Potential Distribution of Tribe Erythroneurini in China Based on the R-Optimized MaxEnt Model, with Implications for Management
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Occurrence Records
2.2. Acquisition and Organization of Environmental Data
2.2.1. Data Acquisition
2.2.2. Data Preprocessing
2.3. Model Optimization, Construction, and Accuracy Assessment
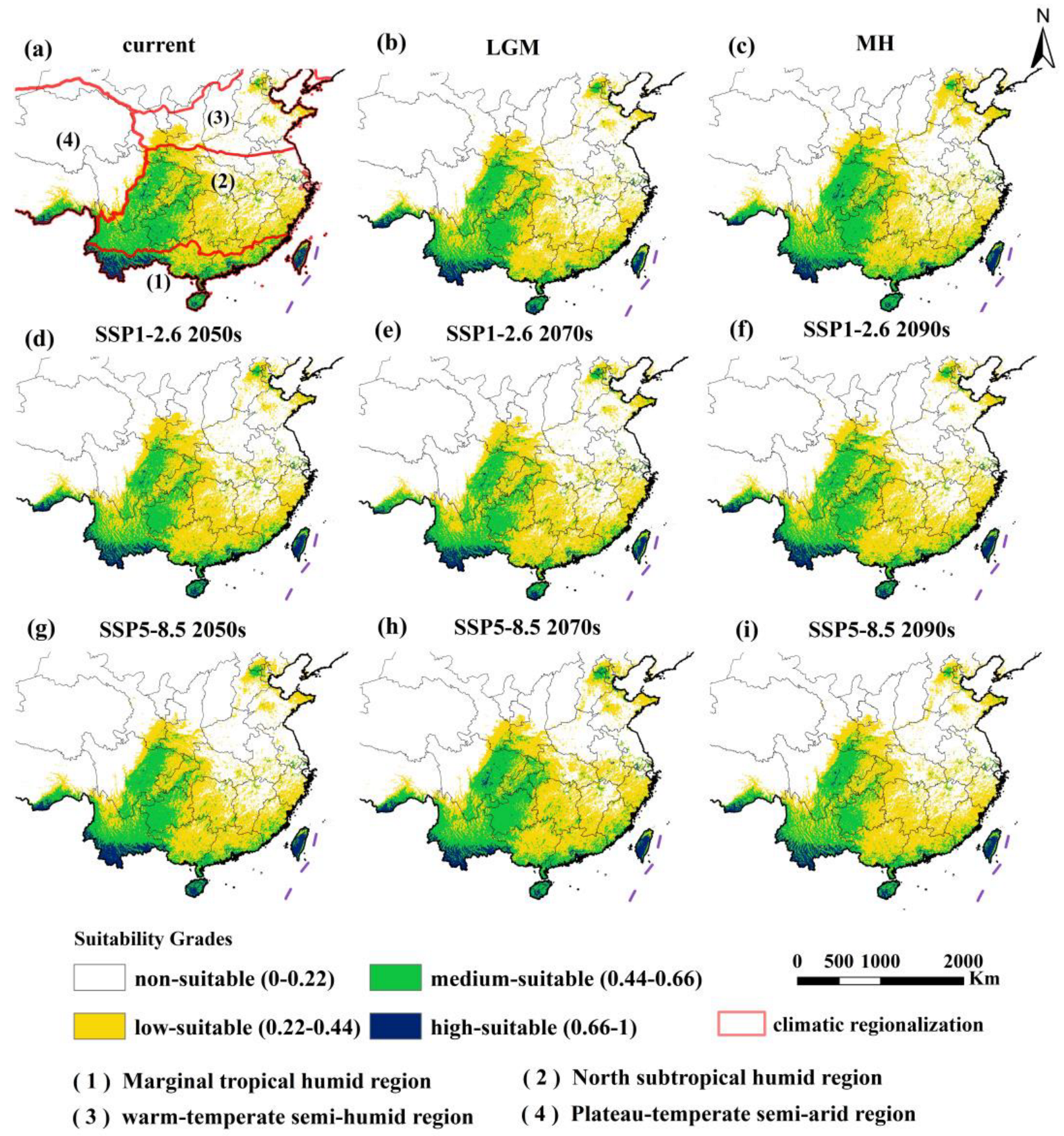
2.4. Division of Suitable Distribution Area
2.5. Dynamic Change in Suitable Areas
3. Results
3.1. Analysis of Dominant Factors in Geographic Distribution Patterns of Erythroneurini
3.2. Current Potential Suitable Areas of Erythroneurini in China
3.3. Spatial Distribution of Erythroneurini in Different Periods
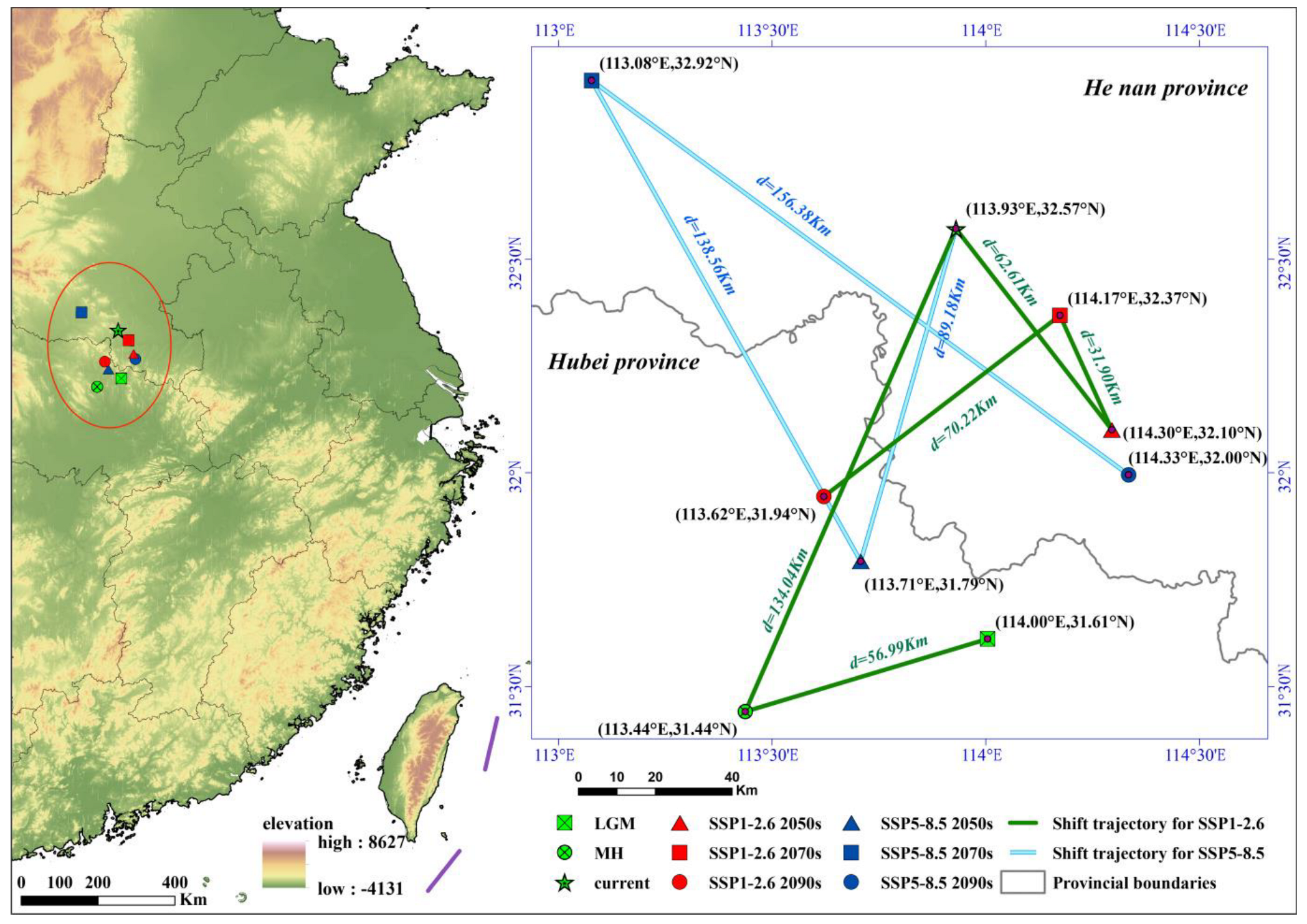
3.4. Potential Distribution and Centroid Dynamic Changes
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Glossary
| MaxEnt Model (Maximum Entropy Model) | The MaxEnt Model is a methodology that utilizes statistics and related algorithms. It constructs models based on the known data of species occurrences and relevant environmental factors to predict the potential distribution of species under different spatiotemporal conditions. Moreover, it serves as an important tool for examining the habitat suitability of species and carrying out pest management. |
| Lumping Method | For the occurrence points of species with related lineages or similar ecological requirements, they can be treated as a whole. By integrating the information on these distribution points, the common ecological characteristics among species can be highlighted more effectively. |
| Lumped Model | The lumped model is a type of model that simplifies complex systems. It assumes that all parts within the system are uniform. It regards the system as a whole and ignores the detailed differences and spatial distribution within the system. |
| GCMs (Global climate models) | GCMs are powerful tools that provide us with quantitative and scientific bases for understanding past, present, and future climate conditions, as well as for assessing the interaction between climate and human activities. They have far-reaching impacts on various aspects such as the sustainable development of human society, ecosystem protection, and responding to climate change. |
| FC (feature combinations) | include linear (L), quadratic (Q), product (P), threshold (T), and hinge (H). |
| PC (Percent contribution) | It is used to measure the relative contribution degree of each feature (variable) to the model’s prediction results. |
| RTG (Regularized Training Gain) | It refers to the gain achieved by the model during the training process after regularization treatment. The training gain reflects the degree of improvement in the model’s performance on the training data. |
| MTSPS | Maximum test sensitivity plus specificity Logistic threshold. The optimal threshold found through MTSPS enables the model to achieve better performance on both the training data and the test data. It can enhance the generalization ability and stability of the model, thereby strengthening the accuracy and reliability of predictions. |
| HSI (Habitat Suitability Index) | HSI is a quantitative indicator that comprehensively evaluates the suitability level of a habitat for a species. Its value range is generally from 0.0 to 1.0. A value of 0.0 represents an unsuitable habitat, meaning that the habitat can hardly meet the basic needs of species for survival, reproduction, and so on. While a value of 1.0 indicates the most suitable habitat, that is, all kinds of environmental conditions in this habitat are quite ideal and can support the survival and reproduction of species to the greatest extent. |
References
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosenzweig, C.; Pounds, J.A. Fingerprints of Global Warming on Wild Animals and Plants. Nature 2003, 421, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid Range Shifts of Species Associated with High Levels of Climate Warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- Gidden, M.J.; Riahi, K.; Smith, S.J.; Fujimori, S.; Luderer, G.; Kriegler, E.; Van Vuuren, D.P.; Van Den Berg, M.; Feng, L.; Klein, D.; et al. Global Emissions Pathways under Different Socioeconomic Scenarios for Use in CMIP6: A Dataset of Harmonized Emissions Trajectories through the End of the Century. Geosci. Model Dev. 2019, 12, 1443–1475. [Google Scholar] [CrossRef]
- Cao, J.; Xu, J.; Pan, X.B.; Monaco, T.A.; Zhao, K.; Wang, D.P.; Rong, Y.P. Potential Impact of Climate Change on the Global Geographical Distribution of the Invasive Species, Cenchrus Spinifex (Field Sandbur, Gramineae). Ecol. Indic. 2021, 131, 108204. [Google Scholar] [CrossRef]
- Skea, J.; Shukla, P.R.; Reisinger, A.; Slade, R.; Pathak, M.; Al Khourdajie, A.; van Diemen, R.; Abdulla, A.; Akimoto, K.; Babiker, M.; et al. Summary for Policymakers. In Climate Change 2022: Mitigation of Climate Change; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar] [CrossRef]
- Dmitriev, D.A. 3I, a New Program for Creating Internet-Accessible Interactive Keys and Taxonomic Databases and Its Application for Taxonomy of Cicadina (Homoptera). Russ. Entomol. J. 2006, 15, 263–268. [Google Scholar]
- Young, D.A. A Reclassification of Western Hemisphere Typhlocybinae (Homoptera, Cicadellidae). Univ. Kans. Sci. Bull. 1952, 35, 3–217. [Google Scholar] [CrossRef]
- Dietrich, C.H.; Dmitriev, D.A. Review of the New World Genera of the Leafhopper Tribe Erythroneurini (Hemiptera: Cicadellidae: Typhlocycbinae). Ill. Nat. Hist. Surv. Bull. 2006, 37, 118–190. [Google Scholar] [CrossRef]
- Luo, G.M.; Pu, T.Y.; Wang, J.Q.; Ran, W.W.; Zhao, Y.Q.; Dietrich, C.H.; Li, C.; Song, Y.H. Genetic Differentiation and Phylogeography of Erythroneurini (Hemiptera, Cicadellidae, Typhlocybinae) in the Southwestern Karst Area of China. Ecol. Evol. 2024, 14, e11264. [Google Scholar] [CrossRef]
- Zhang, Y.L. A Taxonomic Study of Chinese Cicadellidae (Hemiptera); Tianze Eldonejo: Yangling, China, 1990; pp. 1–20, 139–144. (In Chinese) [Google Scholar]
- Ge, Z.L. Chinese Economic Entomological Records (Homoptera: Leafhoppers); Science Press: Beijing, China, 1966; pp. 84–93. (In Chinese) [Google Scholar]
- Song, Y.H.; Li, Z.Z. Erythroneurini and Zyginellini from China (Hemiptera: Cicadellidae: Typhlocybinae); Guizhou Science and Technology Publishing House: Guiyang, China, 2014; pp. 1–206. (In Chinese) [Google Scholar]
- Bai, Y.; Yang, C.; Halitschke, R.; Paetz, C.; Kessler, D.; Burkard, K.; Gaquerel, E.; Baldwin, I.T.; Li, D. Natural History–Guided Omics Reveals Plant Defensive Chemistry against Leafhopper Pests. Science 2022, 375, eabm2948. [Google Scholar] [CrossRef]
- Wu, G.R.; Ruan, Y.L. Bionomics and the appropriate time for chemical control of the white leafhopper thaia subrufa (motschulsky). Acta Entomol. Sin. 1982, 25, 178–184. (In Chinese) [Google Scholar] [CrossRef]
- Cao, Y.H. Taxonomy and Phylogenetic Study of Representative Genera of the World’s Erythroneurini Leafhoppers (Hemiptera: Cicadellidae: Typhlocycbinae). Ph.D. Thesis, Northwest A&F University, Xianyang, China, 2014. (In Chinese). [Google Scholar]
- Wang, L.M.; Liao, Q.R.; Yang, M.F. The occurrence law and control of Erythroneura sp. in Guiyang area. Guizhou Agric. Sci. 1997, 25, 24–27. (In Chinese) [Google Scholar]
- Backus, E.A.; Serrano, M.S.; Ranger, C.M. Mechanisms of Hopperburn: An Overview of Insect Taxonomy, Behavior, and Physiology. Annu. Rev. Entomol. 2005, 50, 125–151. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.H.; Dietrich, C.H.; Kits, J.H.; Dmitriev, D.A.; Richter, R.; Eyres, J.; Dettman, J.R.; Xu, Y.; Huang, M. Phylogenomics of Microleafhoppers (Hemiptera: Cicadellidae: Typhlocybinae): Morphological Evolution, Divergence Times, and Biogeography. Insect Syst. Divers. 2023, 7, 1. [Google Scholar] [CrossRef]
- Neven, L.G. Physiological Responses of Insects to Heat. Postharvest Biol. Technol. 2000, 21, 103–111. [Google Scholar] [CrossRef]
- Daniel, G.T.; Alex, C.A.; Wesley, D.; Andrés, L.N.; Rosa, A.S.G.; Fabricio, V. Insect Responses to Heat: Physiological Mechanisms, Evolution and Ecological Implications in a Warming World. Biol. Rev. 2020, 95, 802–821. [Google Scholar] [CrossRef]
- Carlos, G.R.; Erin, K.K.; Charles, L.S.; Terry, L.E.; Kress, W.J. Limited Tolerance by Insects to High Temperatures across Tropical Elevational Gradients and the Implications of Global Warming for Extinction. Proc. Natl. Acad. Sci. USA 2016, 113, 680–685. [Google Scholar] [CrossRef]
- Bradshaw, W.E.; Holzapfel, C.M. Genetic Shift in Photoperiodic Response Correlated with Global Warming. Proc. Natl. Acad. Sci. USA 2001, 98, 14509–14511. [Google Scholar] [CrossRef]
- Bradshaw, W.E.; Holzapfel, C.M. Evolutionary Response to Rapid Climate Change. Science 2006, 312, 1477–1478. [Google Scholar] [CrossRef]
- Lehmann, P.; Ammunét, T.; Barton, M.; Battisti, A.; Eigenbrode, S.D.; Jepsen, J.U.; Kalinkat, G.; Neuvonen, S.; Niemelä, P.; Terblanche, J.S.; et al. Complex Responses of Global Insect Pests to Climate Warming. Front. Ecol. Environ. 2020, 18, 141–150. [Google Scholar] [CrossRef]
- Hamann, E.; Blevins, C.; Franks, S.J.; Jameel, M.I.; Anderson, J.T. Climate Change Alters Plant–Herbivore Interactions. New Phytol. 2021, 229, 1894–1910. [Google Scholar] [CrossRef]
- Chen, X.X.; Jiang, J.; Zhang, N.; Yang, X.; Chi, Y.K.; Song, Y.H. Effects of Habitat Fragmentation on the Population Structure and Genetic Diversity of Erythroneurini in the Typical Karst Rocky Ecosystem, Southwest China. Insects 2022, 13, 499. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, X.X.; Yuan, Z.W.; Su, D.; Song, Y.H. Community Structure and Dynamics of Leafhopper in Different Land Restoration Types in the Light-Moderate Rocky Desertification Areas. Acta Ecol. Sin. 2022, 42, 6790–6800. [Google Scholar] [CrossRef]
- Wang, J.Q.; Jiang, J.; Chi, Y.; Su, D.; Song, Y.H. Diversity and Community Structure of Typhlocybinae in the Typical Karst Rocky Ecosystem, Southwest China. Diversity 2023, 15, 387. [Google Scholar] [CrossRef]
- Chen, J.J.; Jiang, J.; Zhang, N.; Song, Y.H. Effects of Habitats in Typical Karst Areas of Guizhou on Ultrastructural Morphology of Typhlocybinae. Ecol. Evol. 2023, 13, e10680. [Google Scholar] [CrossRef]
- Cao, Y.H.; Dmitriev, D.A.; Dietrich, C.H.; Zhang, Y.L. New Taxa and New Records of Erythroneurini from China (Hemiptera: Cicadellidae: Typhlocybinae). Acta Entomol. Musei Natl. Pragae 2019, 59, 189–210. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, J.Q.; Pu, T.Y.; Li, C.; Song, Y.H. Two New Species of Erythroneurini (Hemiptera, Cicadellidae, Typhlocybinae) from Southern China Based on Morphology and Complete Mitogenomes. Peerj 2024, 12, e16853. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, X.X.; Li, C.; Song, Y.H. Mitogenome and Phylogenetic Analysis of Typhlocybine Leafhoppers (Hemiptera: Cicadellidae). Sci. Rep. 2021, 11, 10053. [Google Scholar] [CrossRef]
- Chen, X.X.; Li, C.; Song, Y.H. The Complete Mitochondrial Genomes of Two Erythroneurine Leafhoppers (Hemiptera, Cicadellidae, Typhlocybinae, Erythroneurini) with Assessment of the Phylogenetic Status and Relationships of Tribes of Typhlocybinae. ZooKeys 2021, 1037, 137–159. [Google Scholar] [CrossRef]
- Chen, X.X.; Yuan, Z.W.; Li, C.; Dietrich, C.H.; Song, Y.H. Structural Features and Phylogenetic Implications of Cicadellidae Subfamily and Two New Mitogenomes Leafhoppers. PLoS ONE 2021, 16, e0251207. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Elith, J.; Catherine, H.G.; Anderson, P.R.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel Methods Improve Prediction of Species’ Distributions from Occurrence Data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Kumar, S.; Graham, J.; West, A.M.; Evangelista, P.H. Using District-Level Occurrences in MaxEnt for Predicting the Invasion Potential of an Exotic Insect Pest in India. Comput. Electron. Agric. 2014, 103, 55–62. [Google Scholar] [CrossRef]
- Tang, J.H.; Li, J.H.; Lu, H.; Lu, F.P.; Lu, B.Q. Potential Distribution of an Invasive Pest, Euplatypus parallelus, in China as Predicted by Maxent. Pest. Manag. Sci. 2019, 75, 1630–1637. [Google Scholar] [CrossRef]
- Santana, P.A., Jr.; Kumar, L.; Da Silva, R.S.; Pereira, J.L.; Picanço, M.C. Assessing the Impact of Climate Change on the Worldwide Distribution of Dalbulus maidis (DeLong) Using MaxEnt. Pest Manag. Sci. 2019, 75, 2706–2715. [Google Scholar] [CrossRef]
- Gao, R.H.; Liu, L.; Zhao, L.J.; Cui, S.P. Potentially Suitable Geographical Area for Monochamus alternatus under Current and Future Climatic Scenarios Based on Optimized MaxEnt Model. Insects 2023, 14, 182. [Google Scholar] [CrossRef]
- Hosni, E.M.; Al-Khalaf, A.A.; Nasser, M.G.; ElShahed, S.M.; Alashaal, S.A. Locusta migratoria (L.) (Orthoptera) in a Warming World: Unravelling the Ecological Consequences of Climate Change Using GIS. Biodivers. Data J. 2024, 12, e115845. [Google Scholar] [CrossRef]
- Ran, W.W.; Luo, G.M.; Zhao, Y.Q.; Li, C.; Dietrich, C.H.; Song, Y.H. Climate Change May Drive the Distribution of Tribe Zyginelline Pests in China and the Indo-China Peninsula to Shift towards Higher Latitude River-mountain Systems. Pest Manag. Sci. 2023, 80, 613–626. [Google Scholar] [CrossRef]
- Smith, A.B.; Godsoe, W.; Rodríguez-Sánchez, F.; Wang, H.-H.; Warren, D. Niche Estimation Above and Below the Species Level. Trends Ecol. Evol. 2019, 34, 260–273. [Google Scholar] [CrossRef]
- Breiner, F.T.; Guisan, A.; Bergamini, A.; Nobis, M.P. Overcoming Limitations of Modelling Rare Species by Using Ensembles of Small Models. Methods Ecol. Evol. 2015, 6, 1210–1218. [Google Scholar] [CrossRef]
- Hernandez, P.A.; Graham, C.H.; Master, L.L.; Albert, D.L. The Effect of Sample Size and Species Characteristics on Performance of Different Species Distribution Modeling Methods. Ecography 2006, 29, 773–785. [Google Scholar] [CrossRef]
- Peterson, A.T.; Soberón, J.; Pearson, R.G.; Anderson, R.P.; Martínez-Meyer, E.; Nakamura, M.; Araújo, M.B. Ecological Niches and Geographic Distributions (MPB-49). In Ecological Niches and Geographic Distributions (MPB-49); Princeton University Press: Princeton, NJ, USA, 2011; ISBN 978-1-4008-4067-0. [Google Scholar]
- Liu, T.; Liu, H.Y.; Wang, Y.J.; Yang, Y.X. Climate Change Impacts on the Potential Distribution Pattern of Osphya (Coleoptera: Melandryidae), an Old but Small Beetle Group Distributed in the Northern Hemisphere. Insects 2023, 14, 476. [Google Scholar] [CrossRef] [PubMed]
- Ran, W.W.; Chen, J.J.; Zhao, Y.Q.; Zhang, N.; Luo, G.M.; Zhao, Z.B.; Song, Y.H. Global Climate Change-driven Impacts on the Asian Distribution of Limassolla Leafhoppers, with Implications for Biological and Environmental Conservation. Ecol. Evol. 2024, 14, e70003. [Google Scholar] [CrossRef]
- Smith, A.B.; Santos, M.J. Testing the Ability of Species Distribution Models to Infer Variable Importance. Ecography 2020, 43, 1801–1813. [Google Scholar] [CrossRef]
- Dietrich, C.H.; Dmitriev, D.A.; Takiya, D.M.; Thomas, M.J.; Webb, M.D.; Zahniser, J.N.; Zhang, Y. Morphology-Based Phylogenetic Analysis of Membracoidea (Hemiptera: Cicadomorpha) With Placement of Fossil Taxa and Description of a New Subfamily. Insect Syst. Divers. 2022, 6, 7. [Google Scholar] [CrossRef]
- Yuan, Z.W. Response of Leafhopper Community Structure and Biodiversity to Ecological Restoration of Rocky Desertification. Ph.D. Thesis, Guizhou Normal University, Guiyang, China, 2022. [Google Scholar]
- Tan, W.W. Study on the Occurrence Dynamics of Empoasca Onukii in Tea Gardens Under Karst Background and the Influence of Plant Essential Oils on Its Biological Activity. Master’s Thesis, Guizhou Normal University, Guiyang, China, 2023. [Google Scholar]
- Jiang, J.Q. Effects of Habitats in Typical Karst Areas of Guizhou on Species Diversity and Ultrastructural Morphology of Typhlocybinae. Master’s Thesis, Guizhou Normal University, Guiyang, China, 2022. [Google Scholar]
- Zhang, N.; Song, Q.F.; Song, Y.H. A New Leafhopper Genus of Erythroneurini (Hemiptera, Cicadellidae, Typhlocybinae) from Karst Area in Southwestern China. Rev. Bras. Entomol. 2021, 65, e20210041. [Google Scholar] [CrossRef]
- Yackulic, C.B.; Chandler, R.; Zipkin, E.F.; Royle, J.A.; Nichols, J.D.; Campbell Grant, E.H.; Veran, S. Presence-only Modelling Using MaxEnt: When Can We Trust the Inferences? Methods Ecol. Evol. 2013, 4, 236–243. [Google Scholar] [CrossRef]
- Zhu, G.P.; Liu, Q.; Gao, Y.B. Improving Ecological Niche Model Transferability to Predict the Potential Distribution of Invasive Exotic Species. Biodivers. Sci. 2014, 22, 223. [Google Scholar] [CrossRef]
- Rocchini, D.; Tordoni, E.; Marchetto, E.; Marcantonio, M.; Barbosa, A.M.; Bazzichetto, M.; Beierkuhnlein, C.; Castelnuovo, E.; Gatti, R.C.; Chiarucci, A.; et al. A Quixotic View of Spatial Bias in Modelling the Distribution of Species and Their Diversity. Npj Biodivers 2023, 2, 10. [Google Scholar] [CrossRef]
- Harvey, J.A.; Tougeron, K.; Gols, R.; Heinen, R.; Abarca, M.; Abram, P.K.; Basset, Y.; Berg, M.; Boggs, C.; Brodeur, J.; et al. Scientists’ Warning on Climate Change and Insects. Ecol. Monogr. 2023, 93, e1553. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-Km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very High Resolution Interpolated Climate Surfaces for Global Land Areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Poggio, L.; Simonetti, E.; Gimona, A. Enhancing the WorldClim Data Set for National and Regional Applications. Sci. Total Environ. 2018, 625, 1628–1643. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2021—The Physical Science Basis: Working Group I Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, 1st ed.; Cambridge University Press: Cambridge, UK, 2023; ISBN 978-1-00-915789-6. [Google Scholar]
- Wang, L.; Zhang, B.C.; Shi, Y.; Han, Z.; Lu, B. Interpretation of the IPCC AR6 on the Impacts and Risks of Climate Change. Adv. Clim. Chang. Res. 2022, 18, 389. [Google Scholar] [CrossRef]
- Eyring, V.; Bony, S.; Meehl, G.A.; Senior, C.A.; Stevens, B.; Stouffer, R.J.; Taylor, K.E. Overview of the Coupled Model Intercomparison Project Phase 6 (CMIP6) Experimental Design and Organization. Geosci. Model Dev. 2016, 9, 1937–1958. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the Black Box: An Open-Source Release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Peterson, A.T. Predicting Species’ Geographic Distributions Based on Ecological Niche Modeling. Condor 2001, 103, 599–605. [Google Scholar] [CrossRef]
- Mutamiswa, R.; Chikowore, G.; Nyamukondiwa, C.; Mudereri, B.T.; Khan, Z.R.; Chidawanyika, F. Biogeography of Cereal Stemborers and Their Natural Enemies: Forecasting Pest Management Efficacy under Changing Climate. Pest Manag. Sci. 2022, 78, 4446–4457. [Google Scholar] [CrossRef]
- Brown, J.L.; Bennett, J.R.; French, C.M. SDMtoolbox 2.0: The next Generation Python-Based GIS Toolkit for Landscape Genetic, Biogeographic and Species Distribution Model Analyses. Peerj 2017, 5, e4095. [Google Scholar] [CrossRef]
- Graham, M.H. Confronting Multicollinearity in Ecological Multiple Regression. Ecology 2003, 84, 2809–2815. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A Review of Methods to Deal with It and a Simulation Study Evaluating Their Performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Liu, T.; Liu, H.Y.; Tong, J.B.; Yang, Y.X. Habitat Suitability of Neotenic Net-winged Beetles (Coleoptera: Lycidae) in China Using Combined Ecological Models, with Implications for Biological Conservation. Divers. Distrib. 2022, 28, 2806–2823. [Google Scholar] [CrossRef]
- Warren, D.L.; Wright, A.N.; Seifert, S.N.; Shaffer, H.B. Incorporating Model Complexity and Spatial Sampling Bias into Ecological Niche Models of Climate Change Risks Faced by 90 California Vertebrate Species of Concern. Divers. Distrib. 2014, 20, 334–343. [Google Scholar] [CrossRef]
- Wei, B.; Wang, R.L.; Hou, K.; Wang, X.Y.; Wu, W. Predicting the Current and Future Cultivation Regions of Carthamus tinctorius L. Using MaxEnt Model under Climate Change in China. Glob. Ecol. Conserv. 2018, 16, e00477. [Google Scholar] [CrossRef]
- Elith, J.; Kearney, M.; Phillips, S. The Art of Modelling Range-Shifting Species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Naimi, B.; Araújo, M.B. Sdm: A Reproducible and Extensible R Platform for Species Distribution Modelling. Ecography 2016, 39, 368–375. [Google Scholar] [CrossRef]
- Lobo, J.M.; Jiménez-Valverde, A.; Real, R. AUC: A Misleading Measure of the Performance of Predictive Distribution Models. Glob. Ecol. Biogeogr. 2008, 17, 145–151. [Google Scholar] [CrossRef]
- Al-Khalaf, A.A.; Nasser, M.G.; Hosni, E.M. Global Potential Distribution of Sarcophaga Dux and Sarcophaga Haemorrhoidalis under Climate Change. Diversity 2023, 15, 903. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifert, S.N. Ecological Niche Modeling in Maxent: The Importance of Model Complexity and the Performance of Model Selection Criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENMeval: An R Package for Conducting Spatially Independent Evaluations and Estimating Optimal Model Complexity for Maxent Ecological Niche Models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Phillips, S.; Elith, J.; Leathwick, J. Dismo: Species Distribution Modeling 2023. Available online: https://CRAN.R-project.org/package=dismo (accessed on 21 July 2024).
- Radosavljevic, A.; Anderson, R.P. Making Better Maxent Models of Species Distributions: Complexity, Overfitting and Evaluation. J. Biogeogr. 2014, 41, 629–643. [Google Scholar] [CrossRef]
- Wang, Y.J.; Xie, L.Y.; Zhou, X.Y.; Chen, R.F.; Zhao, G.H.; Zhang, F.G. Prediction of the Potentially Suitable Areas of Leonurus japonicus in China Based on Future Climate Change Using the Optimized MaxEnt Model. Ecol. Evol. 2023, 13, e10597. [Google Scholar] [CrossRef]
- Akaike, H. Information Theory and an Extension of the Maximum Likelihood Principle. In Selected Papers of Hirotugu Akaike; Parzen, E., Tanabe, K., Kitagawa, G., Eds.; Springer: New York, NY, USA, 1998; pp. 199–213. [Google Scholar]
- Hanley, J.A.; McNeil, B.J. The Meaning and Use of the Area under a Receiver Operating Characteristic (ROC) Curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A Review of Methods for the Assessment of Prediction Errors in Conservation Presence/Absence Models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Xu, Z.L.; Peng, H.H.; Peng, S.Z. Development of species distribution models and evaluation methods. Acta Ecol. Sin. 2015, 35, 557–567. [Google Scholar]
- Negrini, M.; Fidelis, E.G.; Picanço, M.C.; Ramos, R.S. Mapping of the Steneotarsonemus spinki Invasion Risk in Suitable Areas for Rice (Oryza Sativa) Cultivation Using MaxEnt. Exp. Appl. Acarol. 2020, 80, 445–461. [Google Scholar] [CrossRef]
- Pearson, R.G.; Dawson, T.; Liu, C. Modelling Species Distributions in Britain: A Hierarchical Integration of Climate and Land-cover Data. Ecography 2004, 2, 285–298. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. Environmental Niche Equivalency versus Conservatism: Quantitative Approaches to Niche Evolution. Evolution 2008, 62, 2868–2883. [Google Scholar] [CrossRef]
- Ye, P.C.; Zhang, G.F.; Zhao, X.; Chen, H.; Si, Q.; Wu, J.Y. Potential Geographical Distribution and Environmental Explanations of Rare and Endangered Plant Species through Combined Modeling: A Case Study of Northwest Yunnan, China. Ecol. Evol. 2021, 11, 13052–13067. [Google Scholar] [CrossRef]
- Luo, G.M.; Yuan, Z.W.; Song, Y.H. Species composition and geographical distribution pattern of Erythroneurini worldwide. Southwest China J. Agric. Sci. 2022, 35, 1588–1597. (In Chinese) [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Global Warming of 1.5 °C: IPCC Special Report on Impacts of Global Warming of 1.5 °C Above Pre-Industrial Levels in Context of Strengthening Response to Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar]
- Jiang, M.X.; Zhong, W.Y.; Hu, H.Q.; Zheng, Z.Q.; Chen, Y.T.; You, M.S.; Chen, L.L. Prediction of Potential Suitable Regions of Tea Green Leafhopper in China in the Context of Climate Change. Chin. J. Ecol. 2022, 41, 2008–2016. [Google Scholar] [CrossRef]
- Han, P.; Rodriguez-Saona, C.; Zalucki, M.P.; Liu, S.; Desneux, N. A Theoretical Framework to Improve the Adoption of Green Integrated Pest Management Tactics. Commun. Biol. 2024, 7, 337. [Google Scholar] [CrossRef] [PubMed]
- Dyck, V.A.; Hendrichs, J.; Robinson, A.S. (Eds.) Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Taylor & Francis: Abingdon, UK, 2021. [Google Scholar]
- Bisht, D.S.; Bhatia, V.; Bhattacharya, R. Improving Plant-Resistance to Insect-Pests and Pathogens: The New Opportunities through Targeted Genome Editing. Semin. Cell Dev. Biol. 2019, 96, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.J.; Dudík, M. Modeling of Species Distributions with Maxent: New Extensions and a Comprehensive Evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]



| Variables a/Description | Percent Contribution/% | Permutation Importance/% | Regularized Training Gain |
|---|---|---|---|
| Min Temperature of Coldest Month (BIO6/°C) | 28.1 | 21.4 | 0.98 |
| Mean Diurnal Range (BIO2/°C) | 21.7 | 2.7 | 0.71 |
| Temperature Seasonality (SD × 100) (BIO4) | 19.3 | 41.7 | 0.84 |
| Isothermality (BIO2/BIO7) (×100) (BIO3) | 10.6 | 5.6 | 0.20 |
| Ground height above sea level (Elevation/meter) | 4.2 | 8.2 | 0.18 |
| Normalized Difference Vegetation Index (NDVI) | 4.1 | 7.7 | 0.33 |
| Precipitation Seasonality (BIO15/mm) | 2.9 | 3.4 | 0.20 |
| Annual Precipitation (BIO12/mm) | 2.7 | 3.1 | 0.81 |
| Slope/Degrees | 2.4 | 1.9 | 0.02 |
| Precipitation of Driest Month (BIO14/mm) | 2 | 2.7 | 0.33 |
| Aspect/Degrees | 1.9 | 1.5 | 0.03 |
| Historical Climate Scenarios | Future Climate Scenarios | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Classification Level | Current | LGM | MH | SSP126 2050s | SSP585 2050s | SSP126 2070s | SSP585 2070s | SSP126 2090s | SSP585 2090s |
| None-suitable | 748.56 | 762.70 | 747.40 | 751.94 | 756.68 | 757.51 | 735.39 | 756.77 | 754.88 |
| Low-suitable | 118.80 | 117.77 | 119.44 | 127.46 | 117.31 | 124.68 | 124.76 | 120.54 | 127.78 |
| Medium-suitable | 79.82 | 67.65 | 81.24 | 68.09 | 73.19 | 66.45 | 86.81 | 70.11 | 66.20 |
| High-suitable | 13.37 | 12.44 | 12.48 | 13.08 | 13.38 | 11.93 | 13.61 | 13.14 | 11.70 |
| Total-suitable | 212.00 | 197.86 | 213.16 | 208.62 | 203.88 | 203.05 | 225.17 | 203.79 | 205.68 |
| Period | Area | Gain | Stable | Loss | Range Change (%) | Percentage Loss (%) | Percentage Gain (%) |
|---|---|---|---|---|---|---|---|
| current | 212 | / | / | / | / | / | / |
| LGM | 197.86 | 3.64 | 211.84 | 19.27 | −6.67 | 9.09 | 1.72 |
| MH | 213.16 | 12.18 | 219.08 | 12.02 | +0.55 | 5.67 | 5.74 |
| SSP126 2050s | 208.62 | 6.33 | 220.69 | 10.41 | −1.59 | 4.91 | 2.99 |
| SSP126 2070s | 203.05 | 4.53 | 215.17 | 15.94 | −4.22 | 7.52 | 2.14 |
| SSP126 2090s | 203.79 | 4.53 | 217.66 | 13.45 | −3.87 | 6.34 | 2.14 |
| SSP585 2050s | 203.88 | 4.63 | 217.69 | 13.42 | −3.83 | 6.33 | 2.18 |
| SSP585 2070s | 225.17 | 18.76 | 225.92 | 5.19 | +6.21 | 2.45 | 8.85 |
| SSP585 2090s | 205.68 | 5.55 | 218.33 | 12.78 | −2.98 | 6.03 | 2.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, X.; Ran, W.; Xu, W.; Zhao, Y.; Su, D.; Song, Y. Potential Distribution of Tribe Erythroneurini in China Based on the R-Optimized MaxEnt Model, with Implications for Management. Insects 2025, 16, 450. https://doi.org/10.3390/insects16050450
Yuan X, Ran W, Xu W, Zhao Y, Su D, Song Y. Potential Distribution of Tribe Erythroneurini in China Based on the R-Optimized MaxEnt Model, with Implications for Management. Insects. 2025; 16(5):450. https://doi.org/10.3390/insects16050450
Chicago/Turabian StyleYuan, Xiaojuan, Weiwei Ran, Wenming Xu, Yuanqi Zhao, Di Su, and Yuehua Song. 2025. "Potential Distribution of Tribe Erythroneurini in China Based on the R-Optimized MaxEnt Model, with Implications for Management" Insects 16, no. 5: 450. https://doi.org/10.3390/insects16050450
APA StyleYuan, X., Ran, W., Xu, W., Zhao, Y., Su, D., & Song, Y. (2025). Potential Distribution of Tribe Erythroneurini in China Based on the R-Optimized MaxEnt Model, with Implications for Management. Insects, 16(5), 450. https://doi.org/10.3390/insects16050450






