Ground-Active Arthropod Diversity Under Energycane and Biomass Sorghum Production
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Descriptions
2.2. Energycane and Biomass Sorghum Genotypes
2.3. Experimental Design
2.4. Pitfall Trap Design and Ground-Active Arthropod Sample Collection
2.5. Pitfall Trap Sample Processing
2.6. Data Analysis
3. Results
3.1. Arthropod Abundance Across Sites, Years, and Crop Types
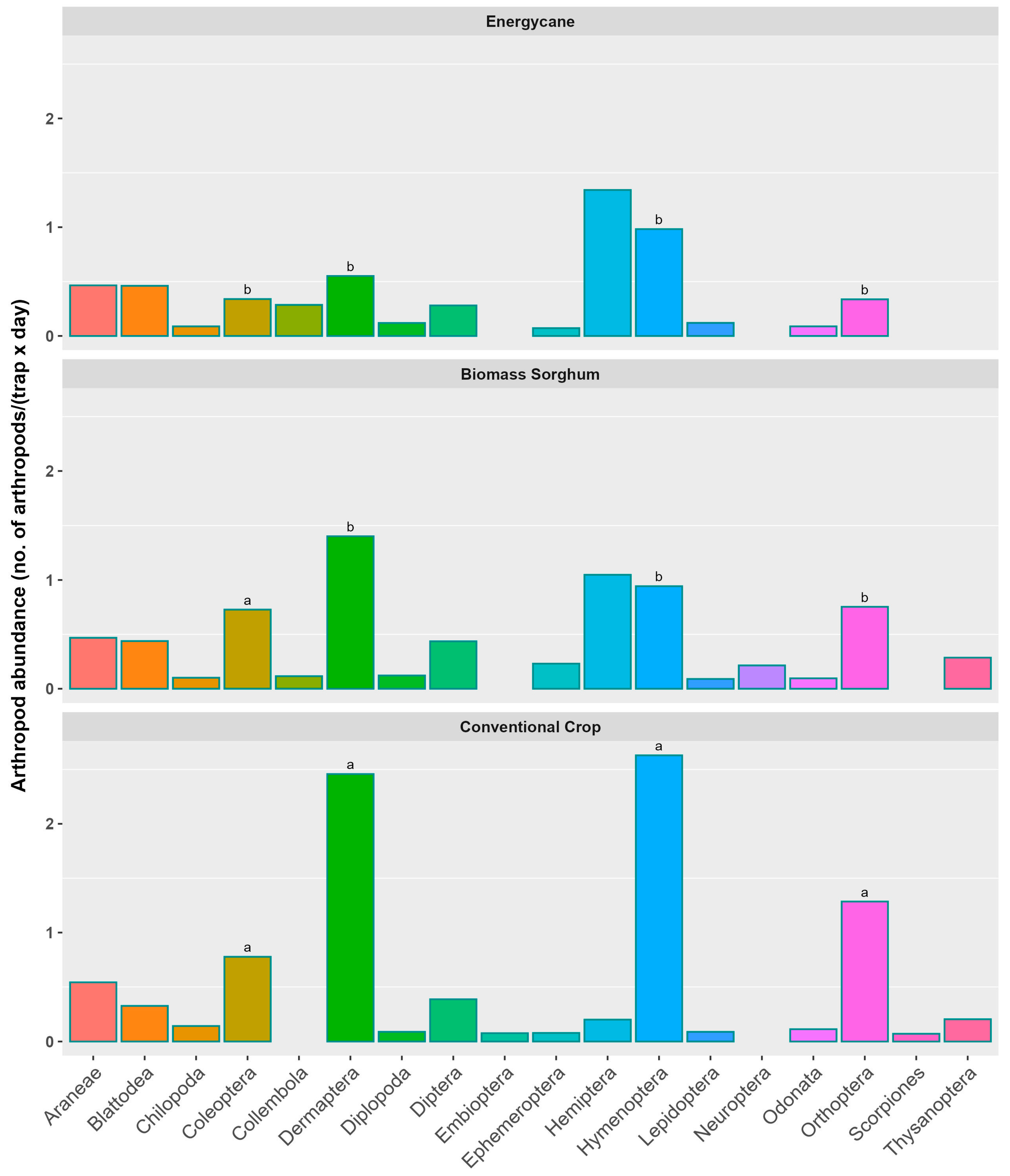
3.2. Arthropod Distribution Across Taxonomic Orders
3.3. Abundance-Based Pairwise Dissimilarity
3.4. Impact of Sites, Years, and Crop Types on Arthropod Abundance
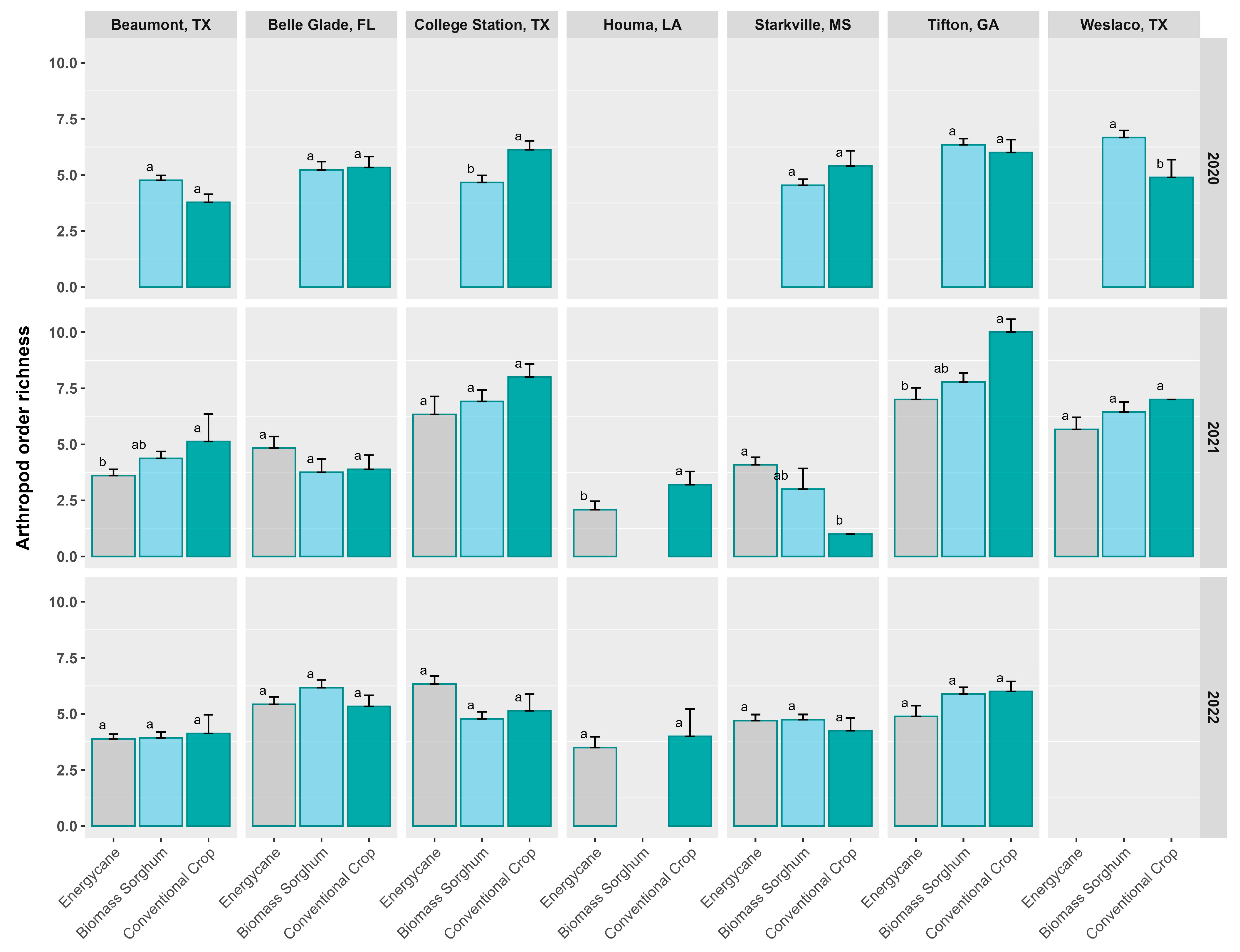
3.5. Order-Based Arthropod Richness, Shannon’s Diversity, and Simpson’s Diversity
3.6. Impact of Sites, Years, and Crop Types on Order-Based Arthropod Diversity
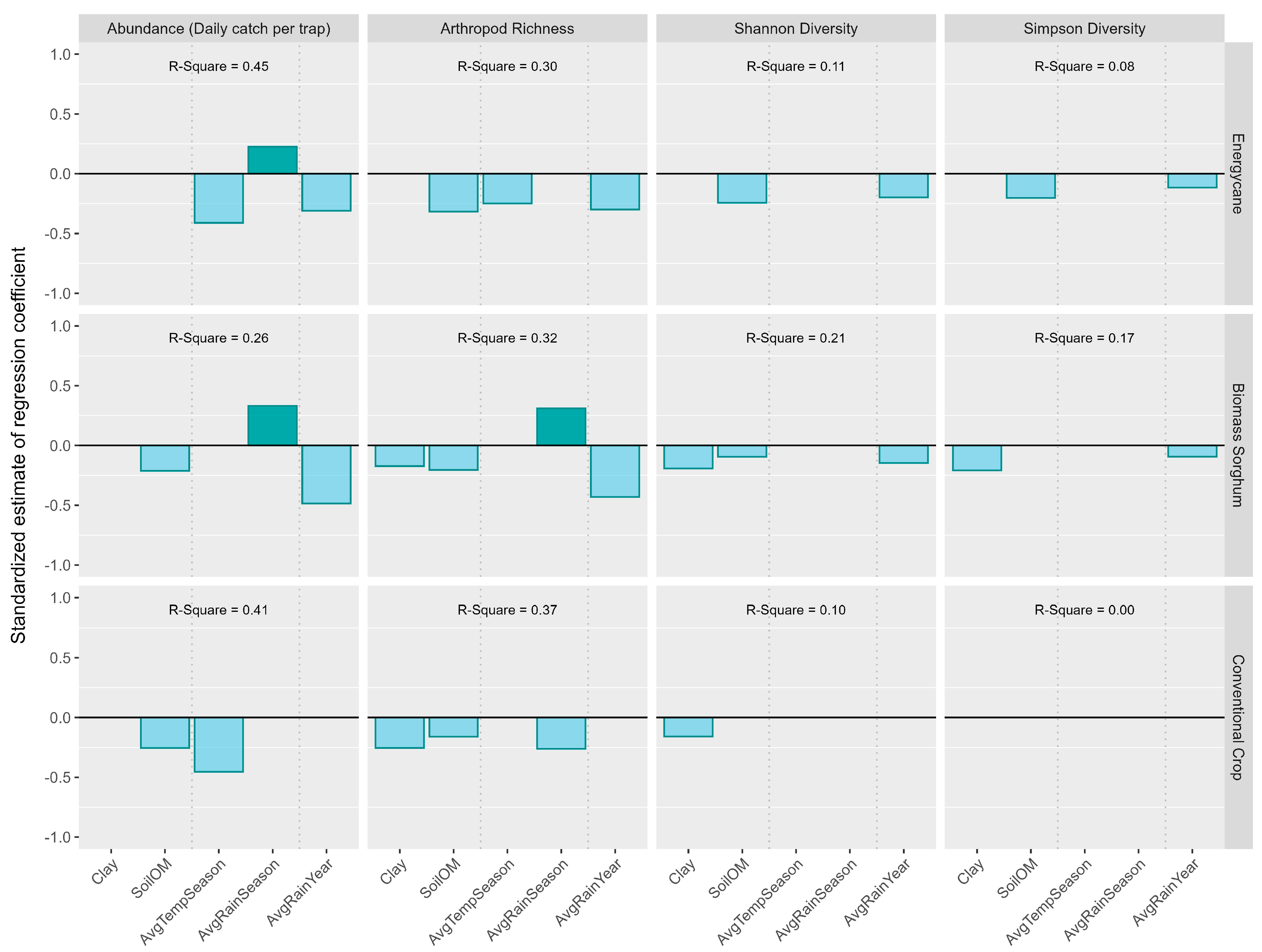
3.7. Impact of Environmental Factors on Arthropod Abundance and Order-Based Arthropod Diversity
4. Discussion
4.1. Cellulosic Energy Crops and Biodiversity
4.2. Biodiversity in Perennial vs. Annual Crops
4.3. Taxonomic Sufficiency and Recognized Taxonomic Units in Biodiversity Analysis
4.4. Ground- and Vegetation-Dwelling Taxa
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Solomon, B.D.; Barnes, J.R.; Halvorsen, K.E. Grain and cellulosic ethanol: History, economics, and energy policy. Biomass Bioenergy 2007, 31, 416–425. [Google Scholar] [CrossRef]
- Mohr, A.; Raman, S. Lessons from first generation biofuels and implications for the sustainability appraisal of second generation biofuels. Energy Policy 2013, 63, 114–122. [Google Scholar] [CrossRef] [PubMed]
- DOE. 2023 Billion-Ton Report: An Assessment of U.S. Renewable Carbon Resources; Langholtz, M.H., Lead; ORNL/SPR-2024/3103; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2024. [CrossRef]
- Langholtz, M.H.; Stokes, B.J.; Eaton, L.M. 2016 Billion-Ton Report: Advancing Domestic Resources for a Thriving Bioeconomy, Volume 1: Economic Availability of Feedstock; Oak Ridge National Laboratory, managed by UT-Battelle, LLC for the US Department of Energy: Oak Ridge, TN, USA, 2016; pp. 1–411.
- USDA. A USDA Regional Roadmap to Meeting the Biofuels Goals of the Renewable Fuels Standard by 2022. 2010. Available online: https://www.usda.gov/sites/default/files/documents/USDA_Biofuels_Report_6232010.pdf (accessed on 17 February 2025).
- Lewandowski, I.; Scurlock, J.M.O.; Lindvallc, E.; Christou, M. The development and current status of perennial rhizomatous grasses as energy crops in the US and Europe. Biomass Bioenergy 2003, 25, 335–361. [Google Scholar] [CrossRef]
- McLaughlin, S.B.; Kszos, L.A. Development of switchgrass (Panicum virgatum) as a bioenergy feedstock in the United States. Biomass Bioenergy 2005, 25, 515–535. [Google Scholar] [CrossRef]
- Heaton, E.; Moore, K.; Salas-Fernandez, M.; Hartzler, B.; Liebman, M.; Barnhart, S. Giant Miscanthus for Biomass Production; AG210; Iowa State University, Department of Agronomy: Ames, IA, USA, 2010; p. 2. [Google Scholar]
- Williams, M.A.; Feest, A. The effect of Miscanthus cultivation on the biodiversity of ground beetles (Coleoptera: Carabidae), spiders and harvestmen (arachnida: Araneae and opiliones). Agric. Sci. 2019, 10, 903–917. [Google Scholar] [CrossRef][Green Version]
- Legendre, B.L.; Burner, D.M. Biomass production of sugarcane cultivars and early-generation hybrids. Biomass Bioenergy 1995, 8, 55–61. [Google Scholar] [CrossRef]
- Fageria, N.K.; Moreira, A.; Moraes, L.A.C.; Hale, A.L.; Viator, R.P. Sugarcane and Energycane. In Biofuel Crops: Production, Physiology and Genetics; Singh, B.P., Ed.; CAB International: Wallingford, UK; Boston, MA, USA, 2013; pp. 151–171. [Google Scholar]
- Prine, G.M.; Stricker, J.A.; McConnell, W.V. Opportunities for bioenergy development in lower South USA. In Proceedings of the Third Biomass Conference of the America, Montreal, QC, Canada, 24–29 August 1997; Volume 1, pp. 227–235. [Google Scholar]
- LSU. Sugarcane Production Handbook; Louisiana State University AgCenter: Baton Rouge, LA, USA, 2014; Pub. 2859; 84p. [Google Scholar]
- Rooney, W.L.; Blumenthal, J.; Bean, B.; Mullet, J.E. Designing sorghum as a dedicated bioenergy feedstock. Biofuels Bioprod. Biorefining 2007, 1, 147–157. [Google Scholar] [CrossRef]
- Olson, S.N.; Ritter, K.; Medley, J.; Wilson, T.; Rooney, W.L.; Mullet, J.E. Energy sorghum hybrids: Functional dynamics of high nitrogen use efficiency. Biomass Bioenergy 2013, 56, 307–316. [Google Scholar] [CrossRef]
- Lee, D.; Aberle, E.; Anderson, E.; Anderson, W.; Baldwin, B.; Baltensperger, D.; Barrett, M.; Bonos, S.; Bouton, J.; Brummer, C.; et al. Biomass production of herbaceous energy crops in the United States: Field trial results and yield potential maps from the multiyear regional feedstock partnership. GCB Bioenergy 2018, 10, 698–716. [Google Scholar] [CrossRef]
- Wright, L.; Turhollow, A. Switchgrass selection as a “model” bioenergy crop: A history of the process. Biomass Bioenergy 2010, 34, 851–868. [Google Scholar] [CrossRef]
- Yang, Y.; Wilson, L.T.; Jifon, J.; Landivar, J.; da Silva, J.; Maeda, M.M.; Wang, J.; Christensen, E. Energycane growth dynamics and potential early harvest penalties along the Texas Gulf Coast. Biomass Bioenergy 2018, 113, 1–14. [Google Scholar] [CrossRef]
- Salassi, M.E.; Brown, K.; Hilbun, B.M.; Deliberto, M.A.; Gravois, K.A.; Mark, T.B.; Falconer, L.L. Farm-Scale Cost of Producing Perennial Energy Cane as a Biofuel Feedstock. Bioenergy Res. 2014, 7, 609–619. [Google Scholar] [CrossRef]
- Salassi, M.E.; Holzapfel, A.; Hilbun, B.M.; Deliberto, M.A.; Gravois, K.A.; Viator, H.P.; Falconer, L.L.; Mark, T.B. Feedstock Crop Production Costs and Biofuel Feedstock Input Costs Associated with the Production of Energy Cane and Sweet Sorghum in the Southeastern USA. Bioenergy Res. 2017, 10, 772–782. [Google Scholar] [CrossRef]
- Hague, M.; Epplin, F.M.; Biermacher, J.T.; Holcomb, R.B.; Kenkel, P.L. Marginal cost of delivering switchgrass feedstock and producing cellulosic ethanol at multiple biorefineries. Biomass Bioenergy 2014, 66, 308–319. [Google Scholar] [CrossRef][Green Version]
- Liebig, M.A.; Schmer, M.R.; Vogel, K.P.; Mitchell, R.B. Soil Carbon Storage by Switchgrass Grown for Bioenergy. Bioenergy Res. 2008, 1, 215–222. [Google Scholar] [CrossRef]
- Sinistore, J.C.; Reinemann, D.J.; Izaurralde, R.C.; Cronin, K.R.; Meier, P.J.; Runge, T.M.; Zhang, X.S. Life Cycle Assessment of Switchgrass Cellulosic Ethanol Production in the Wisconsin and Michigan Agricultural Contexts. Bioenergy Res. 2015, 8, 897–909. [Google Scholar] [CrossRef][Green Version]
- Nocentini, A.; Di Virgilio, N.; Monti, A. Model Simulation of Cumulative Carbon Sequestration by Switchgrass (Panicum virgatum L.) in the Mediterranean Area Using the DAYCENT Model. Bioenergy Res. 2015, 8, 1512–1522. [Google Scholar] [CrossRef]
- Lask, J.; Magenau, E.; Ferrarini, A.; Kiesel, A.; Wagner, M.; Lewandowski, I. Perennial rhizomatous grasses: Can they really increase species richness and abundance in arable land?—A meta-analysis. Glob. Change Biol. Bioenergy 2020, 12, 968–978. [Google Scholar] [CrossRef]
- Immerzeel, D.J.; Verweij, P.A.; Hilst, F.v.d.; Faaij, A.P.C. Biodiversity impacts of bioenergy crop production: A state-of-the-art review. Glob. Change Biol. Bioenergy 2014, 6, 183–209. [Google Scholar] [CrossRef]
- Tudge, S.J.; Purvis, A.; De Palma, A. The impacts of biofuel crops on local biodiversity: A global synthesis. Biodivers. Conserv. 2021, 30, 2863–2883. [Google Scholar] [CrossRef]
- Núñez-Regueiro, M.M.; Siddiqui, S.F.; Fletcher, R.J. Effects of bioenergy on biodiversity arising from land-use change and crop type. Conserv. Biol. 2021, 35, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Kempski, C.M. The Abundance and Biodiversity of Arthropods in Biofuel Crops: Insects and Arachnids in Corn, Switchgrass and Native Mixed Grass Prairie Fields; Environmental Science, College of Science, Rochester Institute of Technology, ProQuest LLC: Ann Arbor, MI, USA, 2013. [Google Scholar]
- Zahorec, A.; Reid, M.L.; Tiemann, L.K.; Landis, D.A. Perennial grass bioenergy cropping systems: Impacts on soil fauna and implications for soil carbon accrual. Glob. Change Biol. Bioenergy 2022, 14, 4–23. [Google Scholar] [CrossRef]
- Semere, T.; Slater, F.M. Invertebrate populations in miscanthus (Miscanthus x giganteus) and reed canary-grass (Phalaris arundinacea) fields. Biomass Bioenergy 2007, 31, 30–39. [Google Scholar] [CrossRef]
- Radzikowski, P.; Matyka, M.; Berbec, A.K. Biodiversity of Weeds and Arthropods in Five Different Perennial Industrial Crops in Eastern Poland. Agriculture 2020, 10, 636. [Google Scholar] [CrossRef]
- Burmeister, J. Promotion of ground beetles by integrating perennial energy crops into existing agricultural landscapes. Biomass Bioenergy 2021, 146, 105973. [Google Scholar] [CrossRef]
- Burmeister, J.; Panassiti, B.; Heine, F.; Wolfrum, S.; Moriniere, J. Perennial alternative crops for biogas production increase arthropod abundance and diversity after harvest-results of suction sampling and metabarcoding. Eur. J. Entomol. 2023, 120, 59–69. [Google Scholar] [CrossRef]
- Gardiner, M.A.; Tuell, J.K.; Isaacs, R.; Gibbs, J.; Ascher, J.S.; Landis, D.A. Implications of Three Biofuel Crops for Beneficial Arthropods in Agricultural Landscapes. Bioenergy Res. 2010, 3, 6–19. [Google Scholar] [CrossRef]
- Werling, B.P.; Dickson, T.L.; Isaacs, R.; Gaines, H.; Gratton, C.; Gross, K.L.; Liere, H.; Malmstrom, C.M.; Meehan, T.D.; Ruan, L.L.; et al. Perennial grasslands enhance biodiversity and multiple ecosystem services in bioenergy landscapes. Proc. Natl. Acad. Sci. USA 2014, 111, 1652–1657. [Google Scholar] [CrossRef]
- Helms, J.A.; Ijelu, S.E.; Wills, B.D.; Landis, D.A.; Haddad, N.M. Ant biodiversity and ecosystem services in bioenergy landscapes. Agric. Ecosyst. Environ. 2020, 290, 106780. [Google Scholar] [CrossRef]
- Haan, N.L.; Benucci, G.N.M.; Fiser, C.M.; Bonito, G.; Landis, D.A. Contrasting effects of bioenergy crops on biodiversity. Sci. Adv. 2023, 9, eadh796. [Google Scholar] [CrossRef]
- Nunes, D.H.; Pasini, A.; Benito, N.P.; Brown, G.G. Earthworm diversity in four land use systems in the region of Jaguapita, Parana State, Brazil. Caribb. J. Sci. 2006, 42, 331–338. [Google Scholar]
- Hale, A.L.; Dufrene, E.O.; Tew, T.L.; Pan, Y.B.; Viator, R.P.; White, P.M.; Veremis, J.C.; White, W.H.; Cobill, R.; Richard, E.P.; et al. Registration of ‘Ho 02-113’ Sugarcane. J. Plant Regist. 2013, 7, 51–57. [Google Scholar] [CrossRef]
- Gordon, V.S.; Comstock, J.C.; Sandhu, H.S.; Gilbert, R.A.; Sood, S.; Korndorfer, P.; El-Hout, N.; Arundale, R. Registration of ‘UFCP 84-1047’ Sugarcane for Use as a Biofuel Feedstock. J. Plant Regist. 2016, 10, 251–257. [Google Scholar] [CrossRef]
- Gordon, V.S.; Comstock, J.C.; Sandhu, H.S.; Gilbert, R.A.; Sood, S.G.; Korndorfer, P.; El-Hout, N.; Arundale, R. Registration of ‘UFCP 87-0053’ Sugarcane for Use as a Biofuel Feedstock. J. Plant Regist. 2016, 10, 258–264. [Google Scholar] [CrossRef]
- Rooney, W.L.; Aydin, S. Genetic control of a photoperiod-sensitive response in Sorghum bicolor (L.) Moench. Crop Sci. 1999, 39, 397–400. [Google Scholar] [CrossRef]
- Brown, G.R.; Matthews, I.M. A review of extensive variation in the design of pitfall traps and a proposal for a standard pitfall trap design for monitoring ground-active arthropod biodiversity. Ecol. Evol. 2016, 6, 3953–3964. [Google Scholar] [CrossRef]
- Borror, D.J.; Triplehorn, C.A.; Johnson, N.F. An Introduction to the Study of Insects, 6th ed.; Saunders College Publishing: Philadelphia, PA, USA; Ft. Worth, TX, USA, 1989. [Google Scholar]
- Baselga, A.; Orme, C.D.L. betapart: An R package for the study of beta diversity. Methods Ecol. Evol. 2012, 3, 808–812. [Google Scholar] [CrossRef]
- Baselga, A. Partitioning abundance-based multiple-site dissimilarity into components: Balanced variation in abundance and abundance gradients. Methods Ecol. Evol. 2017, 8, 799–808. [Google Scholar] [CrossRef]
- Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Hill, M.O. Diversity and Evenness: A Unifying Notation and Its Consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Jost, L. Entropy and diversity. Oikos 2006, 113, 363–375. [Google Scholar] [CrossRef]
- Henderson, P.A.; Southwood, T.R.E. Ecological Methods, 4th ed.; John Wiley & Sons Ltd.: West Sussex, UK, 2016. [Google Scholar]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Paoletti, M.G.; Taylor, R.A.J.; Stinner, B.R.; Stinner, D.H.; Benzing, D.H. Diversity of soil fauna in the canopy and forest floor of a Venezuelan cloud forest. J. Trop. Ecol. 1991, 7, 373–383. [Google Scholar] [CrossRef]
- SAS. SAS STAT 13.2 User’s Guide; SAS Institute: Cary, NC, USA, 2015. [Google Scholar]
- Schreiber-Gregory, D.N. Multicollinearity: What Is It, Why Should We Care, and How Can It Be Controlled? In Proceedings of the SAS Global Forum, Orlando, FL, USA, 2–5 April 2017; pp. 1404–2017. Available online: https://support.sas.com/resources/papers/proceedings17/1404-2017.pdf (accessed on 15 March 2024).
- Wicklin, R. Collinearity in Regression: The COLLIN Option in PROC REG. 2020. Available online: https://blogs.sas.com/content/iml/2020/01/23/collinearity-regression-collin-option.html (accessed on 15 March 2024).
- Baselga, A.; Leprieur, F. Comparing methods to separate components of beta diversity. Methods Ecol. Evol. 2015, 6, 1069–1079. [Google Scholar] [CrossRef]
- Baselga, A. Separating the two components of abundance-based dissimilarity: Balanced changes in abundance vs. abundance gradients. Methods Ecol. Evol. 2013, 4, 552–557. [Google Scholar] [CrossRef]
- Platen, R.; Konrad, J.; Glemnitz, M. Novel energy crops: An opportunity to enhance the biodiversity of arthropod assemblages in biomass feedstock cultures? Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2017, 13, 162–171. [Google Scholar] [CrossRef][Green Version]
- Brooks, D.R.; Bohan, D.A.; Champion, G.T.; Haughton, A.J.; Hawes, C.; Heard, M.S.; Clark, S.J.; Dewar, A.M.; Firbank, L.G.; Perry, J.N.; et al. Invertebrate responses to the management of genetically modified herbicide-tolerant and conventional spring crops.I. Soil-surface-active invertebrates. Philos. Trans. R. Soc. B-Biol. Sci. 2003, 358, 1847–1862. [Google Scholar] [CrossRef]
- Ebeling, A.; Hines, J.; Hertzog, L.R.; Lange, M.; Meyer, S.T.; Simons, N.K.; Weisser, W.W. Plant diversity effects on arthropods and arthropod-dependent ecosystem functions in a biodiversity experiment. Basic Appl. Ecol. 2018, 26, 50–63. [Google Scholar] [CrossRef]
- Batáry, P.; Dicks, L.V.; Kleijn, D.; Sutherland, W.J. The role of agri-environment schemes in conservation and environmental management. Conserv. Biol. 2015, 29, 1006–1016. [Google Scholar] [CrossRef]
- Dauber, J.; Cass, S.; Gabriel, D.; Harte, K.; Åström, S.; O’Rourke, E.; Stout, J.C. Yield-biodiversity trade-off in patchy fields of Miscanthus x giganteus. Glob. Change Biol. Bioenergy 2015, 7, 455–467. [Google Scholar] [CrossRef]
- Tscharntke, T.; Grass, I.; Wanger, T.C.; Westphal, C.; Batary, P. Beyond organic farming—Harnessing biodiversity-friendly landscapes. Trends Ecol. Evol. 2021, 36, 919–930. [Google Scholar] [CrossRef]
- Rosenfield, M.F.; Brown, L.M.; Anand, M. Increasing cover of natural areas at smaller scales can improve the provision of biodiversity and ecosystem services in agroecological mosaic landscapes. J. Environ. Manag. 2022, 303, 114248. [Google Scholar] [CrossRef]
- Bertrand, C.; Burel, F.; Baudry, J. Spatial and temporal heterogeneity of the crop mosaic influences carabid beetles in agricultural landscapes. Landsc. Ecol. 2016, 31, 451–466. [Google Scholar] [CrossRef]
- Marja, R.; Tscharntke, T.; Batary, P. Increasing landscape complexity enhances species richness of farmland arthropods, agri-environment schemes also abundance-A meta-analysis. Agric. Ecosyst. Environ. 2022, 326, 107822. [Google Scholar] [CrossRef]
- Englund, O.; Dimitriou, I.; Dale, V.H.; Kline, K.L.; Mola-Yudego, B.; Murphy, F.; English, B.; McGrath, J.; Busch, G.; Negri, M.C.; et al. Multifunctional perennial production systems for bioenergy: Performance and progress. Wiley Interdiscip. Rev.-Energy Environ. 2020, 9, e375. [Google Scholar] [CrossRef]
- Dauber, J.; Jones, M.B.; Stout, J.C. The impact of biomass crop cultivation on temperate biodiversity. Glob. Change Biol. Bioenergy 2010, 2, 289–309. [Google Scholar] [CrossRef]
- Chmelíková, L.; Wolfrum, S. Mitigating the biodiversity footprint of energy crops—A case study on arthropod diversity. Biomass Bioenergy 2019, 125, 180–187. [Google Scholar] [CrossRef]
- Landis, D.A.; Werling, B.P. Arthropods and biofuel production systems in North America. Insect Sci. 2010, 17, 220–236. [Google Scholar] [CrossRef]
- Timms, L.L.; Bowden, J.J.; Summerville, K.S.; Buddle, C.M. Does species-level resolution matter? Taxonomic sufficiency in terrestrial arthropod biodiversity studies. Insect Conserv. Divers. 2013, 6, 453–462. [Google Scholar] [CrossRef]
- Terlizzi, A.; Anderson, M.J.; Bevilacqua, S.; Fraschetti, S.; Wlodarska-Kowalczuk, M.; Ellingsen, K.E. Beta diversity and taxonomic sufficiency: Do higher-level taxa reflect heterogeneity in species composition? Divers. Distrib. 2009, 15, 450–458. [Google Scholar] [CrossRef]
- Pik, A.J.; Oliver, I.; Beattie, A.J. Taxonomic sufficiency in ecological studies of terrestrial invertebrates. Aust. J. Ecol. 1999, 24, 555–562. [Google Scholar] [CrossRef]
- Oliver, I.; Beattie, A.J. A Possible Method for the Rapid Assessment of Biodiversity. Conserv. Biol. 1993, 7, 562–568. [Google Scholar] [CrossRef]
- Derraik, J.G.B.; Closs, G.P.; Dickinson, K.J.M.; Sirvid, P.; Barratt, B.I.P.; Patrick, B.H. Arthropod morphospecies versus taxonomic species: A case study with Araneae, Coleoptera, and Lepidoptera. Conserv. Biol. 2002, 16, 1015–1023. [Google Scholar] [CrossRef]
- Barratt, B.I.P.; Derraik, J.G.B.; Rufaut, C.G.; Goodman, A.J.; Dickinson, K.J.M. Morphospecies as a substitute for Coleoptera species identification, and the value of experience in improving accuracy. J. R. Soc. N. Z. 2003, 33, 583–590. [Google Scholar] [CrossRef]
- Biaggini, M.; Consorti, R.; Dapporto, L.; Dellacasa, M.; Paggetti, E.; Corti, C. The taxonomic level order as a possible tool for rapid assessment of Arthropod diversity in agricultural landscapes. Agric. Ecosyst. Environ. 2007, 122, 183–191. [Google Scholar] [CrossRef]
- Oliver, I.; Beattie, A.J. Invertebrate morphospecies as surrogates for species: A case study. Conserv. Biol. 1996, 10, 99–109. [Google Scholar] [CrossRef]
- Beattie, A.J.; Oliver, I. Taxonomic minimalism. Trends Ecol. Evol. 1994, 9, 488–490. [Google Scholar] [CrossRef]
- Weisser, W.W.; Roscher, C.; Meyer, S.T.; Ebeling, A.; Luo, G.J.; Allan, E.; Besser, H.; Barnard, R.L.; Buchmann, N.; Buscot, F.; et al. Biodiversity effects on ecosystem functioning in a 15-year grassland experiment: Patterns, mechanisms, and open questions. Basic Appl. Ecol. 2017, 23, 1–73. [Google Scholar] [CrossRef]
- Jonason, D.; Andersson, G.K.S.; Öckinger, E.; Rundlöf, M.; Smith, H.G.; Bengtsson, J. Assessing the effect of the time since transition to organic farming on plants and butterflies. J. Appl. Ecol. 2011, 48, 543–550. [Google Scholar] [CrossRef]
- Hüber, C.; Zettl, F.; Hartung, J.; Müller-Lindenlaut, M. The impact of maize-bean intercropping on insect biodiversity. Basic Appl. Ecol. 2022, 61, 1–9. [Google Scholar] [CrossRef]
- Honek, A. The effect of plant cover and weather on the activity density of ground surface arthropods in a fallow field. Biol. Agric. Hortic. 1997, 15, 203–210. [Google Scholar] [CrossRef]
- Honek, A. The effect of temperature on the activity of Carabidae (Coleoptera) in a fallow field. Eur. J. Entomol. 1997, 94, 97–104. [Google Scholar]




| Site | State | Lat (°N) | Long (°W) | Elevation (m) | Annual Precipitation (mm) | Daily Average | ||
|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | Humidity (%) | Radiation (MJ/m2) | ||||||
| Weslaco | Texas | 26.2 | 97.9 | 20 | 684 | 23.8 | 65.8 | 16.2 |
| Belle Glade | Florida | 26.7 | 80.6 | 3 | 1217 | 23.7 | 68.2 | 16.2 |
| Houma | Louisiana | 29.6 | 90.8 | 1 | 1598 | 21.1 | 67.8 | 16.0 |
| Beaumont | Texas | 30.1 | 94.3 | 10 | 1398 | 21.0 | 79.1 | 16.1 |
| College Station | Texas | 30.5 | 96.4 | 67 | 853 | 21.5 | 65.3 | 16.5 |
| Tifton | Georgia | 31.5 | 83.6 | 107 | 1030 | 20.0 | 67.7 | 14.7 |
| Starkville | Mississippi | 33.4 | 88.7 | 79 | 1123 | 18.0 | 67.5 | 15.4 |
| Location | Total Number of Trap Deployments | Total Number of Captured Arthropods | ||||||
|---|---|---|---|---|---|---|---|---|
| Energycane * | Biomass Sorghum * | Conventional Crop * | Total Traps | Energycane | Biomass Sorghum | Conventional Crop | Total | |
| Beaumont, TX, USA | 91 × 14 | 136 × 14 | 26 × 13 | 253 | 1015 | 2290 | 358 | 3663 |
| Belle Glade, FL, USA | 46 × 15 | 54 × 16 | 23 × 16 | 123 | 2539 | 5248 | 2024 | 9811 |
| College Station, TX, USA | 30 × 14 | 71 × 14 | 20 × 14 | 121 | 3961 | 3752 | 2642 | 10,355 |
| Houma, LA, USA | 26 × 15 | 9 × 15 | 35 | 147 | 100 | 247 | ||
| Starkville, MS, USA | 32 × 16 | 82 × 15 | 15 × 15 | 129 | 887 | 3561 | 555 | 5003 |
| Tifton, GA, USA | 35 × 17 | 53 × 16 | 12 × 14 | 100 | 1405 | 4116 | 1584 | 7105 |
| Weslaco, TX, USA | 18 × 14 | 48 × 15 | 12 × 16 | 78 | 423 | 5804 | 613 | 6840 |
| Total | 278 × 15 | 444 × 15 | 117 × 14 | 839 | 10,377 | 24,771 | 7876 | 43,024 |
| Main Effects and Two-Way Interactions | Arthropod Abundance | Order-Based Arthropod Diversity | ||||
|---|---|---|---|---|---|---|
| DF | (Square Root Transformed) | DF | Arthropod Richness | |||
| p | Variance (%) | p | Variance (%) | |||
| Site | 6 | <0.001 | 21.4 | 6 | <0.001 | 21.0 |
| Year | 2 | <0.001 | 1.6 | 2 | 0.680 | 0.1 |
| Crop Type | 2 | 0.009 | 0.7 | 2 | 0.344 | 0.2 |
| Nitrogen Level | 1 | 0.514 | 0.0 | 1 | 0.080 | 0.2 |
| Season | 1 | 0.530 | 0.0 | 1 | 0.004 | 0.6 |
| Site × Year | 10 | <0.001 | 8.1 | 10 | <0.001 | 7.0 |
| Site × Crop Type | 11 | <0.001 | 4.6 | 11 | <0.001 | 2.5 |
| Site × Nitrogen Level | 1 | 0.967 | 0.0 | 1 | 0.309 | 0.1 |
| Site × Season | 5 | <0.001 | 2.0 | 5 | <0.001 | 6.6 |
| Year × Crop Type | 3 | 0.192 | 0.4 | 3 | 0.414 | 0.2 |
| Year × Nitrogen Level | 2 | 0.169 | 0.3 | 2 | 0.020 | 0.6 |
| Year × Season | 2 | 0.002 | 1.0 | 2 | 0.001 | 1.3 |
| Crop × Nitrogen Level | 1 | 0.298 | 0.1 | 1 | 0.586 | 0.0 |
| Crop × Season | 2 | 0.565 | 0.1 | 2 | 0.005 | 0.8 |
| Nitrogen Level × Season | 1 | 0.979 | 0.0 | 1 | 0.463 | 0.0 |
| Total (Explained) | 40.3 | 41.2 | ||||
| Model DF | 50 | 50 | ||||
| Error DF | 775 | 775 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Bera, T.; Araji, H.; Dou, F.; Wilson, L.T.; Rooney, W.L.; Morrison, J.I.; Baldwin, B.S.; Knoll, J.E.; Jifon, J.L.; et al. Ground-Active Arthropod Diversity Under Energycane and Biomass Sorghum Production. Insects 2025, 16, 442. https://doi.org/10.3390/insects16050442
Yang Y, Bera T, Araji H, Dou F, Wilson LT, Rooney WL, Morrison JI, Baldwin BS, Knoll JE, Jifon JL, et al. Ground-Active Arthropod Diversity Under Energycane and Biomass Sorghum Production. Insects. 2025; 16(5):442. https://doi.org/10.3390/insects16050442
Chicago/Turabian StyleYang, Yubin, Tanumoy Bera, Hamid Araji, Fugen Dou, Lloyd T. Wilson, William L. Rooney, Jesse I. Morrison, Brian S. Baldwin, Joseph E. Knoll, John L. Jifon, and et al. 2025. "Ground-Active Arthropod Diversity Under Energycane and Biomass Sorghum Production" Insects 16, no. 5: 442. https://doi.org/10.3390/insects16050442
APA StyleYang, Y., Bera, T., Araji, H., Dou, F., Wilson, L. T., Rooney, W. L., Morrison, J. I., Baldwin, B. S., Knoll, J. E., Jifon, J. L., Wright, A. L., Odero, D. C., Sandhu, H. S., Hale, A. L., Mula-Michel, H. P., & Wang, J. (2025). Ground-Active Arthropod Diversity Under Energycane and Biomass Sorghum Production. Insects, 16(5), 442. https://doi.org/10.3390/insects16050442






