Acaricide Resistance Monitoring and Structural Insights for Precision Tetranychus urticae Management
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
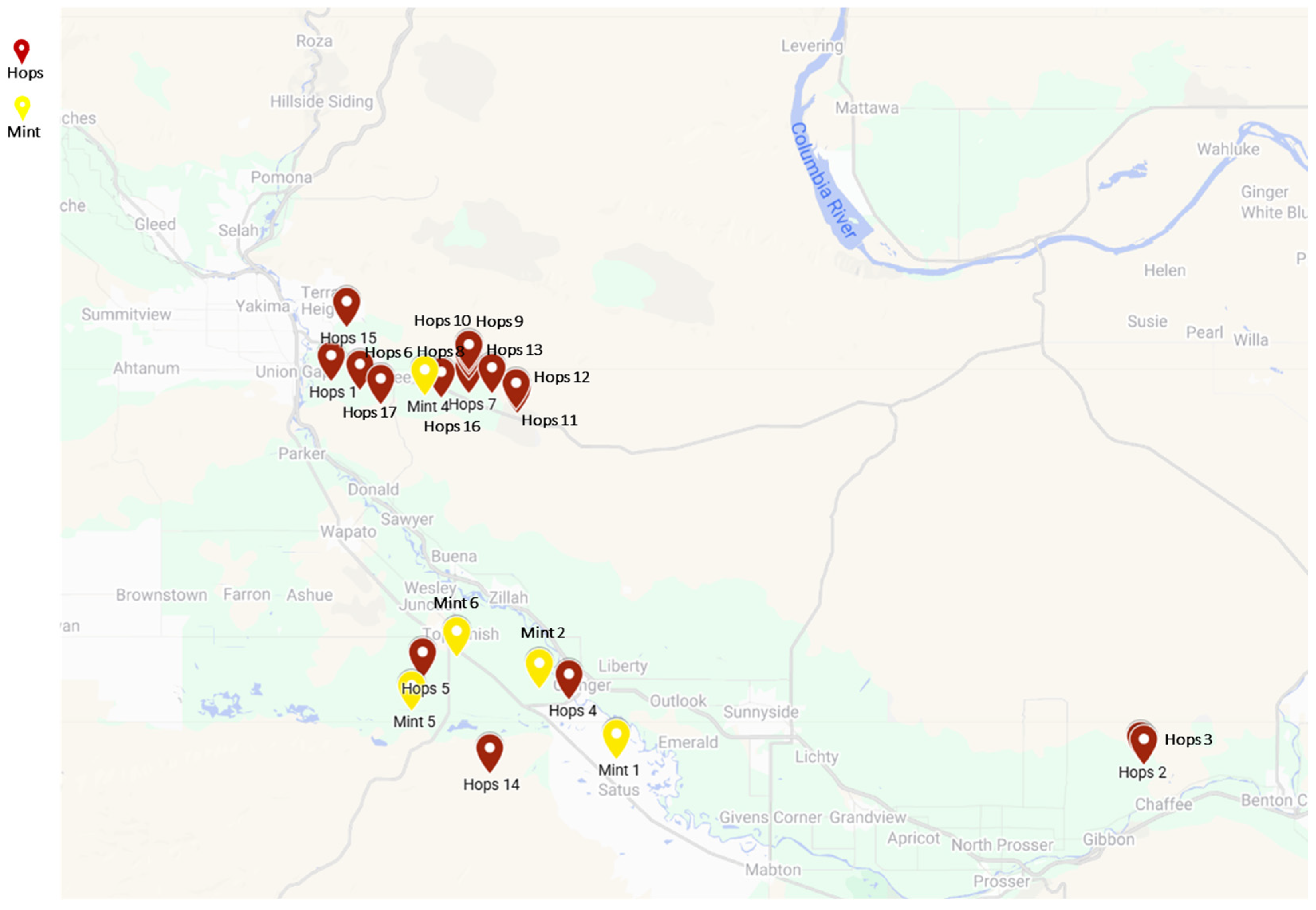
2.1. Mite Populations
2.2. Bioassays
2.3. Detection of Mutations Associated with Acaricide Resistance
2.4. AlphaFold 2 Models and Molecular Docking for Acaricides
3. Results
3.1. Different Resistance Patterns of TSSMs Collected from Hop and Mint Fields
3.2. kdr Mutations Associated with Pyrethroid Resistance in Hop and Mint TSSM Populations
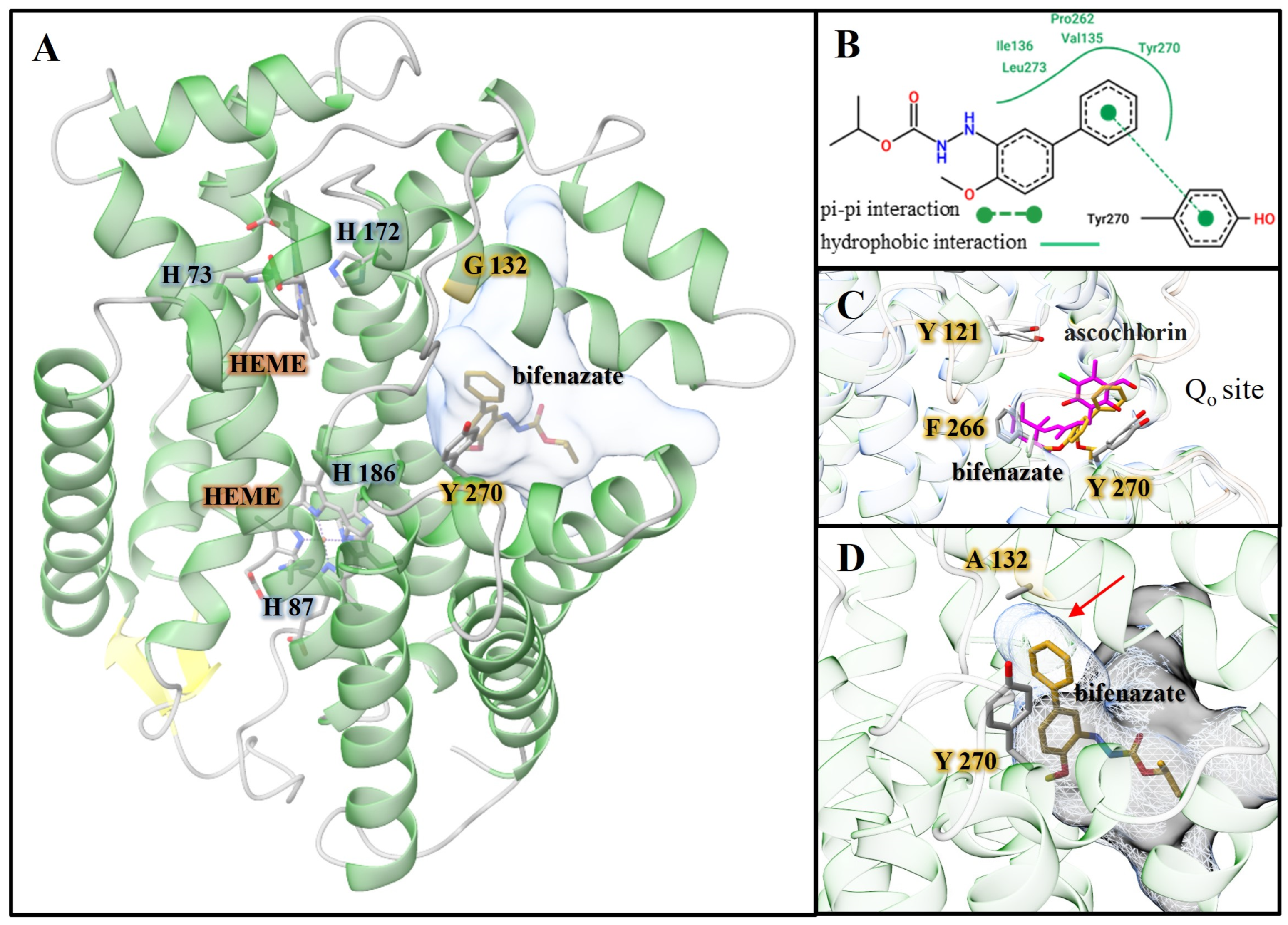
3.3. Molecular Docking of Tucytb with Bifenazate
3.4. Molecular Docking of TuCHS1 with Etoxazole
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Attia, S.; Grissa, K.L.; Lognay, G.; Bitume, E.; Hance, T.; Mailleux, A.C. A review of the major biological approaches to control the worldwide pest Tetranychus urticae (Acari: Tetranychidae) with special reference to natural pesticides. J. Pest Sci. 2013, 86, 361–386. [Google Scholar] [CrossRef]
- Grbić, M.; Van Leeuwen, T.; Clark, R.M.; Rombauts, S.; Rouzé, P.; Grbić, V.; Osborne, E.J.; Dermauw, W.; Thi Ngoc, P.C.; Ortego, F. The genome of Tetranychus urticae reveals herbivorous pest adaptations. Nature 2011, 479, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, T.; Dermauw, W.; Grbic, M.; Tirry, L.; Feyereisen, R. Spider mite control and resistance management: Does a genome help? Pest Manag. Sci. 2013, 69, 156–159. [Google Scholar] [CrossRef]
- Adesanya, A.W.; Lavine, M.D.; Moural, T.W.; Lavine, L.C.; Zhu, F.; Walsh, D.B. Mechanisms and management of acaricide resistance for Tetranychus urticae in agroecosystems. J. Pest Sci. 2021, 94, 639–663. [Google Scholar] [CrossRef]
- Morales, M.A.; Mendoza, B.M.; Lavine, L.C.; Lavine, M.D.; Walsh, D.B.; Zhu, F. Selection of reference genes for expression studies of xenobiotic adaptation in Tetranychus urticae. Int. J. Biol. Sci. 2016, 12, 1129–1139. [Google Scholar] [CrossRef]
- Adesanya, A.W.; Franco, E.; Walsh, D.B.; Lavine, M.; Lavine, L.; Zhu, F. Phenotypic and genotypic plasticity of acaricide resistance in populations of Tetranychus urticae (Acari: Tetranychidae) on peppermint and silage corn in the Pacific Northwest. J. Econ. Entomol. 2018, 111, 2831–2843. [Google Scholar] [CrossRef]
- Park, Y.L.; Lee, J.H. Leaf cell and tissue damage of cucumber caused by twospotted spider mite (Acari: Tetranychidae). J. Econ. Entomol. 2002, 95, 952–957. [Google Scholar] [CrossRef]
- Koirala, B.K.S.; Bhattarai, G.; Adesanya, A.W.; Moural, T.W.; Lavine, L.C.; Walsh, D.B.; Zhu, F. Transcriptome analysis unveils molecular mechanisms of acaricide resistance in two-spotted spider mite populations on hops. Int. J. Mol. Sci. 2024, 25, 13298. [Google Scholar] [CrossRef]
- Piraneo, T.G.; Bull, J.; Morales, M.A.; Lavine, L.C.; Walsh, D.B.; Zhu, F. Molecular mechanisms of Tetranychus urticae chemical adaptation in hop fields. Sci. Rep. 2015, 5, 17090. [Google Scholar] [CrossRef]
- Wu, M.; Adesanya, A.W.; Morales, M.A.; Walsh, D.B.; Lavine, L.C.; Lavine, M.D.; Zhu, F. Multiple acaricide resistance and underlying mechanisms in Tetranychus urticae on hops. J. Pest Sci. 2019, 92, 543–555. [Google Scholar] [CrossRef]
- Okwany, R.O.; Peters, T.R.; Ringer, K.L.; Walsh, D.B.; Rubio, M. Impact of sustained deficit irrigation on spearmint (Mentha spicata L.) biomass production, oil yield, and oil quality. Irrig. Sci. 2011, 30, 213–219. [Google Scholar] [CrossRef]
- McMurtry, J.A.; Croft, B.A. Life-styles of Phytoseiid mites and their roles in biological control. Annu. Rev. Entomol. 1997, 42, 291–321. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, T.; Vontas, J.; Tsagkarakou, A.; Dermauw, W.; Tirry, L. Acaricide resistance mechanisms in the two-spotted spider mite Tetranychus urticae and other important Acari: A review. Insect Biochem. Mol. Biol. 2010, 40, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, T.; Tirry, L.; Yamamoto, A.; Nauen, R.; Dermauw, W. The economic importance of acaricides in the control of phytophagous mites and an update on recent acaricide mode of action research. Pestic. Biochem. Physiol. 2015, 121, 12–21. [Google Scholar] [CrossRef]
- Cruse, C.; Moural, T.W.; Zhu, F. Dynamic roles of insect carboxyl/cholinesterases in chemical adaptation. Insects 2023, 14, 194. [Google Scholar] [CrossRef]
- Abendroth, J.A.; Moural, T.W.; Wei, H.; Zhu, F. Roles of insect odorant binding proteins in communication and xenobiotic adaptation. Front. Insect Sci. 2023, 3, 1274197. [Google Scholar] [CrossRef]
- Zhu, F.; Gujar, H.; Gordon, J.R.; Haynes, K.F.; Potter, M.F.; Palli, S.R. Bed bugs evolved unique adaptive strategy to resist pyrethroid insecticides. Sci. Rep. 2013, 3, 1456. [Google Scholar] [CrossRef]
- Koirala, B.K.S.; Moural, T.; Zhu, F. Functional and structural diversity of insect glutathione S-transferases in xenobiotic adaptation. Int. J. Biol. Sci. 2022, 18, 5713. [Google Scholar] [CrossRef]
- Zhu, F.; Cui, Y.; Walsh, D.B.; Lavine, L.C. Application of RNAi towards insecticide resistance management. In Short Views on Insect Biochemistry and Molecular Biology; Chandrasekar, R., Tyagi, B.K., Gui, Z., Reeck, G.R., Eds.; Academic Publisher: Manhattan, KS, USA, 2014; Volume 2, pp. 595–619. [Google Scholar]
- Moural, T.W.; Koirala, B.K.S.; Bhattarai, G.; He, Z.; Guo, H.; Phan, N.T.; Rajotte, E.G.; Biddinger, D.J.; Hoover, K.; Zhu, F. Architecture and potential roles of a delta-class glutathione S-transferase in protecting honey bee from agrochemicals. Chemosphere 2024, 350, 141089. [Google Scholar] [CrossRef]
- De Rouck, S.; İnak, E.; Dermauw, W.; Van Leeuwen, T. A review of the molecular mechanisms of acaricide resistance in mites and ticks. Insect Biochem. Mol. Biol. 2023, 159, 103981. [Google Scholar] [CrossRef]
- Zhorov, B.S.; Dong, K. Pyrethroids in an AlphaFold2 model of the insect sodium channel. Insects 2022, 13, 745. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Chen, H.; Hu, M.; Wang, X.; Zhang, L. Discovery of novel potential insecticide-resistance mutations in Spodoptera frugiperda. Insects 2024, 15, 186. [Google Scholar] [CrossRef] [PubMed]
- Mirdita, M.; Schütze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making protein folding accessible to all. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 update. Nucleic Acids Res. 2022, 51, D1373–D1380. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New docking methods, expanded force field, and python bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Rosignoli, S.; Paiardini, A. DockingPie: A consensus docking plugin for PyMOL. Bioinformatics 2022, 38, 4233–4234. [Google Scholar] [CrossRef]
- Steinkellner, G.; Rader, R.; Thallinger, G.G.; Kratky, C.; Gruber, K. VASCo: Computation and visualization of annotated protein surface contacts. BMC Bioinform. 2009, 10, 32. [Google Scholar] [CrossRef]
- Hendlich, M.; Rippmann, F.; Barnickel, G. LIGSITE: Automatic and efficient detection of potential small molecule-binding sites in proteins. J. Mol. Graph. Model. 1997, 15, 359–363. [Google Scholar] [CrossRef]
- Lomize, A.L.; Todd, S.C.; Pogozheva, I.D. Spatial arrangement of proteins in planar and curved membranes by PPM 3.0. Protein Sci. 2022, 31, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Stierand, K.; Rarey, M. Drawing the PDB: Protein-ligand complexes in two dimensions. ACS Med. Chem. Lett. 2010, 1, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Stierand, K.; Rarey, M. From modeling to medicinal chemistry: Automatic generation of two-dimensional complex diagrams. ChemMedChem 2007, 2, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Stierand, K.; Maass, P.C.; Rarey, M. Molecular complexes at a glance: Automated generation of two-dimensional complex diagrams. Bioinformatics 2006, 22, 1710–1716. [Google Scholar] [CrossRef]
- Fricker, P.C.; Gastreich, M.; Rarey, M. Automated drawing of structural molecular formulas under constraints. J. Chem. Inf. Comput. Sci. 2004, 44, 1065–1078. [Google Scholar] [CrossRef]
- Goddard, T.D.; Huang, C.C.; Meng, E.C.; Pettersen, E.F.; Couch, G.S.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Meeting modern challenges in visualization and analysis. Protein Sci. 2018, 27, 14–25. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef]
- Meng, E.C.; Goddard, T.D.; Pettersen, E.F.; Couch, G.S.; Pearson, Z.J.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Tools for structure building and analysis. Protein Sci. 2023, 32, e4792. [Google Scholar] [CrossRef]
- Pan, X.; Li, Z.; Huang, X.; Huang, G.; Gao, S.; Shen, H.; Liu, L.; Lei, J.; Yan, N. Molecular basis for pore blockade of human Na(+) channel Na(v)1.2 by the μ-conotoxin KIIIA. Science 2019, 363, 1309–1313. [Google Scholar] [CrossRef]
- Shen, H.; Zhou, Q.; Pan, X.; Li, Z.; Wu, J.; Yan, N. Structure of a eukaryotic voltage-gated sodium channel at near-atomic resolution. Science 2017, 355, eaal4326. [Google Scholar] [CrossRef]
- Shen, H.; Li, Z.; Jiang, Y.; Pan, X.; Wu, J.; Cristofori-Armstrong, B.; Smith, J.J.; Chin, Y.K.Y.; Lei, J.; Zhou, Q.; et al. Structural basis for the modulation of voltage-gated sodium channels by animal toxins. Science 2018, 362, eaau2596. [Google Scholar] [CrossRef] [PubMed]
- de Lera Ruiz, M.; Kraus, R.L. Voltage-gated sodium channels: Structure, function, pharmacology, and clinical indications. J. Med. Chem. 2015, 58, 7093–7118. [Google Scholar] [CrossRef] [PubMed]
- Crofts, A.R. The cytochrome bc1 complex: Function in the context of structure. Annu. Rev. Physiol. 2004, 66, 689–733. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.F.; Wang, F.; Li, H.; Zhu, X.L.; Yang, W.C.; Huang, L.S.; Wu, J.W.; Berry, E.A.; Yang, G.F. Computational discovery of picomolar Q(o) site inhibitors of cytochrome bc1 complex. J. Am. Chem. Soc. 2012, 134, 11168–11176. [Google Scholar] [CrossRef]
- Berry, E.A.; Huang, L.S.; Lee, D.W.; Daldal, F.; Nagai, K.; Minagawa, N. Ascochlorin is a novel, specific inhibitor of the mitochondrial cytochrome bc1 complex. Biochim. Biophys. Acta 2010, 1797, 360–370. [Google Scholar] [CrossRef]
- Van Nieuwenhuyse, P.; Demaeght, P.; Dermauw, W.; Khalighi, M.; Stevens, C.V.; Vanholme, B.; Tirry, L.; Lümmen, P.; Van Leeuwen, T. On the mode of action of bifenazate: New evidence for a mitochondrial target site. Pestic. Biochem. Physiol. 2012, 104, 88–95. [Google Scholar] [CrossRef]
- Van Leeuwen, T.; Vanholme, B.; Van Pottelberge, S.; Van Nieuwenhuyse, P.; Nauen, R.; Tirry, L.; Denholm, I. Mitochondrial heteroplasmy and the evolution of insecticide resistance: Non-Mendelian inheritance in action. Proc. Natl. Acad. Sci. USA 2008, 105, 5980–5985. [Google Scholar] [CrossRef]
- De Beer, B.; Vandenhole, M.; Njiru, C.; Spanoghe, P.; Dermauw, W.; Van Leeuwen, T. High-resolution genetic mapping combined with transcriptome profiling reveals that both target-site resistance and increased detoxification confer resistance to the pyrethroid bifenthrin in the spider mite Tetranychus urticae. Biology 2022, 11, 1630. [Google Scholar] [CrossRef]
- Silver, K.S.; Du, Y.; Nomura, Y.; Oliveira, E.E.; Salgado, V.L.; Zhorov, B.S.; Dong, K. Voltage-gated sodium channels as insecticide targets. Adv. Insect Physiol. 2014, 46, 389–433. [Google Scholar] [CrossRef]
- Dong, K.; Du, Y.; Rinkevich, F.; Nomura, Y.; Xu, P.; Wang, L.; Silver, K.; Zhorov, B.S. Molecular biology of insect sodium channels and pyrethroid resistance. Insect Biochem. Mol. Biol. 2014, 50, 1–17. [Google Scholar] [CrossRef]
- Bajda, S.A.; De Clercq, P.; Van Leeuwen, T. Selectivity and molecular stress responses to classical and botanical acaricides in the predatory mite Phytoseiulus persimilis Athias-Henriot (Acari: Phytoseiidae). Pest Manag. Sci. 2022, 78, 881–895. [Google Scholar] [CrossRef] [PubMed]
- Riga, M.; Bajda, S.; Themistokleous, C.; Papadaki, S.; Palzewicz, M.; Dermauw, W.; Vontas, J.; Leeuwen, T.V. The relative contribution of target-site mutations in complex acaricide resistant phenotypes as assessed by marker assisted backcrossing in Tetranychus urticae. Sci. Rep. 2017, 7, 9202. [Google Scholar] [CrossRef] [PubMed]
- Tsagkarakou, A.; Van Leeuwen, T.; Khajehali, J.; Ilias, A.; Grispou, M.; Williamson, M.S.; Tirry, L.; Vontas, J. Identification of pyrethroid resistance associated mutations in the para sodium channel of the two-spotted spider mite Tetranychus urticae (Acari: Tetranychidae). Insect Mol. Biol. 2009, 18, 583–593. [Google Scholar] [CrossRef]
- Kwon, D.H.; Clark, J.M.; Lee, S.H. Cloning of a sodium channel gene and identification of mutations putatively associated with fenpropathrin resistance in Tetranychus urticae. Pestic. Biochem. Physiol. 2010, 97, 93–100. [Google Scholar] [CrossRef]
- Zong, X.; Song, J.; Huang, X.; Zhu, Y.; Yu, H.; Ning, G.; Zhao, J. Monitoring acaricide resistance and the frequency of associated target-site mutations in Tetranychus urticae populations from rose glasshouses in China. Pest Manag. Sci. 2024, 81, 1579–1591. [Google Scholar] [CrossRef]
- González-Cabrera, J.; Davies, T.G.; Field, L.M.; Kennedy, P.J.; Williamson, M.S. An amino acid substitution (L925V) associated with resistance to pyrethroids in Varroa destructor. PLoS ONE 2013, 8, e82941. [Google Scholar] [CrossRef]
- Morin, S.; Williamson, M.S.; Goodson, S.J.; Brown, J.K.; Tabashnik, B.E.; Dennehy, T.J. Mutations in the Bemisia tabaci para sodium channel gene associated with resistance to a pyrethroid plus organophosphate mixture. Insect Biochem. Mol. Biol. 2002, 32, 1781–1791. [Google Scholar] [CrossRef]
- Rameshgar, F.; Khajehali, J.; Nauen, R.; Bajda, S.; Jonckheere, W.; Dermauw, W.; Van Leeuwen, T. Point mutations in the voltage-gated sodium channel gene associated with pyrethroid resistance in Iranian populations of the European red mite Panonychus ulmi. Pestic. Biochem. Physiol. 2019, 157, 80–87. [Google Scholar] [CrossRef]
- Chen, H.; Li, K.; Wang, X.; Yang, X.; Lin, Y.; Cai, F.; Zhong, W.; Lin, C.; Lin, Z.; Ma, Y. First identification of kdr allele F1534S in VGSC gene and its association with resistance to pyrethroid insecticides in Aedes albopictus populations from Haikou City, Hainan Island, China. Infect. Dis. Poverty 2016, 5, 31. [Google Scholar] [CrossRef]
- Du, Y.; Nomura, Y.; Zhorov, B.S.; Dong, K. Sodium channel mutations and pyrethroid resistance in Aedes aegypti. Insects 2016, 7, 60. [Google Scholar] [CrossRef]
- Modak, M.P.; Saha, D. First report of F1534C kdr mutation in deltamethrin resistant Aedes albopictus from northern part of West Bengal, India. Sci. Rep. 2022, 12, 13653. [Google Scholar] [CrossRef] [PubMed]
- Van Nieuwenhuyse, P.; Van Leeuwen, T.; Khajehali, J.; Vanholme, B.; Tirry, L. Mutations in the mitochondrial cytochrome b of Tetranychus urticae Koch (Acari: Tetranychidae) confer cross-resistance between bifenazate and acequinocyl. Pest Manag. Sci. 2009, 65, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Fotoukkiaii, S.M.; Tan, Z.; Xue, W.; Wybouw, N.; Van Leeuwen, T. Identification and characterization of new mutations in mitochondrial cytochrome b that confer resistance to bifenazate and acequinocyl in the spider mite Tetranychus urticae. Pest Manag. Sci. 2020, 76, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Wybouw, N.; Van Leeuwen, T. The G126S substitution in mitochondrially encoded cytochrome b does not confer bifenazate resistance in the spider mite Tetranychus urticae. Exp. Appl. Acarol. 2021, 85, 161–172. [Google Scholar] [CrossRef]
- Lu, X.; Vandenhole, M.; Tsakireli, D.; Pergantis, S.A.; Vontas, J.; Jonckheere, W.; Van Leeuwen, T. Increased metabolism in combination with the novel cytochrome b target-site mutation L258F confers cross-resistance between the Qo inhibitors acequinocyl and bifenazate in Tetranychus urticae. Pestic. Biochem. Physiol. 2023, 192, 105411. [Google Scholar] [CrossRef]
- Hoy, M.A.; Ouyang, Y.-L. Selectivity of the acaricides clofentezine and hexythiazox to the predator Metaseiulus occidentalis (Acari: Phytoseiidae). J. Econ. Entomol. 1986, 79, 1377–1380. [Google Scholar] [CrossRef]
- Adesanya, A.; Morales, M.; Walsh, D.; Lavine, L.; Lavine, M.; Zhu, F. Mechanisms of resistance to three mite growth inhibitors of Tetranychus urticae in hops. Bull. Entomol. Res. 2018, 108, 23–34. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Merzendorfer, H.; Zhang, W.; Zhang, J.; Muthukrishnan, S. Biosynthesis, turnover, and functions of chitin in insects. Annu. Rev. Entomol. 2016, 61, 177–196. [Google Scholar] [CrossRef]
- Van Leeuwen, T.; Demaeght, P.; Osborne, E.J.; Dermauw, W.; Gohlke, S.; Nauen, R.; Grbic, M.; Tirry, L.; Merzendorfer, H.; Clark, R.M. Population bulk segregant mapping uncovers resistance mutations and the mode of action of a chitin synthesis inhibitor in arthropods. Proc. Natl. Acad. Sci. USA 2012, 109, 4407–4412. [Google Scholar] [CrossRef]
- Demaeght, P.; Osborne, E.J.; Odman-Naresh, J.; Grbić, M.; Nauen, R.; Merzendorfer, H.; Clark, R.M.; Van Leeuwen, T. High resolution genetic mapping uncovers chitin synthase-1 as the target-site of the structurally diverse mite growth inhibitors clofentezine, hexythiazox and etoxazole in Tetranychus urticae. Insect Biochem. Mol. Biol. 2014, 51, 52–61. [Google Scholar] [CrossRef]
- Xue, W.; Snoeck, S.; Njiru, C.; Inak, E.; Dermauw, W.; Van Leeuwen, T. Geographical distribution and molecular insights into abamectin and milbemectin cross-resistance in European field populations of Tetranychus urticae. Pest Manag. Sci. 2020, 76, 2569–2581. [Google Scholar] [CrossRef] [PubMed]
- Dermauw, W.; Ilias, A.; Riga, M.; Tsagkarakou, A.; Grbić, M.; Tirry, L.; Van Leeuwen, T.; Vontas, J. The cys-loop ligand-gated ion channel gene family of Tetranychus urticae: Implications for acaricide toxicology and a novel mutation associated with abamectin resistance. Insect Biochem. Mol. Biol. 2012, 42, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; He, Y.; Zhang, Y.; Xie, W.; Wu, Q.; Wang, S. Status of pesticide resistance and associated mutations in the two-spotted spider mite, Tetranychus urticae, in China. Pestic. Biochem. Physiol. 2018, 150, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Pavlidi, N.; Tseliou, V.; Riga, M.; Nauen, R.; Van Leeuwen, T.; Labrou, N.E.; Vontas, J. Functional characterization of glutathione S-transferases associated with insecticide resistance in Tetranychus urticae. Pestic. Biochem. Physiol. 2015, 121, 53–60. [Google Scholar] [CrossRef]



| Population | TuVGSC II | TuVGSC III | TuVGSC II-III | ||||||
|---|---|---|---|---|---|---|---|---|---|
| M918L | L925M | T929I | L932F | L1014H | L1024V | F1534S | F1538I | A1215D | |
| Susceptible | M | L | T | L | L | L | F | F | A |
| Ph-1 | M | L | T | L | L | L | F | F | A |
| Ph-2 | M | L/M | T | L | L | L | F | F/I | A |
| Ph-3 | M | L/M | T | L | L | L | --- | --- | A |
| Ph-4 | M | L/M | T | L | L | L | --- | --- | A |
| Ph-5 | --- | --- | --- | --- | --- | --- | F/S | F/I | A |
| Ph-6 | M | L/M | T | L | L | L | F | F/I | A |
| Ph-7 | M | L/M | T | L | L | L | F | F/I | A |
| Ph-8 | M | L | T | L | L | L | F | F/I | A |
| Ph-9 | M | L/M | T | L | L | L/V | F | F/I | A |
| Ph-10 | M | L | T | L | L | L | F | F/I | A |
| Ph-11 | M | L | T | L | L | L | F | F/I | A |
| Ph-12 | M | L/M | T | L | L | L | F | I | A |
| Ph-13 | M | L | T | L | L | L | F | F/I | A |
| Ph-14 | M | L | T | L | L | L | F | F/I | A |
| Ph-15 | M | L/M | T | L | L | L | F | F/I | A |
| Ph-16 | M | L | T | L | L | L | F | F/I | A |
| Ph-17 | M | L | T | L | L | L | L | F/I | A |
| Pm-1 | M | L | T | L | L | L | F | F/I | A |
| Pm-2 | M | L | T | L | L | L | F | F/I | A |
| Pm-3 | M | L/M | T | L | L | L/V | F/S | F/I | A |
| Pm-4 | M | L/M | T | L | L | L | F | F/I | A |
| Pm-5 | M | L | T | L | L | L | F | F/I | A |
| Pm-6 | M | L | T | L | L | L | F | F/I | A |
| Population | TuCytb | TuCHS 1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| G126S | G132A | A133T | I136T | S141F | L258F | D161G | P262T | I1017F | |
| Susceptible | G | G | A | I | S | L | D | P | I |
| Ph-1 | G | G | A | I | S | L | D | P | I/F |
| Ph-2 | G | G | A | I | S | L | D | P | I/F |
| Ph-3 | --- | --- | --- | --- | --- | --- | --- | --- | I/F |
| Ph-4 | G | G | A | I | S | L | D | P | I |
| Ph-5 | G | G | A | I | S | L | D | P | I/F |
| Ph-6 | G | G/A | A | I | S | L | D | P | I/F |
| Ph-7 | G | G/A | A | I | S | L | D | P | I/F |
| Ph-8 | G | A | A | I | S | L | D | P | F |
| Ph-9 | G | G/A | A | I | S | L | D | P | F |
| Ph-10 | G | G/A | A | I | S | L | D | P | I/F |
| Ph-11 | G | G/A | A | I | S | L | D | P | F |
| Ph-12 | G | G/A | A | I | S | L | D | P | I/F |
| Ph-13 | G | G/A | A | I | S | L | D | P | I/F |
| Ph-14 | G | G | A | I | S | L | D | P | I/F |
| Ph-15 | G | G/A | A | I | S | L | D | P | F |
| Ph-16 | G | G/A | A | I | S | L | D | P | F |
| Ph-17 | G | G/A | A | I | S | L | D | P | F |
| Pm-1 | G | A | A | I | S | L | D | P | F |
| Pm-2 | G | A | A | I | S | L | D | P | I/F |
| Pm-3 | G | G | A | I | S | L | D | P | I/F |
| Pm-4 | -- | -- | -- | -- | S | L | D | P | I/F |
| Pm-5 | G | G | A | I | S | L | D | P | I/F |
| Pm-6 | G | G | A | I | S | L | D | P | F |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kewedar, S.; Chen, Q.-R.; Moural, T.W.; Lo, C.; Umbel, E.; Forrence, P.J.; Walsh, D.B.; Zhu, F. Acaricide Resistance Monitoring and Structural Insights for Precision Tetranychus urticae Management. Insects 2025, 16, 440. https://doi.org/10.3390/insects16050440
Kewedar S, Chen Q-R, Moural TW, Lo C, Umbel E, Forrence PJ, Walsh DB, Zhu F. Acaricide Resistance Monitoring and Structural Insights for Precision Tetranychus urticae Management. Insects. 2025; 16(5):440. https://doi.org/10.3390/insects16050440
Chicago/Turabian StyleKewedar, Said, Qi-Ren Chen, Timothy W. Moural, Carah Lo, Elsie Umbel, Peter J. Forrence, Douglas B. Walsh, and Fang Zhu. 2025. "Acaricide Resistance Monitoring and Structural Insights for Precision Tetranychus urticae Management" Insects 16, no. 5: 440. https://doi.org/10.3390/insects16050440
APA StyleKewedar, S., Chen, Q.-R., Moural, T. W., Lo, C., Umbel, E., Forrence, P. J., Walsh, D. B., & Zhu, F. (2025). Acaricide Resistance Monitoring and Structural Insights for Precision Tetranychus urticae Management. Insects, 16(5), 440. https://doi.org/10.3390/insects16050440







