From Digestion to Detoxification: Exploring Plant Metabolite Impacts on Insect Enzyme Systems for Enhanced Pest Control
Simple Summary
Abstract
1. Introduction
2. Insect Enzymes
2.1. Insect Digestive Enzymes
2.2. Insect Antioxidative Enzymes
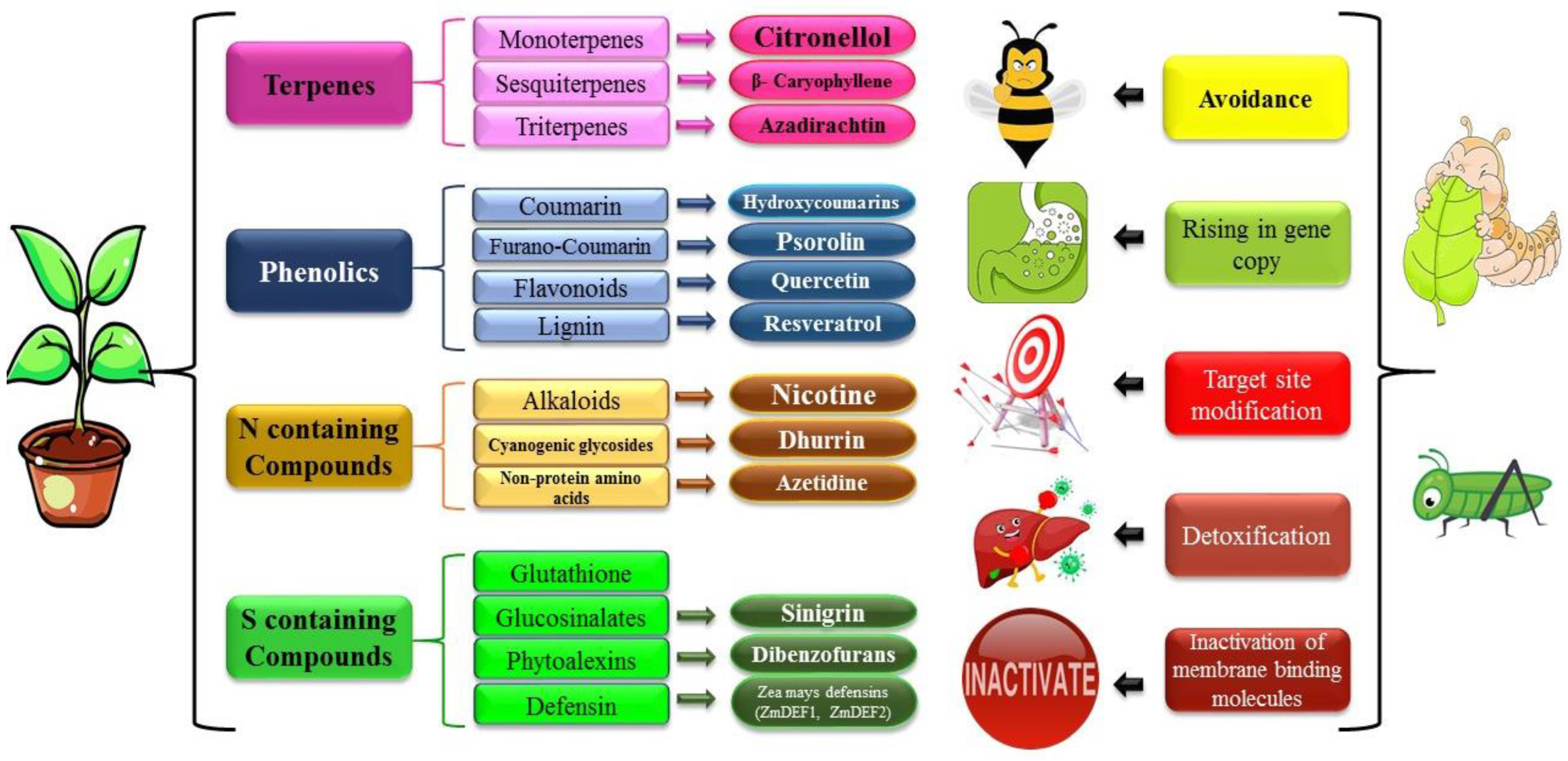
3. Plant Metabolites and Their Impact on Insect Enzymes
3.1. Plant Metabolites and Their Impact on Digestive Enzymes
3.2. Plant Metabolites and Their Impact on Insect Antioxidative Enzymes
4. Antioxidative Enzymes and Oxidative Stress
5. Interplay Between Digestive and Antioxidative Enzymes
6. Regulation of Enzyme Activity in Response to Plant Metabolites
7. Applications and Future Directions
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kutty, N.N.; Mishra, M. Dynamic Distress Calls: Volatile Info Chemicals Induce and Regulate Defense Responses during Herbivory. Front. Plant Sci. 2023, 14, 1135000. [Google Scholar] [CrossRef]
- Beran, F.; Petschenka, G. Sequestration of Plant Defense Compounds by Insects: From Mechanisms to Insect-Plant Coevolution. Annu. Rev. Entomol. 2022, 67, 163–180. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Cao, X.; Jiang, H. Digestion-Related Proteins in the Tobacco Hornworm, Manduca Sexta. Insect Biochem. Mol. Biol. 2020, 126, 103457. [Google Scholar] [CrossRef]
- War, A.R.; Buhroo, A.A.; Hussain, B.; Ahmad, T.; Nair, R.M.; Sharma, H.C. Plant Defense and Insect Adaptation with Reference to Secondary Metabolites. In Co-Evolution of Secondary Metabolites; Reference Series in Phytochemistry; Springer: Berlin/Heidelberg, Germany, 2020; pp. 795–822. [Google Scholar] [CrossRef]
- Pentzold, S.; Zagrobelny, M.; Rook, F.; Bak, S. How Insects Overcome Two-Component Plant Chemical Defence: Plant β-Glucosidases as the Main Target for Herbivore Adaptation. Biol. Rev. 2014, 89, 531–551. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Peter Constabel, C. Tannins in Plant-Herbivore Interactions. Phytochemistry 2011, 72, 1551–1565. [Google Scholar] [CrossRef]
- Felton, G.W.; Summers, C.B. Antioxidant Systems in Insects. Arch. Insect Biochem. Physiol. 1995, 29, 187–197. [Google Scholar] [CrossRef]
- Chamani, M.; Naseri, B.; Rafiee-Dastjerdi, H.; Emaratpardaz, J.; Ebadollahi, A.; Palla, F. Some Physiological Effects of Nanofertilizers on Wheat-Aphid Interactions. Plants 2023, 12, 2602. [Google Scholar] [CrossRef]
- George, D.G.M.; Gatehouse, A.M.R. Original Research Article Oxidative Stress Enzymes in Busseola fusca. Int. J. Curr. Microbiol. Appl. Sci. 2013, 2, 485–495. [Google Scholar]
- Jena, K.; Kar, P.K.; Babu, C.S.; Giri, S.; Singh, S.S.; Prasad, B.C. Comparative Study of Total Hydroperoxides and Antioxidant Defense System in the Indian Tropical Tasar Silkworm, Antheraea mylitta, in Diapausing and Non-Diapausing Generations. J. Insect Sci. 2013, 13, 123. [Google Scholar] [CrossRef]
- Łukasik, I.; Goławska, S. Effect of Host Plant on Levels of Reactive Oxygen Species Andantioxidants in the Cereal Aphids Sitobion avenae and Rhopalosiphum padi. Biochem. Syst. Ecol. 2013, 51, 232–239. [Google Scholar] [CrossRef]
- Senthil-Nathan, S. Physiological and Biochemical Effect of Neem and Other Meliaceae Plants Secondary Metabolites against Lepidopteran Insects. Front. Physiol. 2013, 4, 359. [Google Scholar]
- Bertić, M.; Orgel, F.; Gschwendtner, S.; Schloter, M.; Moritz, F.; Schmitt-Kopplin, P.; Zimmer, I.; Fladung, M.; Schnitzler, J.P.; Schroeder, H.; et al. European Oak Metabolites Shape Digestion and Fitness of the Herbivore Tortrix viridana. Funct. Ecol. 2023, 37, 1476–1491. [Google Scholar] [CrossRef]
- Li, W.; Zou, J.; Yang, X.; Yang, M.; Jiang, P.; Wang, X.; Huang, C.; He, Y. Identification of Metabolizing Enzyme Genes Associated with Xenobiotics and Odorants in the Predatory Stink Bug Arma Custos Based on Transcriptome Analysis. Heliyon 2023, 9, e18657. [Google Scholar]
- BK, S.K.; Moural, T.; Zhu, F. Functional and Structural Diversity of Insect Glutathione S-Transferases in Xenobiotic Adaptation. Int. J. Biol. Sci. 2022, 18, 5713. [Google Scholar]
- Ishaaya, I. Insect Detoxifying Enzymes: Their Importance in Pesticide Synergism and Resistance. Arch. Insect Biochem. Physiol. 1993, 22, 263–276. [Google Scholar]
- Hosseini Mousavi, S.M.; Hemmati, S.A.; Rasekh, A. Feeding Responses and Digestive Function of Spodoptera littoralis (Boisd) on Various Leafy Vegetables Exhibit Possible Tolerance Traits. Bull. Entomol. Res. 2023, 25, 430–438. [Google Scholar] [CrossRef]
- Babamir-Satehi, A.; Habibpour, B.; Aghdam, H.R.; Hemmati, S.A. Interaction between Feeding Efficiency and Digestive Physiology of the Pink Stem Borer, Sesamia cretica Lederer (Lepidoptera: Noctuidae), and Biochemical Compounds of Different Sugarcane Cultivars. Arthropod. Plant. Interact. 2022, 16, 309–316. [Google Scholar] [CrossRef]
- Qi, G.; Gu, Z.; Shen, J.; Wang, C.; Zeng, L.; Reitz, S.R.; Cao, Y. Rosa Chinensis Cultivars Affect Fitness-Related Characteristics and Digestive Physiology of the Western Flower Thrips, Frankliniella occidentalis Pergande (Thysanoptera: Thripidae). J. Econ. Entomol. 2022, 115, 1620–1626. [Google Scholar] [CrossRef]
- Ali, M.; Elshehaby, M.; Abdel-Rahman, M.; Abdelreheem, A. Effect of Secondary Metabolites of Different Cucumber Cultivars on Antioxidant Enzymes of Whitefly, Bemisia tabaci (Gen.), Assiut, Egypt. Egypt. Acad. J. Biol. Sci. A Entomol. 2023, 16, 141–147. [Google Scholar] [CrossRef]
- Johnson, K.S.; Felton, G.W. Plant Phenolics as Dietary Antioxidants for Herbivorous Insects: A Test with Genetically Modified Tobacco. J. Chem. Ecol. 2001, 27, 2579–2597. [Google Scholar] [CrossRef]
- Gatehouse, J.A. Plant Resistance towards Insect Herbivores: A Dynamic Interaction. New Phytol. 2002, 156, 145–169. [Google Scholar] [CrossRef] [PubMed]
- Chamani, M.; Naseri, B.; Rafiee-Dastjerdi, H.; Emaratpardaz, J.; Farshbaf Pourabad, R.; Chenari Bouket, A.; Oszako, T.; Belbahri, L. Examining Innovative Technologies: Nano-Chelated Fertilizers for Management of Wheat Aphid (Schizaphis graminum Rondani). Insects 2024, 15, 209. [Google Scholar] [CrossRef] [PubMed]
- Chamani, M.; Askari, N.; Pourabad, R.F.; Bouket, A.C.; Oszako, T.; Belbahri, L. Potential Biopesticides from Seed Extracts: A Sustainable Way to Protect Co Tt on Crops from Bollworm Damage. Sustainability 2024, 16, 145. [Google Scholar]
- Huang, J.H.; Jing, X.; Douglas, A.E. The Multi-Tasking Gut Epithelium of Insects. Insect Biochem. Mol. Biol. 2015, 67, 15–20. [Google Scholar] [CrossRef]
- Abou El Asrar, R.; Cools, D.; Vanden Broeck, J. Role of Peptide Hormones in Insect Gut Physiology. Curr. Opin. Insect Sci. 2020, 41, 71–78. [Google Scholar] [CrossRef]
- Predel, R.; Brandt, W.; Kellner, R.; Rapus, J.; Nachman, R.J.; Gade, G. Post-Translational Modifications of the Insect Sulfakinins: Sulfation, Pyroglutamate-Formation and O-Methylation of Glutamic Acid. Eur. J. Biochem. 1999, 263, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Ashouri, S.; Farshbaf Pourabad, R. Regulation of Gene Expression Encoding the Digestive α-Amylase in the Larvae of Colorado Potato Beetle, Leptinotarsa decemlineata (Say) in Response to Plant Protein Extracts. Gene 2021, 766, 145159. [Google Scholar] [CrossRef]
- Terra, W.R.; Ferreira, C. Insect Digestive Enzymes: Properties, Compartmentalization and Function. Comp. Biochem. Physiol. Part B Biochem. 1994, 109, 1–62. [Google Scholar] [CrossRef]
- Kessler, A.; Baldwin, I.T. Plant Responses to Insect Herbivory: The Emerging Molecular Analysis. Annu. Rev. Plant Biol. 2002, 53, 299–328. [Google Scholar] [CrossRef]
- Kim, Y.H.; Soumaila Issa, M.; Cooper, A.M.W.; Zhu, K.Y. RNA Interference: Applications and Advances in Insect Toxicology and Insect Pest Management. Pestic. Biochem. Physiol. 2015, 120, 109–117. [Google Scholar] [CrossRef]
- Kuwar, S.S.; Pauchet, Y.; Vogel, H.; Heckel, D.G. Adaptive Regulation of Digestive Serine Proteases in the Larval Midgut of Helicoverpa armigera in Response to a Plant Protease Inhibitor. Insect Biochem. Mol. Biol. 2015, 59, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Rajput, S.; Sharma, P.K.; Malviya, R. Utilization of Herbal Components as Insecticidal and Repellent Effects. Recent Adv. Food Nutr. Agric. 2023, 14, 144–154. [Google Scholar] [CrossRef]
- Soltani, P.; Farshbaf Pourabad, R.; Chamani, M.; Darabi, M.; Naseri, B. Gas-Liquid Chromatography Analysis of Phospholipids and Triglycerides in Plodia interpunctella (Hübner) Larvae: Effect of Different Diets. J. Stored Prod. Res. 2024, 106, 102315. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Dec, K.; Kałduńska, J.; Kawczuga, D.; Kochman, J.; Janda, K. Reactive Oxygen Species-Sources, Functions, Oxidative Damage. Pol. Merkur. Lek. Organ Pol. Tow. Lek. 2020, 48, 124–127. [Google Scholar]
- Choudhary, A.; Kumar, A.; Kaur, N. ROS and Oxidative Burst: Roots in Plant Development. Plant Divers. 2020, 42, 33–43. [Google Scholar] [CrossRef]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox System in Health and Disease: The Latest Update. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef]
- Cecerska-Heryć, E.; Surowska, O.; Heryć, R.; Serwin, N.; Napiontek-Balińska, S.; Dołęgowska, B. Are Antioxidant Enzymes Essential Markers in the Diagnosis and Monitoring of Cancer Patients—A Review. Clin. Biochem. 2021, 93, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Irato, P.; Santovito, G. Enzymatic and Non-Enzymatic Molecules with Antioxidant Function. Antioxidants 2021, 10, 579. [Google Scholar] [CrossRef]
- Colinet, D.; Cazes, D.; Belghazi, M.; Gatti, J.-L.; Poirié, M. Extracellular Superoxide Dismutase in Insects: Characterization, Function, and Interspecific Variation in Parasitoid Wasp Venom. J. Biol. Chem. 2011, 286, 40110–40121. [Google Scholar]
- Ahmad, S.; Pardini, R.S. Mechanisms for Regulating Oxygen Toxicity in Phytophagous Insects. Free Radic. Biol. Med. 1990, 8, 401–413. [Google Scholar]
- Ahmad, S.; Beilstein, M.A.; Pardini, R.S. Glutathione Peroxidase Activity in Insects: A Reassessment. Arch. Insect Biochem. Physiol. 1989, 12, 31–49. [Google Scholar]
- Simon, J.Y. Insect Glutathione S-Transferases. Zool. Stud. 1996, 35, 9–19. [Google Scholar]
- Pang, R.; Xing, K.; Yuan, L.; Liang, Z.; Chen, M.; Yue, X.; Dong, Y.; Ling, Y.; He, X.; Li, X. Peroxiredoxin Alleviates the Fitness Costs of Imidacloprid Resistance in an Insect Pest of Rice. PLoS Biol. 2021, 19, e3001190. [Google Scholar]
- Gřešková, A.; Petřivalský, M. Thioredoxin System in Insects: Uncovering the Roles of Thioredoxins and Thioredoxin Reductase beyond the Antioxidant Defences. Insects 2024, 15, 797. [Google Scholar] [CrossRef]
- Zanganeh, L.; Hassanpour, M.; Madadi, H.; Razmjou, J.; Behnamian, M. Nanofertilizers and Plant Metabolites: Investigating Their Influence on Trophic Interactions in the Cucumber-Melon Aphid-Green Lacewing. Plant Stress 2024, 12, 100436. [Google Scholar]
- Kumar, S.; Park, J.; Kim, E.; Na, J.; Chun, Y.S.; Kwon, H.; Kim, W.; Kim, Y. Oxidative Stress Induced by Chlorine Dioxide as an Insecticidal Factor to the Indian Meal Moth, Plodia interpunctella. Pestic. Biochem. Physiol. 2015, 124, 48–59. [Google Scholar] [CrossRef]
- Palma-Onetto, V.; Oliva, D.; González-Teuber, M. Lethal and Oxidative Stress Side Effects of Organic and Synthetic Pesticides on the Insect Scale Predator Rhyzobius lophanthae. Entomol. Gen. 2021, 41, 345–355. [Google Scholar] [CrossRef]
- Samet, J.M.; Wages, P.A. Oxidative Stress from Environmental Exposures. Curr. Opin. Toxicol. 2018, 7, 60–66. [Google Scholar] [CrossRef]
- Emarat-pardaz, J.; Moharramnejad, S.; Panahandeh, J.; Chamani, M.; Zadeh Esfahlan, M.R.; Karbalaei Khiavi, H. Effect of Light Intensity on Growth Traits, Photosynthetic and Total Hypericin in Topaz Cultivar and Mishu Ecotype of Hypericum perforatum. Eco-Phytochem. J. Med. Plants 2019, 7, 53–63. [Google Scholar]
- Wink, M. Plant Secondary Metabolites Modulate Insect Behavior-Steps toward Addiction? Front. Physiol. 2018, 9, 364. [Google Scholar] [CrossRef]
- Zhu-Salzman, K.; Zeng, R. Insect Response to Plant Defensive Protease Inhibitors. Annu. Rev. Entomol. 2015, 60, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Rane, A.S.; Joshi, R.S.; Giri, A.P. Molecular Determinant for Specificity: Differential Interaction of α-Amylases with Their Proteinaceous Inhibitors. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129703. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, L.; Zhao, J.; Chen, M. Effect of Tannic Acid on Nutrition and Activities of Detoxification Enzymes and Acetylcholinesterase of the Fall Webworm (Lepidoptera: Arctiidae). J. Insect Sci. 2020, 20, 8. [Google Scholar] [CrossRef]
- Rehman, F.; Khan, F.A.; Badruddin, S.M.A. Role of Phenolics in Plant Defense Against Insect Herbivory. In Chemistry of Phytopotentials: Health, Energy and Environmental Perspectives; Springer: Berlin/Heidelberg, Germany, 2012; pp. 309–313. [Google Scholar] [CrossRef]
- Mukherjee, S. Influence of Plant Allelochemicals on Growth Rate, Nutritional Physiology and Mid-Gut Esterase Activity in Fifth Instar Larvae of Spodoptera litura (f.) (Lepidoptera: Noctuidae). Invertebr. Reprod. Dev. 2003, 43, 125–132. [Google Scholar] [CrossRef]
- Mrdaković, M.; Perić Mataruga, V.; Ilijin, L.; Vlahović, M.; Janković Tomanić, M.; Mirčić, D.; Lazarević, J. Response of Lymantria dispar (Lepidoptera: Lymantriidae) Larvae from Differently Adapted Populations to Allelochemical Stress: Effects of Tannic Acid. Eur. J. Entomol. 2013, 110, 55–63. [Google Scholar] [CrossRef]
- Chamani, M.; Farshbaf Pourabad, R.; Valizadeh, M. Lipase Enzyme Activity in Digestive System of Helicoverpa armigera Hb.(Lep.: Noctuidae) and Effect of Some Laboratory Compounds on It. J. Appl. Res. Plant Prot. 2015, 4, 113–129. [Google Scholar]
- Ishaaya, I.; Casida, J.E. Phenyltin Compounds Inhibit Digestive Enzymes of Tribolium confusum Larvae. Pestic. Biochem. Physiol. 1975, 5, 350–358. [Google Scholar] [CrossRef]
- Lazarević, J.; Milanović, S.; Šešlija Jovanović, D.; Janković-Tomanić, M. Temperature- and Diet-Induced Plasticity of Growth and Digestive Enzymes Activity in Spongy moth Larvae. Biomolecules 2023, 13, 821. [Google Scholar] [CrossRef]
- Askari, N.; Farshbaf Pourabad, R.; Mohammadi, D.; Khaghaninia, S. Effects of Some Plants Seed Extracts on Helicoverpa armigera Hübner (Lepidoptera: Noctuidae) Midgut Protease Activity. Munis Entomol. Zool. 2016, 11, 26–32. [Google Scholar]
- Napoleão, T.H.; Albuquerque, L.P.; Santos, N.D.L.; Nova, I.C.V.; Lima, T.A.; Paiva, P.M.G.; Pontual, E.V. Insect Midgut Structures and Molecules as Targets of Plant-Derived Protease Inhibitors and Lectins. Pest Manag. Sci. 2019, 75, 1212–1222. [Google Scholar] [CrossRef]
- Rodrigues Macedo, M.; Das Graças, M.; Freire, M. Insect Digestive Enzymes as a Target for Pest Control. Invertebr. Surviv. J. 2011, 8, 190–198. [Google Scholar]
- Singh, S.; Singh, A.; Kumar, S.; Mittal, P.; Singh, I.K. Protease Inhibitors: Recent Advancement in Its Usage as a Potential Biocontrol Agent for Insect Pest Management. Insect Sci. 2020, 27, 186–201. [Google Scholar] [CrossRef] [PubMed]
- Gatehouse, A. Biotechnological Applications of Plant Genes in the Production of Insect-Resistant Crops. In Global Plant Genetic Resources for Insect-Resistant Crops; CRC Press: Boca Raton, FL, USA, 1998; pp. 263–280. [Google Scholar]
- Divekar, P.A.; Rani, V.; Majumder, S.; Karkute, S.G.; Molla, K.A.; Pandey, K.K.; Behera, T.K.; Govindharaj, G.P.P. Protease Inhibitors: An Induced Plant Defense Mechanism Against Herbivores. J. Plant Growth Regul. 2023, 42, 6057–6073. [Google Scholar] [CrossRef]
- Chung, S.H.; Feng, H.; Jander, G. Engineering Pest Tolerance through Plant-Mediated RNA Interference. Curr. Opin. Plant Biol. 2021, 60, 102029. [Google Scholar] [CrossRef]
- Kolliopoulou, A.; Taning, C.N.T.; Smagghe, G.; Swevers, L. Viral Delivery of DsRNA for Control of Insect Agricultural Pests and Vectors of Human Disease: Prospects and Challenges. Front. Physiol. 2017, 8, 399. [Google Scholar] [CrossRef]
- Mehrabadi, M.; Bandani, A.R.; Saadati, F.; Mahmudvand, M. A-Amylase Activity of Stored Products Insects and Its Inhibition By Medicinal Plant Extracts. J. Agric. Sci. Technol. 2011, 13, 1173–1182. [Google Scholar]
- Sarate, P.J.; Tamhane, V.A.; Kotkar, H.M.; Ratnakaran, N.; Susan, N.; Gupta, V.S.; Giri, A.P. Developmental and Digestive Flexibilities in the Midgut of a Polyphagous Pest, the Cotton Bollworm, Helicoverpa armigera. J. Insect Sci. 2012, 12, 42. [Google Scholar] [CrossRef]
- Napoleão, T.H.; Belmonte, B.d.R.; Pontual, E.V.; de Albuquerque, L.P.; Sá, R.A.; Paiva, L.M.; Breitenbach Barroso Coelho, L.C.; Paiva, P.M.G. Deleterious Effects of Myracrodruon urundeuva Leaf Extract and Lectin on the Maize Weevil, Sitophilus zeamais (Coleoptera, Curculionidae). J. Stored Prod. Res. 2013, 54, 26–33. [Google Scholar] [CrossRef]
- Dastranj, M.; Bandani, A.R.; Mehrabadi, M. Age-Specific Digestion of Tenebrio Molitor (Coleoptera: Tenebrionidae) and Inhibition of Proteolytic and Amylolytic Activity by Plant Proteinaceous Seed Extracts. J. Asia. Pac. Entomol. 2013, 16, 309–315. [Google Scholar] [CrossRef]
- Sami, A.J. Azadirachta Indica Derived Compounds as Inhibitors of Digestive Alpha-Amylase ininsect Pests: Potential Bio-Pesticides in Insect Pest Management. J. Exp. Biol. 2014, 4, 259–264. [Google Scholar]
- Herde, M.; Howe, G.A. Host Plant-Specific Remodeling of Midgut Physiology in the Generalist Insect Herbivore Trichoplusia ni. Insect Biochem. Mol. Biol. 2014, 50, 58–67. [Google Scholar] [CrossRef]
- Oliveira, C.T.; Kunz, D.; Silva, C.P.; Macedo, M.L.R. Entomotoxic Properties of Dioclea Violacea Lectin and Its Effects on Digestive Enzymes of Anagasta kuehniella (Lepidoptera). J. Insect Physiol. 2015, 81, 81–89. [Google Scholar] [CrossRef]
- Jalali, M.; Hosseininaveh, V.; Imani, S. Inhibitory Activity of Proteinaceous Inhibitors from Cassia Angustifolia and Trigonella Foenum-Graecum Seeds against Plodia interpunctella (Lepidoptera: Pyralidae): Interaction of the Inhibitors and the Insect Digestive Enzymes. Arch. Phytopathol. Plant Prot. 2015, 48, 268–276. [Google Scholar] [CrossRef]
- de Oliveira, L.; Fernandes, K.; Souza Padua, D.; Carvalho, A.O.; Lemos, F.; Gomes, V.; Oliveira, A.; da Silva Ferreira, A.; Perales, J. A Trypsin Inhibitor from Clitoria fairchildiana Cotyledons Is Active Against Digestive Enzymes of Aedes Aegypti Larvae. Protein Pept. Lett. 2015, 22, 893–902. [Google Scholar] [CrossRef]
- Li, W.; Zhao, X.; Yuan, W.; Wu, K. Activities of Digestive Enzymes in the Omnivorous Pest Apolygus lucorum (Hemiptera: Miridae). J. Econ. Entomol. 2017, 110, 101–110. [Google Scholar] [CrossRef]
- Shahriari, M.; Sahebzadeh, N.; Zibaee, A. Effect of Teucrium polium (Lamiaceae) Essential Oil on Digestive Enzyme Activities and Energy Reserves of Ephestia kuehniella (Lepidoptera: Pyralidae). Invertebr. Surviv. J. 2017, 14, 182–189. [Google Scholar]
- Bezzar-Bendjazia, R.; Kilani-Morakchi, S.; Maroua, F.; Aribi, N. Azadirachtin Induced Larval Avoidance and Antifeeding by Disruption of Food Intake and Digestive Enzymes in Drosophila Melanogaster (Diptera: Drosophilidae). Pestic. Biochem. Physiol. 2017, 143, 135–140. [Google Scholar] [CrossRef]
- Meriño-Cabrera, Y.; Zanuncio, J.C.; da Silva, R.S.; Solis-Vargas, M.; Cordeiro, G.; Rainha, F.R.; Campos, W.G.; Picanço, M.C.; de Almeida Oliveira, M.G. Biochemical Response between Insects and Plants: An Investigation of Enzyme Activity in the Digestive System of Leucoptera coffeella (Lepidoptera: Lyonetiidae) and Leaves of Coffea arabica (Rubiaceae) after Herbivory. Ann. Appl. Biol. 2018, 172, 236–243. [Google Scholar] [CrossRef]
- Camaroti, J.R.S.L.; de Almeida, W.A.; do Rego Belmonte, B.; de Oliveira, A.P.S.; de Albuquerque Lima, T.; Ferreira, M.R.A.; Paiva, P.M.G.; Soares, L.A.L.; Pontual, E.V.; Napoleão, T.H. Sitophilus zeamais Adults Have Survival and Nutrition Affected by Schinus terebinthifolius Leaf Extract and Its Lectin (SteLL). Ind. Crops Prod. 2018, 116, 81–89. [Google Scholar] [CrossRef]
- Zou, C.S.; Wang, Y.J.; Zou, H.; Ding, N.; Geng, N.N.; Cao, C.W.; Zhang, G.C. Sanguinarine in Chelidonium majus Induced Antifeeding and Larval Lethality by Suppressing Food Intake and Digestive Enzymes in Lymantria dispar. Pestic. Biochem. Physiol. 2019, 153, 9–16. [Google Scholar] [CrossRef]
- Farhoodi, N.; Kazzazi, M.; Hosseininaveh, V.; Arezi, I. Inhibitory Effect of Proteinaceous Seed Extract of Three Iranian Wheat Cultivars on Eurygaster integriceps (Sunn Pest) Digestive Enzymes. Arch. Phytopathol. Plant Prot. 2019, 52, 1177–1192. [Google Scholar] [CrossRef]
- Cantón, P.E.; Bonning, B.C. Transcription and Activity of Digestive Enzymes of Nezara viridula Maintained on Different Plant Diets. Front. Physiol. 2020, 10, 1553. [Google Scholar] [CrossRef]
- Fathipour, Y.; Kianpour, R.; Bagheri, A.; Karimzadeh, J.; Hosseininaveh, V.; Mehrabadi, M. Targeting Plutella xylostella Digestive Enzymes by Applying Resistant Brassicaceae Host Cultivars. J. Crop Prot. 2020, 9, 65–79. [Google Scholar]
- Zhi, J.; Liu, L.; Hou, X.; Xie, W.; Yue, W.; Zeng, G. Role of Digestive Enzymes in the Adaptation of Frankliniella occidentalis to Preferred and Less-Preferred Host Plants. Entomol. Exp. Appl. 2021, 169, 688–700. [Google Scholar] [CrossRef]
- Abdelgaleil, S.A.M.; Abou-Taleb, H.K.; Al-Nagar, N.M.A.; Shawir, M.S. Antifeedant, Growth Regulatory and Biochemical Effects of Terpenes and Phenylpropenes on Spodoptera Littoralis boisduval. Int. J. Trop. Insect Sci. 2020, 40, 423–433. [Google Scholar] [CrossRef]
- Singh, P.; Jayaramaiah, R.H.; Sarate, P.; Thulasiram, H.V.; Kulkarni, M.J.; Giri, A.P. Insecticidal Potential of Defense Metabolites from Ocimum kilimandscharicum against Helicoverpa armigera. PLoS ONE 2014, 9, e104377. [Google Scholar] [CrossRef]
- Mahajan, E.; Singh, S.; Diksha; Kaur, S.; Sohal, S.K. The Genotoxic, Cytotoxic and Growth Regulatory Effects of Plant Secondary Metabolite β-Caryophyllene on Polyphagous Pest Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae). Toxicon 2022, 219, 106930. [Google Scholar] [CrossRef]
- Mikani, A. Effect of Quercetin on Some Digestive Enzyme Activity via Crustacean Cardioactive Peptide (CCAP) Content of the Midgut of the Diamondback Moth, Plutella xylostella (Lepidoptera: Plutellidae). J. Crop Prot. 2019, 8, 33–43. [Google Scholar]
- War, A.R.; Paulraj, M.G.; Hussain, B.; Buhroo, A.A.; Ignacimuthu, S.; Sharma, H.C. Effect of Plant Secondary Metabolites on Legume Pod Borer, Helicoverpa armigera. J. Pest Sci. (2004) 2013, 86, 399–408. [Google Scholar] [CrossRef]
- Abdelsalam, S.A.; Awad, A.M.A.; Abdelrahman, M.A.A.; Nasser, M.A.K.; Abdelhamid, N.M.R. Antioxidant Defense Response of the Green Peach Aphid, Myzus persicae against Secondary Metabolites of the Host Plants Cumin, Anise, and Coriander. J. Agric. Sci. Technol. 2016, 18, 1583–1592. [Google Scholar]
- Shikano, I. Evolutionary Ecology of Multitrophic Interactions between Plants, Insect Herbivores and Entomopathogens. J. Chem. Ecol. 2017, 43, 586–598. [Google Scholar] [CrossRef]
- Li-, Y.U. Advances in the Adaption to Plant Defenses in Phytophagous Insects. Acta Entomol. Sin. 2019, 62, 124–132. [Google Scholar]
- Kant, M.R.; Jonckheere, W.; Knegt, B.; Lemos, F.; Liu, J.; Schimmel, B.C.J.; Villarroel, C.A.; Ataide, L.M.S.; Dermauw, W.; Glas, J.J.; et al. Mechanisms and Ecological Consequences of Plant Defence Induction and Suppression in Herbivore Communities. Ann. Bot. 2015, 115, 1015–1051. [Google Scholar] [CrossRef]
- Gajger, I.T.; Dar, S.A. Plant Allelochemicals as Sources of Insecticides. Insects 2021, 12, 189. [Google Scholar] [CrossRef]
- Bi, J.L.; Felton, G.W. Foliar Oxidative Stress and Insect Herbivory: Primary Compounds, Secondary Metabolites, and Reactive Oxygen Species as Components of Induced Resistance. J. Chem. Ecol. 1995, 21, 1511–1530. [Google Scholar] [CrossRef]
- Lattanzio, V.; Kroon, P.A.; Quideau, S.; Treutter, D. Plant Phenolics—Secondary Metabolites with Diverse Functions. In Recent Advances in Polyphenol Research; Wiley: Hoboken, NJ, USA, 2008; Volume 1, pp. 1–35. ISBN 9781444302400. [Google Scholar]
- Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K.; et al. Plant Secondary Metabolites as Defense Tools against Herbivores for Sustainable Crop Protection. Int. J. Mol. Sci. 2022, 23, 2690. [Google Scholar] [CrossRef]
- Theis, N.; Lerdau, M. The Evolution of Function in Plant Secondary Metabolites. Int. J. Plant Sci. 2003, 164, S93–S102. [Google Scholar] [CrossRef]
- Sun, L.; Hou, W.; Zhang, J.; Dang, Y.; Yang, Q.; Zhao, X.; Ma, Y.; Tang, Q. Plant Metabolites Drive Different Responses in Caterpillars of Two Closely Related Helicoverpa Species. Front. Physiol. 2021, 12, 662978. [Google Scholar] [CrossRef]
- Jiang, D.; Wu, S.; Tan, M.; Wang, Q.; Zheng, L.; Yan, S. chun The High Adaptability of Hyphantria cunea Larvae to Cinnamic Acid Involves in Detoxification, Antioxidation and Gut Microbiota Response. Pestic. Biochem. Physiol. 2021, 174, 104805. [Google Scholar] [CrossRef]
- Goggin, F.L.; Fischer, H.D. Reactive Oxygen Species in Plant Interactions with Aphids. Front. Plant Sci. 2022, 12, 811105. [Google Scholar]
- Premachandran, K.; Srinivasan, T.S.; Alphonse, C.R.W. In Silico Approach on Sequential and Structural Variability in Oryzacystatin and Its Interaction with Cysteine Protease Enzymes of Insect. Phytochemistry 2021, 186, 112728. [Google Scholar]
- Ishii, K.; Hamamoto, H.; Kamimura, M.; Sekimizu, K. Activation of the Silkworm Cytokine by Bacterial and Fungal Cell Wall Components via a Reactive Oxygen Species-Triggered Mechanism. J. Biol. Chem. 2008, 283, 2185–2191. [Google Scholar]
- Subramanyam, S.; Nemacheck, J.A.; Suetsugu, T.E.; Flynn, R.D.; Faik, A. Genetic and Phenotypic Responses of Temperature-Independent Hessian Fly-Resistant Durum Wheat to Larval Attack during Heat Stress. BMC Plant Biol. 2025, 25, 210. [Google Scholar]
- Ahmad, S.; Duval, D.L.; Weinhold, L.C.; Pardini, R.S. Cabbage Looper Antioxidant Enzymes: Tissue Specificity. Insect Biochem. 1991, 21, 563–572. [Google Scholar] [CrossRef]
- Aucoin, R.R.; Philogène, B.J.R.; Arnason, J.T. Antioxidant Enzymes as Biochemical Defenses against Phototoxin-induced Oxidative Stress in Three Species of Herbivorous Lepidoptera. Arch. Insect Biochem. Physiol. 1991, 16, 139–152. [Google Scholar] [CrossRef]
- Zaman, K.; MacGill, R.S.; Johnson, J.E.; Ahmad, S.; Pardini, R.S. An Insect Model for Assessing Mercury Toxicity: Effect of Mercury on Antioxidant Enzyme Activities of the Housefly (Musca domestica) and the Cabbage Looper Moth (Trichoplusia ni). Arch. Environ. Contam. Toxicol. 1994, 26, 114–118. [Google Scholar] [CrossRef]
- Wang, Y.; Oberley, L.W.; Murhammer, D.W. Antioxidant Defense Systems of Two Lipidopteran Insect Cell Lines. Free Radic. Biol. Med. 2001, 30, 1254–1262. [Google Scholar] [CrossRef]
- Barbehenn, R.V. Gut-Based Antioxidant Enzymes in a Polyphagous and a Graminivorous Grasshopper. J. Chem. Ecol. 2002, 28, 1329–1347. [Google Scholar] [CrossRef]
- Cervera, A.; Maymó, A.C.; Martínez-Pardo, R.; Garcerá, M.D. Antioxidant Enzymes in Oncopeltus fasciatus (Heteroptera: Lygaeidae) Exposed to Cadmium. Environ. Entomol. 2003, 32, 705–710. [Google Scholar] [CrossRef]
- Lijun, L.; Xuemei, L.; Yaping, G.; Enbo, M. Activity of the Enzymes of the Antioxidative System in Cadmium-Treated Oxya Chinensis (Orthoptera Acridoidae). Environ. Toxicol. Pharmacol. 2005, 20, 412–416. [Google Scholar] [CrossRef]
- Krishnan, N.; Kodrík, D. Antioxidant Enzymes in Spodoptera littoralis (Boisduval): Are They Enhanced to Protect Gut Tissues during Oxidative Stress? J. Insect Physiol. 2006, 52, 11–20. [Google Scholar] [CrossRef]
- Mittapalli, O.; Neal, J.J.; Shukle, R.H. Antioxidant Defense Response in a Galling Insect. Proc. Natl. Acad. Sci. USA 2007, 104, 1889–1894. [Google Scholar] [CrossRef]
- Hyršl, P.; Büyükgüzel, E.; Büyükgüzel, K. The Effects of Boric Acid-Induced Oxidative Stress on Antioxidant Enzymes and Survivorship in Galleria mellonella. Arch. Insect Biochem. Physiol. 2007, 66, 23–31. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Zhu, Z.; Ma, W.; Lei, C. The Molecular Characterization of Antioxidant Enzyme Genes in Helicoverpa armigera Adults and Their Involvement in Response to Ultraviolet-A Stress. J. Insect Physiol. 2012, 58, 1250–1258. [Google Scholar] [CrossRef]
- Büyükgüzel, E.; Büyükgüzel, K.; Snela, M.; Erdem, M.; Radtke, K.; Ziemnicki, K.; Adamski, Z. Effect of Boric Acid on Antioxidant Enzyme Activity, Lipid Peroxidation, and Ultrastructure of Midgut and Fat Body of Galleria mellonella. Cell Biol. Toxicol. 2013, 29, 117–129. [Google Scholar] [CrossRef]
- Jena, K.; Kumar Kar, P.; Kausar, Z.; Babu, C.S. Effects of Temperature on Modulation of Oxidative Stress and Antioxidant Defenses in Testes of Tropical Tasar Silkworm Antheraea mylitta. J. Therm. Biol. 2013, 38, 199–204. [Google Scholar] [CrossRef]
- Büyükgüzel, E.; Kalender, Y. Penicillin-Induced Oxidative Stress: Effects on Antioxidative Response of Midgut Tissues in Instars of Galleria mellonella. J. Econ. Entomol. 2014, 100, 1533–1541. [Google Scholar]
- Dere, B.; Altuntaş, H.; Nurullahoğlu, Z.U. Insecticidal and Oxidative Effects of Azadirachtin on the Model Organism Galleria mellonella L. (Lepidoptera: Pyralidae). Arch. Insect Biochem. Physiol. 2015, 89, 138–152. [Google Scholar] [CrossRef]
- Wu, G.; Yi, Y. Effects of Dietary Heavy Metals on the Immune and Antioxidant Systems of Galleria mellonella Larvae. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2015, 167, 131–139. [Google Scholar] [CrossRef]
- Li, X.; Liu, Q.; Lewis, E.E.; Tarasco, E. Activity Changes of Antioxidant and Detoxifying Enzymes in Tenebrio Molitor (Coleoptera: Tenebrionidae) Larvae Infected by the Entomopathogenic Nematode Heterorhabditis beicherriana (Rhabditida: Heterorhabditidae). Parasitol. Res. 2016, 115, 4485–4494. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Rashid, M.A.; Huang, Q.Y.; Wong, C.; Lei, C.-L. Response of Antioxidant Enzymes in Mythimna separata (Lepidoptera: Noctuidae) Exposed to Thermal Stress. Bull. Entomol. Res. 2017, 107, 382–390. [Google Scholar]
- Ali, A.; Rashid, M.A.; Huang, Q.Y.; Lei, C.L. Influence of UV-A Radiation on Oxidative Stress and Antioxidant Enzymes in Mythimna separata (Lepidoptera: Noctuidae). Environ. Sci. Pollut. Res. 2017, 24, 8392–8398. [Google Scholar] [CrossRef]
- Dixit, G.; Praveen, A.; Tripathi, T.; Yadav, V.K.; Verma, P.C. Herbivore-Responsive Cotton Phenolics and Their Impact on Insect Performance and Biochemistry. J. Asia. Pac. Entomol. 2017, 20, 341–351. [Google Scholar] [CrossRef]
- Karthi, S.; Vaideki, K.; Shivakumar, M.S.; Ponsankar, A.; Thanigaivel, A.; Chellappandian, M.; Vasantha-Srinivasan, P.; Muthu-Pandian, C.K.; Hunter, W.B.; Senthil-Nathan, S. Effect of Aspergillus Flavus on the Mortality and Activity of Antioxidant Enzymes of Spodoptera litura Fab. (Lepidoptera: Noctuidae) Larvae. Pestic. Biochem. Physiol. 2018, 149, 54–60. [Google Scholar] [CrossRef]
- Islam, S.J.; Manna, P.; Kailta, J.; Unni, B. Higher Concentrations of Heavy Metals Impair Antioxidant Defense Mechanism and Growth Response of Muga Silkworm, Antheraea assamensis (Lepidoptera: Saturniidae). J. Entomol. Zool. Stud. 2019, 7, 715–724. [Google Scholar]
- Ramadan, R.; Abdel-Meguid, A.; Emara, M. Effects of Synthesized Silver and Chitosan Nanoparticles Using Nerium Oleander and Aloe Vera on Antioxidant Enzymes in Musca domestica. Catrina Int. J. Environ. Sci. 2020, 21, 9–14. [Google Scholar] [CrossRef]
- Miao, Z.Q.; Tu, Y.Q.; Guo, P.Y.; He, W.; Jing, T.X.; Wang, J.J.; Wei, D.D. Antioxidant Enzymes and Heat Shock Protein Genes from Liposcelis bostrychophila Are Involved in Stress Defense upon Heat Shock. Insects 2020, 11, 839. [Google Scholar] [CrossRef]
- Farahani, S.; Bandani, A.R.; Alizadeh, H.; Goldansaz, S.H.; Whyard, S. Differential Expression of Heat Shock Proteins and Antioxidant Enzymes in Response to Temperature, Starvation, and Parasitism in the Carob Moth Larvae, Ectomyelois ceratoniae (Lepidoptera: Pyralidae). PLoS ONE 2020, 15, e0228104. [Google Scholar]
- Li, Z.; Hou, M.; Qiu, Y.; Zhao, B.; Nie, H.; Su, S. Changes in Antioxidant Enzymes Activity and Metabolomic Profiles in the Guts of Honey Bee (Apis mellifera) Larvae Infected with Ascosphaera apis. Insects 2020, 11, 419. [Google Scholar] [CrossRef]
- Muhammad, A.; He, J.; Yu, T.; Sun, C.; Shi, D.; Jiang, Y.; Xianyu, Y.; Shao, Y. Dietary Exposure of Copper and Zinc Oxides Nanoparticles Affect the Fitness, Enzyme Activity, and Microbial Community of the Model Insect, Silkworm Bombyx Mori. Sci. Total Environ. 2022, 813, 152608. [Google Scholar] [CrossRef]
- Janković-Tomanić, M.; Petković, B.; Vranković, J.S.; Perić-Mataruga, V. Effects of High Doses of Zearalenone on Some Antioxidant Enzymes and Locomotion of Tenebrio molitor Larvae (Coleoptera: Tenebrionidae). J. Insect Sci. 2024, 24, 6. [Google Scholar]
- Ma, T.; Ding, Y.; Xu, F.; Zhang, C.; Zhou, M.; Tang, Y.; Chen, Y.; Wen, Y.; Chen, R.; Tang, B. Effects of Acute and Chronic Chromium Stress on the Expression of Heat Shock Protein Genes and Activities of Antioxidant Enzymes in Larvae of Orthetrum albistylum. Environ. Pollut. 2024, 340, 122712. [Google Scholar]
- de Sousa, G.; dos Santos, V.C.; de Figueiredo Gontijo, N.; Constantino, R.; de Oliveira Paiva e Silva, G.; Bahia, A.C.; Gomes, F.M.; de Alcantara Machado, E. Morphophysiological Study of Digestive System Litter-Feeding Termite Cornitermes cumulans (Kollar, 1832). Cell Tissue Res. 2017, 368, 579–590. [Google Scholar] [CrossRef]
- Sousa, G.; Gandara, A.C.P.; Oliveira, P.L.; Gomes, F.M.; Bahia, A.C.; Machado, E.A. The Relationship between Oxidant Levels and Gut Physiology in a Litter-Feeding Termite. Sci. Rep. 2019, 9, 670. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Li, J.; Liu, M.; Liu, Z. Midgut Transcriptome of the Cockroach Periplaneta americana and Its Microbiota: Digestion, Detoxification and Oxidative Stress Response. PLoS ONE 2016, 11, e0155254. [Google Scholar] [CrossRef]
- Wu, K.; Zhang, J.; Zhang, Q.; Zhu, S.; Shao, Q.; Clark, K.D.; Liu, Y.; Ling, E. Plant Phenolics Are Detoxified by Prophenoloxidase in the Insect Gut. Sci. Rep. 2015, 5, 16823. [Google Scholar] [CrossRef]
- Latha, B.; Ramakrishnan, M.; Jayaraman, V.; Babu, M. The Efficacy of Trypsin: Chymotrypsin Preparation in the Reduction of Oxidative Damage during Burn Injury. Burns 1998, 24, 532–538. [Google Scholar] [CrossRef]
- Patlevič, P.; Vašková, J.; Švorc, P.; Vaško, L.; Švorc, P. Reactive Oxygen Species and Antioxidant Defense in Human Gastrointestinal Diseases. Integr. Med. Res. 2016, 5, 250–258. [Google Scholar] [CrossRef]
- Stygar, D.; Dolezych, B.; Nakonieczny, M.; Migula, P.; Michalczyk, K.; Zaak, M. Digestive Enzymes Activity in Larvae of Cameraria ohridella (Lepidoptera: Gracillariidae). Comptes Rendus Biol. 2010, 333, 725–735. [Google Scholar] [CrossRef]
- Skowronek, P.; Strachecka, A. Cannabidiol (CBD) Supports the Honeybee Worker Organism by Activating the Antioxidant System. Antioxidants 2023, 12, 279. [Google Scholar] [CrossRef]
- Khochapong, W.; Ketnawa, S.; Ogawa, Y.; Punbusayakul, N. Effect of in Vitro Digestion on Bioactive Compounds, Antioxidant and Antimicrobial Activities of Coffee (Coffea arabica L.) Pulp Aqueous Extract. Food Chem. 2021, 348, 129094. [Google Scholar] [CrossRef] [PubMed]
- Terra, W.R.; Ferreira, C.; Silva, C.P. Mechanisms of Avoiding the Action of Plant Inhibitors on Digestion. In Molecular Physiology and Evolution of Insect Digestive Systems; Springer: Berlin/Heidelberg, Germany, 2023; pp. 165–184. [Google Scholar]
- Mumm, R.; Burow, M.; Bukovinszkine’Kiss, G.; Kazantzidou, E.; Wittstock, U.; Dicke, M.; Gershenzon, J. Formation of Simple Nitriles upon Glucosinolate Hydrolysis Affects Direct and Indirect Defense against the Specialist Herbivore, Pieris rapae. J. Chem. Ecol. 2008, 34, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J.; Nikmaram, N.; Roohinejad, S.; Khelfa, A.; Zhu, Z.; Koubaa, M. Bioavailability of Glucosinolates and Their Breakdown Products: Impact of Processing. Front. Nutr. 2016, 3, 24. [Google Scholar] [CrossRef]
- Chen, W.; Dong, Y.; Saqib, H.S.A.; Vasseur, L.; Zhou, W.; Zheng, L.; Lai, Y.; Ma, X.; Lin, L.; Xu, X. Functions of Duplicated Glucosinolate Sulfatases in the Development and Host Adaptation of Plutella xylostella. Insect Biochem. Mol. Biol. 2020, 119, 103316. [Google Scholar] [CrossRef] [PubMed]
- Appel, H.M.; Martin, M.M. Significance of Metabolic Load in the Evolution of Host Specificity of Manduca Sexta. Ecology 1992, 73, 216–228. [Google Scholar] [CrossRef]
- Li, X.; Berenbaum, M.R.; Schuler, M.A. Plant Allelochemicals Differentially Regulate Helicoverpa zea Cytochrome P450 Genes. Insect Mol. Biol. 2002, 11, 343–351. [Google Scholar]
- Housecroft, C.E. Tolerating Toxins: Grasshoppers That Feast on Pyrrolizidine Alkaloids §: Chemical Education. Chimia 2018, 72, 156. [Google Scholar] [CrossRef]
- Jia, S.; Li, Y.; Dai, X.; Li, X.; Zhou, Y.; Xu, Y.; Wang, H. Physiological Adaptations to Sugar-mimic Alkaloids: Insights from Bombyx Mori for Long-term Adaption and Short-term Response. Ecol. Evol. 2020, 10, 9682–9695. [Google Scholar] [CrossRef]
- Mackay-Smith, A.; Dornon, M.K.; Lucier, R.; Okimoto, A.; Mendonca de Sousa, F.; Rodriguero, M.; Confalonieri, V.; Lanteri, A.A.; Sequeira, A.S. Host-Specific Gene Expression as a Tool for Introduction Success in Naupactus Parthenogenetic Weevils. PLoS ONE 2021, 16, e0248202. [Google Scholar] [CrossRef]
- Li, X.; Shi, L.; Dai, X.; Chen, Y.; Xie, H.; Feng, M.; Chen, Y.; Wang, H. Expression Plasticity and Evolutionary Changes Extensively Shape the Sugar-mimic Alkaloid Adaptation of Nondigestive Glucosidase in Lepidopteran Mulberry-specialist Insects. Mol. Ecol. 2018, 27, 2858–2870. [Google Scholar] [PubMed]
- Zhang, N.; Qian, Z.; He, J.; Shen, X.; Lei, X.; Sun, C.; Fan, J.; Felton, G.W.; Shao, Y. Gut Bacteria of Lepidopteran Herbivores Facilitate Digestion of Plant Toxins. Proc. Natl. Acad. Sci. USA 2024, 121, e2412165121. [Google Scholar] [CrossRef]
- Dayé, C.; Spök, A.; Allan, A.C.; Yamaguchi, T.; Sprink, T. Social Acceptability of Cisgenic Plants: Public Perception, Consumer Preferences, and Legal Regulation. In Cisgenic Crops: Safety, Legal and Social Issues; Springer: Berlin/Heidelberg, Germany, 2023; pp. 43–75. [Google Scholar]
- Zu, P.J.; García-García, R.; Schuman, M.C.; Saavedra, S.; Melián, C.J. Plant–Insect Chemical Communication in Ecological Communities: An Information Theory Perspective. J. Syst. Evol. 2023, 61, 445–453. [Google Scholar] [CrossRef]
- Utsumi, S. Eco-Evolutionary Dynamics in Herbivorous Insect Communities Mediated by Induced Plant Responses. Popul. Ecol. 2011, 53, 23–34. [Google Scholar] [CrossRef]
- Zhou, S.; Jander, G. Engineering Insect Resistance Using Plant Specialized Metabolites. Curr. Opin. Biotechnol. 2021, 70, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Zeier, J. Metabolic Regulation of Systemic Acquired Resistance. Curr. Opin. Plant Biol. 2021, 62, 102050. [Google Scholar] [CrossRef]
- Desmedt, W.; Jonckheere, W.; Nguyen, V.H.; Ameye, M.; De Zutter, N.; De Kock, K.; Debode, J.; Van Leeuwen, T.; Audenaert, K.; Vanholme, B.; et al. The Phenylpropanoid Pathway Inhibitor Piperonylic Acid Induces Broad-Spectrum Pest and Disease Resistance in Plants. Plant Cell Environ. 2021, 44, 3122–3139. [Google Scholar] [CrossRef]

| Researcher | Plant | Plant Utilization Form | Insect | Insect Stage | Investigated Enzymes |
|---|---|---|---|---|---|
| Mehrabadi et al. [70] | Punica granatum L. (Punicaceae) Rheum officinale Baill (Polygonaceae) Rhus coriaria L. (Anacardiaceae) Artemisia sieberi B. (Compositae) Peganum harmala L. (Nitrariaceae) Datura stramonium L. (Solanaceae) Thymus vulgaris L. (Lamiaceae) | Extract | Callosobruchus maculatus F. (Coleoptera: Bruchidae) Rhyzopertha dominica F. (Coleoptera: Bostrichidae) Sitophilus granarius L. (Coleoptera: Curculionidae) Trogoderma granarium E. (Coleoptera: Dermestidae). | Last larval instar | α-amylase |
| Sarate et al. [71] | Chickpea, pigeonpea Tomato, okra Rose, marigold Sorghum, maize | Herbal diet | Helicoverpa armigera | Larve pupa | Amylases Proteases Lipases |
| Napoleão et al. [72] | Myracrodruon urundeuva M.Allemão | Leaf extract and lectin | Sitophilus zeamais Motschulsky | adults | Protease Trypsin-Like Acid Phosphatase Amylase |
| Senthil-Nathan [12] | Meliaceae | Secondary metabolites | Pyralidae, Nuctoidae | (a review) | α-Amylases α and β-glucosidases Lipases Proteases Serine, Cysteine, And Aspartic Proteinases General Esterases (EST) Glutathione S-Transferase (GST) Phosphatases Alkaline Phosphatase Acid Phosphatase |
| Dastranj et al. [73] | wheat cultivars | Seed extract | Tenebrio molitor L. | All larval stages Adult | α-amylases Proteases |
| Sami [74] | Azadirachta indica A.Juss | Azadirachtin and Saponin | Tribolium castaneum Herbst Aulacophora foveicollis Lucas Oxya chinensis Thunberg | Larve Adult | α -Amylases |
| Herde and Howe [75] | Arabidopsis thaliana L. Solanum lycopersicum L. | As a host plants | Trichoplusia ni Hübner | Larve | Phase I and II detoxification enzymes Proteinase Serine Proteases Lipase |
| Oliveira et al. [76] | Dioclea violacea Mart. ex Benth. | Lectin (DVL) | Anagasta kuehniella Zeller | Larve | Trypsin-Like Chymotrypsin-Like α-amylase Proteases |
| Jalali et al. [77] | Cassia angustifolia Mill. Trigonella foenum-graecum L. | Proteinaceous extracts | Plodia interpunctella Hübner (Lep.: Pyralidae) | Larve | trypsin |
| de Oliveira et al. [78] | Clitoria fairchildiana R. A. Howard | trypsin inhibitor derived from the cotyledons | Aedes aegypti Linnaeus in Hasselquist | Larve | Trypsin-Like A-Amylase Serine Proteases Elastase Chymotrypsin Subtilisin |
| Li et al. [79] | green bean pods (Gb) | green bean pods and H. armigera eggs | Apolygus lucorum Meyer-Dür | Adults | Amylase Protease |
| Shahriari et al. [80] | Teucrium polium L. | essential oil | Ephestia kuehniella Z. (Lep.: Pyralidae) | Larve | α-amylase Triacylglycerol Lipase General Protease Serine Proteases (Trypsin and Chymotrypsin-Like) Carboxy- And Aminopeptidases |
| Bezzar-Bendjazia et al. [81] | (Meliaceae) Azadirachta indica | Azadirachtin | Drosophila melanogaster Meigen | Third instars larvae | α-amylase Chitinase Protease Lipase |
| Meriño-Cabrera et al. [82] | Coffea arabica L. | Leaf extract | Leucoptera coffeella Guérin-Méneville (Lepidoptera: Lyonetiidae) | fourth instar larvae | Trypsin Chymotrypsin Cysteine proteases Total protease |
| Camaroti et al. [83] | Schinus terebinthifolius Raddi | saline leaf extract Lectin | Sitophilus zeamais Motsch. (maize weevil) | Adults | Protease Amylase |
| Zou et al. [84] | Chelidonium majus L. | alkaloid | Lymantria dispar | Third instar larvae | α-amylase Lipase Total Protease |
| Farhoodi et al. [85] | Iranian wheat cultivars | Seed proteinaceous extracts | Eurygaster integriceps Puton | Adults | α-amylase α-glucosidase β-glucosidase proteolytic activities |
| Cantón and Bonning [86] | corn green bean | Protein Extracts | Nezara viridula L. | Second instar nymph | Protease Nuclease Serine Proteases |
| Fathipour et al. [87] | Brassicaceae two canola cultivars two cabbage cultivars | Host plant | Plutella xylostella (L.) (Lepidoptera: Plutellidae) | last-larval instars | proteolytic and amylolytic activities α-glucosidase and β-glucosidases |
| Zhi et al. [88] | kidney bean | Host plant | Frankliniella occidentalis (Pergande) | nymph and adult | α-amylase trypsin tryptase |
| Researcher | Issue | Pest | Investigated Enzymes |
|---|---|---|---|
| Ahmad et al. [109] | Antioxidant enzyme activity is elevated in high-metabolism tissues like Malpighian tubules, hindgut, muscles, and gonads. | Trichoplusia ni | superoxide dismutase (SOD) glutathione peroxidase (GPOX) Glutathione-S-transferase (GST) glutathione reductase (GR) |
| Aucoin et al. [110] | biochemical defenses, against oxidative stress from phototoxins | Ostrinia nubilalis Hübner Manduca sexta L., Anaitis plagiata L. | (SOD) catalase (CAT) (GPOX) (GR). |
| [111] | effect of mercury as Hg2Cl2 and HgCl2 | Musca domestica L. Trichoplusia ni | SOD CAT GST peroxidase |
| Wang et al. [112] | Investigation of antioxidant enzyme activities in insect cell lines | Spodoptera frugiperda Smith Trichoplusia ni | ascorbate peroxidase (APOX) MnSOD CuZnSOD (CAT) GR dehydroascorbic acid reductase |
| Barbehenn [113] | Investigation of Gut-based antioxidant | Melanoplus sanguinipes Fabricius Aulocara elliotti Thomas | (SOD) (CAT) (APOX) glutathione transferase peroxidase (GSTPX) |
| Cervera et al. [114] | Investigation of cadmium toxicity | Oncopeltus fasciatus (Dallas) | (CAT) (GR) (GST) thiobarbituric acid reactive substances (TBARS) |
| Lijun et al. [115] | The effect of different concentrations of cadmium (Cd2+) | Oxya chinensis Thunberg (Orthoptera: Acridoidae) | (SOD) (CAT) guaiacol peroxidase (GPx) |
| Krishnan and Kodrík [116] | The effect of host plant and artificial diet on enzyme activity | Spodoptera littoralis | (SOD) (CAT) (APOX) (GSTpx) |
| Mittapalli et al. [117] | Insect antioxidant activity in interaction with host plant | Mayetiola destructor | phospholipid glutathione peroxidases (MdesPHGPX-1 and MdesPHGPX-2) catalases (MdesCAT-1 and MdesCAT-2) (MdesSOD-1 and MdesSOD-2) |
| Hyršl et al. [118] | boric acid-induced oxidative stress | Galleria mellonella (L.) | (SOD) (CAT) (GST) (GPx) |
| Wang et al. [119] | ultraviolet-A stress | Helicoverpa armigera | (Cu/ZnSOD) (CAT) (GPX) |
| Büyükgüzel et al. [120] | Effect of boric acid on antioxidant enzyme activity | Galleria mellonella L. | CAT SOD GST GP |
| Büyükgüzel et al. [120] | Effect of cadmium | Galleria mellonella | lipid peroxidation (MDA) (SOD) (CAT) |
| Jena et al. [121] | Effect of High temperatures | Antheraea mylitta L. | (SOD) (CAT) (GST) ascorbic acid (ASA) |
| Büyükgüzel and Kalender [122] | Penicillin-Induced Oxidative Stress | Galleria mellonella | [SOD] [CAT] [GST] [GPx] |
| Dere et al. [123] | Effect of azadirachtin | Galleria mellonella (Lepidoptera: Pyralidae) | GST |
| Wu and Yi [124] | effects of chromium (Cr) and lead (Pb) | Galleria mellonella | phenoloxidase, PO |
| Li et al. [125] | Effect of Entomopathogenic nematodes (EPNs) Steinernema and Heterorhabditis | Tenebrio molitor L. | (SODs) peroxidases (PODs) (CATs) tyrosinase (TYR) acetylcholinesterase (AChE) carboxylesterase (CarE) (GSTs) |
| Ali et al. [126] | Thermal stress | Mythimna separata Walker (Lepidoptera: Noctuidae) | (SOD) (CAT) (POX) (GSTs) total antioxidant capacity (T-AOC) |
| Ali et al. [127] | Influence of UV-A radiation | Mythimna separata (Lepidoptera: Noctuidae) | SOD CAT POX GST |
| Dixit et al. [128] | Effect of cotton phenolics | Helicoverpa armigera Spodoptera litura | lactate dehydrogenase GST |
| Karthi et al. [129] | Effect of Aspergillus flavus | Spodoptera litura | Phenoloxidase CAT POX SOD Lipid peroxidase Acid phosphatase Alkaline phosphatase |
| Islam et al. [130] | heavy metals stress (Cd, Pb, Mn, and Zn) | Antheraea assamensis Helfer (Lepidoptera: Saturniidae) | GST SOD CAT |
| Ramadan et al. [131] | Effects of synthesized silver and chitosan nanoparticles | Musca domestica | SOD CAT GPx GST |
| Miao et al. [132] | Heat shock | Liposcelis bostrychophila Badonnel | (SOD) (CAT) (POD) (GST) (MDA) |
| Farahani et al. [133] | impact of temperature extremes, starvation, and parasitism by Habroacon hebetor wasps. | Ectomyelois ceratoniae Zeller | (SOD) (CAT) |
| Li et al. [134] | Infection by the fungus Ascosphaera apis | Apis mellifera L. | SOD CAT GST |
| Muhammad et al. [135] | biological impacts linked to dietary exposure to CuO and ZnO nanoparticles | Bombyx mori | SOD GST CAT |
| Chamani et al. [8] | Effect of Zn, Cu, and Fe nanoparticles and Urea | Schizaphis graminum Rondani | SOD CAT POX |
| Janković-Tomanić et al. [136] | Impact of elevated levels of zearalenone | Tenebrio molitor L. (Coleoptera: Tenebrionidae) | (SOD) (GST) |
| Ma et al. [137] | Impacts of acute and chronic exposure to chromium stress | Orthetrum albistylum Selys | SOD CAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamani, M.; Dadpour, M.; Dehghanian, Z.; Panahirad, S.; Chenari Bouket, A.; Oszako, T.; Kumar, S. From Digestion to Detoxification: Exploring Plant Metabolite Impacts on Insect Enzyme Systems for Enhanced Pest Control. Insects 2025, 16, 392. https://doi.org/10.3390/insects16040392
Chamani M, Dadpour M, Dehghanian Z, Panahirad S, Chenari Bouket A, Oszako T, Kumar S. From Digestion to Detoxification: Exploring Plant Metabolite Impacts on Insect Enzyme Systems for Enhanced Pest Control. Insects. 2025; 16(4):392. https://doi.org/10.3390/insects16040392
Chicago/Turabian StyleChamani, Masoud, MohammadReza Dadpour, Zahra Dehghanian, Sima Panahirad, Ali Chenari Bouket, Tomasz Oszako, and Sumit Kumar. 2025. "From Digestion to Detoxification: Exploring Plant Metabolite Impacts on Insect Enzyme Systems for Enhanced Pest Control" Insects 16, no. 4: 392. https://doi.org/10.3390/insects16040392
APA StyleChamani, M., Dadpour, M., Dehghanian, Z., Panahirad, S., Chenari Bouket, A., Oszako, T., & Kumar, S. (2025). From Digestion to Detoxification: Exploring Plant Metabolite Impacts on Insect Enzyme Systems for Enhanced Pest Control. Insects, 16(4), 392. https://doi.org/10.3390/insects16040392










