Biology and Ecology of Lygus pratensis (Linn, 1758) (Heteroptera: Miridae): Towards the Practical Management of Cropping Landscapes in China
Simple Summary
Abstract
1. Introduction
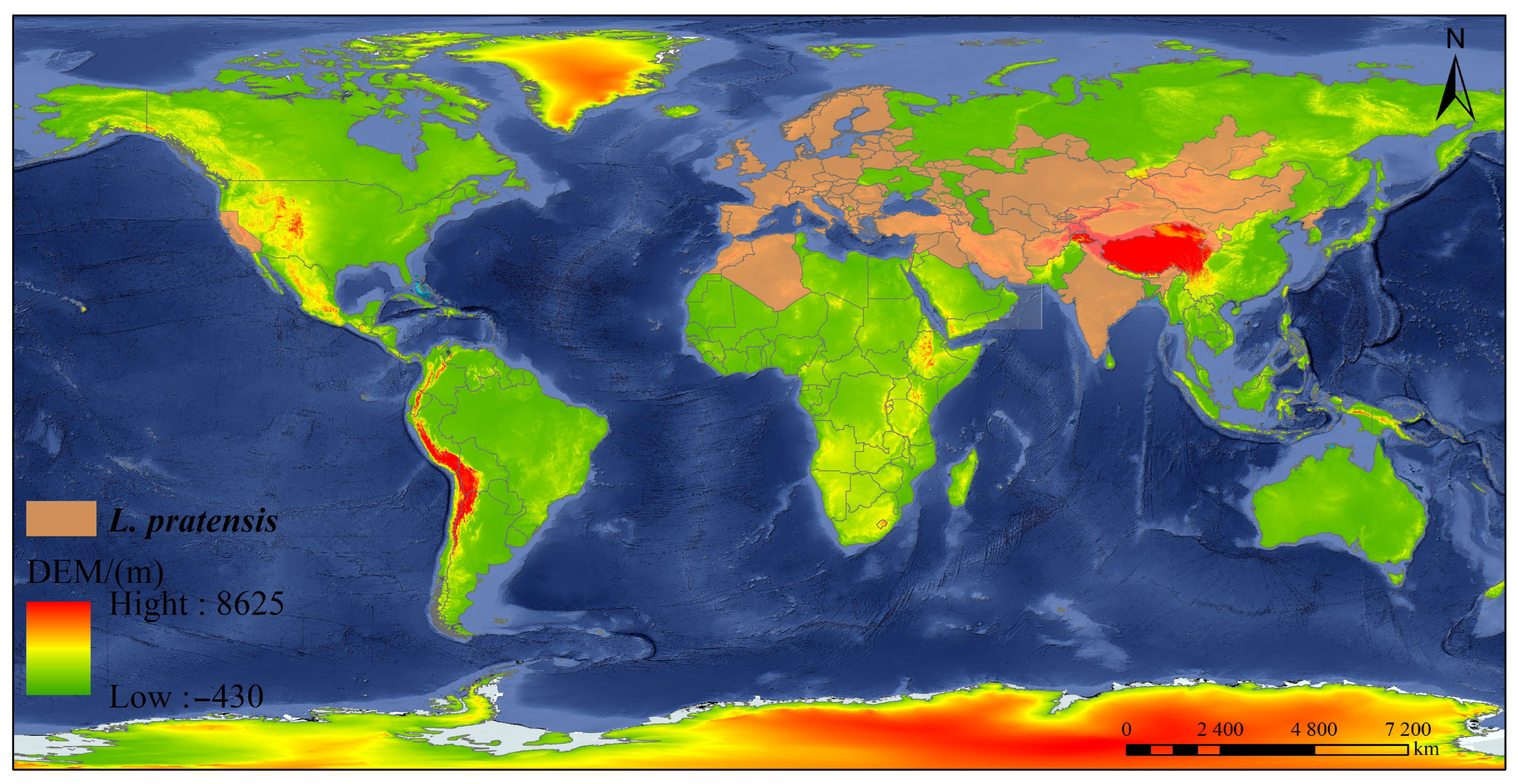
2. Distribution and Damage
| Number | Family Name | Species Quantity | Proportion (%) |
|---|---|---|---|
| 1 | Amaranthaceae | 22 [17,18,82] | 13.8 |
| 2 | Asteraceae | 21 [17,18,27,82] | 12.2 |
| 3 | Fabaceae | 19 [17,18,27,82,83] | 11.9 |
| 4 | Brassicaceae | 15 [17,18,64] | 9.4 |
| 5 | Solanaceae | 10 [17,18,82] | 6.3 |
| 6 | Rosaceae | 9 [17,18] | 5.7 |
| 7 | Chenopodiaceae | 7 [18,27,64,82] | 4.4 |
| 8 | Cucurbitaceae | 7 [17,18] | 4.4 |
| 9 | Malvaceae | 7 [17,18,64,82] | 4.4 |
| 10 | Poaceae | 6 [17,18] | 3.8 |
| 11 | Apiaceae | 5 [17,18] | 3.1 |
| 12 | Convolvulaceae | 4 [17,18] | 2.5 |
| 13 | Polygonaceae | 3 [17,18] | 1.9 |
| 14 | Labiatae | 3 [17,18] | 1.9 |
| 15 | Plantaginaceae | 1 [18] | 0.6 |
| 16 | Zygophyllaceae | 1 [17] | 0.6 |
| 17 | Dioscoreaceae | 1 [18] | 0.6 |
| 18 | Balsaminaceae | 1 [17] | 0.6 |
| 19 | Elaeagnaceae | 1 [17] | 0.6 |
| 20 | Juglandaceae | 1 [17] | 0.6 |
| 21 | Euphorbiaceae | 1 [17] | 0.6 |
| 22 | Nyctaginaceae | 1 [17] | 0.6 |
| 23 | Berberidaceae | 1 [18] | 0.6 |
| 24 | Portulacaceae | 1 [17] | 0.6 |
| 25 | Rhamnaceae | 1 [17] | 0.6 |
| 26 | Tamaricaceae | 1 [18] | 0.6 |
| 27 | Apocynaceae | 1 [17] | 0.6 |
| 28 | Cannabaceae | 1 [17] | 0.6 |
| 29 | Oxalidaceae | 1 [18] | 0.6 |
| 30 | Pedaliaceae | 1 [17] | 0.6 |
| 31 | Moraceae | 1 [18] | 0.6 |
| 32 | Ulmaceae | 1 [18] | 0.6 |
| 33 | Vitaceae | 1 [18] | 0.6 |
| 34 | Oleaceae | 1 [18] | 0.6 |
| 35 | Linaceae | 1 [18] | 0.6 |
3. Biosystematics
3.1. Morphology
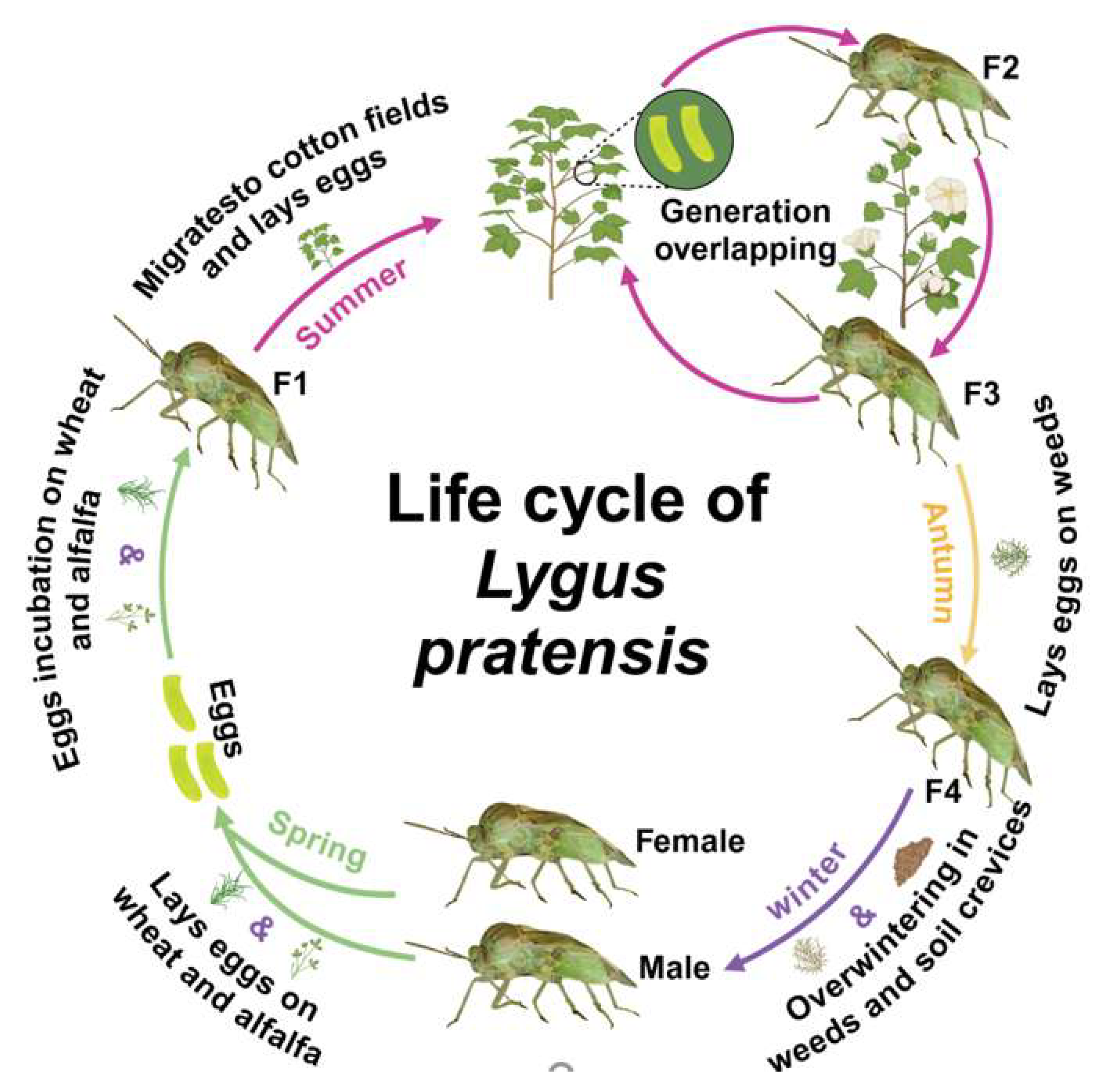
3.2. Life History
3.3. Migration and Dispersion
4. Ecology
4.1. The Occurrence of L. pratensis Is Related to the Environment
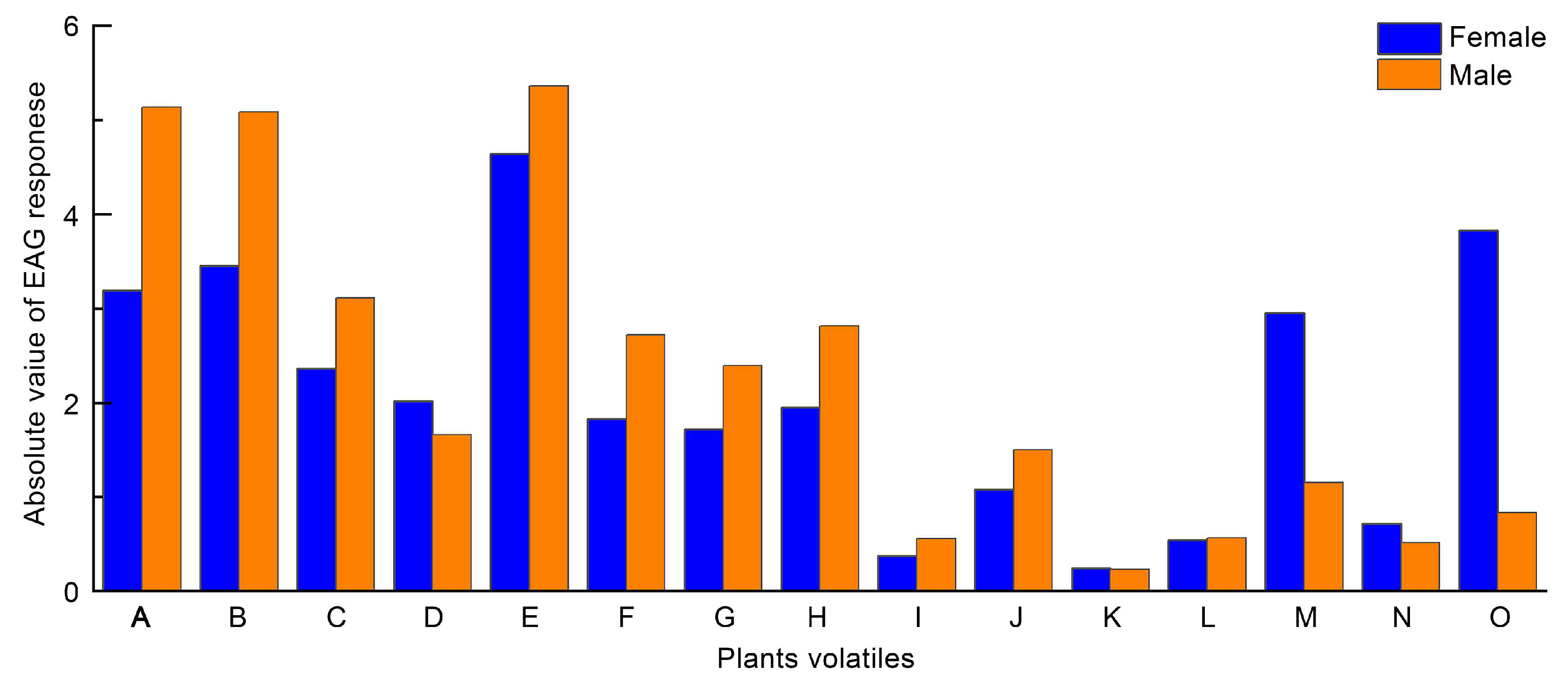
4.2. Influence of Plant Volatiles on L. pratensis
4.3. Impact of Agricultural Landscapes
5. Prevention and Control
5.1. Sampling Survey
5.2. Prevention and Control Indicators
5.3. Ecological Control
5.4. Physical Control
5.5. Agricultural Prevention and Control
5.6. Chemical Control
| Pesticide Name | Pesticide Type | LC50 (ng/cm2) |
|---|---|---|
| Phoxim | Oganophosphorus | 34.61 [146] |
| Methomyl | Carbamatepesticide | 12.64 [146] |
| Imidacloprid | Neonicotinoid | 45.61 [146] |
| High-efficacy cyfluthrin | Pyrethroids | 55.85 [147] |
| Chlorpyrifos | Oganophosphorus | 54.15 [141] |
| Abamectin | Agricultural antibiotic | 40.96 [141] |
| Thiamethoxam | Neonicotinoid | 196.48 [141] |
| Acetamiprid | Neonicotinoid | 178.05 [141] |
| Flupyradifurone | Neonicotinoid | 43.34 [144] |
5.7. Biological Control
6. Conclusions and Prospects
6.1. Conclusions
6.2. Prospects
6.2.1. Ecological Effects of Agricultural Landscapes
6.2.2. Development of New Biological Control Methods
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weirauch, C.; Schuh, R.T. Systematics and evolution of Heteroptera: 25 years of progress. Annu. Rev. Entomol. 2011, 56, 487–510. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, C.W.; Panizzi, A.R. Economic Importance of Heteroptera: A General View, 1st ed.; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Cassis, G.; Schuh, R.T. Systematics, biodiversity, biogeography, and host associations of the Miridae (Insecta: Hemiptera: Heteroptera: Cimicomorpha). Annu. Rev. Entomol. 2012, 57, 77–404. [Google Scholar] [CrossRef] [PubMed]
- Bauer, P.J.; McAlister, D.D., III; Roof, M.E. Evidence that light stink bug damage does not influence open end yarn processing performance. J. Cotton Sci. 2006, 10, 161–167. [Google Scholar]
- Guo, J.Y.; Zhou, H.H.; Wan, F.H.; Liu, X.J.; Han, Z.J. Population dynamics and damage of Lygus lucorum in Bt cotton fields under two control measures. Chin. J. Appl. Entomol. 2005, 42, 424–428. [Google Scholar] [CrossRef]
- Lu, Y.H.; Qiu, F.; Feng, H.Q.; Li, H.B.; Yang, Z.C.; Wyckhuys, K.A.G.; Wu, K.M. Species composition and seasonal abundance of pestiferous plant bugs (Hemiptera: Miridae) on Bt cotton in China. Crop. Prot. 2008, 27, 465–472. [Google Scholar] [CrossRef]
- Jenkins, J.N.; Parrott, W.L.; McCarty, J.C., Jr.; Callahan, F.E.; Berberich, S.A.; Deaton, R.W. Growth and survival of Heliothis virescens (Lepidoptera: Noctuidae) on transgenic cotton containing a truncated form of the delta endotoxin gene from Bacillus thuringiensis. J. Econ. Entomol. 1993, 86, 181–185. [Google Scholar] [CrossRef]
- Luttrell, R.G.; Fitt, G.P.; Ramalho, F.S.; Sugonyaev, E.S. Cotton pest management: Part 1. A worldwide perspective. Annu. Rev. Entomol. 1994, 39, 517–526. [Google Scholar] [CrossRef]
- Gould, F. Sustainability of transgenic insecticidal cultivars: Integrating pest genetics and ecology. Annu. Rev. Entomol. 1998, 43, 701–726. [Google Scholar] [CrossRef]
- Hardee, D.D.; Bryan, W.W. Influence of bacillus thuringiensis transgenic and nectariless cotton on insect populations with emphasis on the tarnished plant bug (heteroptera: Miridae). J. Econ. Entomol. 1997, 90, 663–668. [Google Scholar] [CrossRef]
- Wu, K.; Li, W.; Feng, H.; Guo, Y. Seasonal abundance of the mirids, Lygus lucorum and Adelphocoris spp. (Hemiptera: Miridae) on Bt cotton in Northern China. Crop. Prot. 2002, 21, 997–1002. [Google Scholar] [CrossRef]
- Wu, K.M. Environmental impact and risk management strategies of Bt cotton commercialization in China. Chin. J. Agric. Biotechnol. 2007, 4, 93–97. [Google Scholar] [CrossRef]
- Liu, B.; Li, H.Q.; Ali, A.; Li, H.B.; Liu, J.; Yang, Y.Z.; Lu, Y.H. Effects of temperature and humidity on immature development of Lygus pratensis (L.) (Hemiptera: Miridae). J. Asia-Pacific Entomol. 2015, 18, 139–143. [Google Scholar] [CrossRef]
- Zhang, L.J.; Cai, W.Z.; Luo, J.Y.; Zhang, S.; Wang, C.Y.; Lv, L.M.; Zhu, X.Z.; Wang, L.; Cui, J.J. Phylogeographic patterns of Lygus pratensis (Hemiptera: Miridae): Evidence for weak genetic structure and recent expansion in Northwest China. PLoS ONE 2017, 12, e0174712. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.J.; Li, Y.; Sun, C.Y.; Feng, L.K.; Wang, P.L.; Lu, Y.H. The predation of Lygus pratensis (L.) to Aphis gossypii Glover. J. Environ. Entomol. 2013, 35, 317–321. [Google Scholar] [CrossRef]
- Fitt, G.P.; Mares, C.L.; Llewellyn, D.J. Field evaluation and potential ecological impact of transgenic cottons (Gossypium hirsutum) in Australia. Biocontrol Sci. Technol. 1994, 4, 535–548. [Google Scholar] [CrossRef]
- Zhang, R.F.; Wang, W.; Liu, H.Y.; Yao, J. Host plants species and seasonal succession host feeding of Lygus pratensis (Heteroptera: Miridae). Xinjiang Agric. Sci. 2022, 59, 707–715. [Google Scholar] [CrossRef]
- Cao, N.; Leng, L.Y.; Liu, D.C.; Feng, H.Z. Study on the host plants and diet selection of Lygus pratensis. China Cotton 2017, 44, 27–29+38. [Google Scholar] [CrossRef]
- Sun, P.; Dilinuer, A.; Gou, C.Q.; Feng, H.Z. EAG and olfactory behavioral responses of Lygus pratensis to volatiles from seven host plants species. Chin. J. Appl. Entomol. 2019, 56, 316–326. [Google Scholar] [CrossRef]
- Lu, Y.H.; Liang, G.M.; Zhang, Y.J.; Yang, X.M. Advances in the management of insect pests of cotton in China since the 21st century. Chin. J. Appl. Entomol. 2020, 57, 477–490. [Google Scholar] [CrossRef]
- Wu, K.M.; Guo, Y.Y. The evolution of cotton pest management practices in China. Annu. Rev. Entomol. 2005, 50, 31–52. [Google Scholar] [CrossRef]
- Zhang, X.F. A preliminary report on the study of individual developmental processes of the Lygus pratensis. Shaanxi J. Agric. Sci. 2014, 60, 43–44. [Google Scholar] [CrossRef]
- Yang, M.C.; Yang, T. Hazardous occurrence of Lygus pratensis in Southern Xinjiang and its prevention and control. Plant Prot. 2001, 27, 31–32. [Google Scholar] [CrossRef]
- Ruberson, J.R.; Williams, L.I. Biological control of Lygus spp.: A component of areawide management. Southwest. Entomol. 2000, 23, 96–110. [Google Scholar]
- Lu, Y.H.; Wu, K.M.; Jiang, Y.Y.; Xia, B.; Li, P.; Feng, H.Q.; Wyckhuys, K.A.G.; Guo, Y.Y. Mirid bug outbreaks in multiple crops correlated with wide-scale adoption of Bt cotton in China. Science 2010, 328, 1151–1154. [Google Scholar] [CrossRef]
- Sun, P. Analysis of the Volatiles of Lygus pratensis Host Plants and its Inducement. Master’s Thesis, Tarim University, Alar, China, 2018. [Google Scholar]
- Zhang, R.F.; Wang, W.; Liu, H.Y.; Yao, J. Seasonal host transfer pattern and migration capacity of Lygus pratensis (Hemiptera: Miridae) in Southern Xinjiang oasis cropland. Chin. J. Ecol. 2021, 40, 171–179. [Google Scholar] [CrossRef]
- Greene, J.K.; Turnipseed, S.G.; Sullivan, M.J.; Herzog, G.A. Boll damage by southern green stink bug (Hemiptera: Pentatomidae) and tarnished plant bug (Hemiptera: Miridae) caged on transgenic Bacillus thuringiensis cotton. J. Econ. Entomol. 1999, 92, 941–944. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Y.Q.; Lu, Y.H.; Wu, K.M. Toxicity and persistence of four kinds of insecticides against Apolygus lucorum. China Cotton 2017, 44, 5–7+10. [Google Scholar]
- Tan, Y.; Ma, Y.; Jia, B.; Homem, R.A.; Williamson, M.S.; Gao, S.J.; Han, H.B.; Xiang, K.F.; Sun, X.T.; Gao, X.; et al. Laboratory selection, cross-resistance, risk assessment to lambda-cyhalothrin resistance, and monitoring of insecticide resistance for plant bug lygus pratensis (Hemiptera: Miridae) in farming-pastoral ecotones of Northern China. J. Econ. Entomol. 2021, 114, 891–902. [Google Scholar] [CrossRef]
- Qiu, Y.; Fu, B.J.; Wang, J.; Chen, L.D. Quantitative analysis of relationships between spatial and temporal variation of soil moisture content and environmental factors at a gully catchment of the Loess Plateau. Acta Ecol. Sin. 2000, 20, 741–747. [Google Scholar] [CrossRef]
- Li, Y.L.; Qiao, M.; Yang, X.L.; Zhou, S.B.; Zeng, Y.J. Analysis on land use/cover change and landscape fragmentation in typical watershed of arid zone in last 30 years—A case of Manasi River Watershed, Xinjiang. J. Desert Res. 2008, 28, 1050–1058. [Google Scholar]
- Liu, J.N.; Li, W.Q.; Bao, Z.Y. Application of road network theory in studying ecological effects of landscape fragmentation: A case study with the road network of Zhejiang Province. Acta Ecol. Sin. 2008, 28, 4352–4362. [Google Scholar] [CrossRef]
- Zaller, J.G.; Moser, D.; Drapela, T.; Schmöger, C.; Frank, T. Parasitism of stem weevils and pollen beetles in winter oilseed rape is differentially affected by crop management and landscape characteristics. Biocontrol 2009, 54, 505–514. [Google Scholar] [CrossRef]
- Ou, Y.F.; Zhao, Z.H.; Ge, F. Insect ecological services. Chin. J. Appl. Entomol. 2013, 50, 305–310. [Google Scholar] [CrossRef]
- Bianchi, F.J.J.A.; Booij, C.J.H.; Tscharntke, T. Sustainable pest regulation in agricultural landscapes: A review on landscape composition, biodiversity and natural pest control. Proc. Biol. Sci. 2006, 273, 1715–1727. [Google Scholar] [CrossRef]
- Ou, Y.F.; Ge, F. Effects of agricultural landscape patterns on insects. Chin. J. Appl. Entomol. 2011, 48, 1177–1183. [Google Scholar] [CrossRef]
- Rusch, A.; Chaplin-Kramer, R.; Gardiner, M.M.; Hawro, V.; Holland, J.; Landis, D.; Thies, C.; Tscharntke, T.; Weisser, W.W.; Winqvist, C. Agricultural landscape simplification reduces natural pest control: A quantitative synthesis. Agric. Ecosyst. Environ. 2016, 221, 198–204. [Google Scholar] [CrossRef]
- Gardiner, M.M.; Landis, D.A. Impact of intraguild predation by adult Harmonia axyridis (Coleoptera: Coccinellidae) on Aphis glycines (Hemiptera: Aphididae) biological control in cage studies. Biol. Control. 2007, 40, 386–395. [Google Scholar] [CrossRef]
- Zheng, Y.K.; You, M.S. Biological diversity in support of ecologically-based pest management at landscape level. Acta Ecol. Sin. 2009, 29, 1508–1518. [Google Scholar] [CrossRef]
- Haddad, N.M.; Crutsinger, G.M.; Gross, K.; Haarstad, J.; Tilman, D. Plant diversity and the stability of food webs. Ecol. Lett. 2011, 14, 42–46. [Google Scholar] [CrossRef]
- Vinokurov, N.N.; Kanyukova, E.V.; Golub, V.B. Katalog Poluzhestkokrylykh Nasekomykh (Heteroptera) Aziatskoi Chasti Rossii (Catalogue of Heteroptera of Asian Russia), 1st ed.; Nauka: Novosibirsk, Russia, 2010. [Google Scholar]
- Dilshod, M.U.; Sattorov, N.; Abdullaeva, J.; Makhliyo, M.; Abdirahman, S.; Kholmatov, B.; Asiya, Y.; Maxsetbay, M. Plant bugs (Heteroptera: Miridae) development and damage to cotton crop in Uzbekistan. WSEAS Trans. Environ. Dev. 2023, 19, 329–340. [Google Scholar] [CrossRef]
- Wheeler, A.G. Biology of the Plant Bugs (Hemiptera: Miridae): Pests, Predators, Opportunists, 1st ed.; Cornell University Press: Ithaca, NY, USA, 2001. [Google Scholar]
- Xiao, C.Y.; Men, X.L. Description of Lygus bugs in China botton fields. Acta Zool. Sin. 1963, 3, 439–449. [Google Scholar]
- Wang, J.R. Preliminary Observation of Three Lygus Bugs Damaging Cotton in Xinjiang, 1st ed.; Geology Press: Beijing, China, 1959. [Google Scholar]
- Wang, X.Y.; Chang, G.Y. Analysis of the spatio-temporal evolution of regional specialization of agriculture in the Hexi Corridor between 1998 and 2018. J. Lanzhou Univ. (Nat. Sci.) 2022, 58, 186–194. [Google Scholar] [CrossRef]
- Kelton, L.A. The Lygus bugs (genus Lygus Hahn) of North America (Heteroptera: Miridae). Mem. Entomol. Soc. Can. 1975, 107, 5–101. [Google Scholar] [CrossRef]
- Wang, J.R. Distribution characteristics and occurrence profile of cotton bugs in Xinjiang. Chin. J. Appl. Entomol. 1963, 3, 121–123. [Google Scholar]
- Wang, J.R. Characteristics of cotton bugs and their control strategies in Xinjiang. Xinjiang Agric. Sci. 1964, 4, 133–137. [Google Scholar]
- Wang, J.R.; Ji, R.G.; Meng, Z.J. Relationship between boll shedding and mirid bug infestation in central cotton. Xinjiang Agric. Sci. 1966, 1, 17–19. [Google Scholar]
- Lv, Z.Z.; Chen, J.; Wu, Z.Y.; Ma, G.L. The main pest in Xinjiang cotton region and sustainable development. Arid. Zone Res. 1999, 16, 28–32. [Google Scholar] [CrossRef]
- Li, J.B.; Lv, Z.Z.; Wang, D.Y.; Tian, C.Y. Succession of major pests and their mechanistic analysis in Xinjiang cotton region. Chin. J. Ecol. 2005, 24, 261–264. [Google Scholar] [CrossRef]
- Wu, K.M.; Lu, Y.H.; Feng, H.Q.; Jiang, Y.Y.; Zhao, J.Z. Suppression of cotton bollworm in multiple crops in China in areas with Bt toxin-containing cotton. Science 2008, 321, 1676–1678. [Google Scholar] [CrossRef]
- Zhang, P.; Zhao, Y.H.; Zhang, X.F.; Song, Y.Y.; Zhang, Z.Q.; Liu, F. Field resistance monitoring of Apolygus lucorum (Hemiptera: Miridae) in Shandong, China to seven commonly used insecticides. Crop. Prot. 2015, 76, 127–133. [Google Scholar] [CrossRef]
- An, J.; Liu, C.; Dou, Y.N.; Gao, Z.; Dang, Z.; Yan, X.; Pan, W.; Li, Y. Analysis of differentially expressed transcripts in Apolygus lucorum (Meyer-Dür) exposed to different temperature coefficient insecticides. Int. J. Mol. Sci. 2020, 21, 658. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.H.; Zeng, J.; Jiang, Y.Y.; Wu, K.M. Techniques for surveying mirid bug (Heteroptera: Miridae) populations and assessing crop damage caused by these pests. Chin. J. Appl. Entomol. 2014, 51, 848–852. [Google Scholar] [CrossRef]
- Varis, A.L. Seasonal occurrence of Lygus bugs on field crops in Finland. Agric. Food Sci. 1997, 6, 409–413. [Google Scholar] [CrossRef]
- Lu, Y.H.; Wu, K.M. Biology and Control Methods of the Mirids, 1st ed.; Golden Shield: Beijing, China, 2008. [Google Scholar]
- Solhjouy-Fard, S.; Sarafrazi, A.; Minbashi, M.M.; Ahadiyat, A. Predicting habitat distribution of five heteropteran pest species in Iran. J. Insect Sci. 2013, 13, 116. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, X.F.; Zhou, Z.F.; Liang, G.; Huang, Y.Y.; Cao, J. The occurrence of major insect pests on Medicago sativa in the USA. Plant Quar. 2015, 29, 82–87. [Google Scholar] [CrossRef]
- Jia, B.; Tan, Y.; Fu, X.T.; Han, H.B.; Chang, J.P.; Pang, B.P. Effect of host plants on development, reproduction, and digestive enzyme activity in Lygus pratensis. Pratac. Sci. 2018, 35, 1975–1984. [Google Scholar] [CrossRef]
- Gou, C.Q.; Sun, P.; Liu, D.C.; Dilinuer, A.; Feng, H.Z. Effects of different host plants on the growth and development of Lygus pratensis. J. Envion. Entomol. 2019, 41, 1065–1069. [Google Scholar]
- Xia, X.; Zheng, Y.X.; Yao, C.C.; Gou, C.Q.; Feng, H.Z. Electrophysiological and behavioral responses of Lygus pratensis to plant volatiles and regulation of brassica campestris trapping belt on L. pratensis in cotton fields. Cotton Sci. 2023, 35, 128–137. [Google Scholar] [CrossRef]
- Nieto, D.J.; Hagler, J.R.; Swezey, S.L.; Machtley, S.A.; Bryer, J.A. Immigration of Lygus spp. (Hemiptera: Miridae) and predaceous natural enemies to trap-cropped organic strawberry. Environ. Entomol. 2023, 52, 824–831. [Google Scholar] [CrossRef]
- Tóth, P.; Tóthová, M.; Krchňavá, V.; Ščevková, J. Diversity of true bugs (Hemiptera: Heteroptera) on common ragweed (Ambrosia artemisiifolia) in Southern Slovakia. Diversity 2023, 15, 757. [Google Scholar] [CrossRef]
- Conti, E.; Bin, F. Native Lygus spp. (Heteroptera: Miridae) damaging introduced Hibiscus cannabinus in Italy. J. Econ. Entomol. 2001, 94, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liu, C.; Wang, X. Pests species investigation on Medicago sativa in Gansu province. Grassl. Turf. 2011, 31, 49–55. [Google Scholar]
- Tan, Y.; Jia, B.; Chi, Y.M.; Han, H.B.; Zhou, X.R.; Pang, B.P. The complete mitochondrial genome of the plant bug Lygus pratensis Linnaeus (Hemiptera: Miridae). J. Insect Sci. 2018, 18, 41. [Google Scholar] [CrossRef]
- Wang, Q.; Bao, W.F.; Zhang, Q.; Fu, X.W.; Yang, Y.Z.; Lu, Y.H. Host plant use of a polyphagous mirid, Apolygus lucorum: Molecular evidence from migratory individuals. Ecol. Evol. 2019, 9, 11518–11528. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, R.F.; Liu, H.Y.; Tian, J.C.; Shelton, A.M.; Yao, J. Use of safflower as a trap crop for managing the mirid bug, Lygus pratensis Linnaeus (Hemiptera: Miridae), in cotton fields. Pest Manag. Sci. 2021, 77, 1829–1838. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, R.F.; Liu, H.H.; Zhang, Y.; Yao, J. Control indices for Lygus pratensis (Heteroptera: Miridae) in cotton plantations in Kashgar, Xinjiang. Chin. J. Appl. Entomol. 2016, 53, 1146–1152. [Google Scholar] [CrossRef]
- Zhang, R.F.; Wang, W.; Liu, H.Y.; Guljamal, T.; Yao, J. Comparison of three sampling techniques for estimating the population density of Lygus pratensis in cotton fields. Chin. J. Appl. Entomol. 2018, 55, 310–316. [Google Scholar]
- Jiao, X.D.; Guo, Y.L.; Xia, W.; Song, C.G.; Zhang, J.P. Study on the occurrence characteristics and population dynamics of the main insect pests in Bazhou of Xinjiang. Xinjiang Agric. Sci. 2013, 50, 1254–1259. [Google Scholar]
- Asanova, R.B.; Iskakov, B.V. Vrednye i Poleznye Poluzhestkokrylye Kazakhstana. Opredelitel’ (A Key to Harmful and Beneficial True Bugs of Kazakhstan); Kainar: Alma-Ata, Kazakhstan, 1977. [Google Scholar]
- Sobko, O.A.; Matsishina, N.V.; Fisenko, P.V.; Kim, I.V.; Didora, A.S.; Boginskay, N.G.; Volkov, D.I. Viruses in the agrobiocenosis of the potato fields. IOP Conf. Ser. Earth. Environ. Sci. 2021, 677, 052093. [Google Scholar] [CrossRef]
- Sobko, O.; Matsishina, N.; Fisenko, P.; Kim, I.; Boginskaya, N. Phytoviruses in the potato field tripartite agroecosystem. In Fundamental and Applied Scientific Research in the Development of Agriculture in the Far East (AFE-2021). Lecture Notes in Networks and Systems; Muratov, A., Ignateva, S., Eds.; Springer: Cham, Switzerland, 2022; Volume 353, p. 1176. [Google Scholar]
- Puchkov, V. Glavneishie Klopy-Slepnyaki—Vrediteli Sel’skokhozyaistvennykh Kul’tur (Major Capsid Bugs Important as Agricultural Pests); Naukova Dumka: Kyiv, Ukraine, 1966. [Google Scholar]
- Shackel, K.A.; Celorio-Mancera, M.P.; Ahmadi, H.; Greve, L.C.; Teuber, L.R.; Backus, E.A.; Labavitch, J.M. Micro-injection of lygus salivary gland proteins to simulate feeding damage in alfalfa and cotton flowers. Arch. Insect Biochem. Physiol. 2005, 58, 69–83. [Google Scholar] [CrossRef]
- Yang, X.; Jing, B.F.; Meng, J.W.; Zhu, B. Large occurrence of Lygus pratensis in Southern Xinjiang in 2003. China Cotton 2004, 5, 43. [Google Scholar] [CrossRef]
- Su, L.Y.; Yuan, B.Z. Infestation pattern of Lygus pratensis and control technology. Shanxi Fruits 2012, 5, 48. [Google Scholar] [CrossRef]
- Zhang, R.F.; Wang, W.; Liu, H.Y.; Yao, J. Effects of different summer host plants on the developmental duration and adult longevity of Lygus pratensis Linnaeus (Heteroptera: Miridae). Chin. J. Appl. Entomol. 2022, 59, 124–133. [Google Scholar] [CrossRef]
- Tan, Y.; Qiao, L.; Li, L.; Yang, J.L.; Han, H.B.; Zhu, M.M.; Zhang, Z.Q.; Ni, P. The effect of different color and hanging methods of sticky boards on trapping the tarnished Lygus pratensis (Hemiptera: Miridae) in alfalfa field. Acta Agrestia Sin. 2023, 31, 1226–1233. [Google Scholar] [CrossRef]
- Namyatova, A.A.; Tyts, V.D.; Bolshakova, D.S. Identification and delimitation of the trans-Palearctic Lygus species (Insecta: Heteroptera: Miridae) using integrative approach. Insect Syst. Evol. 2022, 54, 146–192. [Google Scholar] [CrossRef]
- Xia, X.; Bai, S.X.; Yao, C.C.; Gou, C.Q.; Feng, H.Z. Ultrastructural observation on the adult of Lygus pratensis. China Cotton 2022, 49, 22–25. [Google Scholar] [CrossRef]
- Yang, Q. Analysis of Body Colour Variations and Main Energy Substance Contents of Lygus pratensis (L.) in Overwintering Period. Master’s Thesis, Tarim University, Alar, China, 2017. [Google Scholar]
- Asensio de la, S.E. El Lygus pratensis (Linn.) como plaga de la alfalfa para semilla. An. Inst. Nac. Invest. Agrar. Ser. Prot. Veg. 1973, 3, 349–358. [Google Scholar]
- Yang, M.C.; Yang, T. Occurrence of L. pratensis in Southern Xinjiang and its control. Plant Prot. 2001, 5, 31–32. [Google Scholar] [CrossRef]
- Hu, C.X. Northern Overwintering Boundary and Voltinism of the Brown Planthopper Nilaparvata lugens Stål Under Climate Warming Scenarios. Master’s Thesis, Hebei Agricultural University, Baoding, China, 2013. [Google Scholar]
- Lu, Y.H.; Wu, K.M.; Jiang, Y.Y.; Xia, B. Occurrence trend and control strategy of cotton mirids in China. Plant Prot. 2010, 36, 150–153. [Google Scholar] [CrossRef]
- Zheng, Y.X.; Li, P.F.; Li, T.L.; Wang, K.Y.; Gou, C.Q.; Feng, H.Z. Studies on Lygus pratensis (Hemiptera: Miridae) flight ability. Insects 2024, 15, 762. [Google Scholar] [CrossRef]
- Ahmadi, F.; Mikani, A.; Moharramipour, S. Induction of diapause by clock proteins period and timeless via changes in PTTH and ecdysteroid titer in the sugar beet moth, Scrobipalpa ocellatella (Lepidoptera: Gelechiidae). Arch. Insect Biochem. Physiol. 2021, 107, 1790. [Google Scholar] [CrossRef]
- Chen, C.; Wei, X.T.; Xiao, H.J.; He, H.M.; Xia, Q.W.; Xue, F.S. Diapause induction and termination in Hyphantria cunea (Drury) (Lepidoptera: Arctiinae). PLoS ONE 2014, 9, e98145. [Google Scholar] [CrossRef]
- Li, Y.; Yang, A.; Feng, L.K.; Wang, P.L. Effects of different temperatures on the development and eproduction of Lygus pratensis. Plant Prot. 2015, 41, 59–62. [Google Scholar] [CrossRef]
- Gou, C.Q. Effects of Temperature and Photoperiod on Diapause of Lygus pratensis and Diapause-Related Multiomics Analyses. Ph.D. Thesis, Tarim University, Alar, China, 2022. [Google Scholar]
- Yao, C.C. The Overwintering Biological Characteristics and the Effects of Short-Term High Temperature on the Survival and Reproduction of the Lygus pratensis. Master’s Thesis, Tarim University, Alar, China, 2023. [Google Scholar]
- Yao, C.C.; Zheng, Y.X.; Gou, C.Q.; Feng, H.Z. Effects of short-term high temperature exposure on the survival and fecundity of Lygus pratensis. China Cotton 2023, 50, 6–12. [Google Scholar] [CrossRef]
- Yang, M.C.; Yang, T. Occurrence and damage of L. pratensis in southern Xinjiang and its control measures. China Cotton 2001, 3, 39–40. [Google Scholar]
- Zhang, R.F.; Wang, W.; Liu, H.Y.; Yao, J. Effects of the occurrence and damage of Lygus pratensis (Linnaeus) on cotton under almond cotton interplanting. Plant Prot. 2018, 44, 172–176. [Google Scholar] [CrossRef]
- Khuhro, S.A.; Liao, H.; Dong, X.T.; Yu, Q.; Yan, Q.; Dong, S.L. Two general odorant binding proteins display high bindings to both host plant volatiles and sex pheromones in a pyralid moth Chilo suppressalis (Lepidoptera: Pyralidae). J. Asia-Pacific Entomol. 2017, 20, 521–528. [Google Scholar] [CrossRef]
- Khan, Z.R.; Ampong-Nyarko, K.; Chiliswa, P.; Hassanali, A.; Kimani, S.; Lwande, W.; Overholt, W.A.; Picketta, J.A.; Smart, L.E.; Woodcock, C.M. Intercropping increases parasitism of pests. Nature 1997, 388, 631–632. [Google Scholar] [CrossRef]
- Balvanera, P.; Daily, G.C.; Ehrlich, P.R.; Ricketts, T.H.; Bailey, S.A.; Kark, S.; Kremen, C.; Pereira, H. Conserving biodiversity and ecosystem services. Science 2001, 291, 2047. [Google Scholar] [CrossRef]
- Dong, W.X.; Xiao, C.; Li, C.Y. Effect of diversified cropping on insect pests and natural enemies in agroecosystems. Chin. J. Eco-Agric. 2016, 24, 435–442. [Google Scholar] [CrossRef]
- Koricheva, J.; Hayes, D. The relative importance of plant intraspecific diversity in structuring arthropod communities: A meta-analysis. Funct. Ecol. 2018, 32, 1704–1717. [Google Scholar] [CrossRef]
- Landis, D.A.; Wratten, S.D.; Gurr, G.M. Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu. Rev. Entomol. 2000, 45, 175–201. [Google Scholar] [CrossRef]
- Sarkar, S.C.; Wang, E.; Wu, S.; Lei, Z. Application of trap cropping as companion plants for the management of agricultural pests: A review. Insects 2018, 9, 128. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.W. Multitrophic interactions among plants, aphids, alternate prey and shared natural enemies—A review. Eur. J. Entomol. 2013, 105, 369–380. [Google Scholar] [CrossRef]
- Ramsden, M.W.; Menéndez, R.; Leather, S.R.; Wäckers, F. Optimizing field margins for biocontrol services: The relative role of aphid abundance, annual floral resources, and overwinter habitat in enhancing aphid natural enemies. Agric. Ecosyst. Environ. 2015, 199, 94–104. [Google Scholar] [CrossRef]
- Wang, K.T. Effects of Landscape Pattern on the Population Dynamics of Apolygus lucorum (Meyer-Dür) in Aksu Area, Xinjiang. Master’s Thesis, Jilin Agricultural University, Changchun, China, 2022. [Google Scholar]
- Bai, S.X.; Zhao, J.P.; Gou, C.Q.; Yao, C.C.; Feng, H.Z. Effects of farmland landscape pattern on adult population dynamics of Lygus pratensis in Aral Reclamation Area of Xinjiang. Cotton Sci. 2022, 34, 23–532. [Google Scholar] [CrossRef]
- Lu, Y.H.; Wu, K.M.; Cai, X.M.; Liu, Y.Q. A rearing method for mirids using the green beam Phaseolus vulgaris in the laboratory. J. Plant Prot. 2008, 35, 215–219. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, R.F.; Zhang, Y.; Liu, H.Y.; Yao, J. Distribution of Lygus pratensis (Linnaeus) in different organs of the cotton in Xinjiang. Plant Prot. 2016, 42, 177–180. [Google Scholar] [CrossRef]
- Dong, J.W.; Pan, H.S.; Lu, Y.H.; Yang, Y.Z. Nymphal performance correlated with adult preference for flowering host plants in a polyphagous mirid bug, Apolygus lucorum (Heteroptera: Miridae). Arthropod-Plant Interact. 2013, 7, 83–91. [Google Scholar] [CrossRef]
- Pan, H.S.; Lu, Y.H.; Wyckhuys, K.A.; Wu, K.M. Preference of a polyphagous mirid bug, Apolygus lucorum (Meyer-Dür) for flowering host plants. PLoS ONE 2013, 8, e68980. [Google Scholar] [CrossRef]
- Pan, H.S.; Lu, Y.H.; Wyckhuys, K.A. Early-season host switching in Adelphocoris spp. (Hemiptera: Miridae) of differing host breadth. PLoS ONE 2013, 8, e59000. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, W.; Zhang, R.F.; Liu, H.Y.; Zhang, Y.; Yao, J. Spatial distribution pattern of common meadow bug Lygus pratensis (Linnaeus) in Xinjiang cotton fields. J. Plant Prot. 2016, 43, 972–978. [Google Scholar] [CrossRef]
- Zhang, Y. The occurrence and damage of L. pratensis and integrated control technology. Anhui Agri. Sci. Bull. 2010, 16, 95+123. [Google Scholar] [CrossRef]
- Jin, L.G. Occurrence pattern and integrated control of mirid bug in cotton fields. Rural. Sci. Technol. 2009, 8, 47–48. [Google Scholar] [CrossRef]
- Wang, S.S.; Yang, M.C.; Yan, X.J.; Wang, Y.J.; Song, Y.; Liu, Y.Q. Population dynamics of natural enemies of the cotton aphid and control of aphid infestation in the Southern Xinjiang region. China Cotton 2000, 27, 18. [Google Scholar] [CrossRef]
- Zhang, R.F.; Wang, W.; Liu, H.Y.; Wang, D.Y.; Yao, J. Field evaluation of sunflower as a potential trap crop of Lygus pratensis in cotton fields. PLoS ONE 2020, 15, e0237318. [Google Scholar] [CrossRef]
- Martin, E.A.; Reineking, B.; Seo, B.; Steffan-Dewenter, I. Natural enemy interactions constrain pest control in complex agricultural landscapes. Proc. Natl. Acad. Sci. USA 2013, 110, 5534–5539. [Google Scholar] [CrossRef]
- Pretty, J. Intensification for redesigned and sustainable agricultural systems. Science 2018, 362, 6417. [Google Scholar] [CrossRef]
- Haan, N.L.; Zhang, Y.; Landis, D.A. Predicting landscape configuration effects on agricultural pest suppression. Trends Ecol. Evol. 2020, 35, 175–186. [Google Scholar] [CrossRef]
- Stein-Bachinger, K.; Preißel, S.; Kühne, S.; Reckling, M. More diverse but less intensive farming enhances biodiversity. Trends Ecol. Evol. 2022, 37, 395–396. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Liu, J.; Zeng, J.; Lu, Y.H.; Lu, Y.; Xu, H.H.; Liu, D.; Gao, Y.J. Study on the lamp lure effect of insects in Xinjiang cotton field. China Plant Prot. 2017, 37, 49–55. [Google Scholar] [CrossRef]
- Wang, Z.H.; Tian, W.; Feng, Z.C. Occurrence pattern and control of cotton mirids in Kuitun, Xinjiang. China Cotton 2002, 12, 32–33. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, W.B.; Zhang, H.L.; Ma, H.Y.; Han, H.B.; Pang, B.P.; Tan, Y. Cloning of CYP6Al3 and sodium channel gene LPVSSC and their involvement in analysis on the resistance of lambda-cyhalothrin in Lygus pratensis (Hemiptera: Miridae). J. Environ. Entomol. 2022, 44, 1252–1263. [Google Scholar] [CrossRef]
- Legrand, A.; Los, L. Visual responses of Lygus lineolaris and Lygocoris spp. (Hemiptera: Miridae) on peaches. Fla. Entomol. 2003, 86, 424–428. [Google Scholar] [CrossRef]
- Dilinu’er, A.; Liu, D.C.; Feng, H.Z. Attractive effect of differently colored sticky traps on Lygus pratensis. China Cotton 2018, 45, 23–25. [Google Scholar] [CrossRef]
- Zhang, Z.H. Cotton mirids occurrence pattern and control technology. Rural Sci. Technol. Train. 2013, 10, 30–32. [Google Scholar] [CrossRef]
- Zhang, H.S. Occurrence and control of important pests in the cotton field of Xinjiang production and construction corps. Xinjiang Farm Res. Sci. Technol. 2011, 34, 35–36. [Google Scholar] [CrossRef]
- May, W.E.; Soroka, J.J.; Loeppky, H.A.; Murrell, D.C. The effects of trichlorfon and deltamethrin on alfalfa plant bug and lygus bug (Heteroptera: Miridae) populations in alfalfa grown in Canada. Crop. Prot. 2003, 22, 883–889. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.; Ray, I.; Song, M. Transcriptome responses in alfalfa associated with tolerance to intensive animal grazing. Sci. Rep. 2016, 6, 19438. [Google Scholar] [CrossRef]
- Sun, T.; Li, Z. Alfalfa-corn rotation and row placement affects yield, water use, and economic returns in Northeast China. Field Crop. Res. 2019, 241, 107558. [Google Scholar] [CrossRef]
- Sun, P.; Yang, Q.; Liu, R.C.; Gou, C.Q.; Feng, H.Z. Taxis responses of Lygus pratensis to 7 different host plants. Xinjiang Agric. Sci. 2017, 54, 925–930. [Google Scholar]
- Zhen, C.; Gao, X. A point mutation (l1015f) of the voltage-sensitive sodium channel gene associated with lambda-cyhalothrin resistance in Apolygus lucorum (meyerdr) population from the transgenic Bt cotton field of China. Pestic. Biochem. Physiol. 2016, 127, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Zhang, S.; Gao, X.W. Monitoring the insecticide resistance of the cotton bugs Apolygus lucorum and Adelphocoris suturalis. Chin. J. Appl. Entomol. 2012, 49, 348–358. [Google Scholar]
- Zhen, C.; Tan, Y.; Miao, L.; Wu, J.; Gao, X. Overexpression of cytochrome P450s in a lambda-cyhalothrin resistant population of Apolygus lucorum (Meyer-Dür). PLoS ONE 2018, 13, e0198671. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Luttrell, R. Altered gene regulation and potential association with metabolic resistance development to imidacloprid in the tarnished plant bug, Lygus lineolaris. Pest Manag. Sci. 2015, 71, 40–57. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Luttrell, R. Variation of acephate susceptibility and correlation with esterase and glutathione S-transferase activities in field populations of the tarnished plant bug, Lygus lineolaris. Pestic. Biochem. Physiol. 2012, 103, 202–209. [Google Scholar] [CrossRef]
- Da, X.P.; Zhao, J.P.; Zhang, K.K.; Feng, H.Z. Toxicity determination of six insecticides to nymphs of Lygus pratensis in laboratory. China Cotton 2019, 46, 4–6+18. [Google Scholar]
- Bromilow, R.H.; Chamberlain, K.; Evans, A.A. Physicochemical aspects of phloem translocation of herbicides. Weed Sci. 1990, 38, 305–314. [Google Scholar] [CrossRef]
- Yang, J.C.; Li, M.; Chai, B.S.; Liu, C.L. Recent research advances in new neonicotinoids insecticide. Agrochemicals 2007, 46, 433–438. [Google Scholar] [CrossRef]
- Gao, H.L.; Yan, X.; Ye, J.K.; Hu, Y.L.; Wang, Y.R.; Talehar, D.M.; Lu, W. Laboratory activity and field efficacy of 2 insecticides and their mixtures with synergist against Lygus pratensis. Agrochemicals 2024, 63, 675–679+684. [Google Scholar] [CrossRef]
- Wu, L.L.; Ma, S.K. Indoor efficacy tests of several biological agents against L. pratensis. Xinjiang Agric. Sci. Technol. 2018, 1, 50. [Google Scholar] [CrossRef]
- Ma, Y. Resistance of Lygus pratensis to Lambda-Cyhalothrin and its Physiological, Biochemical and Molecular Mechanisms. Master’s Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2013. [Google Scholar]
- Ma, S.K.; Wu, L.L.; Zhang, Z.L.; Chen, Y.; Liu, C.J. Indoor efficacy tests of several agents against L. pratensis. Xinjiang Agric. Sci. Technol. 2015, 6, 14–15. [Google Scholar] [CrossRef]
- Nyffeler, M.; Sunderland, K.D. Composition, abundance and pest control potential of spider communities in agroecosystems: A comparison of European and US studies. Agric. Ecosyst. Environ. 2003, 95, 579–612. [Google Scholar] [CrossRef]
- Yao, J.M.; Xie, C.H.; He, Y.B.; Qiu, B.; Chen, H.Y.; Xu, Z.F. Investigation on hymenopterous parasitoids of Bactrocera dorsalis (Hendel) in Guangdong. J. Environ. Entomol. 2008, 30, 350–356. [Google Scholar] [CrossRef]
- Whitehouse, M.E.; Hardwick, S.; Scholz, B.C.; Annells, A.J.; Ward, A.; Grundy, P.R.; Harden, S. Evidence of a latitudinal gradient in spider diversity in Australian cotton. Austral Ecol. 2009, 34, 10–23. [Google Scholar] [CrossRef]
- Pérez-Guerrero, S.; Gelan-Begna, A.; Vargas-Osuna, E. Impact of Cheiracanthium pelasgicum (Araneae: Miturgidae) and Chrysoperlacarnea (Neuroptera: Chrysopidae) intraguild predation on the potential control of cotton pest Helicoverpa armigera (Lepidoptera: Noctuidae). Biocontrol Sci. Technol. 2014, 24, 216–228. [Google Scholar] [CrossRef]
- Haye, T. Studies on the Ecology of European Peristenus spp. (Hymenoptera: Braconidae) and Their Potential for the Biological Control of Lygus spp. (Hemiptera: Miridae) in Canada. Ph.D. Thesis, Kiel University, Schleswig-Holstein, Germany, 2004. [Google Scholar]
- Day, W.H. Evaluation of biological control of the tarnished plant bug (Hemiptera: Miridae) in alfalfa by the introduced parasite Peristenus digoneutis (Hymenoptera: Braconidae). Environ. Entomol. 1996, 25, 512–518. [Google Scholar] [CrossRef]
- Chen, X.D.; Johnson, D.L.; Cárcamo, H.; Jegatheeswaran, P.; Kawchuk, L.; Jaronski, S. Laboratory testing of a new strain of the insect-pathogenic fungus Metarhizium anisopliae “S54” as a potential control agent for Lygus keltoni (Hemiptera: Miridae), a pest of canola and alfalfa in Western Canada. Biopestic. Int. 2020, 16, 101–110. [Google Scholar]
- Sabbahi, R.; Merzouki, A.; Guertin, C. Efficacy of Beauveria bassiana (Bals.) Vuill. against the tarnished plant bug, Lygus lineolaris L., in strawberries. J. Appl. Entomol. 2008, 132, 124–134. [Google Scholar] [CrossRef]

| Generations | Nymph | Adult | Main Host Plants | ||
|---|---|---|---|---|---|
| Genus | Species | Region of Distribution * | |||
| Overwintering generation | Overwintering adults lay eggs on host plants from late March to mid-April | Halogeton | Halogeton glomeratus (Bieb) C. A. Mey. [17] | Canada, China, America, United Kingdom, France, India, Sweden, Spain, Mongolia, Russia, Kazakhstan, Uzbekistan, Kyrgyzstan, Tajikistan, Turkmenistan, etc. | |
| Suaeda | Suaeda microphylla (C. A. Mey.) Pall [18] | China, Russia, Iran, Kazakhstan, Uzbekistan, Kyrgyzstan, Tajikistan, and Turkmenistan | |||
| Kochia | Kochia prostrata (L.) Schrad. var. prostrate [19] | China, Mongolia, Russia, Kazakhstan, Uzbekistan, Kyrgyzstan, Turkmenistan | |||
| Descurainia | Descurainia sophia (L.) Webb ex Prantl [17] | China, France, Mongolia, Russia, Sweden, Kazakhstan, Uzbekistan, Kyrgyzstan, Turkey, Iran, Afghanistan, America, Canada, Europe, India, etc. | |||
| Brassica | Brassica napus L. [64] | China, America, Russia, United Kingdom, Denmark, Sweden, Germany, Switzerland, the Netherlands, etc. | |||
| Medicago | Medicago sativa L. [17,27] | China, America, France, Belgium, Sweden, Poland, Canada, Russia, Germany, Spain, Ukraine, Switzerland, the Netherlands, Pakistan, India, etc. | |||
| Triticum | Triticum aestivum L. [18] | Every country in the world | |||
| Cirsium | Cirsium arvense var. integrifolium C. Wimm. [17] | China, Russia, America, United Kingdom, Germany, Romania, Kazakhstan, Denmark, etc. | |||
| First generation | Early to mid-May | Late May to mid-June | Chenopodium | Chenopodium glaucum L. [64] | China, America, Canada, United Kingdom, Australia, Sweden, Russia, Slovakia, etc. |
| Amaranthus | Amaranthus retroflexus L. [18] | China, America, Canada, United Kingdom, Belgium, France, Sweden, Poland, Germany, Spain, India, etc. | |||
| Carthamus | Carthamus tinctorius L. [17,27] | China, America, France, United Kingdom, Japan, Sweden, India, Canada, Mexico, Uzbekistan, Slovakia, Slovakia, Greece, etc. | |||
| Kochia | Kochia prostrata (L.) Schrad. var. prostrate [19] | China, Mongolia, Russia, Kazakhstan, Uzbekistan, Kyrgyzstan, and Turkmenistan | |||
| Salsola | Salsola collina Pall. [17] | China, America, Russia, Poland, Czechia, Canada, Estonia, Latvia, Slovakia, Romania, Belarus, etc. | |||
| Bassia | Bassia hyssopifolia (Pall.) O. Kuntze [17] | China, America, Russia, Canada, Belgium, France, Australia, Denmark, Spain, Lithuania, etc. | |||
| Ziziphus | Ziziphus jujuba Mill. [18] | China, America, Russia, Australia, Japan, India, Italy, Croatia, Bulgaria, Libya, Romania, Türkiye, etc. | |||
| Brassica | Brassica napus L. [27,64] | China, America, Russia, United Kingdom, Denmark, Sweden, Germany, Switzerland, the Netherlands, etc. | |||
| Helianthus | Helianthus annuus L. [17,27] | Every country in the world | |||
| Malus | Malus pumila Mill. [17] | Asia, Europe, parts of North and South America, and Africa | |||
| Pyrus | Pyrus brestschneideri Rehd. [18] | China and Pakistan | |||
| Second generation | Mid-late June to early July | Mid- to end of July | Gossypium | Gossypium herbaceum L. [64] | China, Brazil, United States, Australia, India, Uzbekistan, Egypt, etc. |
| Third generation | Early August | Late August | Gossypium | Gossypium herbaceum L. [64] | China, Brazil, United States, Australia, India, Uzbekistan, Egypt, etc. |
| Fourth generation | Mid-September | Late September to mid-late October | Chenopodium | Chenopodium glaucum L. [17] | China, America, Canada, United Kingdom, Australia, Sweden, Russia, Slovakia, etc. |
| Kochia | Kochia prostrata (L.) Schrad. var. prostrate [19] | China, Mongolia, Russia, Kazakhstan, Uzbekistan, Kyrgyzstan, Turkmenistan | |||
| Bassia | Bassia hyssopifolia (Pall.) O. Kuntze [18] | China, America, Russia, Canada, Belgium, France, Australia, Denmark, Spain, Lithuania, etc. | |||
| Salsola | Salsola collina Pall. [17] | China, America, Russia, Poland, Czechia, Canada, Estonia, Latvia, Slovakia, Romania, Belarus, etc. | |||
| Medicago | Medicago sativa L. [17] | China, America, France, Belgium, Sweden, Poland, Canada, Russia, Germany, Spain, Ukraine, Switzerland, the Netherlands, Pakistan, India, etc. | |||
| Plant Volatiles | Source |
|---|---|
| α-Pinene | Chenopodium glaucum L., Convolvulus arvensis L., and Chenopodium serotinum L. [19] |
| 1,6,10-Dodecatriene, 7,11-dimethyl-3-methylene, (E)- | Convolvulus arvensis L. [19] |
| 1-Caryophyllene | Chenopodium glaucum L. and Convolvulus arvensis L. [19] |
| 3-Hexen-1-ol, acetate, (Z)- | Chenopodium glaucum L., Convolvulus arvensis L., and Lycopersicon esculentum Mill [19] |
| Nonanal | Kochia prostrata (L.) Schrad. var. prostrate, Chenopodium glaucum L., and Brassica napus L. [19,64] |
| Dibutyl phthalate | Brassica oleracea var. botrytis L. [19] |
| 1-Hexanol, 2-ethyl | Kochia prostrata (L.) Schrad. var. prostrate and Chenopodium glaucum L. [19] |
| Butylated hydroxytoluene | Brassica oleracea var. botrytis L. [19] |
| 1(3H)-Isobenzofuranone, 6-(dimethylamino)-3,3-bis [4-(dimethylamino) phenyl] | Kochia prostrata (L.) Schrad. var. prostrate and Brassica campestris L. ssp. chinensis Makino [19] |
| Methyl salicylate | Solanum nigrum L. and Brassica napus L. [64] |
| Linalool | Chenopodium glaucum L. [64] |
| 2-Hexen-1-ol, (E)- | Gossypium herbaceum L. [64] |
| Ocimene | Gossypium herbaceum L., Chenopodium glaucum L., and Portulaca oleracea L. [64] |
| Isothiocyanic acid sec-butyl ester | Brassica napus L. [64] |
| Phenylacetaldehyde | Solanum nigrum L. and Brassica napus L. [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Gou, C.; Feng, H. Biology and Ecology of Lygus pratensis (Linn, 1758) (Heteroptera: Miridae): Towards the Practical Management of Cropping Landscapes in China. Insects 2025, 16, 441. https://doi.org/10.3390/insects16050441
Li P, Gou C, Feng H. Biology and Ecology of Lygus pratensis (Linn, 1758) (Heteroptera: Miridae): Towards the Practical Management of Cropping Landscapes in China. Insects. 2025; 16(5):441. https://doi.org/10.3390/insects16050441
Chicago/Turabian StyleLi, Pengfei, Changqing Gou, and Hongzu Feng. 2025. "Biology and Ecology of Lygus pratensis (Linn, 1758) (Heteroptera: Miridae): Towards the Practical Management of Cropping Landscapes in China" Insects 16, no. 5: 441. https://doi.org/10.3390/insects16050441
APA StyleLi, P., Gou, C., & Feng, H. (2025). Biology and Ecology of Lygus pratensis (Linn, 1758) (Heteroptera: Miridae): Towards the Practical Management of Cropping Landscapes in China. Insects, 16(5), 441. https://doi.org/10.3390/insects16050441







