Medical Potential of Insect Symbionts
Simple Summary
Abstract
1. Introduction
2. Medical Potential of Insect Symbionts
2.1. Inhibition of Human Pathogenic Bacteria and Fungi
2.1.1. Fatty Acids
2.1.2. Antibacterial Peptides
2.1.3. Polyene Macrolides
2.1.4. Alkaloids
2.1.5. Roseoflavin
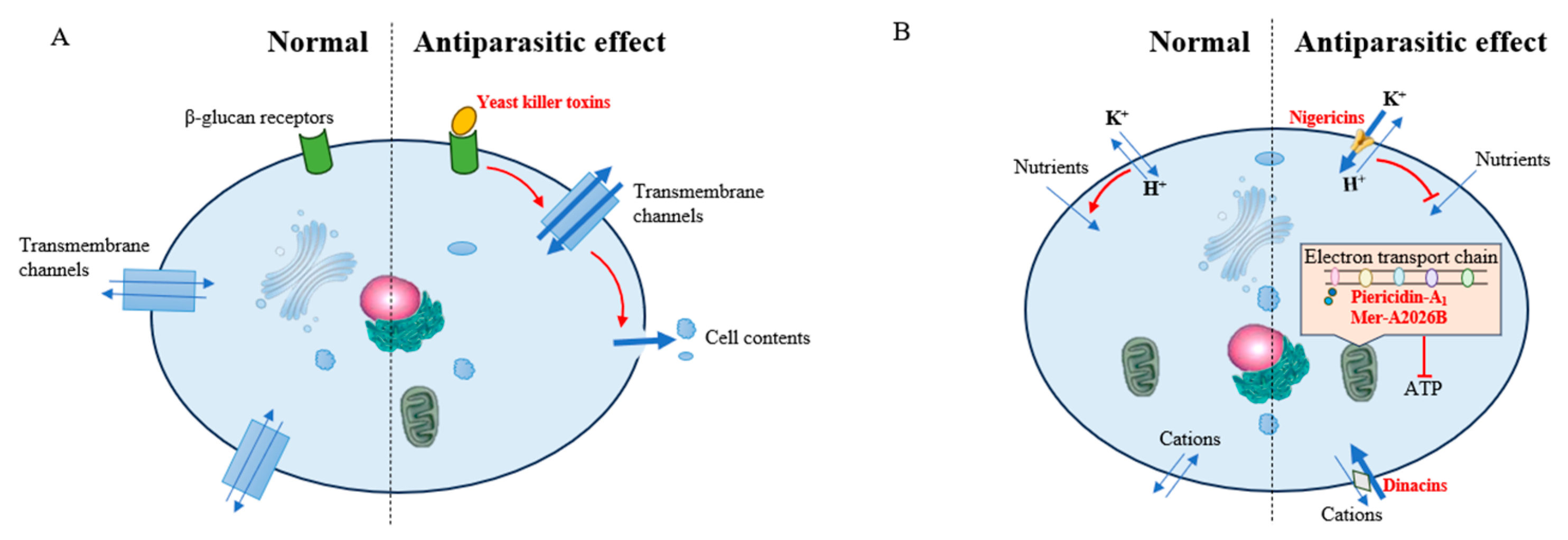
2.2. Inhibition of Parasites
2.2.1. Inhibition of Malaria Parasites
2.2.2. Inhibition of Leishmania donovani
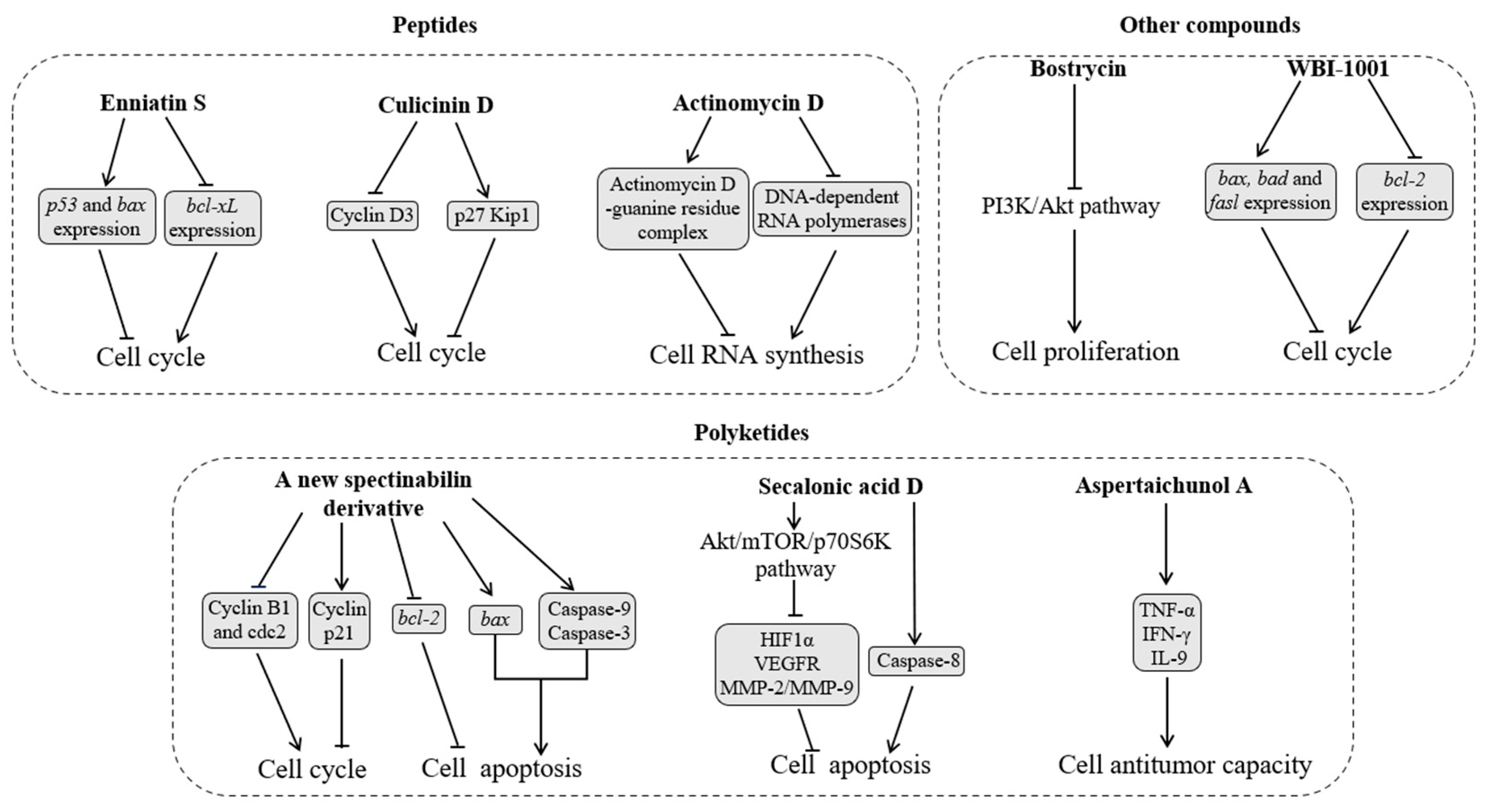
2.3. Inhibition of Tumor Cells
2.3.1. Peptides
2.3.2. Polyketides
2.3.3. Other Compounds
3. Medical Application of Insect Symbionts
3.1. Status Quo and Existing Problems
3.1.1. Application of Symbionts
3.1.2. Application of Symbiont Metabolites
3.2. Possible Solutions
3.2.1. Application of Insect Cell Cultures
3.2.2. Screening of New Symbiont Stains and Metabolites
3.2.3. Combination of Multiple Strains
3.2.4. Combination of Symbionts with Synergists
3.2.5. Adverse Reaction Surveillance and Prediction
4. Summary and Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z.Q. Phylum Arthropoda. Zootaxa 2013, 3703, 17–26. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Chang, Z.Z.; Liu, Z.Y.; Zhang, S. Influences of microbial symbionts on chemoreception of their insect hosts. Insects 2023, 14, 638. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Zhao, Y.R.; Yong, H.Z.; Liu, Z.Y.; Wang, W.F.; Lu, Y.J. The contribution of gut bacteria to pesticide resistance of Tribolium castaneum (Herbst). J. Stored Prod. Res. 2023, 103, 102160. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Yong, H.Z.; Zhang, S.; Liu, Z.Y.; Zhao, Y.R. Colonization resistance of symbionts in their insect hosts. Insects 2023, 14, 594. [Google Scholar] [CrossRef] [PubMed]
- Bisson, L.F.; Walker, G.; Ramakrishnan, V.; Luo, Y.; Fan, Q.; Wiemer, E.; Luong, P.; Ogawa, M.; Joseph, L. The two faces of Lactobacillus kunkeei: Wine spoilage agent and bee probiotic. Am. J. Enol. Vitic. 2017, 1, 1–11. [Google Scholar] [CrossRef]
- Vergalito, F.; Testa, B.; Cozzolino, A.; Letizia, F.; Succi, M.; Lombardi, S.J.; Tremonte, P.; Pannella, G.; Marco, R.D.; Sorrentino, E.; et al. Potential application of Apilactobacillus kunkeei for human use: Evaluation of probiotic and functional properties. Foods 2020, 9, 1535. [Google Scholar] [CrossRef]
- Bhalchandra, P.M.; Pathade, G.R. Antibacterial and cholesterol reducing lactic acid bacteria from silk worm (Bombyx mori) gut environment—A review. Nat. Environ. Pollut. Technol. 2011, 10, 319–326. [Google Scholar]
- Wu, H.M.; Lin, L.P.; Xu, Q.L.; Han, W.B.; Zhang, S.; Liu, Z.W.; Mei, Y.N.; Yao, Z.J.; Tan, R.X. Nodupetide, a potent insecticide and antimicrobial from Nodulisporium sp. associated with Riptortus pedestris. Tetrahedron Lett. 2017, 58, 663–665. [Google Scholar] [CrossRef]
- Isaka, M.; Palasarn, S.; Lapanun, S.; Sriklung, K. Paecilodepsipeptide A, an antimalarial and antitumor cyclohexadepsipeptide from the insect pathogenic fungus Paecilomyces cinnamomeus BCC 9616. J. Nat. Prod. 2007, 70, 675–678. [Google Scholar] [CrossRef]
- Liu, C.X.; Liu, S.H.; Zhao, J.W.; Zhang, J.; Wang, X.J.; Li, J.S.; Wang, J.D.; Xiang, W.S. A new spectinabilin derivative with cytotoxic activity from ant-derived Streptomyces sp. 1H-GS5. J. Asian Nat. Prod. Res. 2016, 19, 924–929. [Google Scholar] [CrossRef]
- Banerjee, S.; Maiti, T.K.; Roy, R.N. Enzyme producing insect gut microbes: An unexplored biotechnological aspect. Crit. Rev. Biotechnol. 2022, 42, 384–402. [Google Scholar] [CrossRef] [PubMed]
- Masson, F.; Lemaitre, B. Growing ungrowable bacteria: Overview and perspectives on insect symbiont culturability. Microbiol. Molec. Biol. Rev. 2020, 84, 4. [Google Scholar] [CrossRef] [PubMed]
- Ailioaie, L.M.; Litscher, G. Probiotics, photobiomodulation, and disease management: Controversies and challenges. Int. J. Mol. Sci. 2021, 22, 4942. [Google Scholar] [CrossRef]
- Huang, R.; Wu, F.; Zhou, Q.; Wei, W.; Yue, J.; Xiao, B.; Luo, Z.H. Lactobacillus and intestinal diseases: Mechanisms of action and clinical applications. Microbiol. Res. 2022, 260, 127019. [Google Scholar] [CrossRef]
- Berasategui, A.; Shukla, S.; Salem, H.; Kaltenpoth, M. Potential applications of insect symbionts in biotechnology. Appl. Microbiol. Biotechnol. 2016, 100, 1567–1577. [Google Scholar] [CrossRef]
- Nadia, A.B.; Jannah, S.N.; Purwantisari, S. Isolation and characterization of lactic acid bacteria from Apis mellifera stomach and their potential as antibacterial using in vitro test against growth of Staphylococcus aureus and Salmonella typhimurium. NICHE J. Trop. Biol. 2020, 3, 35–44. [Google Scholar]
- Elzeini, H.M.; Ali, A.R.A.A.; Nasr, N.F.; Elenany, Y.E.; Hassan, A.A.M. Isolation and identification of lactic acid bacteria from the intestinal tracts of honey bees, Apis mellifera L., in Egypt. J. Apic. Res. 2021, 60, 349–357. [Google Scholar] [CrossRef]
- Fallingborg, J. Intraluminal pH of the human gastrointestinal tract. Dan. Med. Bull. 1999, 46, 183–196. [Google Scholar]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef]
- Carson, D.D.; Daneo-Moore, L. Effects of fatty acids on lysis of Streptococcus faecalis. J. Bacteriol. 1980, 141, 1122–1126. [Google Scholar] [CrossRef]
- Shin, S.Y.; Bajpai, V.K.; Kim, H.R.; Kang, S.C. Antibacterial activity of eicosapentaenoic acid (EPA) against foodborne and food spoilage microorganisms. LWT 2007, 40, 1515–1519. [Google Scholar] [CrossRef]
- Bae, M.; Mevers, E.; Pishchany, G.; Whaley, S.G.; Rock, C.O.; Andes, D.R.; Currie, C.R.; Pupo, M.T.; Clardy, J. Chemical exchanges between multilateral symbionts. Org. Lett. 2021, 23, 1648–1652. [Google Scholar] [CrossRef] [PubMed]
- An, J.S.; Hong, S.H.; Somers, E.; Lee, J.; Kim, B.Y.; Woo, D.; Kim, S.W.; Hong, H.J.; Jo, S.I.; Shin, J.; et al. Lenzimycins A and B, metabolites with antibacterial properties from Brevibacillus sp. associated with the dung beetle Onthophagus lenzii. Front. Microbiol. 2020, 11, 599911. [Google Scholar] [CrossRef] [PubMed]
- Kabara, J.J.; Vrable, R.; Lie Ken Jie, M.S.F. Antimicrobial lipids: Natural and synthetic fatty acids and monoglycerides. Lipids 1977, 12, 753–759. [Google Scholar] [CrossRef]
- Yun, H.M.; Hyun, S. Role of gut commensal bacteria in juvenile developmental growth of the host: Insights from Drosophila studies. Anim. Cells Syst. 2023, 27, 329–339. [Google Scholar] [CrossRef]
- Kaur, S.; Kaur, S. Bacteriocins as potential anticancer agents. Front. Pharmacol. 2015, 6, 272. [Google Scholar] [CrossRef]
- Zhou, L.; Lian, K.; Wang, M.; Jing, X.; Zhang, Y.; Cao, J. The antimicrobial effect of a novel peptide LL-1 on Escherichia coli by increasing membrane permeability. BMC Microbiol. 2022, 22, 220. [Google Scholar] [CrossRef]
- Shin, Y.H.; Bae, S.; Sim, J.; Hur, J.; Jo, S.I.; Shin, J.; Suh, Y.G.; Oh, K.B.; Oh, D.C. Nicrophorusamides A and B, antibacterial chlorinated cyclic peptides from a gut bacterium of the carrion beetle Nicrophorus concolor. J. Nat. Prod. 2017, 80, 2962–2968. [Google Scholar] [CrossRef]
- Magana, M.; Pushpanathan, M.; Santos, A.L.; Leanse, L.; Fernandez, M.; Ioannidis, A.; Giulianotti, M.A.; Apidianakis, Y.; Bradfute, S.; Ferguson, A.L.; et al. The value of antimicrobial peptides in the age of resistance. Lancet Infect. Dis. 2020, 20, e216–e230. [Google Scholar] [CrossRef]
- Yang, B.; Yang, H.; Liang, J.; Chen, J.; Wang, C.; Wang, Y.; Wang, J.C.; Luo, W.H.; Deng, T.; Guo, J.L. A review on the screening methods for the discovery of natural antimicrobial peptides. J. Pharm. Anal. 2024, 15, 101046. [Google Scholar] [CrossRef]
- Zotchev, S.B. Polyene macrolide antibiotics and their applications in human therapy. Curr. Med. Chem. 2003, 10, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Van Arnam, E.B.; Ruzzini, A.C.; Sit, C.S.; Horn, H.; Pinto-Tomas, A.A.; Currie, C.R.; Clardy, J. Selvamicin, an atypical antifungal polyene from two alternative genomic contexts. Proc. Natl. Acad. Sci. USA 2016, 113, 12940–12945. [Google Scholar] [CrossRef] [PubMed]
- Haeder, S.; Wirth, R.; Herz, H.; Spiteller, D. Candicidin-producing Streptomyces support leaf-cutting ants to protect their fungus garden against the pathogenic fungus Escovopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 4742–4746. [Google Scholar] [CrossRef]
- Barke, J.; Seipke, R.F.; Gruschow, S.; Heavens, D.; Drou, N.; Bibb, M.J.; Goss, R.J.M.; Yu, D.W.; Hutchings, M.I. A mixed community of actinomycetes produce multiple antibiotics for the fungus farming ant Acromyrmex octospinosus. BMC Biol. 2010, 8, 109. [Google Scholar] [CrossRef]
- Anderson, T.M.; Clay, M.C.; Cioffi, A.G.; Diaz, K.A.; Hisao, G.S.; Tuttle, M.D.; Nieuwkoop, A.J.; Comellas, G.; Maryum, N.; Wang, S.; et al. Amphotericin forms an extramembranous and fungicidal sterol sponge. Nat. Chem. Biol. 2014, 10, 400–406. [Google Scholar] [CrossRef]
- Yan, Y.; Li, X.; Zhang, C.; Lv, L.; Gao, B.; Li, M. Research progress on antibacterial activities and mechanisms of natural alkaloids: A review. Antibiotics 2021, 10, 318. [Google Scholar] [CrossRef]
- Zhang, Q.; Lyu, Y.; Huang, J.; Zhang, X.; Yu, N.; Wen, Z.; Chen, S. Antibacterial activity and mechanism of sanguinarine against Providencia rettgeri in vitro. PeerJ. 2020, 8, e9543. [Google Scholar] [CrossRef]
- Um, S.; Bach, D.H.; Shin, B.; Ahn, C.H.; Kim, S.H.; Bang, H.S.; Oh, K.B.; Lee, S.K.; Shin, J.; Oh, D.C. Naphthoquinone–oxindole alkaloids, coprisidins A and B, from a gut-associated bacterium in the dung beetle, Copris tripartitus. Org. Lett. 2016, 18, 5792–5795. [Google Scholar] [CrossRef]
- Lee, E.R.; Blount, K.F.; Breaker, R.R. Roseoflavin is a natural antibacterial compound that binds to FMN riboswitches and regulates gene expression. RNA Biol. 2009, 6, 187–194. [Google Scholar] [CrossRef]
- Zhou, L.F.; Wu, J.; Li, S.; Li, Q.; Jin, L.P.; Yin, C.P.; Zhang, Y.L. Antibacterial potential of termite-associated Streptomyces spp. ACS Omega 2021, 6, 4329–4334. [Google Scholar] [CrossRef]
- Miller, L.H.; Good, M.F.; Milon, G. Malaria pathogenesis. Science 1994, 264, 1878–1883. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Bai, L.; Jiang, Y.; Huang, W.; Wang, L.; Li, S.; Zhu, G.D.; Wang, D.Q.; Huang, Z.H.; Li, X.S.; et al. A natural symbiotic bacterium drives mosquito refractoriness to Plasmodium infection via secretion of an antimalarial lipase. Nat. Microbiol. 2021, 6, 806–817. [Google Scholar] [CrossRef] [PubMed]
- Bennink, S.; Kiesow, M.J.; Pradel, G. The development of malaria parasites in the mosquito midgut. Cell Microbiol. 2016, 18, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Balikagala, B.; Fukuda, N.; Ikeda, M.; Katuro, O.T.; Tachibana, S.I.; Yamauchi, M.; Opio, W.; Emoto, S.; Anywar, D.A.; Kimura, E.; et al. Evidence of artemisinin-resistant malaria in Africa. N. Engl. J. Med. 2021, 385, 1163–1171. [Google Scholar] [CrossRef]
- Ricci, I.; Damiani, C.; Scuppa, P.; Mosca, M.; Crotti, E.; Rossi, P.; Rizzi, A.; Capone, A.; Gonella, E.; Ballarini, P.; et al. The yeast Wickerhamomyces anomalus (Pichia anomala) inhabits the midgut and reproductive system of the Asian malaria vector Anopheles stephensi. Environ. Microbiol. 2011, 13, 911–921. [Google Scholar] [CrossRef]
- Valzano, M.; Cecarini, V.; Cappelli, A.; Capone, A.; Bozic, J.; Cuccioloni, M.; Epis, S.; Petrelli, D.; Angeletti, M.; Eleuteri, A.M.; et al. A yeast strain associated to Anopheles mosquitoes produces a toxin able to kill malaria parasites. Malar. J. 2016, 15, 21. [Google Scholar] [CrossRef]
- Cirimotich, C.M.; Dong, Y.; Clayton, A.M.; Sandiford, S.L.; Souza-Neto, J.A.; Mulenga, M.; Dimopoulos, G. Natural microbe-mediated refractoriness to Plasmodium infection in Anopheles gambiae. Science 2011, 332, 855–858. [Google Scholar] [CrossRef]
- Gil, Z.; Martinez-Sotillo, N.; Pinto-Martinez, A.; Mejias, F.; Martinez, J.C.; Galindo, I.; Oldfield, E.; Benaim, G. SQ109 inhibits proliferation of Leishmania donovani by disruption of intracellular Ca2+ homeostasis, collapsing the mitochondrial electrochemical potential (ΔΨm) and affecting acidocalcisomes. Parasitol. Res. 2020, 119, 649–657. [Google Scholar] [CrossRef]
- Debrabant, A.; Joshi, M.B.; Pimenta, P.F.; Dwyer, D.M. Generation of Leishmania donovani axenic amastigotes: Their growth and biological characteristics. Int. J. Parasitol. 2004, 34, 205–217. [Google Scholar] [CrossRef]
- Croft, S.L.; Sundar, S.; Fairlamb, A.H. Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 2006, 19, 111–126. [Google Scholar] [CrossRef]
- Ortega, H.E.; Ferreira, L.L.; Melo, W.G.; Oliveira, A.L.L.; Ramos Alvarenga, R.F.; Lopes, N.P.; Bugni, T.S.; Andricopulo, A.D.; Pupo, M.T. Antifungal compounds from Streptomyces associated with attine ants also inhibit Leishmania donovani. PLoS Neglected Trop. Dis. 2019, 13, e0007643. [Google Scholar] [CrossRef] [PubMed]
- Ortega, H.E.; Lourenzon, V.B.; Chevrette, M.G.; Ferreira, L.L.; Alvarenga, R.F.R.; Melo, W.G.; Venancio, T.; Currie, C.R.; Andricopulo, A.D.; Bugni, T.S.; et al. Antileishmanial macrolides from ant-associated Streptomyces sp. ISID311. Bioorg. Med. Chem. 2021, 32, 116016. [Google Scholar] [CrossRef] [PubMed]
- She, J.L.; Zhou, X.F. New insights into the antitumor potential of natural piericidins. J. Holistic Integr. Pharm. 2021, 2, 153–162. [Google Scholar] [CrossRef]
- Sarkar, D.; Bhaduri, A. Temperature-induced rapid increase in cytoplasmic free Ca2+ in pathogenic Leishmania donovani promastigotes. FEBS Lett. 1995, 375, 83–86. [Google Scholar] [CrossRef]
- Chan-Bacab, M.J.; Reyes-Estebanez, M.M.; Camacho-Chab, J.C.; Ortega-Morales, B.O. Microorganisms as a potential source of molecules to control trypanosomatid diseases. Molecules 2021, 26, 1388. [Google Scholar] [CrossRef]
- Asher, I.M.; Phillies, G.D.J.; Kim, B.J.; Stanley, H.E. Ion complexation in nonactin, monactin, and dinactin: A Raman spectroscopic study. Biopolymers 1977, 16, 157–185. [Google Scholar] [CrossRef]
- Hussain, A.; Rather, M.A.; Dar, M.S.; Dangroo, N.A.; Aga, M.A.; Qayum, A.; Shah, A.M.; Ahmad, Z.; Dar, M.J.; Hassan, Q.P. Streptomyces puniceus strain AS13., production, characterization and evaluation of bioactive metabolites: A new face of dinactin as an antitumor antibiotic. Microbiol. Res. 2018, 207, 196–202. [Google Scholar] [CrossRef]
- Vazquez-Laslop, N.; Mankin, A.S. How macrolide antibiotics work. Trends Biochem. Sci. 2018, 43, 668–684. [Google Scholar] [CrossRef]
- Otto, T.; Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer 2017, 17, 93–115. [Google Scholar] [CrossRef]
- Matthews, H.K.; Bertoli, C.; de Bruin, R.A. Cell cycle control in cancer. Nat. Rev. Mol. Cell Biol. 2022, 23, 74–88. [Google Scholar] [CrossRef]
- Fiandalo, M.V.; Kyprianou, N. Caspase control: Protagonists of cancer cell apoptosis. Exp. Oncol. 2012, 34, 165. [Google Scholar] [PubMed]
- Evan, G.I.; Vousden, K.H. Proliferation, cell cycle and apoptosis in cancer. Nature 2001, 411, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef]
- Dornetshuber, R.; Heffeter, P.; Kamyar, M.R.; Peterbauer, T.; Berger, W.; Lemmens-Gruber, R. Enniatin exerts p53-dependent cytostatic and p53-independent cytotoxic activities against human cancer cells. Chem. Res. Toxicol. 2007, 20, 465–473. [Google Scholar] [CrossRef]
- Tian, J.; Han, J.J.; Zhang, X.; He, L.W.; Zhang, Y.J.; Bao, L.; Liu, H.W. New cyclohexadepsipeptides from an entomogenous fungus Fusarium proliferatum and their cytotoxicity and autophagy-inducing activity. Chem. Biodivers. 2016, 13, 852–860. [Google Scholar] [CrossRef]
- He, H.; Janso, J.E.; Yang, H.Y.; Bernan, V.S.; Lin, S.L.; Yu, K. Culicinin D, an antitumor peptaibol produced by the fungus Culicinomyces clavisporus, strain LL-12I252. J. Nat. Prod. 2006, 69, 736–741. [Google Scholar] [CrossRef]
- Schoenian, I.; Spiteller, M.; Ghaste, M.; Wirth, R.; Herz, H.; Spiteller, D. Chemical basis of the synergism and antagonism in microbial communities in the nests of leaf-cutting ants. Proc. Natl. Acad. Sci. USA 2011, 108, 1955–1960. [Google Scholar] [CrossRef]
- Lu, D.F.; Wang, Y.S.; Li, C.; Wei, G.J.; Chen, R.; Dong, D.M.; Yao, M. Actinomycin D inhibits cell proliferations and promotes apoptosis in osteosarcoma cells. Int. J. Clin. Exp. Med. 2015, 8, 1904. [Google Scholar]
- Gao, X.X.; Yao, H.Z.; Mu, Y.; Guan, P.P.; Li, G.D.; Lin, B.; Jiang, Y.; Han, L.; Huang, X.S.; Jiang, C.L. The antiproliferative effect of spectinabilins from Streptomyces spectabilis on hepatocellular carcinoma cells in vitro and in vivo. Bioorg. Chem. 2019, 93, 103311. [Google Scholar] [CrossRef]
- Li, T.X.; Su, H.Y.; Yu, J.C.; Hao, H.; Jia, X.W.; Shi, F.C.; Xu, C.P. Antibacterial metabolites from the beetle-associated fungus Penicillium chrysogenum. An. Acad. Bras. Cienc. 2023, 95, e20220178. [Google Scholar] [CrossRef] [PubMed]
- Guru, S.K.; Pathania, A.S.; Kumar, S.; Ramesh, D.; Kumar, M.; Rana, S.; Kumar, A.; Malik, F.; Sharma, P.R.; Chandan, B.K.; et al. Secalonic acid-D represses HIF1α/VEGF-mediated angiogenesis by regulating the Akt/mTOR/p70S6K signaling cascade. Cancer Res. 2015, 75, 2886–2896. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Ma, F.P.; Yan, Y.M.; Zhao, W.L.; Shi, J.; Xiao, W.; Bi, E.G.; Luo, Q. Aspertaichunol A, an immunomodulatory polyketide with an uncommon scaffold from the insect-derived endophytic Aspergillus taichungensis SMU01. Org. Lett. 2022, 24, 7405–7409. [Google Scholar] [CrossRef]
- Kalaba, M.H.; Sultan, M.H.; Elbahnasawy, M.A.; El-Didamony, S.E.; El Bakary, N.M.; Sharaf, M.H. First report on isolation of Mucor bainieri from honeybees, Apis mellifera: Characterization and biological activities. Biotechnol. Rep. 2022, 36, e00770. [Google Scholar] [CrossRef]
- Al-Enazi, N.M.; Abdel-Raouf, N.; Alharbi, R.M.; Sholkamy, E.N. Metabolic profiling of Streptomyces sp. strain ESS_AMH1 isolated from Apis mellifera yemintica’s gut microbiome, and its anticancer activity against breast cancer (MCF7) and hepatocarcinoma (HepG2) cell lines, as well as antimicrobial activity. Appl. Sci. 2022, 12, 12257. [Google Scholar] [CrossRef]
- Chen, W.S.; Hou, J.N.; Guo, Y.B.; Yang, H.L.; Xie, C.M.; Lin, Y.C.; She, Z.G. Bostrycin inhibits proliferation of human lung carcinoma A549 cells via downregulation of the PI3K/Akt pathway. J. Exp. Clin. Cancer Res. 2011, 30, 17. [Google Scholar] [CrossRef]
- Wang, W.Y.; Liao, Y.Y.; Tang, C.; Huang, X.M.; Luo, Z.H.; Chen, J.M.; Cai, P. Cytotoxic and antibacterial compounds from the coral-derived fungus Aspergillus tritici SP2-8-1. Mar. Drugs 2017, 15, 348. [Google Scholar] [CrossRef]
- Paul, V.J.; Frautschy, S.; Fenical, W.; Nealson, K.H. Antibiotics in microbial ecology: Isolation and structure assignment of several new antibacterial compounds from the insect-symbiotic bacteria Xenorhabdus spp. J. Chem. Ecol. 1981, 7, 589–597. [Google Scholar] [CrossRef]
- Gao, E.K.; Qian, F.; Jin, Q.L. Tapinarof inhibits proliferation and induces apoptosis of non-small cell lung cancer A549 cell. J. Hainan Med. Univ. 2023, 29, 7–14. [Google Scholar]
- Roos, K.; Simark-Mattsson, C.; Grahn Hakansson, E.; Larsson, L.; Sandberg, T.; Ahren, C. Can probiotic lactobacilli eradicate persistent carriage of meticillin-resistant Staphylococcus aureus? J. Hosp. Infect. 2011, 78, 77–78. [Google Scholar] [CrossRef]
- Yeruva, T.; Vankadara, S.; Ramasamy, S.; Lingaiah, K. Identification of potential probiotics in the midgut of mulberry silkworm, Bombyx mori through metagenomic approach. Probiotics Antimicrob. Proteins 2019, 12, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, T.C.; Butler, E.; Markowicz, P.; Lindholm, C.; Larsson, L.; Vasquez, A. Lactic acid bacterial symbionts in honeybees–An unknown key to honey’s antimicrobial and therapeutic activities. Int. Wound J. 2014, 13, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Vieco-Saiz, N.; Belguesmia, Y.; Raspoet, R.; Auclair, E.; Gancel, F.; Kempf, I.; Drider, D. Benefits and inputs from lactic acid bacteria and their bacteriocins as alternatives to antibiotic growth promoters during food-animal production. Front. Microbiol. 2019, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Prescott, S.L.; Bjorksten, B. Probiotics for the prevention or treatment of allergic diseases. J. Allergy Clin. Immunol. 2007, 120, 255–262. [Google Scholar] [CrossRef]
- Freedman, S.B.; Schnadower, D.; Tarr, P.I. The probiotic conundrum: Regulatory confusion, conflicting studies, and safety concerns. JAMA 2020, 323, 823–824. [Google Scholar] [CrossRef]
- Crits-Christoph, A.; Hallowell, H.A.; Koutouvalis, K.; Suez, J. Good microbes, bad genes? The dissemination of antimicrobial resistance in the human microbiome. Gut Microb. 2022, 14, 2055944. [Google Scholar] [CrossRef]
- Merenstein, D.; Pot, B.; Leyer, G.; Ouwehand, A.C.; Preidis, G.A.; Elkins, C.A.; Hill, C.; Lewis, Z.T.; Shane, A.L.; Zmora, N.; et al. Emerging issues in probiotic safety: 2023 perspectives. Gut Microb. 2023, 15, 2185034. [Google Scholar] [CrossRef]
- Rao, S.S.C.; Yu, S.; Tetangco, E.P.; Yan, Y. Probiotics can cause D-lactic acidosis and brain fogginess: Reply to Quigley et al. Clin. Transl. Gastroenterol. 2018, 9, 187. [Google Scholar] [CrossRef]
- Uusitalo, U.; Andren Aronsson, C.; Liu, X.; Kurppa, K.; Yang, J.M.; Liu, E.; Skidmore, J.; Winkler, C.; Rewers, M.J.; Hagopian, W.A.; et al. Early probiotic supplementation and the risk of celiac disease in children at genetic risk. Nutrients 2019, 11, 1790. [Google Scholar] [CrossRef]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of antibiotics and antibiotic resistance, and their impacts on drug development: A narrative review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef]
- Morelli, L.; Sarra, P.G.; Bottazzi, V. In vivo transfer of pAMβ1 from Lactobacillus reuteri to Enterococcus faecalis. J. Appl. Bacteriol. 1988, 65, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Jigami, Y.; Harada, N.; Uemura, H.; Tanaka, H.; Ishikawa, K.; Nakasato, S.; Kita, H.; Sugiura, M. Identification of a polymyxin produced by a symbiotic microorganism isolated from the brown planthopper, Nilaparavata lugens. Agric. Biol. Chem. 2014, 50, 1637–1639. [Google Scholar] [CrossRef]
- Landman, D.; Georgescu, C.; Martin, D.A.; Quale, J. Polymyxins revisited. Clin. Microbiol. Rev. 2008, 21, 449–465. [Google Scholar] [CrossRef]
- Bissonnette, R.; Bolduc, C.; Maari, C.; Nigen, S.; Webster, J.M.; Tang, L.; Lyle, M. Efficacy and safety of topical WBI-1001 in patients with mild to moderate psoriasis: Results from a randomized double-blind placebo-controlled, phase II trial. J. Eur. Acad. Dermatol. Venereol. 2011, 26, 1516–1521. [Google Scholar] [CrossRef]
- Bissonnette, R.; Gold, L.S.; Rubenstein, D.S.; Tallman, A.M.; Armstrong, A. Tapinarof in the treatment of psoriasis: A review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor–modulating agent. J. Am. Acad. Dermatol. 2021, 84, 1059–1067. [Google Scholar] [CrossRef]
- Liang, S.N.; Wang, X.Y.; Li, C.; Liu, L.B. Biological activity of lactic acid bacteria exopolysaccharides and their applications in the food and pharmaceutical industries. Foods 2024, 13, 1621. [Google Scholar] [CrossRef]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef]
- Vervloet, D.; Durham, S. Adverse reactions to drugs. BMJ 1998, 316, 1511–1514. [Google Scholar] [CrossRef]
- Luo, Y.Z.; Huang, H.; Liang, J.; Wang, M.; Lu, L.; Shao, Z.Y.; Cobb, R.E.; Zhao, H.M. Activation and characterization of a cryptic polycyclic tetramate macrolactam biosynthetic gene cluster. Nat. Commun. 2013, 4, 2894. [Google Scholar] [CrossRef]
- Darby, A.C.; Chandler, S.M.; Welburn, S.C.; Douglas, A.E. Aphid-symbiotic bacteria cultured in insect cell lines. Appl. Environ. Microbiol. 2005, 71, 4833–4839. [Google Scholar] [CrossRef]
- Xu, Y.; Buss, E.A.; Boucias, D.G. Culturing and characterization of gut symbiont Burkholderia spp. from the Southern chinch bug, Blissus insularis (Hemiptera: Blissidae). Appl. Environ. Microbiol. 2016, 82, 3319–3330. [Google Scholar] [CrossRef] [PubMed]
- Malmierca, M.G.; Gonzalez-Montes, L.; Perez-Victoria, I.; Sialer, C.; Brana, A.F.; Garcia Salcedo, R.; Martín, J.; Reyes, F.; Mendez, C.; Olano, C.; et al. Searching for glycosylated natural products in actinomycetes and identification of novel macrolactams and angucyclines. Front. Microbiol. 2018, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, A.C.; Suomalainen, T.; Tolkko, S.; Salminen, S. In vitro adhesion of propionic acid bacteria to human intestinal mucus. Lait 2002, 82, 123–130. [Google Scholar] [CrossRef]
- Timmerman, H.M.; Koning, C.J.M.; Mulder, L.; Rombouts, F.M.; Beynen, A.C. Monostrain, multistrain and multispecies probiotics—A comparison of functionality and efficacy. Int. J. Food Microbiol. 2004, 96, 219–233. [Google Scholar] [CrossRef]
- Sanders, M.E. Impact of probiotics on colonizing microbiota of the gut. J. Clin. Gastroenterol. 2011, 45, S115–S119. [Google Scholar] [CrossRef]
- Drago, L.; Gismondo, M.R.; Lombardi, A.; de Haen, C.; Gozzini, L. Inhibition of in vitro growth of enteropathogens by new Lactobacillus isolates of human intestinal origin. FEMS Microbiol. Lett. 1997, 153, 455–463. [Google Scholar] [CrossRef]
- Pacheco-Ordaz, R.; Wall-Medrano, A.; Goni, M.G.; Ramos-Clamont-Montfort, G.; Ayala-Zavala, J.F.; Gonzalez-Aguilar, G.A. Effect of phenolic compounds on the growth of selected probiotic and pathogenic bacteria. Lett. Appl. Microbiol. 2018, 66, 25–31. [Google Scholar] [CrossRef]
- de Souza, E.L.; de Albuquerque, T.M.R.; Dos Santos, A.S.; Massa, N.M.L.; de Brito Alves, J.L. Potential interactions among phenolic compounds and probiotics for mutual boosting of their health-promoting properties and food functionalities—A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1645–1659. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Wu, Z.; Weng, P. The modulatory effect of anthocyanins from purple sweet potato on human intestinal microbiota in vitro. J. Agric. Food Chem. 2016, 64, 2582–2590. [Google Scholar] [CrossRef]
- Critchley, I.A.; Karlowsky, J.A. Optimal use of antibiotic resistance surveillance systems. Clin. Microbiol. Infect. 2004, 10, 502–511. [Google Scholar] [CrossRef]
- Ashbee, H.R.; Barnes, R.A.; Johnson, E.M.; Richardson, M.D.; Gorton, R.; Hope, W.W. Therapeutic drug monitoring (TDM) of antifungal agents: Guidelines from the British Society for medical mycology. J. Antimicrob. Chemother. 2014, 69, 1162–1176. [Google Scholar] [CrossRef] [PubMed]
- Edwards, I.R.; Aronson, J.K. Adverse drug reactions: Definitions, diagnosis, and management. Lancet 2000, 356, 1255–1259. [Google Scholar] [CrossRef] [PubMed]
- Weinshilboum, R.M.; Wang, L. Pharmacogenomics: Precision medicine and drug response. Mayo Clin. Proc. 2017, 92, 1711–1722. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Q.; Zheng, P.; Guo, Y.; Wang, L.; Zhang, T.C.; Sun, J.B.; Ma, Y.H. Evolving the L-lysine high-producing strain of Escherichia coli using a newly developed high-throughput screening method. J. Ind. Microbiol. Biotechnol. 2016, 43, 1227–1235. [Google Scholar] [CrossRef]
- Cobb, R.E.; Chao, R.; Zhao, H. Directed evolution: Past, present, and future. AIChE J. 2013, 59, 1432–1440. [Google Scholar] [CrossRef]
- Kao, M.R.; Yu, S.M.; Ho, T.H.D. Improvements of the productivity and saccharification efficiency of the cellulolytic β-glucosidase D2-BGL in Pichia pastoris via directed evolution. Biotechnol. Biofuels 2021, 14, 126. [Google Scholar] [CrossRef]
- Bailey, J.E. Toward a science of metabolic engineering. Science 1991, 252, 1668–1675. [Google Scholar] [CrossRef]
- Cabrera-Valladares, N.; Richardson, A.P.; Olvera, C.; Trevino, L.G.; Deziel, E.; Lepine, F.; Soberon-Chavez, G. Monorhamnolipids and 3-(3-hydroxyalkanoyloxy) alkanoic acids (HAAs) production using Escherichia coli as a heterologous host. Appl. Microbiol. Biotechnol. 2006, 73, 187–194. [Google Scholar] [CrossRef]
- Seipke, R.F.; Barke, J.; Ruiz-Gonzalez, M.X.; Orivel, J.; Yu, D.W.; Hutchings, M.I. Fungus-growing Allomerus ants are associated with antibiotic-producing actinobacteria. Antonie Van Leeuwenhoek 2012, 101, 443–447. [Google Scholar] [CrossRef]
- Putri, I.; Jannah, S.N.; Purwantisari, S. Isolation and characterization of lactic acid bacteria from Apis mellifera and their potential as antibacterial using in vitro test against growth of Listeria monocytogenes and Escherichia coli. NICHE J. Trop. Biol. 2020, 3, 26–34. [Google Scholar]
- Rohlfs, M.; Kurschner, L. Saprophagous insect larvae, Drosophila melanogaster, profit from increased species richness in beneficial microbes. J. Appl. Entomol. 2010, 134, 667–671. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, X.; Elhag, O.; Cai, M.; Zheng, L.; Huang, F.; Jordan, H.R.; Tomberlin, J.K.; Sze, S.H.; Yu, Z.N.; et al. Hermetia illucens L. larvae-associated intestinal microbes reduce the transmission risk of zoonotic pathogens in pig manure. Microb. Biotechnol. 2022, 15, 2631–2644. [Google Scholar] [CrossRef] [PubMed]
- Syed Yaacob, S.N.; Huyop, F.; Kamarulzaman Raja Ibrahim, R.; Wahab, R.A. Identification of Lactobacillus spp. and Fructobacillus spp. isolated from fresh Heterotrigona itama honey and their antagonistic activities against clinical pathogenic bacteria. J. Apic. Res. 2018, 57, 395–405. [Google Scholar] [CrossRef]
- Xiao, Y.S.; Zhang, B.; Zhang, M.; Guo, Z.K.; Deng, X.Z.; Shi, J.; Li, W.; Jiao, R.H.; Tan, R.X.; Ge, H.M. Rifamorpholines A–E, potential antibiotics from locust-associated actinobacteria Amycolatopsis sp. Hca4. Org. Biomol. Chem. 2017, 15, 3909–3916. [Google Scholar] [CrossRef]
- Li, J.J.; Sang, M.L.; Jiang, Y.T.; Wei, J.H.; Shen, Y.L.; Huang, Q.H.; Li, Y.Y.; Ni, J.F. Polyene-producing Streptomyces spp. from the fungus-growing termite Macrotermes barneyi exhibit high inhibitory activity against the antagonistic fungus Xylaria. Front. Microbiol. 2021, 12, 649962. [Google Scholar] [CrossRef]
- Frey Tirri, B.; Bitzer, J.; Geudelin, B.; Drewe, J. Safety, tolerability and pharmacokinetics of intravaginal pentamycin. Chemother. 2010, 56, 190–196. [Google Scholar] [CrossRef]
- Beemelmanns, C.; Ramadhar, T.R.; Kim, K.H.; Klassen, J.L.; Cao, S.; Wyche, T.P.; Hou, Y.P.; Poulsen, M.; Bugni, T.S.; Currie, C.R.; et al. Macrotermycins A-D, glycosylated macrolactams from a termite-associated Amycolatopsis sp. M39. Org. Lett. 2017, 19, 1000–1003. [Google Scholar] [CrossRef]
- Um, S.; Fraimout, A.; Sapountzis, P.; Oh, D.C.; Poulsen, M. The fungus-growing termite Macrotermes natalensis harbors bacillaene-producing Bacillus sp. that inhibit potentially antagonistic fungi. Sci. Rep. 2013, 3, 3250. [Google Scholar] [CrossRef]
- Rodriguez-Hernandez, D.; Melo, W.G.; Menegatti, C.; Lourenzon, V.B.; do Nascimento, F.S.; Pupo, M.T. Actinobacteria associated with stingless bees biosynthesize bioactive polyketides against bacterial pathogens. New J. Chem. 2019, 43, 10109–10117. [Google Scholar] [CrossRef]
- Niu, S.W.; Li, S.M.; Chen, Y.C.; Tian, X.P.; Zhang, H.B.; Zhang, G.T.; Zhang, W.M.; Yang, X.H.; Zhang, S.; Ju, J.H.; et al. Lobophorins E and F, new spirotetronate antibiotics from a South China Sea-derived Streptomyces sp. SCSIO 01127. J. Antibiot. 2011, 64, 711–716. [Google Scholar] [CrossRef]
- Guo, H.; Kreuzenbeck, N.B.; Otani, S.; Garcia-Altares, M.; Dahse, H.M.; Weigel, C.; Aanen, D.K.; Hertweck, C.; Poulsen, M.; Beemelmanns, C. Pseudoxylallemycins A-F, cyclic tetrapeptides with rare allenyl modifications isolated from Pseudoxylaria sp. X802: A competitor of fungus-growing termite cultivars. Org. Lett. 2016, 18, 3338–3341. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Kikuchi, Y. Impact of the insect gut microbiota on ecology, evolution, and industry. Curr. Opin. Insect Sci. 2020, 41, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Heise, P.; Liu, Y.; Degenkolb, T.; Vogel, H.; Schaberle, T.F.; Vilcinskas, A. Antibiotic-producing beneficial bacteria in the gut of the burying beetle Nicrophorus vespilloides. Front. Microbiol. 2019, 10, 1178. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.N.; Venkatachalam, S.R. Inhibition of dihydrofolate reductase and cell growth activity by the phenanthroindolizidine alkaloids pergularinine and tylophorinidine: The in vitro cytotoxicity of these plant alkaloids and their potential as antimicrobial and anticancer agents. Toxicol. In Vitro 2000, 14, 53–59. [Google Scholar] [CrossRef]
- Bi, S.F.; Guo, Z.K.; Jiang, N.; Jiao, R.H.; Ge, H.M.; Tan, R.X. New alkaloid from Streptomyces koyangensis residing in Odontotermes formosanus. J. Asian Nat. Prod. Res. 2013, 15, 422–425. [Google Scholar] [CrossRef]
- Koehler, S.; Doubsky, J.; Kaltenpoth, M. Dynamics of symbiont-mediated antibiotic production reveal efficient long-term protection for beewolf offspring. Front. Zool. 2013, 10, 3. [Google Scholar] [CrossRef]
- Madden, A.A.; Grassetti, A.; Soriano, J.A.N.; Starks, P.T. Actinomycetes with antimicrobial activity isolated from paper wasp (Hymenoptera: Vespidae: Polistinae) nests. Environ. Entomol. 2013, 42, 703–710. [Google Scholar] [CrossRef]
- Oh, D.C.; Poulsen, M.; Currie, C.R.; Clardy, J. Sceliphrolactam, a polyene macrocyclic lactam from a wasp-associated Streptomyces sp. Org. Lett. 2011, 13, 752–755. [Google Scholar] [CrossRef]
- Shao, Y.; Chen, B.; Sun, C.; Ishida, K.; Hertweck, C.; Boland, W. Symbiont-derived antimicrobials contribute to the control of the lepidopteran gut microbiota. Cell Chem. Biol. 2017, 24, 66–75. [Google Scholar] [CrossRef]
- Chang, P.T.; Rao, K.; Longo, L.O.; Lawton, E.S.; Scherer, G.; Van Arnam, E.B. Thiopeptide defense by an ant’s bacterial symbiont. J. Nat. Prod. 2020, 83, 725–729. [Google Scholar] [CrossRef]
- Young, T.S.; Walsh, C.T. Identification of the thiazolyl peptide GE37468 gene cluster from Streptomyces ATCC 55365 and heterologous expression in Streptomyces lividans. Proc. Natl. Acad. Sci. USA 2011, 108, 13053–13058. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, F.; Wang, Z.; Luo, Q.; Liu, Z.; Xiao, Y.; Ren, Y. Medical Potential of Insect Symbionts. Insects 2025, 16, 457. https://doi.org/10.3390/insects16050457
Fan F, Wang Z, Luo Q, Liu Z, Xiao Y, Ren Y. Medical Potential of Insect Symbionts. Insects. 2025; 16(5):457. https://doi.org/10.3390/insects16050457
Chicago/Turabian StyleFan, Fanglei, Zhengyan Wang, Qiong Luo, Zhiyuan Liu, Yu Xiao, and Yonglin Ren. 2025. "Medical Potential of Insect Symbionts" Insects 16, no. 5: 457. https://doi.org/10.3390/insects16050457
APA StyleFan, F., Wang, Z., Luo, Q., Liu, Z., Xiao, Y., & Ren, Y. (2025). Medical Potential of Insect Symbionts. Insects, 16(5), 457. https://doi.org/10.3390/insects16050457





