Vitellogenesis and Embryogenesis in Spiders: A Biochemical Perspective
Simple Summary
Abstract
1. Introduction
2. Vitellogenesis in Spiders
2.1. Vitellogenins and Lipovitellins: Protein Composition
2.2. Lipid Composition and Yolk
| LV1 | LV2 | Egg | References | |
|---|---|---|---|---|
| P. pythagoricus (spider) | TAG: 8 CHOL: 8 ES: 16.6 PE: 25.4 PC: 23.8 | TAG: 9.5 CHOL: 3.3 ES: 24.2 PE: 20.1 PC: 17.5 | TAG: 22.9 PE: 48 PC: 22 | [57,110] |
| S. malitiosa (spider) | TAG: 3.1 PE: 16.9 PC: 5.4 SM + LPC: 72.6 | TAG: 0.4 PE: 3.6 PC: 0.8 SM + LPC: 98.1 | TAG: 25.7 PE: 15.0 PC: 32.6 SM + LPC: 18.9 | [55] |
| P. saltans (spider) | TAG: 45.9 PE: 3 PC: 28.4 LPC:5.5 | [54] | ||
| Alpheus saxidomus (crustacean) | TAG: 51.1 PL: 48.9 | [118] | ||
| Palaemonetes schmitti (crustacean) | TAG: 36.1 PL: 63.6 | [118] | ||
| Macrobrachium borellii (crustacean) | TAG: 20.5 PC: 41.9 PE: 15.8 | TAG: 55.4 PE: 13.2 PC: 14.7 | [117,119] | |
| Locusta migratoria (insect) | TAG: 0.4 PC: 62.1 PE: 21.8 | [120] |
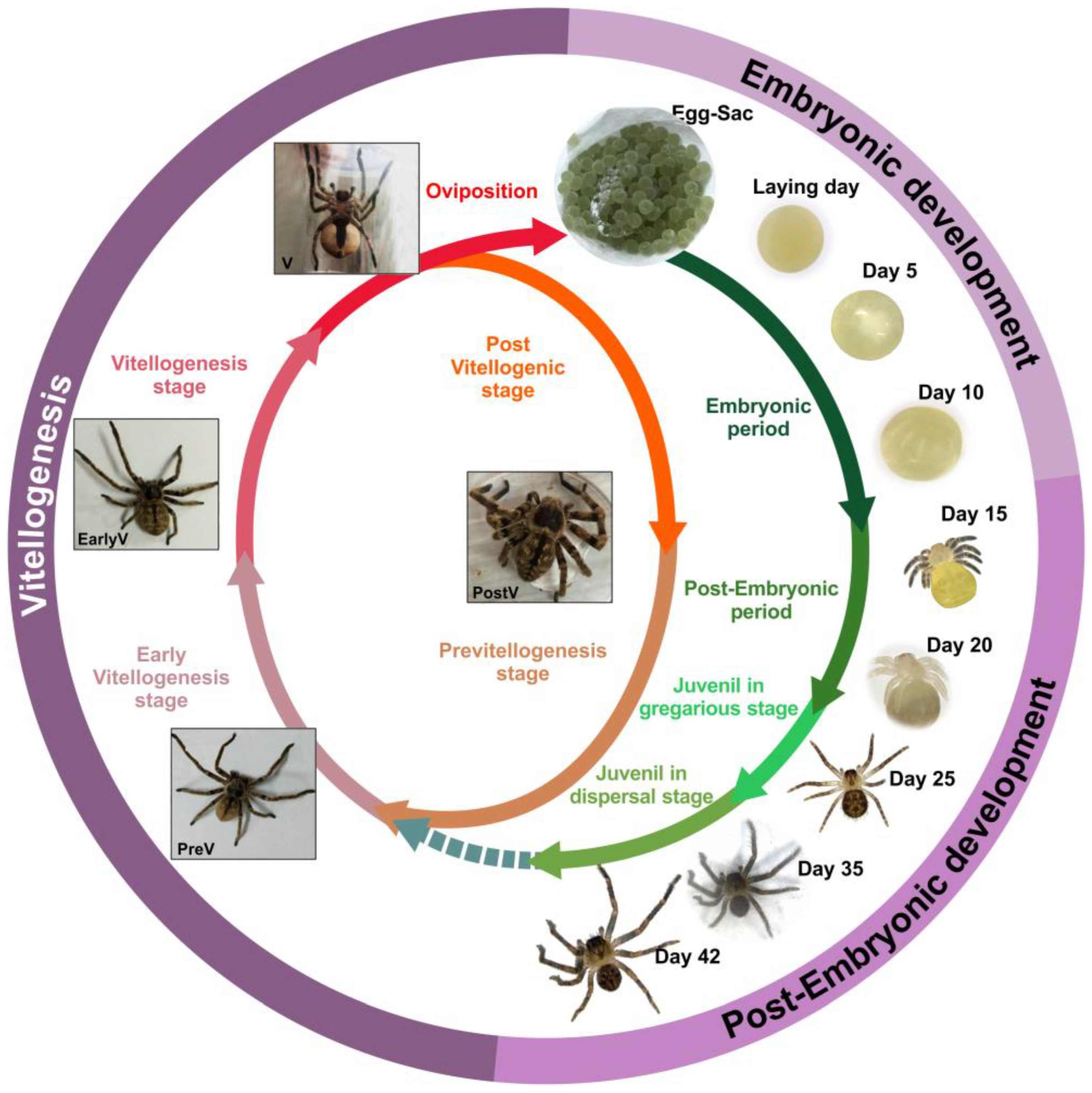
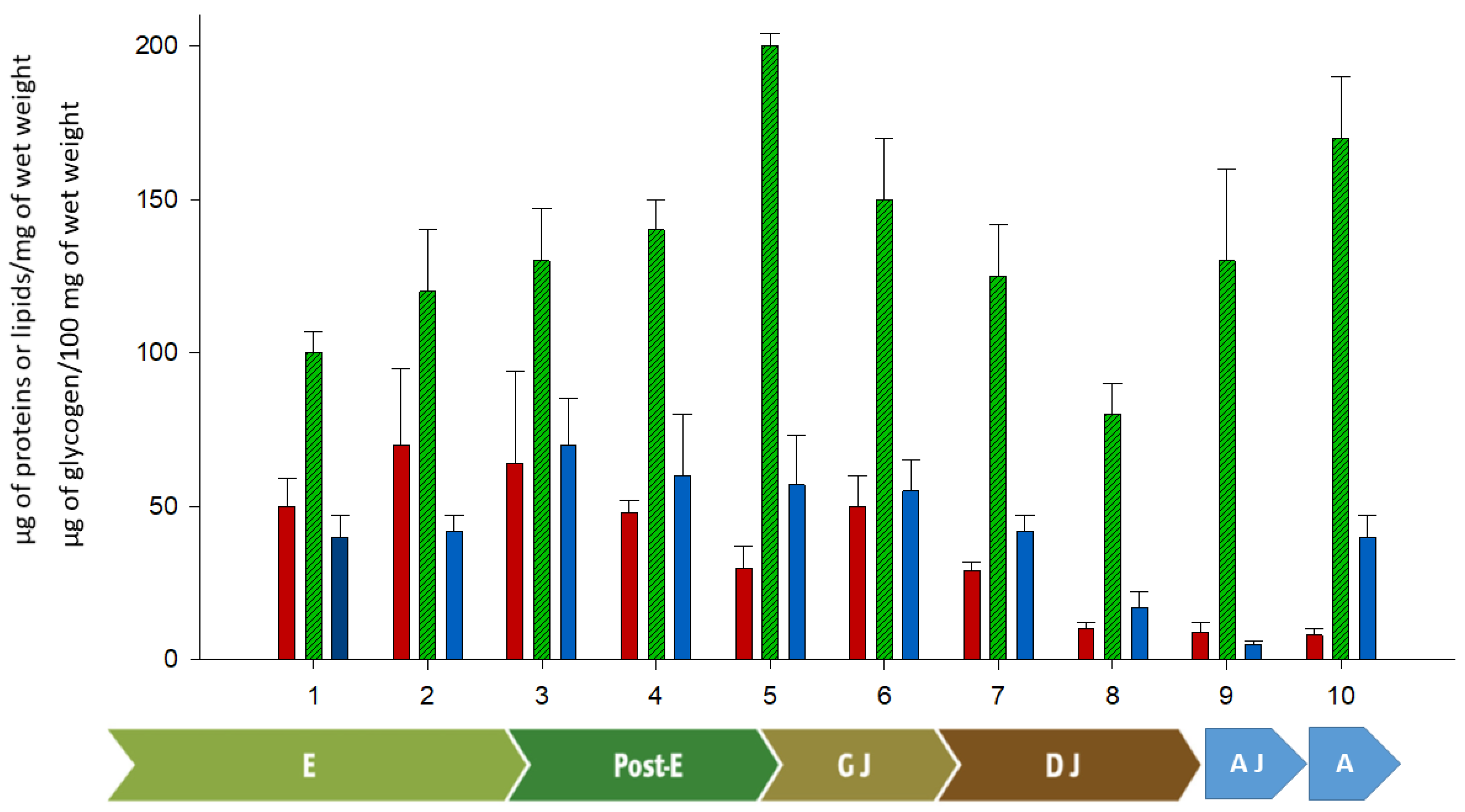
3. Embryonic, Post-Embryonic Development, and Yolk Consumption
4. General Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- World Spider Catalog. Version 26. Natural History Museum Bern. 2025. Available online: http://wsc.nmbe.ch (accessed on 14 February 2025).
- Galiano, M.E. Datos adicionales sobre el ciclo vital de Polybetes pythagoricus (Holmberg, 1874) (Araneae, Eusparassidae). Acta Zool. Lilloana 1979, 35, 75–86. [Google Scholar]
- Robinson, M.H. Courtship and mating behavior in spiders. Ann. Rev. Entomol. 1982, 27, 1–20. [Google Scholar] [CrossRef]
- Costa, F.G.; Pérez-Miles, F. Reproductive biology of uruguayan theraphosids (Araneae, Mygalomorphae). J. Arachnol. 2002, 30, 571–587. [Google Scholar] [CrossRef]
- Ferretti, N.; Pompozzi, G.; Copperi, S.; Pérez-Miles, F.; González, A. Mygalomorph spider community of a natural reserve in a hilly system in central Argentina. J. Insect Sci. 2012, 12, 31. [Google Scholar] [CrossRef]
- Scott, C.; Gerak, C.; McCann, S.; Gries, G. The role of silk in courtship and chemical communication of the false widow spider, Steatoda grossa (Araneae: Theridiidae). J. Ethol. 2018, 36, 191–197. [Google Scholar] [CrossRef]
- Postlethwait, J.H.; Giorgi, F. Vitellogenesis in insects. In Oogenesis; Springer: Boston, MA, USA, 1985; pp. 85–126. [Google Scholar]
- Sappington, T.W.; Raikhel, A.S. Molecular characteristics of insect vitellogenins and vitellogenin receptors. Insect Biochem. Mol. Biol. 1998, 28, 177–300. [Google Scholar] [CrossRef]
- Fruttero, L.L.; Frede, S.; Rubiolo, E.R.; Canavoso, L.E. The storage of nutritional resources during vitellogenesis of Panstrongylus megistus (Hemiptera: Reduviidae): The pathways of lipophorin in lipid delivery to developing oocytes. J. Insect Physiol. 2011, 57, 475–486. [Google Scholar] [CrossRef]
- Romero, S.; Laino, A.; Arrighetti, F.; Cunningham, M.; Garcia, C.F. First study on lipid dynamics during the female reproductive cycle of Polybetes pythagoricus (Araneae Saparassidae). Can. J. Zool. 2018, 96, 847–858. [Google Scholar] [CrossRef]
- Wilder, M.N.; Okumura, T.; Tsutsui, N. Reproductive mechanisms in Crustacea focusing on selected prawn species: Vitellogenin structure, processing and synthetic control. Aqua-BioScience Monogr. 2010, 3, 73–110. [Google Scholar] [CrossRef]
- Byrne, B.M.; Gruber, M.; Ab, G. The evolution of egg yolk proteins. Prog. Biophys. Mol. Biol. 1989, 53, 33–69. [Google Scholar] [CrossRef]
- Choi, Y.S.; Moon, M.J. Fine structure of the ovarian development in the Orb-web Spider, Nephila clavata. Entomol. Res. 2003, 33, 25–32. [Google Scholar] [CrossRef]
- Thompson, M.B.; Russel, K.J. Embryonic energetics in eggs of two species of Australian skink, Morethia boulengeri and Morethia adelaidensis. J. Herpetol. 1999, 33, 291–297. [Google Scholar] [CrossRef]
- Trabalon, M.; Bautz, A.M.; Moriniere, M.; Porcheron, P. Ovarian development and correlated changes in hemolymphatic ecdysteroid levels in two spiders, Coelotes terrestris and Tegenaria domestics (Araneae, Agelenidae). Gen. Comp. Endocrinol. 1992, 88, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Bednarek, A.W.; Sawadro, M.K.; Nicewicz, Ł.; Babczyńska, A.I. Vitellogenins in the spider Parasteatoda tepidariorum–expression profile and putative hormonal regulation of vitellogenesis. BMC Dev. Biol. 2019, 19, 4. [Google Scholar] [CrossRef] [PubMed]
- Stubbendieck, R.M.; Zera, A.J.; Hebets, E.A. No evidence for a relationship between hemolymph ecdysteroid levels and female reproductive behavior in Schizocosa wolf spiders. J. Arachnol. 2013, 41, 349–355. [Google Scholar] [CrossRef]
- Pourié, G.; Trabalon, M. The role of 20-hydroxyecdysone on the control of spider vitellogenesis. Gen. Comp. Endocrinol. 2003, 131, 250–257. [Google Scholar] [CrossRef]
- Sotello, J.R.; Trujillo-Cenoz, O. Electron microscope study of the vitelline body of some spider oocytes. J. Biophys. Biochem. Cytol. 1957, 3, 301–310. [Google Scholar] [CrossRef]
- Osaki, H. Electron microscope studies on developing oocytes of the spider Plexippus paykulli. Annu. Zool. Japan 1972, 45, 187–200. [Google Scholar]
- Jedrzejowska, I.; Kubrakiewicz, J. Yolk nucleus-the complex assemblage of cytoskeleton and ER is a site of lipid droplet formation in spider oocytes. Arthropod Struct. Dev. 2010, 39, 350–359. [Google Scholar] [CrossRef]
- Babczynska, A.; Binkowski, M.; Bednarek, A.; Ogierman, S.; Cibura, D.; Migula, P.; Wilczek, G.; Szulińska, E. X-ray microtomography for imaging of developing spiders inside egg cocoons. Arthropod Struct. Dev. 2014, 43, 595–603. [Google Scholar] [CrossRef]
- Lee, S.M.; Moon, M.J. Fine structural characteristics of the chorionic microspheres on the egg surface of the orb web spider Trichonephila clavata. Appl. Microsc. 2023, 53, 6. [Google Scholar] [CrossRef]
- Yepiz-Plascencia, G.; Vargas-Albores, F.; Higuera-Ciapara, I. Penaeid shrimp hemolymph lipoproteins. Aquaculture 2000, 191, 177–189. [Google Scholar] [CrossRef]
- Tufail, M.; Takeda, M. Molecular characteristics of insect vitellogenins. J. Insect Physiol. 2008, 54, 1447–1458. [Google Scholar] [CrossRef]
- Laino, A.; Cunningham, M.L.; García, F.; Heras, H. First insight into the lipid uptake, storage and mobilization in arachnids: Role of midgut diverticula and lipoproteins. J. Insect Physiol. 2009, 55, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Laino, A.; Cunningham, M.L.; Heras, H.; Garcia, F. In vitro lipid transfer between lipoproteins and midgut-diverticula in the spider Polybetes pythagoricus. Comp. Biochem. Phys. B 2011, 160, 181–186. [Google Scholar] [CrossRef]
- de Oliveira, P.R.; Camargo, M.I.C.; Bechara, G.H. Vitellogenesis in the tick Amblyomma triste (Koch 1844) (Acari: Ixodidae) role for pedicel cells. Vet. Parasitol. 2007, 143, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Boldbaatar, D.; Umemiya-Shirafuji, R.; Liao, M.; Tanaka, T.; Xuan, X.; Fujisaki, K. Multiple vitellogenins from the Haemaphysalis longicornis tick are crucial for ovarian development. J. Insect Physiol. 2010, 56, 1587–1598. [Google Scholar] [CrossRef]
- Bohm, M.K.; Behan, M.; Hagedorn, H.H. Termination of vitellogenin synthesis by mosquito fat body, a programmed response to ecdysteron. Physiol. Entomol. 1978, 3, 17–25. [Google Scholar] [CrossRef]
- García-Orozco, K.D.; Vargas-Albores, F.; Sotelo-Mundo, R.R.; Yepiz-Plascencia, G. Molecular characterization of vitellin from the ovaries of the white shrimp Penaeus (Litopenaeus) vannamei. Comp. Biochem. Physiol. B 2002, 133, 361–369. [Google Scholar]
- Yang, Z.M.; Lu, T.Y.; Wu, Y.; Yu, N.; Xu, G.M.; Han, Q.Q.; Liu, Z.W. The importance of vitellogenin receptors in the oviposition of the pond wolf spider, Pardosa pseudoannulata. Insect Sci. 2022, 29, 443–452. [Google Scholar] [CrossRef]
- Tseng, D.Y.; Chen, Y.N.; Liu, K.F.; Kou, G.H.; Lo, C.F.; Kuo, C.M. Hepatopancreas and ovary are sites of vitellogenin synthesis as determined from partial cDNA encoding of vitellogenin in the marine shrimp, Penaeus vannamei. Invertebr. Reprod. Dev. 2002, 42, 137–143. [Google Scholar] [CrossRef]
- Yang, F.; Xu, H.T.; Dai, Z.M.; Yang, W.J. Molecular characterization and expression analysis of vitellogenin in the marine crab Portunus trituberculatus. Comp. Biochem. Phys. B 2005, 142, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Raikhel, A.S.; Dhadialla, T.S. Accumulation of yolk proteins in insect oocytes. Annu. Rev. Entomol. 1992, 37, 217–251. [Google Scholar] [CrossRef]
- Canavoso, L.E.; Jouni, Z.E.; Karnas, K.J.; Pennington, J.E.; Wells, M.A. Fat metabolism in insects. Ann. Rev. Nutr. 2001, 21, 23–46. [Google Scholar] [CrossRef] [PubMed]
- Snigirevskaya, E.S.; Raikhel, A.S. Receptor-mediated endocytosis of yolk proteins in insect oocytes. In Reproductive Biology of Invertebrates; CRC Press: Boca Raton, FL, USA, 2005; Volume 12, pp. 199–228. [Google Scholar]
- Lamy, M. Vitellogenesis, vitellogenin and vitellin in the males of insects: A review. Int. J. Invertebr. Reprod. Dev. 1984, 7, 311–321. [Google Scholar] [CrossRef]
- Borst, D.W.; Eskew, M.R.; Wagner, S.J.; Shores, K.; Hunter, J.; Luker, L.; Hatle, J.D.; Hecht, L.B. Quantification of juvenile hormone III, vitellogenin, and vitellogenin-mRNA during the oviposition cycle of the lubber grasshopper. Insect Biochem. Mol. Biol. 2000, 30, 813–819. [Google Scholar] [CrossRef]
- Thompson, D.M.; Khalil, S.M.S.; Jeffers, L.A.; Sonenshine, D.E.; Mitchel, R.D.; Osgood, C.J.; Roe, R.M. Sequence and the developmental and tissue-specific regulation of the first complete vitellogenin messenger RNA from ticks responsible for heme sequestration. Insect Biochem. Mol. Biol. 2007, 37, 363–374. [Google Scholar] [CrossRef]
- Chinzei, Y.; Chino, H.; Takahashi, K. Purification and properties of vitellogenin and vitellin from a tick, Ornithodoros moubata. J. Comp. Physiol. 1983, 152, 13–21. [Google Scholar] [CrossRef]
- Hagedorn, H.H.; Kunkel, J.G. Vitellogenin and vitellin in insects. Ann. Rev. Entomol. 1979, 24, 475–505. [Google Scholar] [CrossRef]
- Longyant, S.; Sithigorngul, P.; Thammapalerd, N.; Sithigorngul, W.; Menasveta, P. Characterization of vitellin and vitellogenin of giant tiger prawn Penaeus monodon using monoclonal antibodies specific to vitellin subunits. Invertebr. Reprod. Dev. 2000, 37, 211–221. [Google Scholar] [CrossRef]
- Houlihan, D.F.; Livingstone, D.R.; Lee, R.F.; Lee, R.F. Lipoproteins from the hemolymph and ovaries of marine invertebrates. Adv. Comp. Environ. Physiol. 1991, 7, 187–207. [Google Scholar]
- Jasmani, S.; Kawazoe, I.; Shih, T.W.; Suzuki, Y.; Aida, K. Hemolymph vitellogenin levels during ovarian development in the kuruma prawn Penaeus japonicus. Fish. Sci. 2000, 66, 535–539. [Google Scholar] [CrossRef]
- Lee, F.Y.; Shih, T.W.; Chang, C.F. Isolation and Characterization of the Female-Specific Protein (Vitellogenin) in Mature Female Hemolymph of the Freshwater Prawn, Macrobrachium rosenbergii: Comparison with Ovarian Vitellin. Gen. Comp. Endocrinol. 1997, 108, 406–415. [Google Scholar] [CrossRef]
- Pateraki, L.E.; Stratakis, E. Characterization of vitellogenin and vitellin from land crab Potamon potamios: Identification of a precursor polypeptide in the molecule. J. Exp. Zool. 1997, 279, 597–608. [Google Scholar] [CrossRef]
- Garcia, F.; Cunningham, M.L.; Garda, H.; Heras, H. Embryo lipoproteins and yolk lipovitellin consumption during embryogenesis in Macrobrachium borellii (Crustacea: Palaemonidae). Comp. Biochem. Phys. B 2008, 151, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Lubzens, E.; Ravid, T.; Khayat, M.; Daube, N.; Tietz, A. Isolation and characterization of the high-density lipoproteins from the hemolymph and ovary of the penaeid shrimp Penaeus semisulcatus (de Haan): Apoproteins and lipids. J. Exp. Zool. 1997, 278, 339–348. [Google Scholar] [CrossRef]
- Browdy, C.L.; Fainzilber, M.; Tom, M.; Loya, Y.; Lubzens, E. Vitellin synthesis in relation to oogenesis in vitro incubated ovaries of Penaeus semisculatus (crustacea, decapoda, penaeidae). J. Exp. Zool. 1990, 255, 205–215. [Google Scholar] [CrossRef]
- Fainzilber, M.; Tom, M.; Shafir, S.; Applebaum, S.W.; Lubzens, E. Is There Extraovarian Synth. Vitellogenin Penaeid Shrimp? Biol. Bull. 1992, 183, 233–241. [Google Scholar] [CrossRef]
- Schneider, W.J. Lipoprotein receptors in oocyte growth. Clin. Investig. 1992, 70, 385–390. [Google Scholar] [CrossRef]
- Qiu, Y.W.; Ng, T.B.; Chu, K.H. Purification and characterization of vitellin from the ovaries of the shrimp Metapenaeus ensis (Crustacea: Decapoda: Penaeidae). Invertebr. Reprod. Dev. 1997, 31, 217–223. [Google Scholar] [CrossRef]
- Trabalon, M.; Ruhland, F.; Laino, A.; Cunningham, M.; Garcia, F. Embryonic and post-embryonic development inside wolf spiders’ egg sac with special emphasis on the vitellus. J. Comp. Physiol. B 2017, 188, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Laino, A.; Cunningham, M.; Costa, F.G.; Garcia, C.F. Energy sources from the eggs of the wolf spider Schizocosa malitiosa: Isolation and characterization of lipovitellins. Comp. Biochem. Phys. B 2013, 165, 172–180. [Google Scholar] [CrossRef]
- Romero, S.; Laino, A.; Arrighetti, F.; Garcia, C.F.; Cunningham, M. Vitellogenesis in spiders: First analysis of protein changes in different reproductive stages of Polybetes pythagoricus. J. Comp. Physiol. B. 2019, 189, 335–350. [Google Scholar] [CrossRef]
- Laino, A.; Cunningham, M.L.; Heras, H.; Garcia, F. Isolation and characterization of two vitellins from eggs of the spider Polybetes pythagoricus (Araneae: Sparassidae). Comp. Biochem. Phys. B 2011, 158, 142–148. [Google Scholar] [CrossRef] [PubMed]
- James, A.M.; Oliver, J.H., Jr. Purification and partial characterization of vitellin from the black-legged tick, Ixodes scapularis. Insect Biochem. Mol. Biol. 1997, 27, 639–649. [Google Scholar] [CrossRef] [PubMed]
- James, A.M.; Zhu, X.X.; Oliver JR, J.H. Vitellogenin and ecdysteroid titers in Ixodes scapularis during vitellogenesis. J. Parasitol. 1997, 83, 559–563. [Google Scholar] [CrossRef]
- Shanbaky, N.M.; Mansour, M.M.; Main, A.J.; El-Said, A.; Helmy, N. Hormonal control of vitellogenesis in Argas hermanni (Acari: Argasidae). J. Med. Entomol. 1990, 27, 968–974. [Google Scholar] [CrossRef]
- Taylor, D.; Chinzei, Y.; Miura, K.; Ando, K. Vitellogenin synthesis, processing and hormonal regulation in the tick, Ornithodoros parkeri (Acari: Argasidae). Insect Biochem. 1991, 21, 723–733. [Google Scholar] [CrossRef]
- Rosell, R.; Coons, L.B. The role of the fat body, midgut and ovary in vitellogenin production and vitellogenesis in the female tick, Dermacentor variabilis. Int. J. Parasitol. 1992, 22, 341–349. [Google Scholar] [CrossRef]
- Logullo, C.; Moraes, J.; Dansa-Petretski, M.; Vaz, I.S.; Masuda, A.; Sorgine, M.H.F.; Braz, G.R.; Masuda, H.; Oliveira, P.L. Binding and storage of heme by vitellin from the cattle tick, Boophilus microplus. Insect Biochem. Mol. Biol. 2002, 32, 1805–1811. [Google Scholar] [CrossRef]
- Tellam, R.L.; Kemp, D.; Riding, G.; Briscoe, S.; Smith, D.; Sharp, P.; Willadsen, P. Reduced oviposition of Boophilus microplus feeding on sheep vaccinated with vitellin. Vet. Parasitol. 2002, 103, 141–156. [Google Scholar] [CrossRef]
- Silveira, A.B.; Castro-Santos, J.; Senna, R.; Logullo, C.; Fialho, E.; Silva-Neto, M.A. Tick vitellin is dephosphorylated by a protein tyrosine phosphatase during egg development: Effect of dephosphorylation on VT proteolysis. Insect Biochem. Mol. Biol. 2006, 36, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Ramírez Rodríguez, P.B.; Rosario Cruz, R.; Domínguez García, D.I.; Hernández Gutiérrez, R.; Lagunes Quintanilla, R.E.; Ortuño Sahagún, D.; González Castillo, C.; Gutiérrez Ortega, A.; Herrera Rodríguez, S.E.; Vallejo Cardona, A.; et al. Identification of immunogenic proteins from ovarian tissue and recognized in larval extracts of Rhipicephalus (Boophilus) microplus, through an immunoproteomic approach. Exp. Parasitol. 2016, 170, 227–235. [Google Scholar] [CrossRef]
- Cabrera, A.R.; Donohue, K.V.; Roe, R.M. Regulation of female reproduction in mites: A unifying model for the Acari. J. Insect Physiol. 2009, 55, 1079–1090. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, Y.; Goto, S.G.; Ito, K.; Numata, H. Suppression of ovarian development and vitellogenin gene expression in the adult diapause of the two-spotted spider mite Tetranychus urticae. J. Insect Physiol. 2009, 55, 70–77. [Google Scholar] [CrossRef]
- Yang, C.; Pan, H.; Liu, Y.; Zhou, X. Stably expressed housekeeping genes across developmental stages in the two-spotted spider mite, Tetranychus urticae. PLoS ONE 2015, 10, e0120833. [Google Scholar] [CrossRef]
- Guo, J.; Wang, L.; Wu, H.; Cao, Y.; Xiao, R.; Lai, X.; Zhang, G. Molecular characterization and expression of vitellogenin genes from the wolf spider Pardosa pseudoannulata (Araneae: Lycosidae). Physiol. Entomol. 2018, 43, 295–305. [Google Scholar] [CrossRef]
- Dadzie, S.; Wangila, B.C.C. Reproductive biology, length–weight relationship and relative condition of pond raised Tilapia zilli (Gervais). J. Fish. Biol. 1980, 17, 243–253. [Google Scholar] [CrossRef]
- Fatima, H.; Ayub, Z.; Ali, S.A.; Siddiqui, G. Biochemical composition of the hemolymph, hepatopancreas, ovary, and muscle during ovarian maturation in the penaeid shrimps Fenneropenaeus merguiensis and F. penicillatus (Crustacea: Decapoda). Turk. J. Zool. 2013, 37, 334–347. [Google Scholar] [CrossRef]
- Ahmadi, A.; Ghanem, S. Growth Pattern, Gonadal Maturity, Condition Factor and Gill Net Selectivity of the Hard-Lipped Barb (Osteochilus hasselti CV) from Sungai Batang River, Indonesia. Egypt. J. Aquat. Biol. Fish. 2025, 29, 1–26. [Google Scholar] [CrossRef]
- Di Cosmo, A.; Di Cristo, C.; Paolucci, M. 2001. Sex steroid hormone fluctuations and morphological changes of the reproductive system of the female of Octopus vulgaris throughout the annual cycle. J. Exp. Zool. A 2001, 289, 33–47. [Google Scholar] [CrossRef]
- Iyapparaj, P.; Revathi, P.; Ramasubburayan, R.; Prakash, S.; Anantharaman, P.; Immanuel, G.; Palavesam, A. Antifouling activity of the methanolic extract of Syringodium isoetifolium, and its toxicity relative to tributyltin on the ovarian development of brown mussel Perna indica. Ecotoxicol. Environ. Saf. 2013, 89, 231–238. [Google Scholar] [CrossRef]
- Alvarez-Garcia, I.L.; Abadia-Chanona, Q.Y.; Arellano-Martinez, M.; Avila-Poveda, O.H. Maximum gonad investment reveals male bias when temperature decreases or latitude increases for a broadcast-spawning intertidal chiton (Polyplacophora: Chitonida). Hydrobiologia 2024, 852, 69–88. [Google Scholar] [CrossRef]
- Yuan, H.X.; Xu, X.; Sima, Y.H.; Xu, S.Q. Reproductive toxicity effects of 4-nonylphenol with known endocrine disrupting effects and induction of vitellogenin gene expression in silkworm, Bombyx mori. Chemosphere 2013, 93, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Sujatha, G.S.; Sagar, D.; Kumar, H. Effects of short-and long-term thermal stress on developmental stages and adults vis-à-vis reproductive physiology of Spodoptera litura. Anim. Biol. 2024, 75, 1–21. [Google Scholar]
- Boucard, C.G.V.; Levy, P.; Ceccaldi, H.J.; Brogren, C.H. Developmental changes in concentrations of vitellin, vitellogenin, and lipids in hemolymph, hepatopancreas, and ovaries from different ovarian stages of Indian white prawn Fenneropenaeus indicus. J. Exp. Mar. Biol. Ecol. 2002, 281, 63–75. [Google Scholar] [CrossRef]
- Garcia, C.F.; Heras, H. Vitellogenin and lipovitellin from the prawn Macrobrachium borellii as hydrocarbon pollution biomarker. Mar. Pollut. Bull. 2012, 64, 1631–1636. [Google Scholar] [CrossRef]
- Vivas, M.; Garcia, E.; Muñoz-Vera, A.; Barcala, E.; Guijarro, E. Effect of the invasive blue crab (Callinectes sapidus Rathbun, 1896) in a protected coastal lagoon. Estuaries Coasts 2025, 48, 9. [Google Scholar] [CrossRef]
- Marangos, C.; Ramos, L.; Oliva, M. Variations in protein levels in the hemolymph, hepatopancreas and ovary of Penaeus schmitti during ovarian maturation (Crustacea, Decapoda, Peneidae). Arch. Int. Physiol. Biochim. 1988, 96, 179–190. [Google Scholar]
- Auttarat, J.; Phiriyangkul, P.; Utarabhand, P. Characterization of vitellin from the ovaries of the banana shrimp Litopenaeus merguiensis. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2006, 143, 27–36. [Google Scholar] [CrossRef]
- Ziegler, R.; van Antwerpen, R. Lipid uptake by insect oocytes. Insect Biochem. Mol. 2006, 36, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Lubzens, E.; Tietz, A.; Pines, M.; Applebaum, S.W. Lipid accumulation in oocytes of Locusta migratoria migratiroides. Insect Biochem. 1981, 11, 323–329. [Google Scholar] [CrossRef]
- Grapes, M.; Whiting, P.; Dinan, L. Fatty acid and lipid analysis of the house cricket, Acheta domesticus. Insect Biochem. 1989, 19, 767–774. [Google Scholar] [CrossRef]
- Ravid, T.; Tietz, A.; Khayat, M.; Boehm, E.; Michelis, R.; Lubzens, E. Lipid accumulation in the ovaries of a marine shrimp Penaeus semisulcatus (De Haan). J. Exp. Biol. 1999, 202, 1819–1829. [Google Scholar] [CrossRef]
- Warburg, M.R. Reviewing the structure and function of the scorpion’s hepatopancreas. Arthropods 2012, 1, 79. [Google Scholar]
- Teshima, S.; Kanazawa, A. Digestibility of dietary lipids in the prawn. Bull. Jpn. Soc. Sci. Fish. 1983, 49, 963–966. [Google Scholar] [CrossRef]
- Castille, F.L.; Lawrence, A.L. Relationship between maturation and biochemical composition of the gonads and digestive glands of the shrimps Penaeus aztecus Ives and Penaeus setiferus (L.). J. Crustac. Biol. 1989, 9, 202–211. [Google Scholar] [CrossRef]
- Ruhland, F.; Pétillon, J.; Trabalon, M. Physiological costs during the first maternal care in the wolf spider Pardosa saltans (Araneae, Lycosidae). J. Insect Physiol. 2016, 95, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Galiano, M.E. El desarrollo postembrionario larval en especies de genero Polybetes Simon, 1897 (Araneae, Sparassidae). Acta Zool. Lilloana 1971, 28, 221–225. [Google Scholar]
- Millamena, O.M.; Pascual, F.P. Tissue lipid content and fatty acid composition of Penaeus monodon Fabricius broodstock from the wild. J. World Aquacult. Soc. 1990, 21, 116–121. [Google Scholar] [CrossRef]
- Charniaux-Cotton, H. Controle de la differenciation sexuelle de l’appareil genital chez les Crustaces Malacostraces; comparison avec les Insectes. Bull. Soc. Zool. Fr. 1976, 101, 68–76. [Google Scholar]
- Galois, R.G. Variations de la composition lipidique tissulaire au cours de la vitellogenèse chez la crevette Penaeus indicus. J. Exp. Mar. Biol. Ecol. 1984, 84, 155–166. [Google Scholar] [CrossRef]
- Andre, J.; Rouiller, C. The ultrastructure of the vitelline body in theoocyte of the spider Tegenaria parietina. J. Biophys. Biochem. Cytol. 1957, 3, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Warburg, M.R.; Elias, R.; Rosenberg, M. Ovariuterus and oocyte dimensions in the female buthid scorpion, Leiurus quinquestriatus, H. & E. (Scorpiones: Buthidae), and the effect of higher temperature. Invertebr. Reprod. Dev. 1995, 27, 21–28. [Google Scholar]
- Cunningham, M.; Pollero, R. Characterization of lipoprotein fraction with high content of hemocyanin in the hemolimphatic plasma of Polybetes pythagoricus. J. Exp. Zool. 1996, 274, 275–280. [Google Scholar] [CrossRef]
- Cunningham, M.L.; Gonzalez, A.; Pollero, R.J. Characterization of lipoproteins isolated from the hemolymph of the spider Latrodectus Mirabilis. J. Arachnol. 2000, 28, 49–55. [Google Scholar] [CrossRef]
- Haunerland, N.H.; Bowers, W.S. Lipoprotein from the tarantula, Eurypelma Californicum. Comp. Biochem. Physiol. 1987, 86, 571–574. [Google Scholar]
- Kucharski, L.C.R.; Da Silva, R.S.M. Effect of diet composition on the carbohydrate and lipid metabolism in an estuarine crab, Chasmagnathus granulata (Dana, 1851). Comp. Biochem. Physiol. Part A 1991, 99, 215–218. [Google Scholar] [CrossRef]
- Vinagre, A.S.; Da Silva, R.S. Effects of starvation on the carbohydrate and lipid metabolism in crabs previously maintained on a high protein or carbohydrate-rich diet. Comp. Biochem. Physiol. Part A 1992, 102, 579–583. [Google Scholar] [CrossRef]
- Carvalho, M.; Sampaio, J.L.; Palm, W.; Brankatschk, M.; Eaton, S.; Shevchenko, A. Effects of diet and development on the Drosophila lipidome. Mol. Syst. Biol. 2012, 8, 600. [Google Scholar] [CrossRef]
- Uscian, J.M.; Stanley-Samuelson, D.W. Fatty acid compositions of phospholipids and triacylglycerols from selected terrestrial arthropods. Comp. Biochem. Physiol. B 1994, 107, 371–379. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Gustafsson, I.B.; Grimelius, L.; Vessby, B. The lipid composition of the haemolymph and hepatopancreas of the scorpion (Buthus quinquestriatus). Comp. Biochem. Physiol. B 1981, 69, 873–876. [Google Scholar] [CrossRef]
- Laino, A.; Mattoni, C.; Ojanguren-Affilastro, A.; Cunningham, M.; Garcia, C.F. Analysis of lipid and fatty acid composition of three species of scorpions with relation to different organs. Comp. Biochem. Phys. B 2015, 190, 27–36. [Google Scholar] [CrossRef]
- Yamashita, O.; Indrasith, L.S. Metabolic fates of yolk proteins during embryogenesis in arthropods: (Arthropods/embryogenesis/yolk proteins/limited proteolysis/protease). Dev. Growth Differ. 1988, 30, 337–346. [Google Scholar] [CrossRef]
- Terwilliger, N.B. Hemocyanins in crustacean oöcytes and embryos. In Crustacean Egg Production; CRC Press: Boca Raton, FL, USA, 2020; pp. 31–39. [Google Scholar]
- Foelix, R. Biology of Spiders; Oxford University Press: Oxford, UK, 2011; p. 428. [Google Scholar]
- Romero, S.; Laino, A.; Molina, G.; Cunningham, M.; Garcia, C.F. Embryonic and post-embryonic development of the spider Polybetes pythagoricus (Sparassidae): A biochemical point of view. An. Acad. Bras. Cienc. 2022, 94, e20210159. [Google Scholar] [CrossRef]
- von Wittich, W.H. Observationes Quaedam de Aranearum ex ovo Evolutione; Formis eressum Ploetzianis: Halle-Wittenberg, Germany, 1845. [Google Scholar]
- Kloc, M.; Jedrzejowska, I.; Tworzydlo, W.; Bilinski, S.M. Balbiani body, nuage and sponge bodies–the germ plasm pathway players. Arthropod Struct. Dev. 2014, 43, 341–348. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, H.; Zhou, Z.; Li, K.; Li, K.; Deng, G.Y.; Liu, Z. Purification of vitellin from the ovary of Chinese mitten-handed crab (Eriocheir sinensis) and development of an antivitellin ELISA. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2004, 138, 305–311. [Google Scholar] [CrossRef]
- Garcia, C.F.; Cunningham, M.; Soulages, J.L.; Garda, H.A.; Pollero, R. Structural characterization of the lipovitellin from the shrimp Macrobrachium borellii. Comp. Biochem. Physiol. B 2006, 145, 365–370. [Google Scholar] [PubMed]
- Salerno, A.P.; Dansa-Petretski, M.; Silva-Neto, M.A.C.; Coelho, H.S.L.; Masuda, H. Rhodnius prolixus vitellin is composed of three different populations: Comparison with vitellogenin. Insect Biochem. Mol. Biol. 2002, 32, 709–717. [Google Scholar] [CrossRef]
- Walker, A.; Ando, S.; Smith, G.D.; Lee, R.F. The utilization of lipovitellin during blue crab (Callinectes sapidus) embryogenesis. Comp. Biochem. Phys. B 2006, 143, 201–208. [Google Scholar] [CrossRef]
- Heras, H.; González-Baró, M.D.R.; Pollero, R.J. Lipid and fatty acid composition and energy partitioning during embryo development in the shrimp Macrobrachium borelli. Lipids 2000, 35, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Wehrtmann, I.S.; Graeve, M. Lipid composition and utilization in developing eggs of two tropical marine caridean shrimps (Decapoda: Caridea: Alpheidae, Palaemonidae). Comp. Biochem. Physio. Part B Biochem. Mol. Biol. 1998, 121, 457–463. [Google Scholar] [CrossRef]
- Garcia, F.; Gonzalez-Baró, M.R.; Garda, H.; Cunningham, M.; Pollero, R. Fenitrothion-induced structural and functional perturbations in the yolk lipoproteins of the shrimp Macrobrachium borellii. Lipids 2004, 39, 389–396. [Google Scholar] [CrossRef]
- Chinzei, Y.; Chino, H.; Wyatt, G.R. Purification and properties of vitellogenin and vitellin from Locusta migratoria. Insect Biochem. 1981, 11, 1–7. [Google Scholar] [CrossRef]
- Schie, I.W.; Nolte, L.; Pedersen, T.L.; Smith, Z.; Wu, J.; Yahiatene, I.; Newman, J.W.; Huser, T. Direct comparison of fatty acid ratios in single cellular lipid droplets as determined by comparative Raman spectroscopy and gas chromatography. Analyst 2013, 138, 6662–6670. [Google Scholar] [CrossRef]
- Walther, T.C.; Farese, R.V., Jr. Lipid droplets and cellular lipid metabolism. Ann. Rev. Biochem. 2012, 81, 687–714. [Google Scholar] [CrossRef]
- Slotte, J.P. Biological functions of sphingomyelins. Prog. Lipid Res. 2013, 52, 424–437. [Google Scholar] [CrossRef]
- Slotte, J.P.; Ramstedt, B. The functional role of sphingomyelin in cell membranes. Eur. J. Lipid Sci. Technol. 2007, 109, 977–981. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, T.; Gu, J.; Li, X.; Yang, X.; Gao, X.; Liu, F.; Wang, J. Identification of five anti-lipopolysaccharide factors in oriental river prawn, Macrobrachium nipponense. Fish. Shellfish. Immun. 2015, 46, 252–260. [Google Scholar] [CrossRef]
- Ikon, N.; Ryan, R.O. Cardiolipin and mitochondrial cristae organization. Biochim. Biophys. Acta 2017, 1859, 1156–1163. [Google Scholar] [CrossRef]
- Tasseva, G.; Bai, H.D.; Davidescu, M.; Haromy, A.; Michelakis, E.; Vance, J.E. Phosphatidylethanolamine deficiency in mammalian mitochondria impairs oxidative phosphorylation and alters mitochondrial morphology. J. Biol. Chem. 2013, 288, 4158–4173. [Google Scholar] [CrossRef] [PubMed]
- Teague, W.E.; Soubias, O.; Petrache, H.; Fuller, N.; Hines, K.G.; Rand, R.P.; Gawrisch, K. Elastic properties of polyunsaturated phosphatidylethanolamines influence rhodopsin function. Faraday Discuss. 2013, 161, 383–395. [Google Scholar] [CrossRef]
- Hagen, W.; Van Vleet, E.S.; Kattner, G. Seasonal lipid storage as overwintering strategy of Antarctic krill. Mar. Ecol. Prog. Ser. 1996, 134, 85–89. [Google Scholar] [CrossRef]
- Mayzaud, P.; Boutoute, M.; Alonzo, F. Lipid composition of the euphausiids Euphausia vallentini and Thysanoessa macrura during summer in the Southern Indian Ocean. Antarct. Sci. 2003, 15, 463. [Google Scholar] [CrossRef]
- Sibert, V.; Ouellet, P.; Brêthes, J.C. Changes in yolk total proteins and lipid components and embryonic growth rates during lobster (Homarus americanus) egg development under a simulated seasonal temperature cycle. Mar. Biol. 2004, 144, 1075–1086. [Google Scholar] [CrossRef]
- Zeng, X.; Li, Z.; Zhang, Z.; Shi, X.; Wang, Y. Variations in lipid composition of ovaries and hepatopancreas during vitellogenesis in the mud crab Scylla paramamosain: Implications of lipid transfer from hepatopancreas to ovaries. Aquac. Rep. 2024, 35, 102008. [Google Scholar] [CrossRef]
- Jayadev, S.; Liu, B.; Bielawska, A.E.; Lee, J.Y.; Nazaire, F.; Pushkareva, M.Y.; Obeid, L.M.; Hannun, Y.A. Role for ceramide in cell cycle arrest. J. Biol. Chem. 1995, 270, 2047–2052. [Google Scholar] [CrossRef]
- Orlati, S.; Porcelli, A.M.; Hrelia, S.; Lorenzini, A.; Rugolo, M. Intracellular calcium mobilization and phospholipid degradation in sphingosylphosphorylcholine-stimulated human airway epithelial cells. Biochem. J. 1998, 334, 641–649. [Google Scholar] [CrossRef]
- Yunoki, K.; Ogawa, T.; Ono, J.; Miyashita, R.; Aida, K.; Oda, Y.J.; Ohnishi, M. Analysis of sphingolipid classes and their contents in meals. Biosci. Biotechnol. Biochem. 2008, 72, 222–225. [Google Scholar] [CrossRef]
- An, D.; Na, C.; Bielawski, J.; Hannun, Y.A.; Kasper, D.L. Membrane sphingolipids as essential molecular signals for Bacteroides survival in the intestine. Proc. Natl. Acad. Sci. USA 2011, 108, 4666–4671. [Google Scholar] [CrossRef]
- Hall, M.; Vanheusden, M.C.; Soderhall, K. Identification of the major lipoproteins in crayfish hemolymph as proteins involved in immune recognition and clotting. Biochem. Biophys. Res. Commun. 1995, 216, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Jaenicke, E.; Decker, H. Kinetic properties of catecholoxidase activity of tarantula hemocyanin. FEBS J. 2008, 275, 1518–1528. [Google Scholar] [CrossRef]
- Jaenicke, R. Stability and folding of domain proteins. Prog. Biophys. Mol. Biol. 1999, 71, 155–241. [Google Scholar] [CrossRef] [PubMed]
- Laino, A.; Lavarías, S.; Suárez, G.; Lino, A.; Cunningham, M. Characterization of phenoloxidase activity from spider Polybetes pythagoricus hemocyanin. J. Exp. Zool. Part A 2015, 323, 547–555. [Google Scholar]
- Laino, A.; Cunningham, M.; Suarez, G.; Garcia, C.F. Identification and characterization of the lipid transport system in the tarantula Grammostola rosea. Open J. Anim. Sci. 2015, 5, 9–20. [Google Scholar] [CrossRef]
- Riciluca, K.C.T.; Sayegh, R.S.R.; Melo, R.L.D.; Silva, P.I., Jr. Rondonin an antifungal peptide from spider (Acanthoscurria rondoniae) haemolymph. Results Immunol. 2012, 2, 66–71. [Google Scholar] [CrossRef]
- Decker, H.; Hellmann, N.; Jaenicke, E.; Lieb, B.; Meissner, U.; Markl, J. Minireview: Recent progress in hemocyanin research. Integr. Comp. Biol. 2007, 47, 631–644. [Google Scholar] [CrossRef]
- Coates, C.J.; Nairn, J. Diverse immune functions of hemocyanins. Dev. Comp. Inmunol. 2014, 45, 43–55. [Google Scholar] [CrossRef]
- Cunningham, M.; Laino, A.; Romero, S.; Garcia, C.F. Arachnid Hemocyanins. In Vertebrate and Invertebrate Respiratory Proteins, Lipoproteins and other Body Fluid Proteins; Hoeger, U., Harris, J.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 219–231. [Google Scholar]
- Pick, C.; Schneuer, M.; Burmester, T. Ontogeny of hemocyanin in the ovoviviparous cockroach Blaptica dubia suggests an embryo-specific role in oxygen supply. J. Insect Physiol. 2010, 56, 455–460. [Google Scholar] [CrossRef]
- Chen, B.; Ma, R.; Ding, D.; Wei, L.; Kang, L. Aerobic respiration by haemocyanin in the embryo of the migratory locust. Insect Mol. Biol. 2017, 26, 461–468. [Google Scholar] [CrossRef]
- Scherbaum, S.; Hellmann, N.; Fernández, R.; Pick, C.; Burmester, T. Diversity, evolution, and function of myriapod hemocyanins. BMC Evol. Biol. 2018, 18, 107. [Google Scholar] [CrossRef]
- Terwilliger, N.; Dumler, K. Ontogeny of decapod crustacean hemocyanin: Effects of temperature and nutrition. J. Exp. Biol. 2001, 204, 1013–1020. [Google Scholar] [CrossRef]
- Lipovsek, S.; Leitinger, G.; Kossel, P.; Daris, B.; Perc, M.; Devetak, D.; Weiland, N.; Novak, T. Towards understanding partial adaptation to the subterranean habitat in the European cave spider, Meta menardi. Ecocytological Approach. Sci. Rep. 2019, 9, 9121. [Google Scholar] [CrossRef] [PubMed]
- Ghiretti-Magaldi, A.; MILANESI, C.; SALVATO, B. Identification of hemocyanin in the cyanocytes of Carcinus maenas. Experientia 1973, 29, 1265–1267. [Google Scholar] [CrossRef] [PubMed]
- Ghiretti-Magaldi, A.; Milanesi, C.; Tognon, G. Hemopoiesis in Crustacea Decapoda: Origin and evolution of hemocytes and cyanocytes of Carcinus maenas. Cell Differ. 1977, 6, 167–186. [Google Scholar] [CrossRef]
- Trabalon, M.; Garcia, C.F. Transport pathways of hydrocarbon and free fatty acids to the cuticle in arthropods and hypothetical models in spiders. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2021, 252, 110541. [Google Scholar] [CrossRef]
- Markl, J. Evolution of molluscan hemocyanin structures. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2013, 1834, 1840–1852. [Google Scholar] [CrossRef] [PubMed]
- Fagotto, F. Yolk degradation in tick eggs: I. Occurrence of a cathepsin L-like acid proteinase in yolk spheres. Arch. Insect Biochem. Physiol. 1990, 14, 217–235. [Google Scholar] [CrossRef]
- Cherry, L.M. The accumulation and utilization of food reserves by adult female cattle tick, Boophilus microplus (Canestrine). Aust. J. Zool. 1973, 21, 403–412. [Google Scholar] [CrossRef]
- Sanches, G.S.; Araujo, A.M.; Martins, T.F.; Bechara, G.H.; Labruna, M.B.; Camargo-Mathias, M.I. Morphological records of oocyte maturation in the parthenogenetic tick Amblyomma rotundatum Koch, 1844 (Acari: Ixodidae). Ticks Tick-Borne Dis. 2012, 3, 59–64. [Google Scholar] [CrossRef]
- Sharifian, S.; Kamrani, E.; Safaie, M.; Sharifian, S. Oogenesis and ovarian development in the freshwater Crab Sodhiana iranica (Decapoda: Gecarcinuaidae) from the south of Iran. Tissue Cell 2015, 47, 213–220. [Google Scholar] [CrossRef]
- Rodrigues, M.M.; Lopez Greco, L.S.; De Almeida, L.C.F.; Bertini, G. Histological and Histochemical Dynamism of Oogenesis in the Cinnamon River Prawn Macrobrachium acanthurus (Caridea: Palaemonidae) Induced by Eyestalk Ablation. An. Acad. Bras. Cienc. 2022, 94, e20211294. [Google Scholar] [CrossRef]
- Chippendale, G.M. Carbohydrates in reproduction and embryonic development. In Rockstein M. Biochemistry of Insects; Academic Press: New York, NY, USA, 1978; pp. 42–45. [Google Scholar]
- Anderson, J.F. Energy content of spider eggs. Oecologia 1978, 37, 41–57. [Google Scholar] [CrossRef]
- Schaefer, M. An analysis of diapause and resistance in the egg stage of Floronia bucculenta (Araneida: Linyphiidae). Oecologia 1976, 25, 155–174. [Google Scholar] [CrossRef] [PubMed]
- Rowe, C.L. Standard metabolic rates of early life stages of the diamond back terrapin (Malaclemys terrapin), an estuarine turtle, suggest correlates between life history changes and the metabolic economy of hatchlings. Zoology 2018, 127, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Babczynska, A.; Sułowicz, S.; Talik, E.; Hermyt, M.; Bednarek, A.; Sawadro, M.; Molenda, A. Sterile capsule–egg cocoon covering constitutes an antibacterial barrier for spider Parasteatoda tepidariorum embryos. Physiol. Biochem. Zool. 2019, 92, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Canard, A. Analyse nouvelle du développement postembryonnaire des araignées. Rev. Arachnol. 1987, 7, 91–128. [Google Scholar]
- Canard, A.; Stockmann, R. Comparative postembryonic development of arachnids. Mem. Qld. Mus. 1993, 33, 461–468. [Google Scholar]
- Downes, M.F. A proposal for standardization of the terms used to describe the early development of spiders, based on a study of Theridion rufipes Lucas (Araneae: Theridiidae). Bull. Br. Arachnol. Soc. 1987, 7, 187–193. [Google Scholar]
- Mittmann, B.; Wolff, C. Embryonic development and staging of the cobweb spider Parasteatoda tepidariorum C. L. Koch, 1841 (syn.: Achaearanea tepidariorum; Araneomorphae; Theridiidae). Dev. Genes Evol. 2012, 222, 189–216. [Google Scholar] [CrossRef]
- Wolff, C.; Hilbrant, M. The embryonic development of the Central American wandering spider Cupiennius salei. Front. Zool. 2011, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Vachon, M. Contribution à l’étude du développement postembryonnaire des araignées. Première note. Généralites et nomenclature des stades. Bull. Soc. Zool. Fr. 1957, 82, 337–354. [Google Scholar]
- Vachon, M. Contribution à l’étude du développement postembryonnaire des araignées. Deuxième note. Ortognathes. Bull. Soc. Zool. Fr. 1958, 83, 429–461. [Google Scholar]
- Wurdak, E.; Ramousse, R. Organisation sensorielle de la larve et de la première nymphe chez l’araignée Araneus suspicax (O. Pickard-Cambridge). Rev. Arachnol. 1984, 5, 287–299. [Google Scholar]
- Oda, H.; Akiyama-Oda, Y. The common house spider Parasteatoda tepidariorum. EvoDevo 2020, 11, 6. [Google Scholar] [CrossRef]
- Akiyama-Oda, Y.; Oda, H. Early patterning of the spider embryo: A cluster of mesenchymal cells at the cumulus produces Dpp signals received by germ disc epithelial cells. Development 2003, 130, 1735–1747. [Google Scholar] [CrossRef]
- Chaw, R.C.; Vance, E.; Black, S.D. Gastrulation in the spider Zygiella x- notate involves three distinct phases of cell internalization. Dev. Dyn. 2007, 236, 3484–3495. [Google Scholar] [CrossRef]
- Suzuki, H.; Kondo, A. Early Embryonic Development, Including Germ-Disk Stage, in the Theridiid Spider Achaearanea japonica. J. Morphol. 1995, 224, 147–157. [Google Scholar] [CrossRef]
- Rempel, J.G. The embryology of the black widow spider, Latrodectus mactans. Can. J. Zool. 1957, 35, 35–74. [Google Scholar] [CrossRef]
- McGregor, A.P.; Hilbrant, M.; Pechmann, M.; Schwager, E.E.; Prpic, N.M.; Damen, W.G.M. Cupiennius salei and Achaearanea tepidariorum: Spider models for investigating evolution and development. BioEssays 2008, 30, 487–498. [Google Scholar] [CrossRef]
- Kanayama, M.; Akiyama-Oda, Y.; Oda, H. Early embryonic development in the spider Achaearanea tepidariorum: Microinjection verifies that cellularization is complete before the blastoderm stage. Arthropod Struct. Dev. 2010, 39, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Pechmann, M. Embryonic development and secondary axis induction in the Brazilian white knee tarantula Acanthoscurria geniculata, C.L. Koch, 1841 (Araneae; Mygalomorphae; Theraphosidae). Dev. Genes Evol. 2020, 230, 75–94. [Google Scholar] [CrossRef] [PubMed]
- Prpic, N.-M.; Pechmann, M. Extraembryonic tissue in chelicerates: A review and outlook. Phil. Trans. R. Soc. B 2022, 377, 20210269. [Google Scholar] [CrossRef]
- Turetzek, N.; Prpic, N.-M. Observations on germ band development in the cellar spider Pholcus phalangioides. Dev. Genes Evol. 2016, 226, 413–422. [Google Scholar] [CrossRef]
- Edgar, A.; Bates, C.; Larkin, K.; Black, S. Gastrulation occurs in multiple phases at two distinct sites in Latrodectus and Cheiracanthium spiders. EvoDevo 2015, 6, 33. [Google Scholar] [CrossRef]
- Liu, Y.; Maas, A.; Waloszek, D. Early development of the anterior body region of the grey widow spider Latrodectus geometricus Koch, 1841 (Theridiidae, Araneae). Arthropod Struct. Dev. 2009, 38, 401–416. [Google Scholar] [CrossRef]
- Hilbrant, M.; Damen, W.G.M.; McGregor, A.P. Evolutionary crossroads in developmental biology: The spider Parasteatoda tepidariorum. Development 2012, 139, 2655–2662. [Google Scholar] [CrossRef] [PubMed]
- Hinton, H.E. Biology of Insect Eggs; Pergammon Press: Oxford, UK, 1981. [Google Scholar]
- van Handel, E. Fuel metabolism of the mosquito (Culex quinquefasciatus) embryo. J. Insect Physiol. 1993, 39, 831–833. [Google Scholar] [CrossRef]
- Campos, E.; Moraes, J.; Facanha, A.R.; Moreira, E.; Valle, D.; Abreu, L.; Manso, P.P.A.; Nascimento, A.; Pelajo-Machado, M.; Lenzi, H.; et al. Kinetics of energy source utilization in Boophilus microplus (Canestrini, 1887) (Acari: Ixodidae) embryonic development. Vet. Parasitol. 2006, 138, 349–357. [Google Scholar] [CrossRef]
- Geister, T.L.; Lorenz, M.W.; Hoffmann, K.H.; Fischer, K. Energetics of embryonic development: Effects of temperature on egg and hatchling composition in a butterfly. J. Comp. Physiol. B 2009, 179, 87–98. [Google Scholar] [CrossRef]
- Santos, R.; Rosas-Oliveira, R.; Saraiva, F.B.; Majerowicz, D.; Gondim, K.C. Lipid accumulation and utilization by oocytes and eggs of Rhodnius prolixus. Arch. Insect Biochem. 2011, 77, 1–16. [Google Scholar] [CrossRef]
- Santana, C.C.; do Nascimento, J.S.; Costa, M.M.; da Silva, A.T.; Dornelas, C.B.; Grillo, L.A.M. Embryonic Development of Rhynchophorus palmarum (Coleoptera: Curculionidae): Dynamics of Energy Source Utilization. J. Insect Sci. 2014, 14, 280. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.A. Alpha-Amylase from developing embryos of the camel tick Hyalomma dromedarii. Comp. Biochem. Phys. B 2000, 126, 99–108. [Google Scholar] [CrossRef]
- Yip, E.C.; Rayor, L.S. Maternal care and subsocial behaviour in spiders. Biol. Rev. 2014, 89, 427–449. [Google Scholar] [CrossRef] [PubMed]
- Laino, A.; Cunningham, M.; Garcia, F.; Trabalon, M. Residual vitellus and energetic state of wolf spiderlings Pardosa saltans after emergence from egg-sac until first predation. J. Comp. Physiol. B 2020, 90, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Boctor, F.N.; Kamel, M.Y. Purification and characterization of two lipovitellins from eggs of the tick, Dermacentor andersoni. Insect Biochem. 1976, 6, 233–240. [Google Scholar] [CrossRef]
- Kamel, M.Y.; Shalaby, F.Y.; Ghazy, A.E.H.M. Biochemical studies of tick embryogenesis DNA, RNA, haemoprotein, guanosine and guanine in developing eggs of Hyalomma Dromedarii. Insect. Biochem. 1982, 12, 15–23. [Google Scholar] [CrossRef]
- Angrell, I.P.; Lundquist, A.M. Physiological and biochemical changes during insect development. In The Physiology of Insecta; Rockstein, M., Ed.; Academic Press: Cambridge, MA, USA, 1973; Volume 1, pp. 159–247. [Google Scholar]
- Kamel, M.Y.; Ragga, R.H. Purification and characterization of pyrophosphatase from developing embryos of Hyalomma dromedarii. Insect Biochem. 1981, 11, 691–698. [Google Scholar] [CrossRef]
- Irie, K.; Yamashita, O. Changes in vitellin and other yolk proteins during embryonic development in the silkworm, Bombix mori. J. Insect Physiol. 1980, 26, 811–817. [Google Scholar] [CrossRef]
- Oliveira, P.L.; Dansa-Petretski, M.; Hatisaburo, M. Vitellin processing and degradation during embryogenesis in Rhodnius Prolixus. Insect Biochem. 1989, 19, 489–498. [Google Scholar] [CrossRef]
- Starrett, J.; Hedin, M.; Ayoub, N.; Hayashi, C.Y. Hemocyanin gene family evolution in spiders (Araneae), with implications for phylogenetic relationships and divergence times in the infraorder Mygalomorphae. Gene 2013, 524, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Sugita, H.; Sekiguchi, K. Protein components in the perivitelline fluid of the embryo of the horseshoe crab, Tachypleus tridentatus. Dev. Biol. 1979, 73, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Ma, R.; Ma, G.; Guo, X.; Tong, X.; Tang, G.; Kang, L. Haemocyanin is essential for embryonic development and survival in the migratory locust. Insect Mol. Biol. 2015, 24, 517–527. [Google Scholar] [CrossRef]
- Leite, D.J.; Schönauer, A.; Blakeley, G.; Harper, A.; Garcia Castro, H.; Baudouin Gonzalez, L.; Wang, R.; Sarkis, N.; Günther Nikola, A.; Koka, V.S.P.; et al. An atlas of spider development at single-cell resolution provides new insights into arthropod embryogenesis. EvoDevo 2024, 15, 5. [Google Scholar] [CrossRef] [PubMed]
- Rakshpal, R. Diapause in the eggs of Gryllus pennsylvanicus Burmeister (Orthoptera: Gryllidae). Can. J. Zool. 1962, 40, 179–194. [Google Scholar] [CrossRef]
- Ingrisch, S. Oxygen consumption by developing and diapausing eggs of Eupholidoptera smyrnensis (Orthoptera: Tettigoniidae). J. Insect Physiol. 1987, 33, 861–865. [Google Scholar] [CrossRef]
- Ludwig, D.; Ramazzotto, L.J. Energy sources during embryogenesis of the yellow mealworm, Tenebrio molitor. Ann. Entomol. Soc. Am. 1965, 58, 543–546. [Google Scholar] [CrossRef]
- Waltero, C.; Martins, R.; Calixto, C.; da Fonseca, R.N.; de Abreu, L.A.; da Silva Vaz, I., Jr.; Logullo, C. The hallmarks of GSK-3 in morphogenesis and embryonic development metabolism in arthropods. Insect Biochem. Mol. Biol. 2020, 118, 103307. [Google Scholar] [CrossRef]
- Yamazaki, H.; Nusse, R. Identification of DCAP, a drosophila homolog of a glucose transport regulatory complex. Mech. Develop. 2002, 119, 115–119. [Google Scholar] [CrossRef]
- Chippendale, G.M. Insect embryogenesis, morphology, physiology, genetical and molecular aspects. In Comprehensive Insect Physiology, Biochemistry and Pharmacology; Pergamon Press: Oxford, UK, 1985; pp. 319–385. [Google Scholar]
- Sloggett, J.J.; Lorenz, M.W. Egg composition and reproductive investment in aphidophagous ladybird beetles (Coccinellidae: Coccinellini): Egg development and interspecific variation. Physiol. Entomol. 2008, 33, 200–208. [Google Scholar] [CrossRef]
- Cunningham, M.; Gonzalez, A.; Dreon, M.; Castro, D.; Pollero, R. Lipid and protein composition at different developmental stages of Pediculus capitis (Arthropoda, Phthiraptera). J. Parasitol. 2001, 87, 1251–1254. [Google Scholar] [CrossRef] [PubMed]
- Rey, B.; Pélisson, P.F.; Bel-Venner, M.C.; Voituron, Y.; Venner, S. Revisiting the link between breeding effort and oxidative balance through field evaluation of two sympatric sibling insect species. Evolution 2015, 69, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Rosa, R.; Calado, R.; Andrade, A.M.; Narciso, L.; Nunes, M.L. Changes in amino acids and lipids during embryogenesis of European lobster, Homarus gammarus (Crustacea: Decapoda). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2005, 140, 241–249. [Google Scholar] [CrossRef]
- Lease, H.M.; Wolf, B.O. Lipid content of terrestrial arthropods in relation to body size, phylogeny, ontogeny and sex. Physiol. Entomol. 2011, 36, 29–38. [Google Scholar] [CrossRef]
- Schartau, W.; Leidescher, T. Composition of the hemolymph of the tarantula Eurypelma californicum. J. Comp. Physiol. 1983, 152, 73–77. [Google Scholar] [CrossRef]
- Albessard, E.; Mayzaud, P.; Cuzin-Roudy, J. Variation of lipid classes among organs of the northern krill Meganyctiphanes norvegica, with respect to reproduction. Comp. Biochem. Phys. A 2001, 129, 373–390. [Google Scholar] [CrossRef]
- Clarke, A.; Skadsheim, A.; Holmes, L.J. Lipid biochemistry and reproductive biology in two species of Gammaridae (Crustacea: Amphipoda). Mar. Biol. 1985, 88, 247–263. [Google Scholar] [CrossRef]
- Vance, J.E.; Tasseva, G. Formation and function of phosphatidylserine and phosphatidylethanolamine in mammalian cells. Biochim. Biophys. Acta (BBA)—Mol. Cell. Biol. Lipids 2013, 1831, 543–554. [Google Scholar] [CrossRef]
- Tocher, D.R.; Fraser, A.J.; Sargent, J.R.; Gamble, J.C. Fatty acid composition of phospholipids and neutral lipids during embryonic and early larval development in Atlantic herring (Clupea harengus, L.). Lipids 1985, 20, 69. [Google Scholar] [CrossRef]
- Merrill, A.H., Jr.; Sandhoff, K. Sphingolipids: Metabolism and cell signaling. In New Comprehensive Biochemistry; Vance, D.E., Vance, J.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2002; Volume 36, pp. 373–407. [Google Scholar]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.L.; Souza, C.M.; Pichler, H.; Dewhurst, G.; Schaad, O.; Kajiwara, K.; Wakabayashi, H.; Ivanova, T.; Castillon, G.A.; Piccolis, M.; et al. Functional interactions between sphingolipids and sterols in biological membranes regulating cell physiology. Mol. Biol. Cell 2009, 20, 2083–2095. [Google Scholar] [CrossRef]
- González-Baró, M.R.; Heras, H.; Pollero, R.J. Enzyme activities involved in lipid metabolism during embryonic development of Macrobrachium borellii. J. Exp. Zool. 2000, 286, 231–237. [Google Scholar] [CrossRef]
- Amsler, M.O.; George, R.Y. Changes in the biochemical composition of Euphausia superba Dana embryos during early development. Polar Biol. 1985, 4, 61–63. [Google Scholar] [CrossRef]
- Needham, J. Biochemistry and Morphogenesis; Cambridge University Press: Cambridge, UK, 1950. [Google Scholar]
- Petersen, S.; Anger, K. Chemical and physiological changes during the embryonic development of the spider crab, Hyas araneus L. (Decapoda: Majidae). Comp. Biochem. Physiol. B 1997, 117, 299–306. [Google Scholar] [CrossRef]
- Lecuona, R.; Riba, G.; Cassier, P.; Clement, J.L. Alterations of insect epicuticular hydrocarbons during infection with Beauveria bassiana or B. brongniartii. J. Invertebr. Pathol. 1991, 58, 10–18. [Google Scholar] [CrossRef]
- Hadley, N.F. Cuticular lipids of terrestrial plants and arthropods: A comparison of their structure, composition, and waterproofing function. Biol. Rev. 1981, 56, 23–47. [Google Scholar] [CrossRef]
- Gibbs, A.G. Water-proofing properties of cuticular lipids. Am. Zool. 1998, 38, 471–482. [Google Scholar] [CrossRef]
- Blomquist, G.J.; Bagnères, A.G. Insect Hydrocarbons: Biology, Biochemistry, and Chemical Ecology; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Fan, Y.; Eliyahu, D.; Schal, C. Cuticular hydrocarbons as maternal provisions in embryos and nymphs of the cockroach Blattella germanica. J. Exp. Biol. 2008, 211, 548–554. [Google Scholar] [CrossRef]
- Andersen, S.O. Biochemistry of insect cuticle. Annu. Rev. Entomol. 1979, 24, 29–59. [Google Scholar] [CrossRef]
- Merzendorfer, H.; Zimoch, L. Chitin metabolism in insects: Structure, function and regulation of chitin synthases and chitinases. J. Exp. Biol. 2003, 206, 4393–4412. [Google Scholar] [CrossRef] [PubMed]
- Martin-Creuzburg, D.; Westerlund, S.A.; Hoffmann, K.H. Ecdysteroid levels in Daphnia magna during a molt cycle: Determination by radioimmunoassay (RIA) and liquid chromatography–mass spectrometry (LC–MS). Gen. Comp. Endocr. 2007, 151, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Sargent, J.R.; Henderson, R.J.; Tocher, D.R. The Lipids in Fish Nutrition; Academic Press: NewYork, NY, USA, 1989; pp. 153–218. [Google Scholar]
- Morais, S.; Narciso, L.; Calado, R.; Nunes, M.L.; Rosa, R. Lipid dynamics during the embryonic development of Plesionika martia martia (Decapoda; Pandalidae), Palaemon serratus and P. elegans (Decapoda; Palaemonidae): Relation to metabolic consumption. Mar. Ecol. Prog. Ser. 2002, 242, 195–204. [Google Scholar] [CrossRef]
- Rosa, R.; Calado, R.; Narciso, L.; Nunes, M.L. Embryogenesis of decapod crustaceans with different life history traits, feeding ecologies and habitats: A fatty acid approach. Mar. Biol. 2007, 151, 935–947. [Google Scholar] [CrossRef]
- Hoppe, K.T.; Hadley, N.F.; Trelease, R.N. Changes in lipid and fatty acid composition of eggs during development of the beet armyworm, Spodoptera exigua. J. Insect Physiol. 1975, 21, 1427–1430. [Google Scholar] [CrossRef]
- Figueiredo, J.; Lin, J.; Anto, J.; Narciso, L. The consumption of DHA during embryogenesis as an indicative of the need to supply DHA during early larval development: A review. J. Aquac. Res. Dev. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Heimer, S. Wunderbare Welt der Spinnen, Urania; Verlag Leipzig Jena Berlin (Urania): Berlin, Germany, 1988. [Google Scholar]
- Tahir, H.M.; Zahra, K.; Zaheer, A.; Samiullah, K. Spider silk: An excellent biomaterial for medical science and industry. Punjab Univ. J. Zool. 2017, 32, 143–154. [Google Scholar]
- Malcicka, M.; Visser, B.; Ellers, J. An evolutionary perspective on linoleic acid synthesis in animals. Evol. Biol. 2018, 45, 15–26. [Google Scholar] [CrossRef]
- D’Abramo, L.R.; Conklin, D.E.; Akiyama, D.M. Crustacean Nutrition: Advances in World Aquaculture; World Aquaculture Society: San Diego, CA, USA, 1997; Volume 6, 587p. [Google Scholar]
- González-Félix, M.L.; Gatlin, D.M.; Lawrence, A.L.; Perez-Velazquez, M. Nutritional evaluation of fatty acids for the open thelycum shrimp, Litopenaeus vannamei: II. Effect of dietary n-3 and n-6 polyunsaturated and highly unsaturated fatty acids on juvenile shrimp growth, survival, and fatty acid composition. Aquac. Nutr. 2003, 9, 115–122. [Google Scholar] [CrossRef]
- González-Félix, M.L.; Lawrence, A.L.; Gatlin, D.M.; Perez-Velazquez, M. Nutritional evaluation of fatty acids for the open thelycum shrimp, Litopenaeus vannamei: I. Effect of dietary linoleic and linolenic acids at different concentrations and ratios on juvenile shrimp growth, survival and fatty acid composition. Aquac. Nutr. 2003, 9, 105–113. [Google Scholar] [CrossRef]
- Meijer, L.; Brash, A.R.; Bryant, R.W.; Ng, K.; Maclouf, J.; Sprecher, H. Stereospecific induction of starfish oocyte maturation by (8R)-hydroxyeicosatetraenoic acid. J. Biol. Chem. 1986, 261, 17040–17047. [Google Scholar] [CrossRef] [PubMed]
- Stanley-Samuelson, D.W.; Jensen, E.; Nickerson, K.W.; Tiebel, K.; Ogg, C.L.; Howard, R.W. Insect immune response to bacterial infection is mediated by eicosanoids. Proc. Natl. Acad. Sci. USA 1991, 88, 1064–1068. [Google Scholar] [CrossRef] [PubMed]
- Petzel, D.H. Prostanoids and fluid balance in insects. In Insect Lipids: Chemistry, Biochemistry and Biology; Stanley-Samuelson, D., Nelson, D.R., Eds.; University of Nebraska Press: Lincoln, RI, USA, 1993; pp. 139–178. [Google Scholar]
- Stanley-Samuelson, D.W.; Pedibhotla, V.K. What can we learn from prostaglandins and related eicosanoids in insects? Insect Biochem. Mol. 1996, 26, 223–234. [Google Scholar] [CrossRef]
- Reddy, R.D.; Keshavan, M.S.; Yao, J.K. Reduced red blood cell membrane essential polyunsaturated fatty acids in first episode schizophrenia at neuroleptic-naive baseline. Schizophr. Bull. 2004, 30, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Morishima, I.; Yamano, Y.; Inoue, K.; Matsuo, N. Eicosanoids mediate induction of immune genes in the fat body of the silkworm, Bombyx mori. FEBS Lett. 1997, 419, 83–86. [Google Scholar] [CrossRef]
- Park, Y.; Kim, Y.; Putnam, S.M.; Stanley, D.W. The bacterium Xenorhabdus nematophilus depresses nodulation reactions to infection by inhibiting eicosanoid biosynthesis in tobacco hornworms, Manduca sexta. Arch. Insect Biochem. 2003, 52, 71–80. [Google Scholar] [CrossRef]
- Medina-Jimenez, B.I.; Budd, G.E.; Janssen, R. Single-cell RNA sequencing of mid-to-late-stage spider embryos: New insights into spider development. BMC Genom. 2024, 25, 150. [Google Scholar] [CrossRef]

| Species of Arachnids | Number of Majority Eggs Proteins and Their Molecular Weight (kDa) | Presence of Vgs | Vg Origin or Detection Place |
|---|---|---|---|
| Tegenaria atrica [18] (spider) | 47- | x | Detection: hemolymph and ovaries. |
| Schizocosa malitiosa [55] (spider) | SmLV1: 116, 87 and 42 SmLV2: 135, 126, 109 and 70 | - | Detection: eggs |
| Pardosa saltans [54] (spider) | 116, 87, 70 and 42 | - | Detection: eggs |
| Polybetes pythagoricus [56,57] (spider) | LV1: 120, 75, 46 and 30 LV2: 170, 120, 109, 75, 67 and 30 | - | Detection: eggs |
| Parasteatoda tepidariorum [16] (spider) | Vg 250, 47 and 30 | Origen: midgut glands, ovaries and hemolymph. | |
| Ixodes scapularis [58,59] (acari) | 154, 135, 87, 78, 67, 64 and 35 | x | Origin: fat body Detection: fat bod and hemolymph |
| Ornithodoros moubata [41] (acari) | 160, 140, 125, 100, 64 and 50 | x | Detection: fat body, midgut and hemolymph |
| Argas hermanni [60] (acari) | Exogenous: 66.2 to >200 Endogenous: 22 to 59 Eggs-specific 35.5 and 47.2 | x | Detection: hemolymph and ovary |
| Ornithodoros parkeri [61] (acari) | 160, 140, 125, 100, 64 and 50 | x | Origin: fat body Detection: fat body and hemolymph |
| Dermacentor variabilis [62] (acari) | VnA: 135, 110, 98, 80, 67, 50, 45 and 35 VnB: Identical to VnA with the addition of 93 kDa subunit. | x | Origin: fat body and midgut Detection: fat body and midgut, hemolymph, and ovary |
| Rhipicephalus microplus (formerly Boophilus microplus) [63,64,65,66] (acari) | 107, 102, 87, 67, 65 and 44 | Multiples Vgs | Origin: ovaries and extraovarian tissues. Detection: hemolymph and ovary |
| Tetranychus urticae [67,68] (acari) | (Multiple subunits) 3.6 to 290 | - | - |
| Haemaphysalis longicornis [29,69] (acari) | 203, 147, 126, 82, 74, 70, 61, 47 and 31 | Multiples Vgs | Detection: fat body, hemolymph, and ovary |
| Species of Arachnids | Molecular Weight | N-Terminal Sequence | Detection Place | Observations |
|---|---|---|---|---|
| P. pythagoricus [56] | 75 kDa | AEKMADW(S)KYLKE | Egg | |
| 67 kDa | VVKEKEDRILEXFE | Egg | ||
| 46 kDa | SIMYNEKDDIXVENR | Egg | ||
| 30 KDa | (G)PFQRQSQXAT(R) | Egg | ||
| Tegenaria atrica [18] | 47 kDa | XVEDIEGEVQERLRE | Hemolymph and ovarian | |
| Pardosa pseudoannulata [70] | cDNAs encoding vitellogenins (PpVg1, PpVg2 and PpVg3) | |||
| Parasteatoda tepidariorum [16] | Two genes encoding Vg (PtVg4 and PtVg6) |
| Protein (µg/mg Eggs) | Lipid (µg/mg Eggs) | Carbohydrates (µg/mg Eggs) | References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LV1 | LV2 | Total | LV1 | LV2 | Total | LV1 | LV2 | Total | ||
| Polybetes pythagoricus (spider) | 13.1 | 11.1 | 87.5 | 13.2 | 1.2 | 50 | 0.3 | 0.08 | 0.3 (glycogen) | [57,110] |
| Schizocosa malitiosa (spider) | 3.9 | 3.3 | 12.6 | 1.6 | 0.3 | 8.1 | 0.1 | 0.2 | 4.5 | [55] |
| Pardosa saltans (spider) | 7.7 | 22.1 | 0.8 | [54] | ||||||
| Adalia bipunctata (insect) | 130 | 100 | 1.8 | [211] | ||||||
| Adalia decempunctata (insect) | 145 | 115 | 2.4 | [211] | ||||||
| Anisosticta novemdecimpunctata (insect) | 145 | 125 | 1.3 | [211] | ||||||
| Macrobrachium borellii (crustacean) | 9.2 | 2.7 | [48,119] | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, C.F.; Laino, A.; Cunningham, M. Vitellogenesis and Embryogenesis in Spiders: A Biochemical Perspective. Insects 2025, 16, 398. https://doi.org/10.3390/insects16040398
Garcia CF, Laino A, Cunningham M. Vitellogenesis and Embryogenesis in Spiders: A Biochemical Perspective. Insects. 2025; 16(4):398. https://doi.org/10.3390/insects16040398
Chicago/Turabian StyleGarcia, Carlos Fernando, Aldana Laino, and Mónica Cunningham. 2025. "Vitellogenesis and Embryogenesis in Spiders: A Biochemical Perspective" Insects 16, no. 4: 398. https://doi.org/10.3390/insects16040398
APA StyleGarcia, C. F., Laino, A., & Cunningham, M. (2025). Vitellogenesis and Embryogenesis in Spiders: A Biochemical Perspective. Insects, 16(4), 398. https://doi.org/10.3390/insects16040398







