Azoxystrobin-Induced Physiological and Biochemical Alterations in Apis mellifera Workers of Different Ages
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Honey Bee (A. mellifera) Rearing
2.2. Fungicide
2.3. Azoxystrobin Treatment
2.4. Biochemical Tests on Enzyme Activity
2.5. Gene Expression Tests for Immunity and Nutrition Metabolism
2.6. Statistical Analysis
3. Results
3.1. Azoxystrobin Decreased Survival Rate of Workers
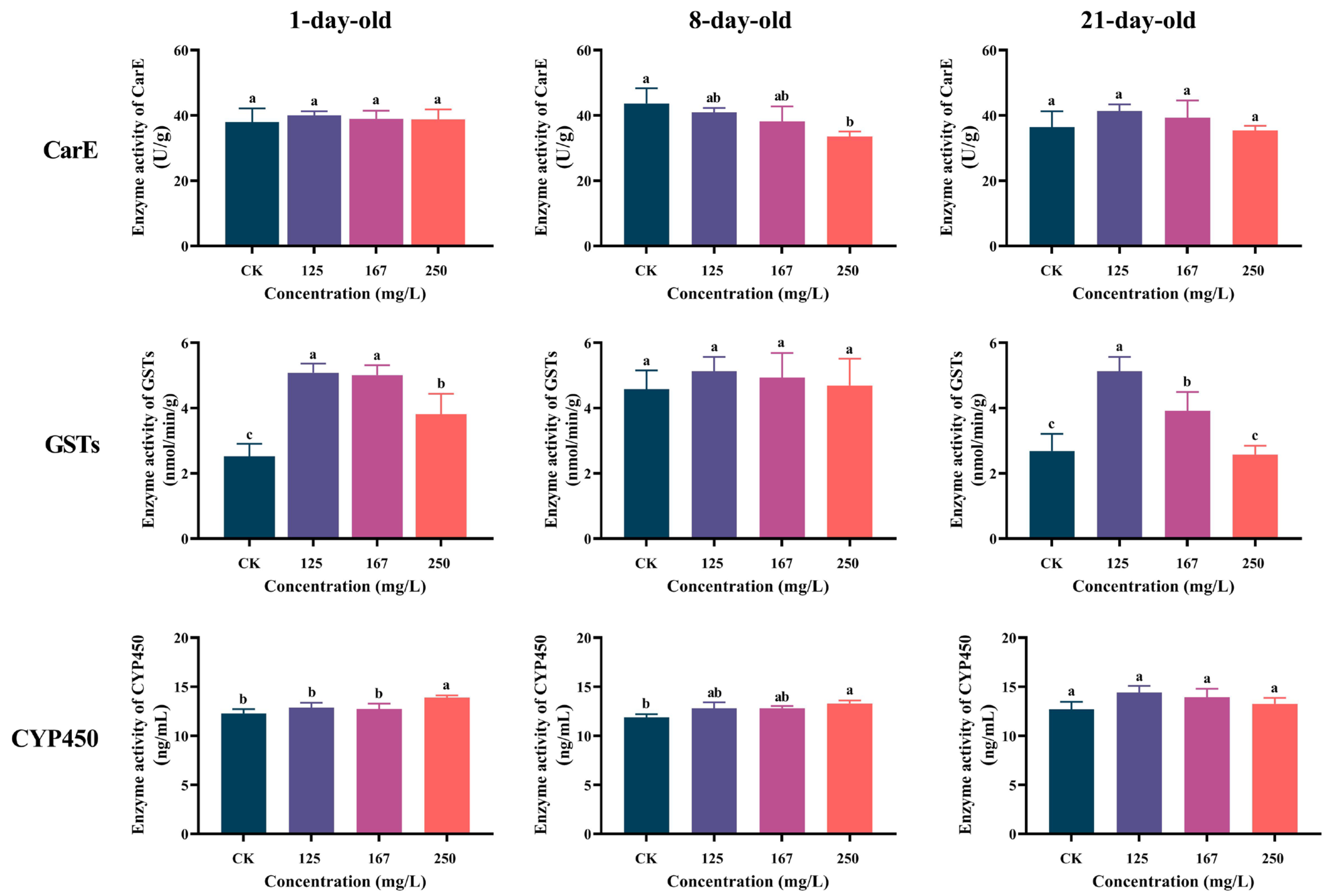
3.2. Azoxystrobin Interfered with Detoxification Enzymes Activities of Workers
3.3. Azoxystrobin Altered Protective Enzymes Activities of Workers
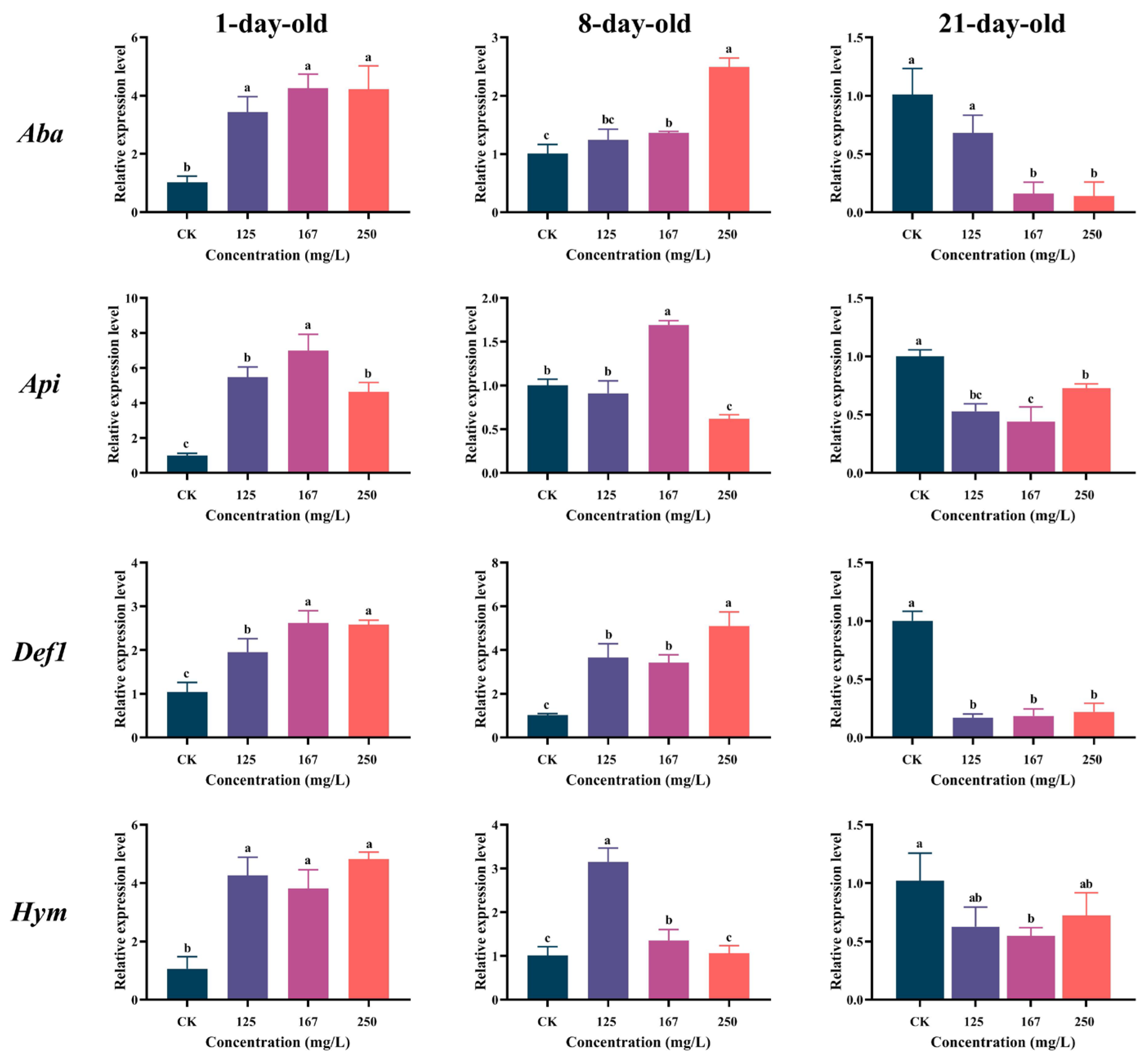
3.4. Azoxystrobin Disrupted Immune Response of Workers
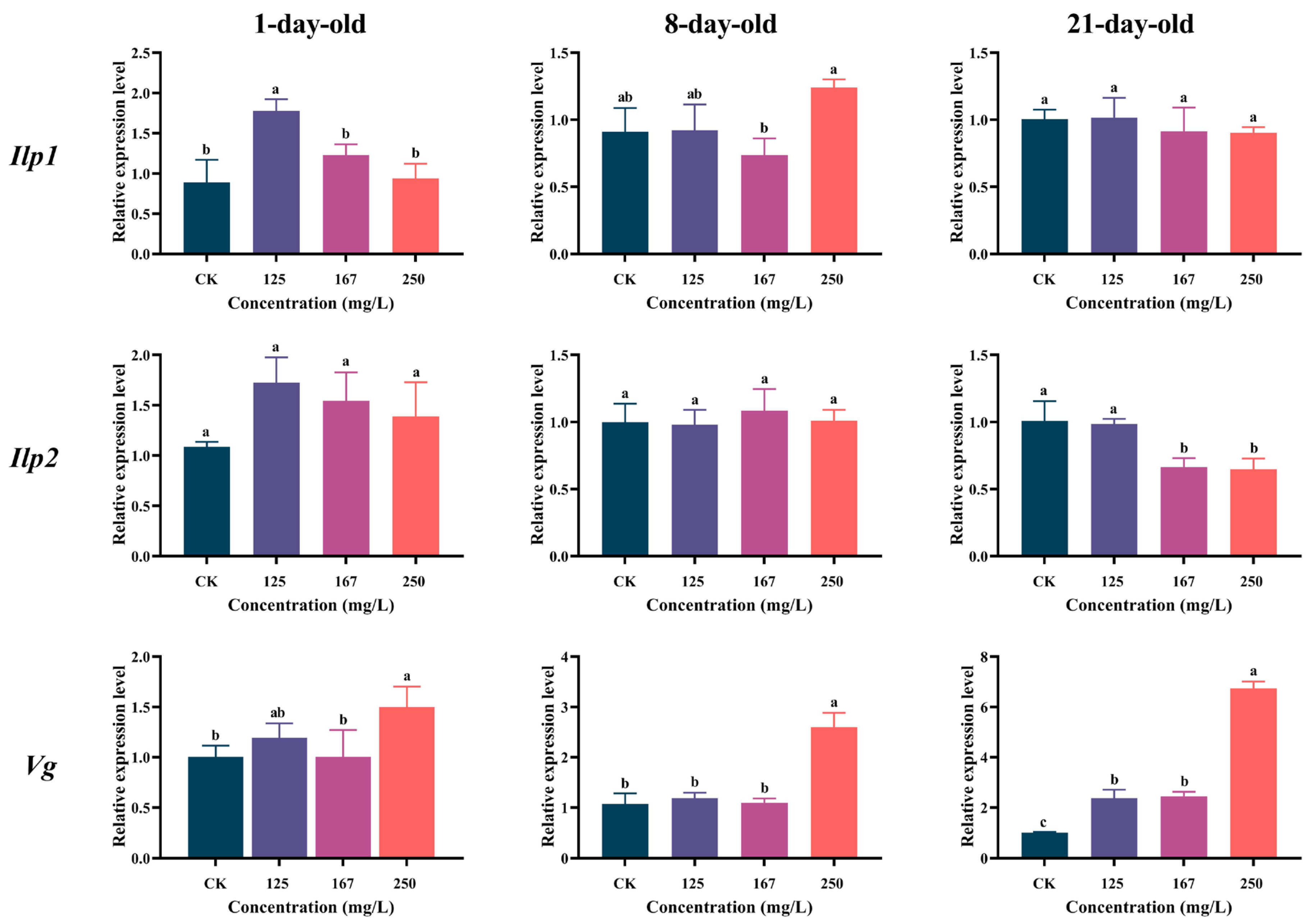
3.5. Azoxystrobin Perturbed Nutrition Metabolism of Workers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng, Q.; Miao, J.Q.; Lu, X.L. Major molecular targets and current registration and resistance status of commercial systemic fungicides. Modern Agrochem. 2024, 23, 1–12+38. [Google Scholar]
- Bartlett, D.W.; Clough, J.M.; Godwin, J.R.; Hall, A.A.; Hamer, M.; Parr-Dobrzanski, B. The strobilurin fungicides. Pest Manag. Sci. 2002, 58, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Russell, P.E. A century of fungicide evolution. J. Agric. Sci. 2005, 143, 11–25. [Google Scholar] [CrossRef]
- Dong, J.; Huang, M.; Guo, H.; Zhang, J.; Tan, X.; Wang, D. Ternary mixture of azoxystrobin, boscalid and pyraclostrobin disrupts the gut microbiota and metabolic balance of honeybees (Apis cerana cerana). Int. J. Mol. Sci. 2023, 24, 5354. [Google Scholar] [CrossRef]
- Fisher, A.; Degrandi-Hoffman, G.; Liao, L.H. The challenge of balancing fungicide use and pollinator health. In Advances in Insect Physiology; Harrison, J.F., Ed.; Academic Press: Cambridge, MA, USA, 2023; Volume 64, pp. 117–190. [Google Scholar]
- Ostiguy, N.; Drummond, F.A.; Aronstein, K.; Eitzer, B.; Ellis, J.D.; Spivak, M.; Sheppard, W.S. Honey bee exposure to pesticides: A four-year nationwide study. Insects 2019, 10, 13. [Google Scholar] [CrossRef]
- Huang, M.; Dong, J.; Yang, S.; Xiao, M.; Guo, H.; Zhang, J.; Wang, D. Ecotoxicological effects of common fungicides on the eastern honeybee Apis cerana cerana (Hymenoptera). Sci. Total Environ. 2023, 868, 161637. [Google Scholar] [CrossRef]
- Egan, P.A.; Dicks, L.V.; Hokkanen, H.M.T.; Stenberg, J.A. Delivering integrated pest and pollinator management (IPPM). Trends Plant Sci. 2020, 25, 577–589. [Google Scholar] [CrossRef]
- Xiong, M.; Qin, G.; Wang, L.; Wang, R.; Zhou, R.; Luo, X.; Lou, Q.; Huang, S.; Li, J.; Duan, X. Field recommended concentrations of pyraclostrobin exposure disturb the development and immune response of worker bees (Apis mellifera L.) larvae and pupae. Front. Physiol. 2023, 14, 1137264. [Google Scholar] [CrossRef]
- Tadei, R.; Domingues, C.E.C.; Malaquias, J.B.; Camilo, E.V.; Malaspina, O.; Silva-Zacarin, E.C.M. Late effect of larval co-exposure to the insecticide clothianidin and fungicide pyraclostrobin in Africanized Apis mellifera. Sci. Rep. 2019, 9, 3277. [Google Scholar] [CrossRef]
- Fisher, A.; Cogley, T.; Ozturk, C.; DeGrandi-Hoffman, G.; Smith, B.H.; Kaftanoglu, O.; Fewell, J.H.; Harrison, J.F. The active ingredients of a mitotoxic fungicide negatively affect pollen consumption and worker survival in laboratory-reared honey bees (Apis mellifera). Ecotoxicol. Environ. Saf. 2021, 226, 112841. [Google Scholar] [CrossRef]
- DeGrandi-Hoffman, G.; Chen, Y.; Simonds, R. The effects of pesticides on queen rearing and virus titers in honey bees (Apis mellifera L.). Insects 2013, 4, 71–89. [Google Scholar] [CrossRef]
- Zhu, W.; Schmehl, D.R.; Mullin, C.A.; Frazier, J.L. Four common pesticides, their mixtures and a formulation solvent in the hive environment have high oral toxicity to honey bee larvae. PLoS ONE 2014, 9, e77547. [Google Scholar] [CrossRef] [PubMed]
- Rondeau, S.; Raine, N.E. Fungicides and bees: A review of exposure and risk. Environ. Int. 2022, 165, 107311. [Google Scholar] [CrossRef]
- IPBES. The Assessment Report of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services on Pollinators, Pollination and Food Production; Potts, S.G., Imperatriz-Fonseca, V.L., Ngo, H.T., Eds.; Secretariat of the Intergovernmental Science-Policy Platform on Biodiveristy and Ecosystem Services: Bonn, Germany, 2016; pp. 23–25. [Google Scholar]
- Duan, X.; Wang, L.; Wang, R.; Xiong, M.; Qin, G.; Huang, S.; Li, J. Variation in the physiological response of adult worker bees of different ages (Apis mellifera L.) to pyraclostrobin stress. Ecotoxicol. Environ. Saf. 2024, 269, 115754. [Google Scholar] [CrossRef]
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Aizen, M.A.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J.; et al. Safeguarding pollinators and their values to human well-being. Nature 2016, 540, 220–229. [Google Scholar] [CrossRef]
- Papa, G.; Maier, R.; Durazzo, A.; Lucarini, M.; Karabagias, I.K.; Plutino, M.; Bianchetto, E.; Aromolo, R.; Pignatti, G.; Ambrogio, A.; et al. The honey bee Apis mellifera: An insect at the interface between human and ecosystem health. Biology 2022, 11, 233. [Google Scholar] [CrossRef]
- Zioga, E.; White, B.; Stout, J.C. Pesticide mixtures detected in crop and non-target wild plant pollen and nectar. Sci. Total. Environ. 2023, 879, 162971. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Dai, P.; Li, R.; Chen, Z.; Liang, P.; Xie, X.; Zhen, C.; Gao, X. The sulfoximine insecticide sulfoxaflor exposure reduces the survival status and disrupts the intestinal metabolism of the honeybee Apis mellifera. J. Hazard Mater. 2023, 442, 130109. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; et al. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef]
- Zattara, E.E.; Aizen, M.A. Worldwide occurrence records suggest a global decline in bee species richness. One Earth 2021, 4, 114–123. [Google Scholar] [CrossRef]
- Duan, X.; Yao, H.; Tong, W.; Xiong, M.; Huang, S.; Li, J. Azoxystrobin exposure impacts on development status and physiological responses of worker bees (Apis mellifera L.) from larval to pupal stages. Int. J. Mol. Sci. 2024, 25, 11806. [Google Scholar] [CrossRef] [PubMed]
- Nebauer, C.A.; Prucker, P.; Ruedenauer, F.A.; Kollmann, J.; Leonhardt, S.D. Bumblebees under stress: Interacting effects of pesticides and heatwaves on colony development and longevity. iScience 2024, 27, 111050. [Google Scholar] [CrossRef]
- Cedergreen, N. Quantifying synergy: A systematic review of mixture toxicity studies within environmental toxicology. PLoS ONE 2014, 9, e96580. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liao, C.; Li, Z.; Shi, X.; Wu, X. Synergistic resistance of honeybee (Apis mellifera) and their gut microorganisms to fluvalinate stress. Pestic. Biochem. Physiol. 2024, 201, 105865. [Google Scholar] [CrossRef] [PubMed]
- Pineaux, M.; Grateau, S.; Lirand, T.; Aupinel, P.; Richard, F.J. Honeybee queen exposure to a widely used fungicide disrupts reproduction and colony dynamic. Environ. Pollut. 2023, 322, 121131. [Google Scholar] [CrossRef]
- Cang, T.; Lou, Y.; Zhu, Y.C.; Li, W.; Weng, H.; Lv, L.; Wang, Y. Mixture toxicities of tetrachlorantraniliprole and tebuconazole to honey bees (Apis mellifera L.) and the potential mechanism. Environ. Int. 2023, 172, 107764. [Google Scholar] [CrossRef]
- Inoue, L.V.B.; Domingues, C.E.C.; Gregorc, A.; Silva-Zacarin, E.C.M.; Malaspina, O. Harmful effects of pyraclostrobin on the fat body and pericardial cells of foragers of africanized honey bee. Toxics 2022, 10, 530. [Google Scholar] [CrossRef]
- DesJardins, N.S.; Fisher, A.; Ozturk, C.; Fewell, J.H.; DeGrandi-Hoffman, G.; Harrison, J.F.; Smith, B.H. A common fungicide, Pristine®, impairs olfactory associative learning performance in honey bees (Apis mellifera). Environ. Pollut. 2021, 288, 117720. [Google Scholar] [CrossRef]
- DesJardins, N.S.; Smith, B.H.; Harrison, J.F. A mitotoxic fungicide alters post-ingestive glucose signals necessary for associative learning in honey bees. J. Insect Physiol. 2023, 149, 104554. [Google Scholar] [CrossRef]
- Kakumanu, M.L.; Reeves, A.M.; Anderson, T.D.; Rodrigues, R.R.; Williams, M.A. Honey Bee gut microbiome is altered by in-hive pesticide exposures. Front. Microbiol. 2016, 7, 1255. [Google Scholar] [CrossRef]
- Han, W.; Ye, Z.; Gu, Y.; Zhong, Y.; Gao, J.; Zhao, S.; Wang, S. Gut microbiota composition and gene expression changes induced in the Apis cerana exposed to acetamiprid and difenoconazole at environmentally realistic concentrations alone or combined. Front. Physiol. 2023, 14, 1174236. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Conclusion on the Peer Review of the Pesticide Risk Assessment of the Active Substance Azoxystrobin. Available online: https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/j.efsa.2010.1542 (accessed on 20 February 2010).
- Zhang, C.; Zhou, T.T.; Xu, Y.Q.; Du, Z.K.; Li, B.; Wang, J.J.; Wang, J.; Zhu, L.S. Ecotoxicology of strobilurin fungicides. Sci. Total. Environ. 2020, 742, 140611. [Google Scholar] [CrossRef] [PubMed]
- China Pesticide Information Network. Pesticide Registration Database. Available online: http://www.chinapesticide.org.cn/ (accessed on 20 February 2024).
- Ray, A.; Dutta, D.; Ghosh, B.; Bahadur, M. Azoxystrobin induced genotoxicity in Pethia conchonius, a freshwater fish of river Teesta, India. Aquat. Toxicol. 2024, 277, 107135. [Google Scholar] [CrossRef] [PubMed]
- Campani, T.; Casini, S.; Maccantelli, A.; Tosoni, F.; D’Agostino, A.; Caliani, I. Oxidative stress and DNA alteration on the earthworm Eisenia fetida exposed to four commercial pesticides. Environ. Sci. Pollut. Res. Int. 2024, 31, 35969–35978. [Google Scholar] [CrossRef]
- Naggar, Y.; Singavarapu, B.; Paxton, R.J.; Wubet, T. Bees under interactive stressors: The novel insecticides flupyradifurone and sulfoxaflor along with the fungicide azoxystrobin disrupt the gut microbiota of honey bees and increase opportunistic bacterial pathogens. Sci. Total Environ. 2022, 25, 157941. [Google Scholar] [CrossRef]
- Straw, E.A.; Brown, M.J.F. Co-formulant in a commercial fungicide product causes lethal and sub-lethal effects in bumble bees. Sci. Rep. 2021, 11, 21653. [Google Scholar] [CrossRef]
- Serra, R.S.; Martínez, L.C.; Cossolin, J.F.S.; Resende, M.T.C.S.; Carneiro, L.S.; Fiaz, M.; Serrão, J.E. The fungicide azoxystrobin causes histopathological and cytotoxic changes in the midgut of the honey bee Apis mellifera (Hymenoptera: Apidae). Ecotoxicology 2023, 32, 234–242. [Google Scholar] [CrossRef]
- Christen, V.; Krebs, J.; Bünter, I.; Fent, K. Biopesticide spinosad induces transcriptional alterations in genes associated with energy production in honey bees (Apis mellifera) at sublethal concentrations. J. Hazard. Mater. 2019, 378, 120736. [Google Scholar] [CrossRef]
- Moritz, R.F.A.; Fuchs, S. Organization of honeybee colonies: Characteristics and consequences of a superorganism concept. Apidologie 1998, 29, 7–21. [Google Scholar] [CrossRef]
- Deseyn, J.; Billen, J. Age-dependent morphology and ultrastructure of the hypopharyngeal gland of Apis mellifera workers (Hymenoptera, Apidae). Apidologie 2005, 36, 49–57. [Google Scholar] [CrossRef]
- Ihle, K.E.; Baker, N.A.; Amdam, G.V. Insulin-like peptide response to nutritional input in honey bee workers. J. Insect Physiol. 2014, 69, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using realtime quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Seeley, T.D. Honey bee foragers as sensory units of their colonies. Behav. Ecol. Sociobiol. 1994, 34, 51–62. [Google Scholar] [CrossRef]
- Zaluski, R.; Justulin, L.A., Jr.; Orsi, R.O. Field-relevant doses of the systemic insecticide fipronil and fungicide pyraclostrobin impair mandibular and hypopharyngeal glands in nurse honeybees (Apis mellifera). Sci. Rep. 2017, 7, 15217. [Google Scholar] [CrossRef]
- Domingues, C.E.D.; Inoue, L.V.B.; da Silva-Zacarin, E.C.M.; Malaspina, O. Foragers of Africanized honeybee are more sensitive to fungicide pyraclostrobin than newly emerged bees. Environ. Pollut. 2020, 266, 115267. [Google Scholar] [CrossRef]
- Rix, R.R.; Cutler, G.C. Review of molecular and biochemical responses during stress induced stimulation and hormesis in insects. Sci. Total Environ. 2022, 827, 154085. [Google Scholar] [CrossRef]
- García-Caparrós, P.; Filippis, L.D.; Gul, A.; Hasanuzzaman, M.; Özturk, M.; Altay, V.; Lao, M.T. Oxidative stress and antioxidant metabolism under adverse environmental conditions: A review. Bot. Rev. 2020, 87, 421–466. [Google Scholar] [CrossRef]
- Krishnan, N.; Kodrík, D. Antioxidant enzymes in Spodoptera littoralis (Boisduval): Are they enhanced to protect gut tissues during oxidative stress? J. Insect Physiol. 2006, 52, 11–20. [Google Scholar] [CrossRef]
- Xu, Y.; Li, B.; Hou, K.; Du, Z.; Allen, S.C.; Zhu, L.; Li, W.; Zhu, L.; Wang, J.; Wang, J. Ecotoxicity evaluation of azoxystrobin on Eisenia fetida in different soils. Environ. Res. 2021, 194, 110705. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, R.; Li, C.; Duan, M.; Cao, N.; Jin, Q.; Chen, X.; Li, L.; Li, X.; Pang, S. Environmental levels of azoxystrobin disturb male zebrafish behavior: Possible roles of oxidative stress, cholinergic system, and dopaminergic system. Ecotoxicol. Environ. Saf. 2024, 269, 115744. [Google Scholar] [CrossRef]
- Li, N.; Li, Y.; Zhang, S.; Fan, Y.; Liu, T. Effect of elevated CO2 concentration and temperature on antioxidant capabilities of multiple generations of Bemisia tabaci MEAM1 (Hemiptera: Aleyrodidae). J. Insect Physiol. 2017, 103, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, M.; He, J.; Zhao, X.; Chaimanee, V.; Huang, W.F.; Nie, H.; Zhao, Y.; Su, S. Differential physiological effects of neonicotinoid insecticides on honey bees: A comparison between Apis mellifera and Apis cerana. Pestic. Biochem. Physiol. 2017, 140, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Velki, M.; Meyer-Alert, H.; Seiler, T.B.; Hollert, H. Enzymatic activity and gene expression changes in zebrafish embryos and larvae exposed to pesticides diazinon and diuron. Aquat. Toxicol. 2017, 193, 187–200. [Google Scholar] [CrossRef]
- Bass, C.; Field, L.M. Gene amplification and insecticide resistance. Pest Manag. Sci. 2011, 67, 886–890. [Google Scholar] [CrossRef]
- Gu, Z.Y.; Sun, S.S.; Wang, Y.H.; Wang, B.B.; Xie, Y.; Ma, L.; Wang, J.M.; Shen, W.D.; Li, B. Transcriptional characteristics of gene expression in the midgut of domestic silkworms (Bombyx mori) exposed to phoxim. Pestic. Biochem. Physiol. 2013, 105, 36–43. [Google Scholar] [CrossRef]
- Kshatriya, K.; Gershenzon, J. Disarming the defenses: Insect detoxification of plant defense-related specialized metabolites. Curr. Opin. Plant Biol. 2024, 81, 102577. [Google Scholar] [CrossRef]
- Nauen, R.; Bass, C.; Feyereisen, R.; Vontas, J. The role of cytochrome P450s in insect toxicology and resistance. Annu. Rev. Entomol. 2022, 67, 105–124. [Google Scholar] [CrossRef]
- Cruse, C.; Moural, T.W.; Zhu, F. Dynamic roles of insect carboxyl/cholinesterases in chemical adaptation. Insects 2023, 14, 194. [Google Scholar] [CrossRef]
- Koirala, B.K.S.; Moural, T.; Zhu, F. Functional and structural diversity of insect glutathione S-transferases in xenobiotic adaptation. Int. J. Biol. Sci. 2022, 18, 5713–5723. [Google Scholar] [CrossRef]
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive oxygen species signaling and oxidative stress: Transcriptional regulation and evolution. Antioxidants 2024, 3, 312. [Google Scholar] [CrossRef]
- Maiwald, F.; Haas, J.; Hertlein, G.; Lueke, B.; Roesner, J.; Nauen, R. Expression profile of the entire detoxification gene inventory of the western honeybee, Apis mellifera across life stages. Pestic. Biochem. Physiol. 2023, 192, 105410. [Google Scholar] [CrossRef] [PubMed]
- Badiou-Bénéteau, A.; Benneveau, A.; Géret, F.; Delatte, H.; Becker, N.; Brunet, J.L.; Reynaud, B.; Belzunces, L.P. Honeybee biomarkers as promising tools to monitor environmental quality. Environ. Int. 2013, 60, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Hua, D.; Li, X.; Yuan, J.; Tao, M.; Zhang, K.; Zheng, X.; Wan, Y.; Gui, L.; Zhang, Y.; Wu, Q. Fitness cost of spinosad resistance related to vitellogenin in Frankliniella occidentalis (Pergande). Pest Manag. Sci. 2023, 79, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.D.; Aronstein, K.; Chen, Y.P.; Hetru, C.; Imler, J.L.; Jiang, H.; Kanost, M.; Thompson, G.J.; Zou, Z.; Hultmark, D. Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect Mol. Biol. 2006, 15, 645–656. [Google Scholar] [CrossRef]
- Danihlík, J.; Aronstein, K.; Petřivalský, M. Antimicrobial peptides: A key component of honey bee innate immunity. J. Apic. Res. 2015, 54, 123–136. [Google Scholar] [CrossRef]
- Duan, X.L.; Zhao, B.A.; Jin, X.; Cheng, X.F.; Huang, S.K.; Li, J.H. Antibiotic treatment decrease the fifitness of honeybee (Apis mellifera) larvae. Insects 2021, 12, 301. [Google Scholar] [CrossRef]
- Vilcinskas, A. Evolutionary plasticity of insect immunity. J. Insect Physiol. 2013, 59, 123–129. [Google Scholar] [CrossRef]
- Cizelj, I.; Glavan, G.; Božič, J.; Oven, I.; Mrak, V.; Narat, M. Prochloraz and coumaphos induce different gene expression patterns in three developmental stages of the Carniolan honey bee (Apis mellifera carnica Pollmann). Pestic. Biochem. Physiol. 2016, 128, 68–75. [Google Scholar] [CrossRef]
- Chaimanee, V.; Pettis, J.S. Gene expression, sperm viability, and queen (Apis mellifera) loss following pesticide exposure under laboratory and field conditions. Apidologie 2019, 50, 304–316. [Google Scholar] [CrossRef]
- Spremo, J.; Purać, J.; Čelić, T.; Đorđievski, S.; Pihler, I.; Kojić, D.; Vukašinović, E. Assessment of oxidative status, detoxification capacity and immune responsiveness in honey bees with ageing. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2024, 298, 111735. [Google Scholar] [CrossRef]
- Azevedo, S.V.; Hartfelder, K. The insulin signaling pathway in honey bee (Apis mellifera) caste development-differential expression of insulin-like peptides and insulin receptors in queen and worker larvae. J. Insect Physiol. 2008, 54, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Mengoni Goñalons, C.; Guiraud, M.; de Brito Sanchez, M.G.; Farina, W.M. Insulin effects on honeybee appetitive behaviour. J. Exp. Biol. 2016, 219, 3003–3008. [Google Scholar] [CrossRef] [PubMed]
- Oldham, S.; Hafen, E. Insulin/IGF and target of rapamycin signaling: A TOR de force in growth control. Trends Cell Biol. 2003, 13, 79–85. [Google Scholar] [CrossRef]
- Wheeler, D.E.; Buck, N.A.; Evans, J.D. Expression of insulin/insulin-like signalling and TOR pathway genes in honey bee caste determination. Insect Mol. Biol. 2014, 23, 113–121. [Google Scholar] [CrossRef]
- Kocher, S.; Kingwell, C. The Molecular substrates of insect eusociality. Annu. Rev. Genet. 2024, 58, 273–295. [Google Scholar] [CrossRef]
- Wheeler, D.E.; Buck, N.; Evans, J.D. Expression of insulin pathway genes during the period of caste determination in the honey bee, Apis mellifera. Insect Mol. Biol. 2006, 15, 597–602. [Google Scholar] [CrossRef]
- Ament, S.A.; Corona, M.; Pollock, H.S.; Robinson, G.E. Insulin signaling is involved in the regulation of worker division of labor in honey bee colonies. Proc. Natl. Acad. Sci. USA 2008, 105, 4226–4231. [Google Scholar] [CrossRef]
- Nilsen, K.A.; Ihle, K.E.; Frederick, K.; Fondrk, M.K.; Smedal, B.; Hartfelder, K.; Amdam, G.V. Insulin-like peptide genes in honey bee fat body respond differently to manipulation of social behavioral physiology. J. Exp. Biol. 2011, 214, 1488–1497. [Google Scholar] [CrossRef]
- Tadei, R.; Castor, R.E.S.; Malaspina, O.; Mathias da Silva, E.C. Effect of neonicotinoid and fungicide strobilurin in neotropical solitary bee Centris analis. Environ. Pollut. 2024, 360, 124712. [Google Scholar] [CrossRef]
- Smith, D.B.; Arce, A.N.; Ramos Rodrigues, A.; Bischoff, P.H.; Burris, D.; Ahmed, F.; Gill, R.J. Insecticide exposure during brood or early-adult development reduces brain growth and impairs adult learning in bumblebees. Proceedings. Proc. Biol. Sci. 2020, 287, 20192442. [Google Scholar] [CrossRef]
- Chen, X.; Li, A.; Yin, L.; Ke, L.; Dai, P.; Liu, Y.J. Early-life sublethal thiacloprid exposure to honey bee larvae: Enduring effects on adult bee cognitive abilities. Toxics 2023, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Fent, K.; Haltiner, T.; Kunz, P.; Christen, V. Insecticides cause transcriptional alterations of endocrine related genes in the brain of honey bee foragers. Chemosphere 2020, 260, 127542. [Google Scholar] [CrossRef] [PubMed]
- Denison, R.; Raymond-Delpech, V. Insights into the molecular basis of social behaviour from studies on the honeybee, Apis mellifera. Invert. Neurosci. 2008, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Heinze, J.; Schrempf, A. Aging and reproduction in social insects-a mini-review. Gerontology 2008, 54, 160–167. [Google Scholar] [CrossRef]
- Salmela, H.; Harwood, G.P.; Münch, D.; Elsik, C.G.; Herrero-Galán, E.; Vartiainen, M.K.; Amdam, G.V. Nuclear translocation of vitellogenin in the honey bee (Apis mellifera). Apidologie 2022, 53, 13. [Google Scholar] [CrossRef]
- Conradie, T.A.; Lawson, K.; Allsopp, M.; Jacobs, K. Exploring the impact of fungicide exposure and nutritional stress on the microbiota and immune response of the Cape honey bee (Apis mellifera capensis). Microbiol. Res. 2024, 280, 127587. [Google Scholar] [CrossRef]
- Kato, A.Y.; Freitas, T.A.L.; Gomes, C.R.A.; Alves, T.R.R.; Ferraz, Y.M.M.; Trivellato, M.F.; De Jong, D.; Biller, J.D.; Nicodemo, D. Bixafen, prothioconazole, and trifloxystrobin alone or in combination have a greater effect on health related gene expression in honey bees from nutritionally deprived than from protein supplemented colonies. Insects 2024, 15, 523. [Google Scholar] [CrossRef]
- Sun, J.; Wu, J.; Zhang, X.; Wei, Q.; Kang, W.; Wang, F.; Liu, F.; Zhao, M.; Xu, S.; Han, B. Enantioselective toxicity of the neonicotinoid dinotefuran on honeybee (Apis mellifera) larvae. Sci. Total. Environ. 2024, 944, 174014. [Google Scholar] [CrossRef]
- Simone, M.; Evans, J.D.; Spivak, M. Resin collection and social immunity in honey bees. Evolution 2009, 63, 3016–3022. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, X.; Tong, W.; Tao, B.; Yao, H.; Xiong, M.; Liu, H.; Huang, S.; Li, J. Azoxystrobin-Induced Physiological and Biochemical Alterations in Apis mellifera Workers of Different Ages. Insects 2025, 16, 449. https://doi.org/10.3390/insects16050449
Duan X, Tong W, Tao B, Yao H, Xiong M, Liu H, Huang S, Li J. Azoxystrobin-Induced Physiological and Biochemical Alterations in Apis mellifera Workers of Different Ages. Insects. 2025; 16(5):449. https://doi.org/10.3390/insects16050449
Chicago/Turabian StyleDuan, Xinle, Wenlong Tong, Bingfang Tao, Huanjing Yao, Manqiong Xiong, Huiping Liu, Shaokang Huang, and Jianghong Li. 2025. "Azoxystrobin-Induced Physiological and Biochemical Alterations in Apis mellifera Workers of Different Ages" Insects 16, no. 5: 449. https://doi.org/10.3390/insects16050449
APA StyleDuan, X., Tong, W., Tao, B., Yao, H., Xiong, M., Liu, H., Huang, S., & Li, J. (2025). Azoxystrobin-Induced Physiological and Biochemical Alterations in Apis mellifera Workers of Different Ages. Insects, 16(5), 449. https://doi.org/10.3390/insects16050449






