (HDPE)—Polyethylene Terephthalate (PET)—Rubber—High Impact Polystyrene (HIPS)—Calibre—Acrylonitrile Butadiene Styrene (ABS)—General Purpose Polystyrene (GPPS)—Poly (Methyl Methacrylate) (PMMA). Since bees carry a positive charge, it is plausible that they preferentially attract MPs/NPs originating from the most negatively charged plastic materials in this triboelectric series.
Regarding the shape of MPs/NPs, it may determine the strength of electrostatic force, considering that pollen adhesion to bees is influenced by its morphology (see Figure 2). Therefore, it is plausible that the sharpest and/or thinnest MPs/NPs, such as fibers, are the most involved in electrostatic adhesion due to the point discharge effect (see Figure 2). This hypothesis may partially explain why fibers were the most abundant MPs/NPs shape in the study by Cortés-Corrales et al. (2024) [], although Edo et al. (2021) [] primarily found fragments. However, these contrasting results could be attributed to differences in sampling sites [] or to the high irregularity and sharpness of the collected fragments, which may enhance point discharge, and consequently, electrostatic adhesion (see Figure 2). Indeed, most MPs/NPs dispersed in the environment exhibit an irregular shape, as they originate from the degradation of plastic materials []. Additionally, certain MPs/NPs, such as glitters, feature highly irregular and angular geometries (e.g., snowflakes, squares, stars, triangles) [], which may further enhance point discharge and, therefore, electrostatic adhesion.
Lastly, the size of MPs/NPs may influence their susceptibility to electrostatic force and the interplay between electrostatic and van der Waals forces, similarly to what occurs with pollen grains. Additionally, the weight of MPs/NPs may affect their resistance to electrostatic adhesion. As observed for certain pollen grains, smaller and lighter MPs/NPs are more likely to adhere electrostatically to bees. Indeed, Zeghloul et al. (2017) [] stated that the size of MPs/NPs influences their electrostatic separation.
4.4. Pathogens
Pathogens—including fungi, bacteria, and viruses—are of significant interest to humans due to their direct impact on human health [] and their indirect effects on society []. Additionally, many pathogens affect domestic animals and cultivated plants, leading to individual losses, reduced product quality, significant damage to agricultural production, and the transmission of zoonotic diseases [,]. The threat of pathogen spread is even extended to bees. Beyond the honey bee mite Varroa destructor [], several pathogens affect these insects, causing behavioral and metabolic changes and, in severe cases, death [,,,,].
Consequently, in recent years, the emergence and spread of pathogens have been widely studied due to their high impact on both humans and other organisms, including bees. The recent global SARS-CoV-2 pandemic has underscored the urgency of constantly monitoring the presence of airborne pathogens in the environment. However, unlike foodborne and waterborne pathogens, research of airborne pathogen detection remains limited []. A promising approach for detecting airborne pathogens and studying their emergence trends is bee biomonitoring. Indeed, bees have been successfully employed to study the environmental presence of airborne entomo-, human-, and phytopathogens, including bacteria [,,], fungi and oomycetes [,], and viruses [,,]. Pathogen biomonitoring through bees is feasible because these insects can passively transport various microorganisms on their bodies, contributing to plant infections as well [,,,]. It is likely that pathogens adhere directly to bees at least partially due to electrostatic force. Indeed, electrostatic force—along with van der Waals forces and other mechanisms—has been shown to play a role in the adhesion of bacteria [,,,,,], fungi [,,,], and viruses [,,,,] to surfaces. Furthermore, since bacteria [], fungi [], and viruses [] can be transmitted via pollen [,], pollen attached to bees may electrostatically adsorb pathogens on its surface, facilitating their secondary transport to bees. Therefore, for pathogens, indirect and combined electrostatic adhesion via pollen may be particularly relevant, although further research is needed to confirm this hypothesis.
Regarding the physicochemical properties that may facilitate electrostatic pathogen adhesion to bees, chemical composition could be relevant, at least for viruses. In fact, the electrostatic adhesion of viruses to pollen grains may involve the proteins forming the viral capsid—an external protein shell that protects the genome []—since, as mentioned earlier, sporopollenin has been shown to electrostatically adsorb proteins []. Moreover, morphology may also play a crucial role, as certain pathogen structures could enhance point discharge and, consequently, electrostatic pathogen adhesion to bees (see Figure 2). For instance, the external ornamentations of some fungal spores, suggested to facilitate adhesion to arthropod vectors [,], could be among these candidate structures. Another example is the spike proteins forming small protrusions on the capsid of SARS-CoV-2 and other coronaviruses []. These glycoproteins mediate a complex process of host cell recognition, binding, membrane fusion, and viral entry []. In this process, electrostatic force arising from the charges accumulated on spike proteins, along with van der Waals forces, is also involved [,,,]. Therefore, the spherical and crown-like SARS-CoV-2 virions (i.e., individual viral particles []), which may be compared to pollen grains with echinate sculptures, could electrostatically adhere to bees due to point discharge effects resulting from their morphology (see Figure 2). This hypothesis may partially explain the successful detection of SARS-CoV-2 through bee biomonitoring, as reported by Cilia et al. (2022) [].
4.5. Pesticides
Pesticides pose a significant threat to the environment and living organisms due to their chemical composition and their extensive, unselective, excessive, and incorrect use []. They are often toxic to a wide range of non-target organisms, causing poisoning and metabolic disturbances, and they can be transferred through food chains []. In particular, inorganic pesticides are especially hazardous, as they frequently contain heavy metals that can be released into the environment []. Even pollinators, such as bees, are not exempt from the harmful effect of pesticides, both directly—since many pesticides are toxic to these insects—and indirectly, as herbicides can lead to the destruction of plant communities [,].
Due to their high toxicity, which endangers pollinator diversity and their crucial ecological role, pesticides have been extensively monitored through bee biomonitoring studies [,,,,,,,]. In particular, the direct adhesion of pesticides to bees may, at least for certain compound classes, occur partially through electrostatic force. For instance, in the 1970s, the insecticide Penncap M, composed of capsules the size of pollen grains, electrostatically adhered to bees, until its subsequent ban []. Furthermore, in an effort to reduce dosage requirements, many pesticides have been optimized through air-assisted electrostatic spraying techniques, which enhance their adhesion to target surfaces [,,,,]. However, this process may also result in the unintended electrostatic adhesion of electrified pesticide droplets to bees and other non-target organisms []. This effect may be further intensified by point discharge (see Figure 2), as droplets can preferentially adhere to the narrowest plant surfaces, such as floral structures visited by bees []. Moreover, chemical composition may play a relevant role in the indirect and combined electrostatic adhesion of pesticides to bees. Certain pesticide chemical components, such as heavy metals [], may be electrostatically adsorbed by pollen grains, which specific functional groups may ionize and generate a charge, similarly to the functional groups of sporopollenin []. Finally, many pesticides, such as tetraconazole, fipronil, and deltamethrin, have been found to adsorb onto atmospheric particles [], potentially leading to their electrostatic adhesion to bees via these substances.
4.6. Radionuclides
Having an excess number of neutrons or protons, some atoms, called radionuclides, randomly decay into more stable atoms by releasing ionizing radiation [,]. This phenomenon is referred to as radioactivity or radioactive decay [,]. While radionuclides are a natural occurrence, various anthropogenic sources—including mining, nuclear power plants, and the historical use of nuclear weapons—now contribute to radionuclide pollution [,]. This type of pollution is particularly hazardous for organisms, as ionizing radiation acts as a powerful mutagen, inducing heritable mutations in the genome [,], which can lead to cancers and teratologies [,]. Additionally, some radionuclides can enter organisms and be transferred along food chains [,].
Due to the significant risks associated with radionuclides, advancing detection methods for these substances in the environment is crucial. Biomonitoring using bees has emerged as a promising approach, as bees actively collect environmental particles over large areas and can serve as effective indicators of contamination. Several studies have demonstrated the potential of this method for radionuclide detection [,,,,,,,,]. Radionuclide bee biomonitoring may be facilitated by electrostatic adhesion of radionuclides to bees or, more likely, of particles containing them. Indeed, radionuclides are often found in many atmospheric particles [], and their radioactivity is known to induce a charge on these particles, thereby enhancing electrostatic particle interactions [,,,,,,,]. Radionuclides may also reach bees via pollen through electrostatic adhesion, and their radioactivity may increase the charge of contaminated pollen grains, facilitating their electrostatic adhesion to bees. This hypothesis is supported by the findings of Tschiersch et al. (1999) [], who observed high concentrations of 137Cs on pine pollen grains after the Chernobyl nuclear accident. Therefore, for radionuclides, indirect and combined electrostatic adhesion to bees may be particularly relevant.
4.7. Volatile Organic Compounds
VOCs are characterized by low boiling points, which allows many of them to easily evaporate into the atmosphere [,]. This class of inert compounds includes acids, alcohols, aliphatics, aldehydes, ethers, hydrocarbons, and ketones []. While these substances can evaporate from natural sources, anthropogenic sources—such as gas and petroleum extraction, industrial and residential coal burnings, means of transport, paints, printers [,,], and silage []—release a relevant amount of VOCs [], which can be toxic to organisms [,]. For instance, in humans, their toxicity is associated with metabolic [,] and respiratory illnesses [,,], reproductive defects [], and even cancer [,,,,]. Additionally, VOCs emissions have an indirect effect on climate change, as they contribute to the reduction of stratospheric ozone—an important greenhouse gas—and participate in photochemical reactions that produce tropospheric ozone [,,].
Due to their substantial impact on the environment and organisms, VOCs have been extensively biomonitored, and several studies have utilized bees as bioindicators for these substances [,,,,,,,,]. For VOCs, indirect and combined electrostatic adhesion to bees may be the most significant mode of adhesion, considering that some VOCs, such as aromatic and aliphatic hydrocarbons, are associated with atmospheric particles [,], which may transport them to bees. Chemical composition may also be a relevant factor for their electrostatic adhesion to bees. Specifically, this phenomenon may occur if certain functional groups of these substances, such as amino and carboxyl groups, ionize, thereby generating a charge. This mechanism may be similar to the ionization of functional groups observed in sporopollenin [] and hypothesized for MPs/NPs and pesticides.
5. Conclusions
This review highlights the crucial role of bees as environmental bioindicators, with a particular emphasis on their ability to collect particulate matter through electrostatic forces. By analyzing the mechanisms of electrostatic adhesion and its implications, the review illustrates how various types of particulate matter—including heavy metals, MPs/NPs, pathogens, pesticides, radionuclides, and VOCs—can adhere to bees and, consequently, be used for monitoring environmental quality.
One of the key findings is that electrostatic adhesion is influenced by multiple factors, including the electric charge acquired by bees during flight and the specific physicochemical properties of each type of particulate matter. The significance of these properties may vary for different types of particulate matter. For instance, in the case of heavy metals, shape, size, and weight may be less critical than their ionic properties. Hypothetically, each type of particulate matter may follow a distinct mode of electrostatic adhesion: direct, indirect, and combined electrostatic adhesion may be more relevant for heavy metals, MPs/NPs, pathogens, and pesticides, whereas indirect and combined electrostatic adhesion may be more relevant for radionuclides and VOCs.
However, the true relevance of physicochemical properties in particulate matter electrostatic adhesion remains unknown and should be investigated through experimental studies. Meaningful experiments should involve particles from the same category with varying properties as well as particles from different categories, employing a multidisciplinary approach combining Ecology, Physics, and Chemistry. This will enable the testing of the hypotheses proposed in this review, which may serve as a valuable starting point for understanding particulate matter electrostatic adhesion to bees and its potential applications.
This knowledge may open new perspectives for biomonitoring by leveraging the natural interactions between bees and airborne pollutants, potentially providing a more refined and sensitive approach compared to traditional monitoring methods. The proposed methodology may be essential for refining detection methods, identifying new environmental contaminants, and improving biomonitoring accuracy by enhancing pollutant detection, especially for substances difficult to monitor conventionally. Moreover, it may provide a better understanding of pollution sources and their distribution, improving risk assessments for environmental and human health. However, given the wide ecological, behavioral, and morphological differences among bee species, their effectiveness in biomonitoring through electrostatic force must be investigated to identify the most suitable species for the detection of each type of particulate matter. In this way, alongside the practical and cost-effective use of honey bees, particulate matter biomonitoring could be extended through the use of various wild bee species, each potentially capable of collecting different types of particulate matter. Ideally, using a combination of different bee species as particulate matter bioindicators may offer a more comprehensive understanding of the pollution levels in a given area.
Furthermore, these findings carry important implications for biodiversity conservation. This approach could be particularly valuable for bee conservation, as many types of particulate matter can negatively impact these pollinators. For example, exposure to heavy metals and pesticides may disrupt bee behavior, reduce reproductive success, and undermine their ecological role in pollination. However, the severity of these effects can vary among bee species, as each species—due to its unique ecological, behavioral, and morphological traits—may exhibit different levels of resistance and face varying degrees of exposure to particulate matter. Pollutants not only affect pollinators directly but can have cascading effects across ecosystems. The decline of honey bee and wild bee populations due to pollutant exposure could reduce floral diversity and decrease agricultural yields dependent on pollination. Additionally, radionuclides and VOCs may disrupt plant-pollinator attraction dynamics, undermining the effectiveness of pollination as a critical ecosystem service. Plus, studies suggest that MPs/NPs accumulation in terrestrial ecosystems could degrade soil quality, affecting food sources for pollinators and disrupting trophic networks. In this context, by identifying areas with high pollution exposure, this approach could help locate regions where wild pollinators are most at risk, guiding targeted conservation efforts. Given the threats posed by pollutants like pesticides and heavy metals to pollinators, incorporating electrostatic biomonitoring into environmental assessments can contribute to more effective mitigation measures.
In conclusion, the use of bees as bioindicators represents a valuable opportunity for both pollution monitoring and biodiversity conservation. Bees can not only provide crucial data on the presence and spread of environmental contaminants, but also help identify high-risk areas where biodiversity is particularly threatened. Therefore, integrating electrostatic principles into biomonitoring presents a promising opportunity to refine detection methodologies and identify new classes of environmental contaminants, thereby improving monitoring accuracy with significant implications for ecosystem health and sustainability. However, further experimental studies are required to optimize sampling protocols and translate these hypotheses into effective biomonitoring and conservation strategies.
Author Contributions
Conceptualization, S.R. and S.M.; writing—original draft preparation, S.M.; writing—review and editing, L.C., E.S. and S.R.; project administration, S.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Orr, M.C.; Hughes, A.C.; Chesters, D.; Pickering, J.; Zhu, C.-D.; Ascher, J.S. Global Patterns and Drivers of Bee Distribution. Curr. Biol. 2021, 31, 451–458.e4, Correction: Curr. Biol. 2023, 33, 1624. https://doi.org/10.1016/j.cub.2023.03.058. [Google Scholar] [CrossRef] [PubMed]
- Henríquez-Piskulich, P.; Hugall, A.F.; Stuart-Fox, D. A Supermatrix Phylogeny of the World’s Bees (Hymenoptera: Anthophila). Mol. Phylogenet. Evol. 2024, 190, 107963. [Google Scholar] [CrossRef]
- Danforth, B.N.; Sipes, S.; Fang, J.; Brady, S.G. The History of Early Bee Diversification Based on Five Genes plus Morphology. Proc. Natl. Acad. Sci. USA 2006, 103, 15118–15123. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, T.H.; Regetz, J.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Bogdanski, A.; Gemmill-Herren, B.; Greenleaf, S.S.; Klein, A.M.; Mayfield, M.M.; et al. Landscape Effects on Crop Pollination Services: Are There General Patterns? Ecol. Lett. 2008, 11, 499–515. [Google Scholar] [CrossRef]
- Kennedy, C.M.; Lonsdorf, E.; Neel, M.C.; Williams, N.M.; Ricketts, T.H.; Winfree, R.; Bommarco, R.; Brittain, C.; Burley, A.L.; Cariveau, D.; et al. A Global Quantitative Synthesis of Local and Landscape Effects on Wild Bee Pollinators in Agroecosystems. Ecol. Lett. 2013, 16, 584–599. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global Pollinator Declines: Trends, Impacts and Drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Wood, T.J.; Michez, D.; Paxton, R.J.; Drossart, M.; Neumann, P.; Gérard, M.; Vanderplanck, M.; Barraud, A.; Martinet, B.; Leclercq, N.; et al. Managed Honey Bees as a Radar for Wild Bee Decline? Apidologie 2020, 51, 1100–1116. [Google Scholar] [CrossRef]
- Reyes-Novelo, E.; Ramírez, V.M.; González, H.D.; Ayala, R. Abejas Silvestres (Hymenoptera: Apoidea) Como Bioindicadores en el Neotrópico. Trop. Subtrop. Agroecosystems 2009, 10, 1–13. [Google Scholar]
- Conte, Y.L.; Navajas, M. Climate Change: Impact on Honey Bee Populations and Diseases. Rev. Sci. Tech. Off. Int. Epizoot. 2008, 27, 499–510. [Google Scholar]
- Brown, M.J.F.; Paxton, R.J. The Conservation of Bees: A Global Perspective. Apidologie 2009, 40, 410–416. [Google Scholar] [CrossRef]
- Schindler, M.; Diestelhorst, O.; Haertel, S.; Saure, C.; Scharnowski, A.; Schwenninger, H.R. Monitoring Agricultural Ecosystems by Using Wild Bees as Environmental Indicators. BioRisk 2013, 8, 53–71. [Google Scholar] [CrossRef]
- Negri, I.; Mavris, C.; Di Prisco, G.; Caprio, E.; Pellecchia, M. Honey Bees (Apis mellifera, L.) as Active Samplers of Airborne Particulate Matter. PLoS ONE 2015, 10, e0132491. [Google Scholar] [CrossRef]
- Edo, C.; Fernández-Alba, A.R.; Vejsnæs, F.; Van Der Steen, J.J.M.; Fernández-Piñas, F.; Rosal, R. Honeybees as Active Samplers for Microplastics. Sci. Total Environ. 2021, 767, 144481. [Google Scholar] [CrossRef]
- Cunningham, M.M.; Tran, L.; McKee, C.G.; Ortega Polo, R.; Newman, T.; Lansing, L.; Griffiths, J.S.; Bilodeau, G.J.; Rott, M.; Marta Guarna, M. Honey Bees as Biomonitors of Environmental Contaminants, Pathogens, and Climate Change. Ecol. Indic. 2022, 134, 108457. [Google Scholar] [CrossRef]
- Cane, J. Global Warming, Advancing Bloom and Evidence for Pollinator Plasticity from Long-Term Bee Emergence Monitoring. Insects 2021, 12, 457. [Google Scholar] [CrossRef]
- Turley, N.E.; Biddinger, D.J.; Joshi, N.K.; López-Uribe, M.M. Six Years of Wild Bee Monitoring Shows Changes in Biodiversity within and across Years and Declines in Abundance. Ecol. Evol. 2022, 12, e9190. [Google Scholar] [CrossRef]
- Porrini, C.; Sabatini, A.G.; Girotti, S.; Fini, F.; Monaco, L.; Celli, G.; Bortolotti, L.; Ghini, S. The Death of Honey Bees and Environmental Pollution by Pesticides: The Honey Bees as Biological Indicators. Bull. Insectology 2003, 56, 147–152. [Google Scholar]
- Porrini, C.; Caprio, E.; Tesoriero, D.; Prisco, G.D. Using Honey Bee as Bioindicator of Chemicals in Campanian Agroecosystems (South Italy). Bull. Insectology 2014, 67, 137–146. [Google Scholar]
- Skorbiłowicz, M.; Skorbiłowicz, E.; Cieśluk, I. Bees as Bioindicators of Environmental Pollution with Metals in an Urban Area. J. Ecol. Eng. 2018, 19, 229–234. [Google Scholar] [CrossRef]
- Girotti, S.; Ghini, S.; Ferri, E.; Bolelli, L.; Colombo, R.; Serra, G.; Porrini, C.; Sangiorgi, S. Bioindicators and Biomonitoring: Honeybees and Hive Products as Pollution Impact Assessment Tools for the Mediterranean Area. Euro Mediterr. J. Environ. Integr. 2020, 5, 62. [Google Scholar] [CrossRef]
- Devillers, J.; Pham-Delègue, M.-H. Honey Bees: Estimating the Environmental Impact of Chemicals; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Bargańska, Ż.; Ślebioda, M.; Namieśnik, J. Honey Bees and Their Products: Bioindicators of Environmental Contamination. Crit. Rev. Environ. Sci. Technol. 2016, 46, 235–248. [Google Scholar] [CrossRef]
- Salkova, D.; Panayotova-Pencheva, M. Honey Bees and Their Products as Indicators of Environmental Pollution: A Review. Agric. Sci. Technol. 2016, 8, 175–182. [Google Scholar] [CrossRef]
- Resci, I.; Cilia, G. The Use of Honey Bee (Apis mellifera L.) as Biological Monitors for Pathogenic Bacteria and Antimicrobial Resistance: A Systematic Review. Environ. Pollut. 2023, 333, 122120. [Google Scholar] [CrossRef] [PubMed]
- Resci, I.; Zavatta, L.; Piva, S.; Mondo, E.; Albertazzi, S.; Nanetti, A.; Bortolotti, L.; Cilia, G. Predictive Statistical Models for Monitoring Antimicrobial Resistance Spread in the Environment Using Apis mellifera (L. 1758) Colonies. Environ. Res. 2024, 248, 118365. [Google Scholar] [CrossRef] [PubMed]
- Celli, G.; Maccagnani, B. Honey Bees as Bioindicators of Environmental Pollution. Bull. Insectology 2003, 56, 137–139. [Google Scholar]
- Gutiérrez, M.; Molero, R.; Gaju, M.; Van Der Steen, J.; Porrini, C.; Ruiz, J.A. Assessing Heavy Metal Pollution by Biomonitoring Honeybee Nectar in Córdoba (Spain). Environ. Sci. Pollut. Res. 2020, 27, 10436–10448. [Google Scholar] [CrossRef]
- Mair, K.S.; Irrgeher, J.; Haluza, D. Elucidating the Role of Honey Bees as Biomonitors in Environmental Health Research. Insects 2023, 14, 874. [Google Scholar] [CrossRef]
- Ward, L.T.; Hladik, M.L.; Guzman, A.; Winsemius, S.; Bautista, A.; Kremen, C.; Mills, N.J. Pesticide Exposure of Wild Bees and Honey Bees Foraging from Field Border Flowers in Intensively Managed Agriculture Areas. Sci. Total Environ. 2022, 831, 154697. [Google Scholar] [CrossRef]
- Zioga, E.; White, B.; Stout, J.C. Honey Bees and Bumble Bees May Be Exposed to Pesticides Differently When Foraging on Agricultural Areas. Sci. Total Environ. 2023, 896, 166214. [Google Scholar] [CrossRef]
- Raine, N.E.; Rundlöf, M. Pesticide Exposure and Effects on Non- Apis Bees. Annu. Rev. Entomol. 2024, 69, 551–576. [Google Scholar] [CrossRef]
- Antoine, C.M.; Forrest, J.R.K. Nesting Habitat of Ground-Nesting Bees: A Review. Ecol. Entomol. 2021, 46, 143–159. [Google Scholar] [CrossRef]
- Moritz, R.F.A.; Härtel, S.; Neumann, P. Global Invasions of the Western Honeybee (Apis mellifera) and the Consequences for Biodiversity. Écoscience 2005, 12, 289–301. [Google Scholar] [CrossRef]
- Breidenbach, L.R.; Benner, L.; Roß-Nickoll, M.; Linnemann, V.; Schäffer, A. Monitoring Metal Patterns from Urban and Agrarian Sites Using the Bumblebee Bombus terrestris as a Bioindicator. Environ. Sci. Pollut. Res. 2023, 30, 119947–119960. [Google Scholar] [CrossRef]
- Peterson, E.M.; Thompson, K.N.; Shaw, K.R.; Tomlinson, C.; Longing, S.D.; Smith, P.N. Use of Nest Bundles to Monitor Agrochemical Exposure and Effects among Cavity Nesting Pollinators. Environ. Pollut. 2021, 286, 117142. [Google Scholar] [CrossRef]
- Bonmatin, J.-M.; Giorio, C.; Girolami, V.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; Long, E.; Marzaro, M.; Mitchell, E.A.D.; et al. Environmental Fate and Exposure; Neonicotinoids and Fipronil. Environ. Sci. Pollut. Res. 2015, 22, 35–67. [Google Scholar] [CrossRef] [PubMed]
- Martinello, M.; Manzinello, C.; Dainese, N.; Giuliato, I.; Gallina, A.; Mutinelli, F. The Honey Bee: An Active Biosampler of Environmental Pollution and a Possible Warning Biomarker for Human Health. Appl. Sci. 2021, 11, 6481. [Google Scholar] [CrossRef]
- Corbet, S.A.; Beament, J.; Eisikowitch, D. Are Electrostatic Forces Involved in Pollen Transfer? Plant Cell Environ. 1982, 5, 125–129. [Google Scholar] [CrossRef]
- Vaknin, Y.; Gan-Mor, S.; Bechar, A.; Ronen, B.; Eisikowitch, D. The Role of Electrostatic Forces in Pollination. Plant Syst. Evol. 2000, 222, 133–142. [Google Scholar] [CrossRef]
- Clarke, D.; Whitney, H.; Sutton, G.; Robert, D. Detection and Learning of Floral Electric Fields by Bumblebees. Science 2013, 340, 66–69. [Google Scholar] [CrossRef]
- Clarke, D.; Morley, E.; Robert, D. The Bee, the Flower, and the Electric Field: Electric Ecology and Aerial Electroreception. J. Comp. Physiol. A 2017, 203, 737–748. [Google Scholar] [CrossRef]
- Montgomery, C.; Koh, K.; Robert, D. Measurement of Electric Charges on Foraging Bumblebees (Bombus terrestris). J. Phys. Conf. Ser. 2019, 1322, 012002. [Google Scholar] [CrossRef]
- Khatawkar, D.S.; James, S.P.; Dhalin, D. Role of Electrostatics in Artificial Pollination and Future Agriculture. Curr. Sci. 2021, 120, 484–491. [Google Scholar]
- Li, W.; Lu, L.; Liu, G.; Zhang, C.; Loos, K.; Pei, Y. Honeybee-Inspired Electrostatic Microparticle Manipulation System Based on Triboelectric Nanogenerator. Nano Energy 2022, 104, 107901. [Google Scholar] [CrossRef]
- England, S.J.; Robert, D. The Ecology of Electricity and Electroreception. Biol. Rev. 2022, 97, 383–413. [Google Scholar] [CrossRef] [PubMed]
- Greggers, U.; Koch, G.; Schmidt, V.; Dürr, A.; Floriou-Servou, A.; Piepenbrock, D.; Göpfert, M.C.; Menzel, R. Reception and Learning of Electric Fields in Bees. Proc. R. Soc. B Biol. Sci. 2013, 280, 20130528. [Google Scholar] [CrossRef]
- Diaz, A.F.; Felix-Navarro, R.M. A Semi-Quantitative Tribo-Electric Series for Polymeric Materials: The Influence of Chemical Structure and Properties. J. Electrost. 2004, 62, 277–290. [Google Scholar] [CrossRef]
- Palmer, R.A.; O’Reilly, L.J.; Carpenter, J.; Chenchiah, I.V.; Robert, D. An Analysis of Time-Varying Dynamics in Electrically Sensitive Arthropod Hairs to Understand Real-World Electrical Sensing. J. R. Soc. Interface 2023, 20, 20230177. [Google Scholar] [CrossRef]
- Bowker, G.E.; Crenshaw, H.C. Electrostatic Forces in Wind-Pollination—Part 1: Measurement of the Electrostatic Charge on Pollen. Atmos. Environ. 2007, 41, 1587–1595. [Google Scholar] [CrossRef]
- Murray, J.S.; Politzer, P. The Electrostatic Potential: An Overview. WIREs Comput. Mol. Sci. 2011, 1, 153–163. [Google Scholar] [CrossRef]
- Gadre, S.R.; Bhadane, P.K. Electrostatics in Chemistry: 1. Basic Principles. Resonance 1999, 4, 8–19. [Google Scholar] [CrossRef]
- Inchaussandague, M.; Skigin, D.; Dolinko, A.; Tellería, M.C.; Barreda, V.; Palazzesi, L. Spines, Microspines and Electric Fields: A New Look at the Possible Significance of Sculpture in Pollen of Basal and Derived Asteraceae. Biol. J. Linn. Soc. 2018, 125, 794–801. [Google Scholar] [CrossRef]
- Lee, P.F.; Cheong, K.K.; Hong, Y.S.; Chong, Y.Z. Surface Charge Study on Pollen with a Simple Microelectrophoresis Instrumentation Setup. In Proceedings of the 2010 IEEE EMBS Conference on Biomedical Engineering and Sciences (IECBES), Kuala Lumpur, Malaysia, 30 November–2 December 2010; pp. 364–368. [Google Scholar]
- Vaknin, Y.; Gan-mor, S.; Bechar, A.; Ronen, B.; Eisikowitch, D. Are Flowers Morphologically Adapted to Take Advantage of Electrostatic Forces in Pollination? New Phytol. 2001, 152, 301–306. [Google Scholar] [CrossRef]
- Ariizumi, T.; Toriyama, K. Genetic Regulation of Sporopollenin Synthesis and Pollen Exine Development. Annu. Rev. Plant Biol. 2011, 62, 437–460. [Google Scholar] [CrossRef] [PubMed]
- Gezici, O.; Küçükosmanoğlu, M.; Ayar, A. The Adsorption Behavior of Crystal Violet in Functionalized Sporopollenin-Mediated Column Arrangements. J. Colloid Interface Sci. 2006, 304, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.-F.; Xiang, S.; Li, L.; Xie, X.-W.; Chai, A.-L.; Shi, Y.-X.; Liu, N.; Abdukerim, R.; Ma, J.-Y.; Shi, J.; et al. Fabrication of Ultrafine Sporopollenin Particles and Its Application as Pesticide Carrier. Appl. Mater. Today 2022, 27, 101454. [Google Scholar] [CrossRef]
- Uddin, M.J.; Liyanage, S.; Warzywoda, J.; Abidi, N.; Gill, H.S. Role of Sporopollenin Shell Interfacial Properties in Protein Adsorption. Langmuir 2022, 38, 2763–2776. [Google Scholar] [CrossRef]
- Matehkolaee, M.J.; Asrami, A.N. The Review on The Charge Distribution on The Conductor Surface. Eur. J. Phys. Educ. 2013, 4, 1–6. [Google Scholar]
- Chaloner, W.G. Elecrostatic Forces in Insect Pollination and Their Significance in Exine Ornament. In Linnean Society Symposium Series; Academic Press: London, UK, 1986; Volume 12, pp. 103–108. [Google Scholar]
- Bowling, R.A. A Theoretical Review of Particle Adhesion. In Particles on Surfaces 1; Mittal, K.L., Ed.; Springer: Boston, MA, USA, 1988; pp. 129–142. ISBN 978-1-4615-9533-5. [Google Scholar]
- Timerman, D.; Barrett, S.C.H. The Biomechanics of Pollen Release: New Perspectives on the Evolution of Wind Pollination in Angiosperms. Biol. Rev. 2021, 96, 2146–2163. [Google Scholar] [CrossRef]
- Hermann, J.; DiStasio, R.A.; Tkatchenko, A. First-Principles Models for van Der Waals Interactions in Molecules and Materials: Concepts, Theory, and Applications. Chem. Rev. 2017, 117, 4714–4758. [Google Scholar] [CrossRef]
- Lin, H.; Gomez, I.; Meredith, J.C. Pollenkitt Wetting Mechanism Enables Species-Specific Tunable Pollen Adhesion. Langmuir 2013, 29, 3012–3023. [Google Scholar] [CrossRef]
- Boi, M. Pollen Attachment in Common Materials. Aerobiologia 2015, 31, 261–270. [Google Scholar] [CrossRef]
- Thio, B.J.R.; Lee, J.-H.; Meredith, J.C. Characterization of Ragweed Pollen Adhesion to Polyamides and Polystyrene Using Atomic Force Microscopy. Environ. Sci. Technol. 2009, 43, 4308–4313. [Google Scholar] [CrossRef]
- Ito, S.; Gorb, S.N. Attachment-Based Mechanisms Underlying Capture and Release of Pollen Grains. J. R. Soc. Interface 2019, 16, 20190269. [Google Scholar] [CrossRef]
- Shi, Y.; Li, C.; Fang, M.; Cen, J.; Wang, Q.; Yan, K. Numerical Investigation of Particle Re-Entrainment Mechanism and Its Suppression Strategy in the High-Temperature Electrostatic Precipitator. Powder Technol. 2024, 437, 119538. [Google Scholar] [CrossRef]
- Feng, J.Q.; Hays, D.A. Relative Importance of Electrostatic Forces on Powder Particles. Powder Technol. 2003, 135, 65–75. [Google Scholar] [CrossRef]
- Hao, K.; Tian, Z.-X.; Wang, Z.-C.; Huang, S.-Q. Pollen Grain Size Associated with Pollinator Feeding Strategy. Proc. R. Soc. B Biol. Sci. 2020, 287, 20201191. [Google Scholar] [CrossRef] [PubMed]
- Zeghloul, T.; Mekhalef Benhafssa, A.; Richard, G.; Medles, K.; Dascalescu, L. Effect of Particle Size on the Tribo-Aero-Electrostatic Separation of Plastics. J. Electrost. 2017, 88, 24–28. [Google Scholar] [CrossRef]
- Buchmann, S.L.; Hurley, J.P. A Biophysical Model for Buzz Pollination in Angiosperms. J. Theor. Biol. 1978, 72, 639–657. [Google Scholar] [CrossRef]
- Buchmann, S.L. Buzz Pollination in Angiosperms. In Handbook of Experimental Pollination Biology: 73; Jones, C.E., Little, R.J., Eds.; Scientific and Academic Editions: New York, NY, USA, 1983; pp. 73–113. [Google Scholar]
- Portman, Z.M.; Orr, M.C.; Griswold, T. A Review and Updated Classification of Pollen Gathering Behavior in Bees (Hymenoptera, Apoidea). J. Hymenopt. Res. 2019, 71, 171–208. [Google Scholar] [CrossRef]
- Southwick, E.E. Bee Hair Structure and the Effect of Hair on Metabolism at Low Temperature. J. Apic. Res. 1985, 24, 144–149. [Google Scholar] [CrossRef]
- Amador, G.J.; Matherne, M.; Waller, D.; Mathews, M.; Gorb, S.N.; Hu, D.L. Honey Bee Hairs and Pollenkitt Are Essential for Pollen Capture and Removal. Bioinspir. Biomim. 2017, 12, 026015. [Google Scholar] [CrossRef]
- Koh, K.; Robert, D. Bumblebee Hairs as Electric and Air Motion Sensors: Theoretical Analysis of an Isolated Hair. J. R. Soc. Interface 2020, 17, 20200146. [Google Scholar] [CrossRef]
- Roquer-Beni, L.; Rodrigo, A.; Arnan, X.; Klein, A.; Fornoff, F.; Boreux, V.; Bosch, J. A Novel Method to Measure Hairiness in Bees and Other Insect Pollinators. Ecol. Evol. 2020, 10, 2979–2990. [Google Scholar] [CrossRef]
- Devarrewaere, W.; Foqué, D.; Nicolai, B.; Nuyttens, D.; Verboven, P. Eulerian-Lagrangian CFD Modelling of Pesticide Dust Emissions from Maize Planters. Atmos. Environ. 2018, 184, 304–314. [Google Scholar] [CrossRef]
- Leite, M.O.G.; Alves, D.A.; Lecocq, A.; Malaquias, J.B.; Delalibera, I.; Jensen, A.B. Laboratory Risk Assessment of Three Entomopathogenic Fungi Used for Pest Control toward Social Bee Pollinators. Microorganisms 2022, 10, 1800. [Google Scholar] [CrossRef]
- Kakutani, K.; Matsuda, Y.; Haneda, K.; Nonomura, T.; Kimbara, J.; Kusakari, S.; Osamura, K.; Toyoda, H. Insects Are Electrified in an Electric Field by Deprivation of Their Negative Charge. Ann. Appl. Biol. 2012, 160, 250–259. [Google Scholar] [CrossRef]
- Perugini, M.; Manera, M.; Grotta, L.; Abete, M.C.; Tarasco, R.; Amorena, M. Heavy Metal (Hg, Cr, Cd, and Pb) Contamination in Urban Areas and Wildlife Reserves: Honeybees as Bioindicators. Biol. Trace Elem. Res. 2011, 140, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, H.; Guimarães, F.; Duque, L.; Noronha, F.; Abreu, I. Characterisation of Particulate Matter on Airborne Pollen Grains. Environ. Pollut. 2015, 206, 7–16. [Google Scholar] [CrossRef]
- Okuyama, Y.; Matsumoto, K.; Okochi, H.; Igawa, M. Adsorption of Air Pollutants on the Grain Surface of Japanese Cedar Pollen. Atmos. Environ. 2007, 41, 253–260. [Google Scholar] [CrossRef]
- Zaynab, M.; Al-Yahyai, R.; Ameen, A.; Sharif, Y.; Ali, L.; Fatima, M.; Khan, K.A.; Li, S. Health and Environmental Effects of Heavy Metals. J. King Saud Univ. Sci. 2022, 34, 101653. [Google Scholar] [CrossRef]
- Brennecke, D.; Duarte, B.; Paiva, F.; Caçador, I.; Canning-Clode, J. Microplastics as Vector for Heavy Metal Contamination from the Marine Environment. Estuar. Coast. Shelf Sci. 2016, 178, 189–195. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Kiran; Bharti, R.; Sharma, R. Effect of Heavy Metals: An Overview. Mater. Today Proc. 2022, 51, 880–885. [Google Scholar] [CrossRef]
- Duruibe, J.O.; Ogwuegbu, M.O.C.; Egwurugwu, J.N. Heavy Metal Pollution and Human Biotoxic Effects. Int. J. Phys. Sci. 2007, 2, 112–118. [Google Scholar]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy Metals, Occurrence and Toxicity for Plants: A Review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Singh, R.; Gautam, N.; Mishra, A.; Gupta, R. Heavy Metals and Living Systems: An Overview. Indian J. Pharmacol. 2011, 43, 246. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Molero, R.; Gaju, M.; Van Der Steen, J.; Porrini, C.; Ruiz, J.A. Assessment of Heavy Metal Pollution in Córdoba (Spain) by Biomonitoring Foraging Honeybee. Environ. Monit. Assess. 2015, 187, 651. [Google Scholar] [CrossRef]
- Veleminsky, M.; Láznička, P.; Starý, P. Honeybees (Apis mellifera) as Environmental Monitors of Heavy Metals. Acta Entomol. Bohemoslov 1990, 87, 37–44. [Google Scholar]
- Leita, L.; Muhlbachova, G.; Cesco, S.; Barbattini, R.; Mondini, C. Investigation of the Use of Honey Bees and Honey Bee Products to Assess Heavy Metals Contamination. Environ. Monit. Assess. 1996, 43, 1–9. [Google Scholar] [CrossRef]
- Conti, M.E.; Botrè, F. Honeybees and Their Products as Potential Bioindicators of Heavy Metals Contamination. Environ. Monit. Assess. 2001, 69, 267–282. [Google Scholar] [CrossRef]
- Roman, A. Levels of Copper, Selenium, Lead, and Cadmium in Forager Bees. Pol. J. Environ. Stud. 2010, 19, 663–669. [Google Scholar]
- Van Der Steen, J.J.M.; De Kraker, J.; Grotenhuis, T. Spatial and Temporal Variation of Metal Concentrations in Adult Honeybees (Apis mellifera L.). Environ. Monit. Assess. 2012, 184, 4119–4126. [Google Scholar] [CrossRef] [PubMed]
- Al Naggar, Y.A.; Naiem, E.-S.A.; Seif, A.I.; Mona, M.H. Honey Bees and Their Products as a Bio-in-Dicator of Environmental Pollution with Heavy Metals. Mellifera 2013, 13, 1–20. [Google Scholar]
- Ruschioni, S.; Riolo, P.; Minuz, R.L.; Stefano, M.; Cannella, M.; Porrini, C.; Isidoro, N. Biomonitoring with Honeybees of Heavy Metals and Pesticides in Nature Reserves of the Marche Region (Italy). Biol. Trace Elem. Res. 2013, 154, 226–233. [Google Scholar] [CrossRef]
- Herrero-Latorre, C.; Barciela-García, J.; García-Martín, S.; Peña-Crecente, R.M. The Use of Honeybees and Honey as Environmental Bioindicators for Metals and Radionuclides: A Review. Environ. Rev. 2017, 25, 463–480. [Google Scholar] [CrossRef]
- Zarić, N.M.; Ilijević, K.; Stanisavljević, L.; Gržetić, I. Use of Honeybees (Apis mellifera L.) as Bioindicators for Assessment and Source Appointment of Metal Pollution. Environ. Sci. Pollut. Res. 2017, 24, 25828–25838. [Google Scholar] [CrossRef]
- Costa, A.; Veca, M.; Barberis, M.; Tosti, A.; Notaro, G.; Nava, S.; Lazzari, M.; Agazzi, A.; Tangorra, F.M. Heavy Metals on Honeybees Indicate Their Concentration in the Atmosphere. A Proof of Concept. Ital. J. Anim. Sci. 2019, 18, 309–315. [Google Scholar] [CrossRef]
- Di Fiore, C.; Nuzzo, A.; Torino, V.; De Cristofaro, A.; Notardonato, I.; Passarella, S.; Di Giorgi, S.; Avino, P. Honeybees as Bioindicators of Heavy Metal Pollution in Urban and Rural Areas in the South of Italy. Atmosphere 2022, 13, 624. [Google Scholar] [CrossRef]
- Zarić, N.M.; Brodschneider, R.; Goessler, W. Honey Bees as Biomonitors—Variability in the Elemental Composition of Individual Bees. Environ. Res. 2022, 204, 112237. [Google Scholar] [CrossRef]
- Di Fiore, C.; De Cristofaro, A.; Nuzzo, A.; Notardonato, I.; Ganassi, S.; Iafigliola, L.; Sardella, G.; Ciccone, M.; Nugnes, D.; Passarella, S.; et al. Biomonitoring of Polycyclic Aromatic Hydrocarbons, Heavy Metals, and Plasticizers Residues: Role of Bees and Honey as Bioindicators of Environmental Contamination. Environ. Sci. Pollut. Res. 2023, 30, 44234–44250. [Google Scholar] [CrossRef]
- Meza-Figueroa, D.; Berrellez-Reyes, F.; Schiavo, B.; Morton-Bermea, O.; Gonzalez-Grijalva, B.; Inguaggiato, C.; Silva-Campa, E. Tracking Fine Particles in Urban and Rural Environments Using Honey Bees as Biosamplers in Mexico. Chemosphere 2024, 363, 142881. [Google Scholar] [PubMed]
- Wang, J.; Chen, C. Biosorbents for Heavy Metals Removal and Their Future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of Heavy Metal Ions from Wastewater: A Comprehensive and Critical Review. Npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Zeng, G.; He, Y.; Liang, D.; Wang, F.; Luo, Y.; Yang, H.; Wang, Q.; Wang, J.; Gao, P.; Wen, X.; et al. Adsorption of Heavy Metal Ions Copper, Cadmium and Nickel by Microcystis Aeruginosa. Int. J. Environ. Res. Public. Health 2022, 19, 13867. [Google Scholar] [CrossRef]
- Hale, R.C.; Seeley, M.E.; La Guardia, M.J.; Mai, L.; Zeng, E.Y. A Global Perspective on Microplastics. J. Geophys. Res. Ocean. 2020, 125, e2018JC014719. [Google Scholar] [CrossRef]
- Lai, H.; Liu, X.; Qu, M. Nanoplastics and Human Health: Hazard Identification and Biointerface. Nanomaterials 2022, 12, 1298. [Google Scholar] [CrossRef]
- Luo, H.; Zhao, Y.; Li, Y.; Xiang, Y.; He, D.; Pan, X. Aging of Microplastics Affects Their Surface Properties, Thermal Decomposition, Additives Leaching and Interactions in Simulated Fluids. Sci. Total Environ. 2020, 714, 136862. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Liu, F.; Cryder, Z.; Huang, D.; Lu, Z.; He, Y.; Wang, H.; Lu, Z.; Brookes, P.C.; Tang, C.; et al. Microplastics in the Soil Environment: Occurrence, Risks, Interactions and Fate—A Review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2175–2222. [Google Scholar] [CrossRef]
- Lin, Z.; Jin, T.; Zou, T.; Xu, L.; Xi, B.; Xu, D.; He, J.; Xiong, L.; Tang, C.; Peng, J.; et al. Current Progress on Plastic/Microplastic Degradation: Fact Influences and Mechanism. Environ. Pollut. 2022, 304, 119159. [Google Scholar] [CrossRef]
- Zhang, K.; Hamidian, A.H.; Tubić, A.; Zhang, Y.; Fang, J.K.H.; Wu, C.; Lam, P.K.S. Understanding Plastic Degradation and Microplastic Formation in the Environment: A Review. Environ. Pollut. 2021, 274, 116554. [Google Scholar] [CrossRef]
- Prata, J.C.; Da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental Exposure to Microplastics: An Overview on Possible Human Health Effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef]
- Prata, J.C.; Da Costa, J.P.; Lopes, I.; Andrady, A.L.; Duarte, A.C.; Rocha-Santos, T. A One Health Perspective of the Impacts of Microplastics on Animal, Human and Environmental Health. Sci. Total Environ. 2021, 777, 146094. [Google Scholar] [CrossRef]
- Dong, X.; Liu, X.; Hou, Q.; Wang, Z. From Natural Environment to Animal Tissues: A Review of Microplastics (Nanoplastics) Translocation and Hazards Studies. Sci. Total Environ. 2023, 855, 158686. [Google Scholar] [CrossRef] [PubMed]
- Jemec Kokalj, A.; Kuehnel, D.; Puntar, B.; Žgajnar Gotvajn, A.; Kalčikova, G. An Exploratory Ecotoxicity Study of Primary Microplastics versus Aged in Natural Waters and Wastewaters. Environ. Pollut. 2019, 254, 112980. [Google Scholar] [CrossRef] [PubMed]
- Akhbarizadeh, R.; Moore, F.; Keshavarzi, B. Investigating Microplastics Bioaccumulation and Biomagnification in Seafood from the Persian Gulf: A Threat to Human Health? Food Addit. Contam. Part A 2019, 36, 1696–1708. [Google Scholar] [CrossRef] [PubMed]
- Krause, S.; Baranov, V.; Nel, H.A.; Drummond, J.D.; Kukkola, A.; Hoellein, T.; Sambrook Smith, G.H.; Lewandowski, J.; Bonet, B.; Packman, A.I.; et al. Gathering at the Top? Environmental Controls of Microplastic Uptake and Biomagnification in Freshwater Food Webs. Environ. Pollut. 2021, 268, 115750. [Google Scholar] [CrossRef]
- Harrison, J.P.; Hoellein, T.J.; Sapp, M.; Tagg, A.S.; Ju-Nam, Y.; Ojeda, J.J. Microplastic-Associated Biofilms: A Comparison of Freshwater and Marine Environments. Freshw. Microplastics Emerg. Environ. Contam. 2018, 58, 181–201. [Google Scholar]
- McCormick, A.; Hoellein, T.J.; Mason, S.A.; Schluep, J.; Kelly, J.J. Microplastic Is an Abundant and Distinct Microbial Habitat in an Urban River. Environ. Sci. Technol. 2014, 48, 11863–11871. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Li, Y.; Powell, T.; Wang, X.; Wang, G.; Zhang, P. Microplastics as Contaminants in the Soil Environment: A Mini-Review. Sci. Total Environ. 2019, 691, 848–857. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Q.; Jia, W.; Yan, C.; Wang, J. Agricultural Plastic Mulching as a Source of Microplastics in the Terrestrial Environment. Environ. Pollut. 2020, 260, 114096. [Google Scholar] [CrossRef]
- Ya, H.; Jiang, B.; Xing, Y.; Zhang, T.; Lv, M.; Wang, X. Recent Advances on Ecological Effects of Microplastics on Soil Environment. Sci. Total Environ. 2021, 798, 149338. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Corrales, L.; Flores, J.J.; Rosa, A.; Van Der Steen, J.J.M.; Vejsnæs, F.; Roessink, I.; Martínez-Bueno, M.J.; Fernández-Alba, A.R. Evaluation of Microplastic Pollution Using Bee Colonies: An Exploration of Various Sampling Methodologies. Environ. Pollut. 2024, 350, 124046. [Google Scholar] [CrossRef] [PubMed]
- Schiano, M.E.; D’Auria, L.J.; D’Auria, R.; Seccia, S.; Rofrano, G.; Signorelli, D.; Sansone, D.; Caprio, E.; Albrizio, S.; Cocca, M. Microplastic Contamination in the Agri-Food Chain: The Case of Honeybees and Beehive Products. Sci. Total Environ. 2024, 948, 174698. [Google Scholar] [CrossRef]
- Chen, G.; Fu, Z.; Yang, H.; Wang, J. An Overview of Analytical Methods for Detecting Microplastics in the Atmosphere. TrAC Trends Anal. Chem. 2020, 130, 115981. [Google Scholar] [CrossRef]
- Liebezeit, G.; Liebezeit, E. Origin of Synthetic Particles in Honeys. Pol. J. Food Nutr. Sci. 2015, 65, 143–147. [Google Scholar] [CrossRef]
- Al Naggar, Y.; Brinkmann, M.; Sayes, C.M.; AL-Kahtani, S.N.; Dar, S.A.; El-Seedi, H.R.; Grünewald, B.; Giesy, J.P. Are Honey Bees at Risk from Microplastics? Toxics 2021, 9, 109. [Google Scholar] [CrossRef]
- Buteler, M.; Villalobos, E.; Alma, A.M.; Silva, L.; Tomba, J.P. Management Practice for Small Hive Beetle as a Source of Microplastic Contamination in Honey and Honeybee Colonies. Environ. Pollut. 2023, 334, 122151. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, P.; Lin, S.; Turner, J.P.; Ke, P.C. Physical Adsorption of Charged Plastic Nanoparticles Affects Algal Photosynthesis. J. Phys. Chem. C 2010, 114, 16556–16561. [Google Scholar] [CrossRef]
- Kalčíková, G. Aquatic Vascular Plants—A Forgotten Piece of Nature in Microplastic Research. Environ. Pollut. 2020, 262, 114354. [Google Scholar] [CrossRef]
- Mizukami-Murata, S.; Suzuki, Y.; Sakurai, K.; Yamashita, H. Freshwater Alga Raphidocelis subcapitata Undergoes Metabolomic Changes in Response to Electrostatic Adhesion by Micrometer-Sized Nylon 6 Particles. Environ. Sci. Pollut. Res. 2021, 28, 66901–66913. [Google Scholar] [CrossRef]
- Kalčíková, G. Beyond Ingestion: Adhesion of Microplastics to Aquatic Organisms. Aquat. Toxicol. 2023, 258, 106480. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Xiang, L.; Bian, Y.; Wang, Z.; Srivastava, P.; Jiang, X.; Xing, B. Attachment of Positively and Negatively Charged Submicron Polystyrene Plastics on Nine Typical Soils. J. Hazard. Mater. 2022, 431, 128566. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Park, J.K.; Jeon, H.S.; Chun, B.C. Triboelectric Series and Charging Properties of Plastics Using the Designed Vertical-Reciprocation Charger. J. Electrost. 2008, 66, 578–583. [Google Scholar] [CrossRef]
- Zou, H.; Zhang, Y.; Guo, L.; Wang, P.; He, X.; Dai, G.; Zheng, H.; Chen, C.; Wang, A.C.; Xu, C.; et al. Quantifying the Triboelectric Series. Nat. Commun. 2019, 10, 1427. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dou, M.; Ren, P.; Sun, B.; Jia, R.; Zhou, Y. Settling Velocity of Irregularly Shaped Microplastics under Steady and Dynamic Flow Conditions. Environ. Sci. Pollut. Res. 2021, 28, 62116–62132. [Google Scholar] [CrossRef]
- Yurtsever, M. Glitters as a Source of Primary Microplastics: An Approach to Environmental Responsibility and Ethics. J. Agric. Environ. Ethics 2019, 32, 459–478. [Google Scholar] [CrossRef]
- Balloux, F.; Van Dorp, L. Q&A: What Are Pathogens, and What Have They Done to and for Us? BMC Biol. 2017, 15, 91. [Google Scholar] [CrossRef]
- Scholthof, K.-B.G. The Disease Triangle: Pathogens, the Environment and Society. Nat. Rev. Microbiol. 2007, 5, 152–156. [Google Scholar] [CrossRef]
- Tomley, F.M.; Shirley, M.W. Livestock Infectious Diseases and Zoonoses. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2637–2642. [Google Scholar] [CrossRef]
- Karesh, W.B.; Dobson, A.; Lloyd-Smith, J.O.; Lubroth, J.; Dixon, M.A.; Bennett, M.; Aldrich, S.; Harrington, T.; Formenty, P.; Loh, E.H.; et al. Ecology of Zoonoses: Natural and Unnatural Histories. Lancet 2012, 380, 1936–1945. [Google Scholar] [CrossRef]
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and Control of Varroa destructor. J. Invertebr. Pathol. 2010, 103, S96–S119. [Google Scholar] [CrossRef]
- Colla, S.R.; Otterstatter, M.C.; Gegear, R.J.; Thomson, J.D. Plight of the Bumble Bee: Pathogen Spillover from Commercial to Wild Populations. Biol. Conserv. 2006, 129, 461–467. [Google Scholar] [CrossRef]
- Genersch, E. Honey Bee Pathology: Current Threats to Honey Bees and Beekeeping. Appl. Microbiol. Biotechnol. 2010, 87, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.M.; Loh, E.H.; Rostal, M.K.; Zambrana-Torrelio, C.M.; Mendiola, L.; Daszak, P. Pathogens, Pests, and Economics: Drivers of Honey Bee Colony Declines and Losses. EcoHealth 2013, 10, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Fünfhaus, A.; Ebeling, J.; Genersch, E. Bacterial Pathogens of Bees. Curr. Opin. Insect Sci. 2018, 26, 89–96. [Google Scholar] [CrossRef]
- Straub, L.; Strobl, V.; Yañez, O.; Albrecht, M.; Brown, M.J.F.; Neumann, P. Do Pesticide and Pathogen Interactions Drive Wild Bee Declines? Int. J. Parasitol. Parasites Wildl. 2022, 18, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Fronczek, C.F.; Yoon, J.-Y. Biosensors for Monitoring Airborne Pathogens. SLAS Technol. 2015, 20, 390–410. [Google Scholar] [CrossRef]
- Ghini, S.; Girotti, S.; Calzolari, A.; Sabatini, A.G.; Alessandrini, A.; Zeri, L.; Porrini, C. Use of Honeybees (Apis mellifera L.) as Indicators of the Presence of the Phytopathogenic Bacteria Erwinia amylovora. In Freshwater Microplastics: Emerging Environmental Contaminants? Wagner, M., Lambert, S., Eds.; Springer: Cham, Switzerland, 2002; pp. 69–77. [Google Scholar]
- Sabatini, A.G.; Alexandrova, M.; Carpana, E.; Medrzycki, P.; Bortolotti, L.; Ghini, S.; Girotti, S.; Porrini, C.; Bazzi, C.; Baroni, F.; et al. Relationships between Apis mellifera and Erwinia amylovora: Bioindication, Bacterium Dispersal and Quarantine Procedures. Acta Hortic. 2006, 704, 155–162. [Google Scholar] [CrossRef]
- Cilia, G.; Resci, I.; Scarpellini, R.; Zavatta, L.; Albertazzi, S.; Bortolotti, L.; Nanetti, A.; Piva, S. Antimicrobial-Resistant Environmental Bacteria Isolated Using a Network of Honey Bee Colonies (Apis mellifera L. 1758). Transbound. Emerg. Dis. 2023, 2023, 1–10. [Google Scholar] [CrossRef]
- Tremblay, É.D.; Duceppe, M.O.; Thurston, G.B.; Gagnon, M.C.; Côté, M.J.; Bilodeau, G.J. High-resolution Biomonitoring of Plant Pathogens and Plant Species Using Metabarcoding of Pollen Pellet Contents Collected from a Honey Bee Hive. Environ. DNA 2019, 1, 155–175. [Google Scholar] [CrossRef]
- Bilodeau, G.J.; Rott, M.; Guarna, M.M.; Pernal, S.F.; Griffiths, J. High-Throughput Sequencing (HTS) of Bees and Pollen for Biosurveillance of Agricultural Pathogens and Invasive Species. In Proceedings of the Plant Health 2020, Online, 10–14 August 2020. [Google Scholar]
- Roberts, J.M.K.; Ireland, K.B.; Tay, W.T.; Paini, D. Honey Bee-assisted Surveillance for Early Plant Virus Detection. Ann. Appl. Biol. 2018, 173, 285–293. [Google Scholar] [CrossRef]
- Cilia, G.; Bortolotti, L.; Albertazzi, S.; Ghini, S.; Nanetti, A. Honey Bee (Apis mellifera L.) Colonies as Bioindicators of Environmental SARS-CoV-2 Occurrence. Sci. Total Environ. 2022, 805, 150327. [Google Scholar] [CrossRef]
- Roberts, J.M.K.; Jooste, A.E.C.; Pretorius, L.-S.; Geering, A.D.W. Surveillance for Avocado Sunblotch Viroid Utilizing the European Honey Bee (Apis mellifera). Phytopathology 2023, 113, 559–566. [Google Scholar] [CrossRef]
- Brunt, A.A.; Crabtree, K.; Dallwitz, M.J.; Gibbs, A.J.; Watson, L. Viruses of Plants; CAB International: Wallingford, UK, 1996; p. 1484. [Google Scholar]
- Card, S.D.; Pearson, M.N.; Clover, G.R.G. Plant Pathogens Transmitted by Pollen. Australas. Plant Pathol. 2007, 36, 455. [Google Scholar] [CrossRef]
- Pattemore, D.E.; Goodwin, R.M.; McBrydie, H.M.; Hoyte, S.M.; Vanneste, J.L. Evidence of the Role of Honey Bees (Apis mellifera) as Vectors of the Bacterial Plant Pathogen Pseudomonas syringae. Australas. Plant Pathol. 2014, 43, 571–575. [Google Scholar] [CrossRef]
- Van Loosdrecht, M.C.M.; Lyklema, J.; Norde, W.; Zehnder, A.J.B. Bacterial Adhesion: A Physicochemical Approach. Microb. Ecol. 1989, 17, 1–15. [Google Scholar] [CrossRef]
- Poortinga, A.T.; Smit, J.; Van Der Mei, H.C.; Busscher, H.J. Electric Field Induced Desorption of Bacteria from a Conditioning Film Covered Substratum. Biotechnol. Bioeng. 2001, 76, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.; Yiacoumi, S.; Lee, I.; Tsouris, C. The Role of the Electrostatic Force in Spore Adhesion. Environ. Sci. Technol. 2010, 44, 6209–6214. [Google Scholar] [CrossRef]
- Kalasin, S.; Dabkowski, J.; Nüsslein, K.; Santore, M.M. The Role of Nano-Scale Heterogeneous Electrostatic Interactions in Initial Bacterial Adhesion from Flow: A Case Study with Staphylococcus aureus. Colloids Surf. B Biointerfaces 2010, 76, 489–495. [Google Scholar] [CrossRef]
- Hori, K.; Matsumoto, S. Bacterial Adhesion: From Mechanism to Control. Biochem. Eng. J. 2010, 48, 424–434. [Google Scholar] [CrossRef]
- Zhao, W.; Walker, S.L.; Huang, Q.; Cai, P. Adhesion of Bacterial Pathogens to Soil Colloidal Particles: Influences of Cell Type, Natural Organic Matter, and Solution Chemistry. Water Res. 2014, 53, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.; O’Shea, P. The Electrostatic Nature of the Cell Surface of Candida albicans: A Role in Adhesion. Exp. Mycol. 1994, 18, 111–120. [Google Scholar] [CrossRef]
- Dunlap, C.A.; Biresaw, G.; Jackson, M.A. Hydrophobic and Electrostatic Cell Surface Properties of Blastospores of the Entomopathogenic Fungus Paecilomyces fumosoroseus. Colloids Surf. B Biointerfaces 2005, 46, 261–266. [Google Scholar] [CrossRef]
- Wargenau, A.; Fleißner, A.; Bolten, C.J.; Rohde, M.; Kampen, I.; Kwade, A. On the Origin of the Electrostatic Surface Potential of Aspergillus niger Spores in Acidic Environments. Res. Microbiol. 2011, 162, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Vasselli, J.G.; Shaw, B.D. Fungal Spore Attachment to Substrata. Fungal Biol. Rev. 2022, 41, 2–9. [Google Scholar] [CrossRef]
- Dika, C.; Ly-Chatain, M.H.; Francius, G.; Duval, J.F.L.; Gantzer, C. Non-DLVO Adhesion of F-Specific RNA Bacteriophages to Abiotic Surfaces: Importance of Surface Roughness, Hydrophobic and Electrostatic Interactions. Colloids Surf. Physicochem. Eng. Asp. 2013, 435, 178–187. [Google Scholar] [CrossRef]
- Xie, L.; Liu, F.; Liu, J.; Zeng, H. A Nanomechanical Study on Deciphering the Stickiness of SARS-CoV-2 on Inanimate Surfaces. ACS Appl. Mater. Interfaces 2020, 12, 58360–58368. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Batys, P.; Barbasz, J. SARS-CoV-2 Virion Physicochemical Characteristics Pertinent to Abiotic Substrate Attachment. Curr. Opin. Colloid Interface Sci. 2021, 55, 101466. [Google Scholar] [CrossRef]
- Gan, H.H.; Zinno, J.; Piano, F.; Gunsalus, K.C. Omicron Spike Protein Has a Positive Electrostatic Surface That Promotes ACE2 Recognition and Antibody Escape. Front. Virol. 2022, 2, 894531. [Google Scholar] [CrossRef]
- Lauster, D.; Osterrieder, K.; Haag, R.; Ballauff, M.; Herrmann, A. Respiratory Viruses Interacting with Cells: The Importance of Electrostatics. Front. Microbiol. 2023, 14, 1169547. [Google Scholar] [CrossRef]
- Tontou, R.; Giovanardi, D.; Stefani, E. Pollen as a Possible Pathway for the Dissemination of Pseudomonas syringae Pv. Actinidiae and Bacterial Canker of Kiwifruit. Phytopathol. Mediterr. 2014, 53, 333–339. [Google Scholar]
- Bhat, A.I.; Rao, G.P. Characterization of Plant Viruses: Methods and Protocols; Springer Protocols Handbooks; Springer: New York, NY, USA, 2020; ISBN 978-1-07-160333-8. [Google Scholar]
- Mateu, M.G. Assembly, Stability and Dynamics of Virus Capsids. Arch. Biochem. Biophys. 2013, 531, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Halbwachs, H. Gone with the Wind—A Review on Basidiospores of Lamellate Agarics. Mycosphere 2015, 6, 78–112. [Google Scholar] [CrossRef]
- Calhim, S.; Halme, P.; Petersen, J.H.; Læssøe, T.; Bässler, C.; Heilmann-Clausen, J. Fungal Spore Diversity Reflects Substrate-Specific Deposition Challenges. Sci. Rep. 2018, 8, 5356. [Google Scholar] [CrossRef]
- Sternberg, A.; Naujokat, C. Structural Features of Coronavirus SARS-CoV-2 Spike Protein: Targets for Vaccination. Life Sci. 2020, 257, 118056. [Google Scholar] [CrossRef]
- Vogt, V.M. Retroviral Virions and Genomes. Retroviruses 1997, 1, 27–69. [Google Scholar]
- Hashimi, M.H.; Hashimi, R.; Ryan, Q. Toxic Effects of Pesticides on Humans, Plants, Animals, Pollinators and Beneficial Organisms. Asian Plant Res. J. 2020, 5, 37–47. [Google Scholar] [CrossRef]
- Hassan, A.S. Inorganic-Based Pesticides: A Review Article. Egypt Sci. J. Pestic. 2019, 5, 39–52. [Google Scholar]
- Abati, R.; Sampaio, A.R.; Maciel, R.M.A.; Colombo, F.C.; Libardoni, G.; Battisti, L.; Lozano, E.R.; Ghisi, N.D.C.; Costa-Maia, F.M.; Potrich, M. Bees and Pesticides: The Research Impact and Scientometrics Relations. Environ. Sci. Pollut. Res. 2021, 28, 32282–32298. [Google Scholar] [CrossRef]
- Niell, S.; Jesús, F.; Pérez, N.; Pérez, C.; Pareja, L.; Abbate, S.; Carrasco-Letelier, L.; Díaz, S.; Mendoza, Y.; Cesio, V.; et al. Neonicotinoids Transference from the Field to the Hive by Honey Bees: Towards a Pesticide Residues Biomonitor. Sci. Total Environ. 2017, 581, 25–31. [Google Scholar] [CrossRef]
- Niell, S.; Jesús, F.; Díaz, R.; Mendoza, Y.; Notte, G.; Santos, E.; Gérez, N.; Cesio, V.; Cancela, H.; Heinzen, H. Beehives Biomonitor Pesticides in Agroecosystems: Simple Chemical and Biological Indicators Evaluation Using Support Vector Machines (SVM). Ecol. Indic. 2018, 91, 149–154. [Google Scholar] [CrossRef]
- Šlachta, M.; Erban, T.; Votavová, A.; Bešta, T.; Skalský, M.; Václavíková, M.; Halešová, T.; Edwards-Jonášová, M.; Včeláková, R.; Cudlín, P. Domestic Gardens Mitigate Risk of Exposure of Pollinators to Pesticides—An Urban-Rural Case Study Using a Red Mason Bee Species for Biomonitoring. Sustainability 2020, 12, 9427. [Google Scholar] [CrossRef]
- Jurak, G.; Bosnir, J.; Racz, A.; Brkic, D.; Prskalo, I.; Kis, D.; Ozimec, S.; Kalambura, S. Bioindicator Detection of Pesticide Residues in the Environment Using Honey Bees. Environ. Prot. Ecol. 2021, 22, 458–466. [Google Scholar]
- Hooven, L.A.; Chakrabarti, P.; Harper, B.J.; Sagili, R.R.; Harper, S.L. Potential Risk to Pollinators from Nanotechnology-Based Pesticides. Molecules 2019, 24, 4458. [Google Scholar] [CrossRef]
- Whitmore, L.F.; Hughes, J.F.; Harrison, N.; Abela, M.; O’Rourke, P. Enhanced Efficiency of Electrostatically Charged Insecticide Aerosols. Pest Manag. Sci. 2001, 57, 432–436. [Google Scholar] [CrossRef]
- Zhao, S.; Castle, G.S.P.; Adamiak, K. Factors Affecting Deposition in Electrostatic Pesticide Spraying. J. Electrost. 2008, 66, 594–601. [Google Scholar] [CrossRef]
- Latheef, M.A.; Carlton, J.B.; Kirk, I.W.; Hoffmann, W.C. Aerial Electrostatic-charged Sprays for Deposition and Efficacy against Sweet Potato Whitefly (Bemisia tabaci) on Cotton. Pest Manag. Sci. 2009, 65, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Appah, S.; Wang, P.; Ou, M.; Gong, C.; Jia, W. Review of Electrostatic System Parameters, Charged Droplets Characteristics and Substrate Impact Behavior from Pesticides Spraying. Int. J. Agric. Biol. Eng. 2019, 12, 1–9. [Google Scholar] [CrossRef]
- Socorro, J.; Durand, A.; Temime-Roussel, B.; Gligorovski, S.; Wortham, H.; Quivet, E. The Persistence of Pesticides in Atmospheric Particulate Phase: An Emerging Air Quality Issue. Sci. Rep. 2016, 6, 33456. [Google Scholar] [CrossRef]
- Smith, B.T. Introduction to Radioactivity and Radioactive Decay. In Nuclear Pharmacy: Concepts and Applications in Nuclear Pharmacy; Pharmaceutical Press: London, UK, 2010. [Google Scholar]
- Atwood, D.A. Radionuclides in the Environment; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Kovler, K. Radioactive Materials. In Toxicity of Building Materials; Elsevier: Amsterdam, The Netherlands, 2012; pp. 196–240. [Google Scholar]
- Hu, Q.-H.; Weng, J.-Q.; Wang, J.-S. Sources of Anthropogenic Radionuclides in the Environment: A Review. J. Environ. Radioact. 2010, 101, 426–437. [Google Scholar] [CrossRef]
- Chandra, K.; Proshad, R.; Dey, H.C.; Idris, A.M. A Review on Radionuclide Pollution in Global Soils with Environmental and Health Hazards Evaluation. Environ. Geochem. Health 2023, 45, 9245–9266. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Yu, K.N. Ionizing Radiation, DNA Double Strand Break and Mutation. Adv. Genet. Res. 2010, 4, 197–210. [Google Scholar]
- Møller, A.P.; Mousseau, T.A. Strong Effects of Ionizing Radiation from Chernobyl on Mutation Rates. Sci. Rep. 2015, 5, 8363. [Google Scholar] [CrossRef]
- De Santis, M.; Di Gianantonio, E.; Straface, G.; Cavaliere, A.F.; Caruso, A.; Schiavon, F.; Berletti, R.; Clementi, M. Ionizing Radiations in Pregnancy and Teratogenesis. Reprod. Toxicol. 2005, 20, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K.; Ozasa, K.; Akiba, S.; Niwa, O.; Kodama, K.; Takamura, N.; Zaharieva, E.K.; Kimura, Y.; Wakeford, R. Long-Term Effects of Radiation Exposure on Health. Lancet 2015, 386, 469–478. [Google Scholar] [CrossRef]
- Stewart, G.M.; Fowler, S.W.; Fisher, N.S. The Bioaccumulation of U-and Th-Series Radionuclides in Marine Organisms. Radioact. Environ. 2008, 13, 269–305. [Google Scholar]
- Carvalho, F.P. Radionuclide Concentration Processes in Marine Organisms: A Comprehensive Review. J. Environ. Radioact. 2018, 186, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Fresquez, P.R.; Armstrong, D.R.; Pratt, L.H. Radionuclides in Bees and Honey within and around Los Alamos National Laboratory. J. Environ. Sci. Health Part Environ. Sci. Eng. Toxicol. 1997, 32, 1309–1323. [Google Scholar] [CrossRef]
- Haarmann, T.K. Honey Bees as Indicators of Radionuclide Contamination: Exploring Colony Variability and Temporal Contaminant Accumulation. J. Apic. Res. 1997, 36, 77–87. [Google Scholar] [CrossRef]
- Haarmann, T.K. Honey Bees as Indicators of Radionuclide Contamination: Comparative Studies of Contaminant Levels in Forager and Nurse Bees and in the Flowers of Three Plant Species. Arch. Environ. Contam. Toxicol. 1998, 35, 287–294. [Google Scholar] [CrossRef]
- Haarmann, T.K. Honey Bees (Hymenoptera: Apidae) as Indicators of Radionuclide Contamination: Investigating Contaminant Redistribution Using Concentrations in Water, Flowers, and Honey Bees. J. Econ. Entomol. 1998, 91, 1072–1077. [Google Scholar] [CrossRef]
- Haarmann, T.K.; Fresquez, P.R. Radionuclide Concentrations in Honey Bees from Area G at TA-54 During 1999; Los Alamos National Lab: Los Alamos, NM, USA, 2000.
- Haarmann, T.K. Honey Bees as Indicators of Radionuclide Contamination: A Truly Useful Biomonitor? In Honey Bees; CRC Press: Boca Raton, FL, USA, 2002; pp. 132–150. [Google Scholar]
- Porrini, C.; Sabatini, A.G.; Girotti, S.; Ghini, S.; Medrzycki, P.; Grillenzoni, F.; Bortolotti, L.; Gattavecchia, E.; Celli, G. Honey Bees and Bee Products as Monitors of the Environmental Contamination. Apiacta 2003, 38, 63–70. [Google Scholar]
- Agnello, L.; Comodo, N. Radioactive Nuclide Monitoring Using Bees as Biological Indicators. Probl. Health Ecol. 2011, 27, 130. [Google Scholar]
- Steinhauser, G. Anthropogenic Radioactive Particles in the Environment. J. Radioanal. Nucl. Chem. 2018, 318, 1629–1639. [Google Scholar] [CrossRef]
- Yeh, H.C.; Newton, G.J.; Raabe, O.G.; Boor, D.R. Self-Charging of 198 Au-Labeled Monodisperse Gold Aerosols Studied with a Miniature Electrical Mobility Spectrometer. J. Aerosol Sci. 1976, 7, 245–253. [Google Scholar] [CrossRef]
- Clement, C.F.; Harrison, R.G. The Charging of Radioactive Aerosols. J. Aerosol Sci. 1992, 23, 481–504. [Google Scholar] [CrossRef]
- Gensdarmes, F.; Boulaud, D.; Renoux, A. Electrical Charging of Radioactive Aerosols—Comparison of the Clement–Harrison Models with New Experiments. J. Aerosol Sci. 2001, 32, 1437–1458. [Google Scholar] [CrossRef]
- Walker, M.E.; McFarlane, J.; Glasgow, D.C.; Chung, E.; Taboada-Serrano, P.; Yiacoumi, S.; Tsouris, C. Influence of Radioactivity on Surface Interaction Forces. J. Colloid Interface Sci. 2010, 350, 595–598. [Google Scholar] [CrossRef]
- Kweon, H.; Yiacoumi, S.; Lee, I.; McFarlane, J.; Tsouris, C. Influence of Surface Potential on the Adhesive Force of Radioactive Gold Surfaces. Langmuir 2013, 29, 11876–11883. [Google Scholar] [CrossRef]
- Kim, Y.; Yiacoumi, S.; Lee, I.; McFarlane, J.; Tsouris, C. Influence of Radioactivity on Surface Charging and Aggregation Kinetics of Particles in the Atmosphere. Environ. Sci. Technol. 2014, 48, 182–189. [Google Scholar] [CrossRef]
- Kim, Y.; Yiacoumi, S.; Tsouris, C. Surface Charge Accumulation of Particles Containing Radionuclides in Open Air. J. Environ. Radioact. 2015, 143, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Jang, G.G.; Wiechert, A.I.; Kim, Y.-H.; Ladshaw, A.P.; Spano, T.; McFarlane, J.; Myhre, K.; Song, J.J.; Yiacoumi, S.; Tsouris, C. Charging of Radioactive and Environmental Airborne Particles. J. Environ. Radioact. 2022, 248, 106887. [Google Scholar] [CrossRef] [PubMed]
- Tschiersch, J.; Frank, G.; Roth, P.; Wagenpfeil, F.; Watterson, J. Enhanced Airborne Radioactivity during a Pine Pollen Release Episode. Radiat. Environ. Biophys. 1999, 38, 139–145. [Google Scholar] [CrossRef]
- Montero-Montoya, R.; López-Vargas, R.; Arellano-Aguilar, O. Volatile Organic Compounds in Air: Sources, Distribution, Exposure and Associated Illnesses in Children. Ann. Glob. Health 2018, 84, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Yadav, R. A Review on Volatile Organic Compounds (VOCs) as Environmental Pollutants: Fate and Distribution. Int. J. Plant Environ. 2018, 4, 14–26. [Google Scholar] [CrossRef]
- Liu, Y.; Shao, M.; Fu, L.; Lu, S.; Zeng, L.; Tang, D. Source Profiles of Volatile Organic Compounds (VOCs) Measured in China: Part I. Atmos. Environ. 2008, 42, 6247–6260. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, X.; Wang, C.; Zhou, H. Environmental and Human Health Impacts of Volatile Organic Compounds: A Perspective Review. Chemosphere 2023, 313, 137489. [Google Scholar] [CrossRef]
- Hafner, S.D.; Howard, C.; Muck, R.E.; Franco, R.B.; Montes, F.; Green, P.G.; Mitloehner, F.; Trabue, S.L.; Rotz, C.A. Emission of Volatile Organic Compounds from Silage: Compounds, Sources, and Implications. Atmos. Environ. 2013, 77, 827–839. [Google Scholar] [CrossRef]
- Meng, T.T. Volatile Organic Compounds of Polyethylene Vinyl Acetate Plastic Are Toxic to Living Organisms. J. Toxicol. Sci. 2014, 39, 795–802. [Google Scholar] [CrossRef]
- Mangotra, A.; Singh, S.K. Volatile Organic Compounds: A Threat to the Environment and Health Hazards to Living Organisms—A Review. J. Biotechnol. 2024, 382, 51–69. [Google Scholar] [CrossRef]
- Lee, I.; Park, H.; Kim, M.J.; Kim, S.; Choi, S.; Park, J.; Cho, Y.H.; Hong, S.; Yoo, J.; Cheon, G.J.; et al. Exposure to Polycyclic Aromatic Hydrocarbons and Volatile Organic Compounds Is Associated with a Risk of Obesity and Diabetes Mellitus among Korean Adults: Korean National Environmental Health Survey (KoNEHS) 2015–2017. Int. J. Hyg. Environ. Health 2022, 240, 113886. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.H.; Spengler, J.D.; Neas, L.M.; Samet, J.M.; Wagner, G.R.; Coultas, D.; Ozkaynak, H.; Schwab, M. Respiratory and Irritant Health Effects of Ambient Volatile Organic Compounds. Am. J. Epidemiol. 1993, 137, 1287–1301. [Google Scholar] [CrossRef] [PubMed]
- Pappas, G.P.; Herbert, R.J.; Henderson, W.; Koenig, J.; Stover, B.; Barnhart, S. The Respiratory Effects of Volatile Organic Compounds. Int. J. Occup. Environ. Health 2000, 6, 1–8. [Google Scholar] [CrossRef]
- Janfaza, S.; Khorsand, B.; Nikkhah, M.; Zahiri, J. Digging Deeper into Volatile Organic Compounds Associated with Cancer. Biol. Methods Protoc. 2019, 4, bpz014. [Google Scholar] [CrossRef]
- Hussain, M.S.; Gupta, G.; Mishra, R.; Patel, N.; Gupta, S.; Alzarea, S.I.; Kazmi, I.; Kumbhar, P.; Disouza, J.; Dureja, H.; et al. Unlocking the Secrets: Volatile Organic Compounds (VOCs) and Their Devastating Effects on Lung Cancer. Pathol. Res. Pract. 2024, 255, 155157. [Google Scholar] [CrossRef]
- Mellouki, A.; Wallington, T.J.; Chen, J. Atmospheric Chemistry of Oxygenated Volatile Organic Compounds: Impacts on Air Quality and Climate. Chem. Rev. 2015, 115, 3984–4014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, L.; Huang, L.; Wang, Y.; Huo, J.; Duan, Y.; Wang, Y.; Fu, Q. The Impact of Volatile Organic Compounds on Ozone Formation in the Suburban Area of Shanghai. Atmos. Environ. 2020, 232, 117511. [Google Scholar] [CrossRef]
- Murcia-Morales, M.; Tzanetou, E.N.; García-Gallego, G.; Kasiotis, K.M.; Vejsnaes, F.; Brodschneider, R.; Hatjina, F.; Machera, K.; Van Der Steen, J.J.M. Environmental Assessment of PAHs through Honey Bee Colonies—A Matrix Selection Study. Heliyon 2024, 10, e23564. [Google Scholar] [CrossRef]
- Amorena, M.; Visciano, P.; Giacomelli, A.; Marinelli, E.; Sabatini, A.G.; Medrzycki, P.; Oddo, L.P.; De Pace, F.M.; Belligoli, P.; Di Serafino, G.; et al. Monitoring of Levels of Polycyclic Aromatic Hydrocarbons in Bees Caught from Beekeeping: Remark 1. Vet. Res. Commun. 2009, 33, 165–167. [Google Scholar] [CrossRef]
- Perugini, M.; Di Serafino, G.; Giacomelli, A.; Medrzycki, P.; Sabatini, A.G.; Persano Oddo, L.; Marinelli, E.; Amorena, M. Monitoring of Polycyclic Aromatic Hydrocarbons in Bees (Apis mellifera) and Honey in Urban Areas and Wildlife Reserves. J. Agric. Food Chem. 2009, 57, 7440–7444. [Google Scholar] [CrossRef]
- Kargar, N.; Matin, G.; Matin, A.A.; Buyukisik, H.B. Biomonitoring, Status and Source Risk Assessment of Polycyclic Aromatic Hydrocarbons (PAHs) Using Honeybees, Pine Tree Leaves, and Propolis. Chemosphere 2017, 186, 140–150. [Google Scholar] [CrossRef]
- Zięba, K.; Szostak, E.; Czekońska, K.; Miśkowiec, P.; Moos-Matysik, A.; Nyczyk-Malinowska, A.; Szentgyörgyi, H. Usefulness of Bee Bread and Capped Brood for the Assessment of Monocyclic Aromatic Hydrocarbon Levels in the Environment. Environ. Pollut. 2020, 265, 114882. [Google Scholar] [CrossRef]
- Cochard, P.; Laurie, M.; Veyrand, B.; Le Bizec, B.; Poirot, B.; Marchand, P. PAH7 Concentration Reflects Anthropization: A Study Using Environmental Biomonitoring with Honeybees. Sci. Total Environ. 2021, 751, 141831. [Google Scholar] [CrossRef] [PubMed]
- Ilić, D.; Brkić, B.; Sekulić, M.T. Biomonitoring: Developing a Beehive Air Volatiles Profile as an Indicator of Environmental Contamination Using a Sustainable In-Field Technique. Sustainability 2024, 16, 1713. [Google Scholar] [CrossRef]
- Lambert, O.; Veyrand, B.; Durand, S.; Marchand, P.; Bizec, B.L.; Piroux, M.; Puyo, S.; Thorin, C.; Delbac, F.; Pouliquen, H. Polycyclic Aromatic Hydrocarbons: Bees, Honey and Pollen as Sentinels for Environmental Chemical Contaminants. Chemosphere 2012, 86, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Odabasi, M.; Ongan, O.; Cetin, E. Quantitative Analysis of Volatile Organic Compounds (VOCs) in Atmospheric Particles. Atmos. Environ. 2005, 39, 3763–3770. [Google Scholar] [CrossRef]
- Schnellekreis, J.; Sklorz, M.; Peters, A.; Cyrys, J.; Zimmermann, R. Analysis of Particle-Associated Semi-Volatile Aromatic and Aliphatic Hydrocarbons in Urban Particulate Matter on a Daily Basis. Atmos. Environ. 2005, 39, 7702–7714. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).