Simple Summary
Insects like the Trichogramma dendrolimi are essential for controlling harmful pests in agriculture. However, pesticides used to kill these pests can also harm beneficial insects like T. dendrolimi. This study examined the effects of a common pesticide, acetamiprid, on T. dendrolimi. It found that even small amounts of acetamiprid can reduce the ability of T. dendrolimi to lay eggs and survive, without necessarily killing them. By using a statistical model, the study also showed that acetamiprid could affect a large portion of similar beneficial insects at commonly used field concentrations. These findings highlight the importance of considering not just whether a pesticide kills insects, but also its subtle effects on beneficial insects that help control pests naturally. This information can help improve pest management strategies to better protect both crops and beneficial insects.
Abstract
Trichogramma dendrolimi, a key egg parasitoid for lepidopteran pest control, faces potential risks from neonicotinoid insecticides like acetamiprid used in integrated pest management (IPM). This study evaluated acetamiprid’s acute and sublethal toxicity to T. dendrolimi and assessed population-level risks via species sensitivity distribution (SSD). Acute toxicity assays using glass-vial residues revealed a 24 h LC50 of 0.12 mg a.i. L−1 for adults, three orders of magnitude below the maximum field rate (100 mg a.i. L−1). Sublethal exposure (1/2–1/100 LC50) significantly reduced parasitism and emergence rates (NOEC = 2.3 μg a.i. L−1) but did not affect offspring survival. Acetamiprid also shortened offspring development at 11.5–57.5 μg a.i. L−1. SSD analysis identified T. dendrolimi as the most sensitive parasitoid to acetamiprid (HC5/HC50) = 0.11/5.88 mg a.i. L−1), with field rates (30–100 mg a.i. L−1) indicating a potentially affected fraction (PAF) of 76.8–97.9%. These findings underscore the need to integrate sublethal effects into pesticide regulations to conserve parasitoid-mediated ecosystem services.
1. Introduction
Integrated pest management (IPM) strategies have evolved to emphasize the synergistic use of biological and chemical controls, aiming to maximize pest suppression while minimizing environmental impacts [1]. Chemical pesticides continue to play a pivotal role in pest management, yet their application often poses risks to beneficial organisms, such as parasitic natural enemies [2,3,4,5]. This is particularly true for neonicotinoid insecticides like acetamiprid, which are widely used in agriculture but have been implicated in disrupting the normal physiological functions of insects, including parasitoids. Parasitoid wasps of Trichogramma (Hymenoptera: Trichogrammatidae) are keystone biocontrol agents, with over 219 recognized species [6,7,8]. Their application in pest control has emerged as a cornerstone of integrated pest management (IPM) strategies across numerous countries [7,9,10,11,12]. In northeastern China, T. dendrolimi dominates corn ecosystems, comprising 45–97% of natural parasitoid communities [13], and is extensively used against Conogethes punctiferalis [14,15]. However, the efficacy of these beneficial insects is threatened by the widespread use of chemical pesticides, particularly neonicotinoids like acetamiprid, which can affect non-target organisms, including parasitoids [16,17,18].
Neonicotinoids, acting as nicotinic acetylcholine receptor (nAChR) antagonists, have been implicated in disrupting the normal physiological functions of insects, including parasitoids [19,20]. Prior studies have quantified the acute toxicity of neonicotinoids to various Trichogramma species [3,21], yet the sublethal impacts and population-level sensitivity variations remain understudied. Recent research has shed light on the compatibility of novel pesticides with parasitoids, demonstrating varying degrees of toxicity [22,23].
To fully understand the ecological risks associated with pesticide use, it is crucial to evaluate both the acute and sublethal effects on non-target organisms. Sublethal doses of pesticides can induce physiological and behavioral changes that may significantly impact parasitism rates, developmental periods, fecundity, and offspring emergence [24,25,26,27]. These sublethal effects are often overlooked in traditional toxicity assessments but can have profound implications for biological control services. Therefore, a comprehensive assessment of pesticide impacts on parasitoids is essential for developing effective and ecologically sound pest management strategies.
Advances in the assessment of pesticide impacts on ecosystems in China have been integrated into comprehensive prevention strategies, driven by a deeper understanding of chemical pesticide toxicity and the establishment of standardized protocols, such as China’s Guidelines on Environmental Safety Assessment for Chemical Pesticides (Standard No. HJ/T 88-2016) [28]. However, there remains a gap in identifying indicator species among parasitic natural enemies within these guidelines. Species sensitivity distributions (SSDs) have emerged as a valuable tool for estimating the sensitivity of aquatic organisms to stressors and have the potential to be applied more broadly in terrestrial ecological scenarios [29,30,31,32,33].
The primary objectives of the present study were as follows: (1) to evaluate the acute toxicity of acetamiprid residues to adult T. dendrolimi, including determining the lethal concentration (LC50) and associated toxicity, (2) to investigate the long-term sublethal effects (here defined as continuous exposure spanning at least one full generation or 10–14 days, covering critical life stages) of acetamiprid exposure on key biological traits of T. dendrolimi, including parasitism percentage, emergence percentage, offspring survival rate, development period of surviving female parasitoids and identify the no-observed-effect concentrations (NOEC) for acetamiprid in T. dendrolimi under sublethal exposure conditions, (3) to assess the sensitivity distribution of T. dendrolimi among parasitic natural enemies under acetamiprid stress using species sensitivity distribution (SSD) models, and (4) to compare the relative sensitivity of T. dendrolimi with other parasitoid species. This study provides critical insights into the ecological risks of acetamiprid to T. dendrolimi and its role as a potential bio-indicator for assessing pesticide impacts on parasitoid communities. The findings help to develop pest management strategies that are more effective and ecologically sound.
2. Materials and Methods
2.1. Insects
T. dendrolimi colonies were maintained at the Beijing Academy of Agricultural and Forestry Science (BAAFS). Corcyra cephalonica Stainton (Lepidoptera: Pyralidae) eggs were used as the substrate for colony rearing. Prior to use, the eggs were subjected to UV sterilization under a UV lamp emitting at 254 nm for 30 min to ensure no contamination. Subsequently, the disinfected eggs were glued onto cardboard strips (dimensions. 1.5 cm × 6 cm) using an adhesive agent. These eggs were then exposed to newly emerged (24–48 h old) T. dendrolimi females in glass tubes (dimensions: 9.5 height × 2.2 cm diameter, covered with black cloth) for a period of 24 h to ensure near-complete parasitism (approximately 100%).
After 24 h of exposure, the cardboard strips were transferred to new containers. A cotton thread soaked in a 10–15% honey solution was provided in the glass tubes to serve as a food source for the newly emerged wasps. All parasitoids were maintained collectively in controlled conditions (25 ± 1 °C, 60 ± 10% RH, and 14:10 photoperiod) in an incubator. Newly emerged females (24–48 h) were used for bioassays.
2.2. Pesticides
For this investigation, two insecticides were identified as active candidates: acetamiprid (97% purity, Qingdao Hailir Pesticides and Chemicals Group Co., Ltd., Qingdao, China) serving as the experimental agent and dimethoate (98% purity, Qingdao Hailir Pesticides and Chemicals Group Co., Ltd.) designated as the reference standard. Additionally, they represent distinct chemical classes, providing a comprehensive perspective on the behavior of different pesticide types. The active ingredients of the pesticides were used rather than their commercial formulations to eliminate potential interference from other components in the formulations. Due to their low solubility in water, the stock solutions were prepared by dissolving the pesticides in analytical-grade acetone before each experiment. Seven doses of acetamiprid, ranging from a low concentration to the highest dose (water, 0, 0.23, 1.15, 2.3, 11.5, 57.5 μg a.i. L−1) in a geometric series, were carefully measured and prepared in the stock solutions. These doses approximated the intended concentrations, ensuring accuracy.
2.3. Acute Contact Toxicity Testing
The experimental design was structured to assess the effects of pesticide residues on adult parasitoid wasps (T. dendrolimi) in a dose-dependent manner. Glass-vial residue assays were conducted with seven acetamiprid concentrations (geometric series) applied to a tube (height: 8.0 × 2.5 cm). Dimethoate was included solely as a positive control (validating assay sensitivity via high mortality at a fixed concentration. For solution application, 1 mL of each pesticide solution was uniformly applied to ensure complete coverage of the internal glass surface. A solvent control group used 1 mL of pure acetone, while a blank control group used the same of deionized water. The tubes were rotated with a pipe roller until no droplets remained on the glass wall and were left at room temperature for 1 h to ensure complete evaporation of acetone. For parasitoid exposure, 100 ± 10 adult parasitoids were placed in each tube, which contained a cotton wool plug soaked with a 10–15% honey solution. The tubes were covered with fine gauze to allow air circulation and maintained under controlled environmental conditions. Mortality assessment was conducted after 24 h of exposure, with the number of dead wasps counted based on the absence of movement. Three independent replicates were conducted for each concentration of each compound, and data were recorded and analyzed to calculate mortality rates. This experimental design ensured precise control of exposure conditions while maintaining consistency across treatments, providing robust data for assessing pesticide effects on adult parasitoids.
2.4. Effects of Sublethal Treatment of Pesticides on the Development of T. dendrolimi
The sublethal toxicity test was conducted using a modified version of the acute contact toxicity test protocol, with adjustments in experimental design and measurement endpoints to assess the sublethal effects of acetamiprid on the development and reproductive success of T. dendrolimi. The experiment utilized five sublethal application rates of acetamiprid, determined as fractions (1/2, 1/5, 1/10, 1/50, and 1/100) of the acute 24 h LC50 value obtained from a previously described acute contact toxicity test (method adapted from Yu et al., 2014 who tested imidacloprid) [34]. These fractions were chosen to encompass a broad range of sublethal effects, allowing for a comprehensive evaluation of the impact of acetamiprid on T. dendrolimi. Solvent and blank controls were included to ensure accurate baseline measurements. Each treatment group consisted of 50 ± 10 adult wasps, with five independent replicates per treatment. LC50 values were calculated using probit analysis with 95% confidence intervals. Data were presented following OECD Guideline 245 format. The NOEC was determined as the highest concentration demonstrating no statistically significant difference from controls in survival/reproduction endpoints. Adult wasps were exposed to the respective acetamiprid concentrations for one hour in glass tubes under controlled conditions (25 ± 1 °C, 70 ± 10% RH, 14:10 L:D photoperiod). After the exposure period, a cardstock strip (1 × 7 cm) containing 300 freshly laid, untreated host eggs (Corcyra cephalonica Stainton) was introduced into each glass tube to allow parasitism. The eggs were not irradiated or cold-stored before being used in the experiment.
Following a three-day exposure period, the parasitized eggs were transferred into clean, pesticide-free glass tubes. All experimental units were maintained in climate-controlled incubators under the same conditions as previously described. The number of successfully parasitized eggs was counted daily, and these eggs were deemed successfully parasitized if they exhibited a black and plump appearance. It is important to note that due to the potential for superparasitism in T. dendrolimi, where multiple wasps may parasitize the same egg, the number of parasitized eggs observed does not necessarily equal the number of emerging adult wasps. To account for this, the percentage of parasitized eggs that successfully developed into adult wasps was calculated based on the number of emerging adults observed in each replicate, rather than the initial count of parasitized eggs. Newly emerged adult wasps from each replicate (typically cohorts of 10–20 individuals) were collectively transferred into clean, chemical-free glass tubes (2.5 × 15 cm) containing fresh honey solution (10–15%). This group transfer approach maintained treatment-specific cohorts while minimizing handling stress, with all tubes replaced daily to ensure hygiene. Survival rates were monitored daily over two weeks. The duration from egg parasitism to adult emergence was recorded for each replicate.
2.5. Species Sensitivity Distribution
SSDs were established to evaluate the acute toxicity biological parameters of acetamiprid against T. dendrolimi and other eleven parasitoid wasps, including Peristenus spretus, Trichogramma ostriniae, Trichogramma confusum, Encarsia formosa, Ganaspis brasiliensis (Ihering), Ganaspis cf. Brasiliensis, Trichogramma nubilale, Trichogramma evanescens, Trichogramma japonicum, Cotesia flavipes, providing a comparative framework for assessing species sensitivity. The acute toxicity data were compiled from multiple sources, including the US-EPA ECOTOX database (https://cfpub.epa.gov/ecotox/search.cfm), peer-reviewed literature, and draft assessment reports of acetamiprid. Recently published studies [35,36,37] were also included to ensure the analysis captures the latest advancements. The analysis assumed a log-normal distribution of the toxicity data, which was verified using the Anderson–Darling test within the ETX software package, version 2.0 [38]. This statistical approach allowed for the 5th percentile (HC5) and 50th percentile (HC50), along with their confidence limits, following the methodology outlined by Aldenberg and Jaworska (2000) [39]. The significance level for the Anderson–Darling test was set at 5%. PAF values were derived from the SSD curves by interpolating the fraction of species expected to be affected at a given concentration. The SSD curves were constructed using the log-logistic distribution model, which provided the HC5 and HC50 values. PAF values at the field-recommended concentration range (30–100 mg a.i. L−1) were then calculated by determining the fraction of species with acute toxicity endpoints below these concentrations. This calculation allowed us to estimate the potential impact of acetamiprid on parasitoid communities under field conditions. This approach provided a robust framework for evaluating species sensitivity to acetamiprid, enabling the identification of critical thresholds for ecological risk assessment.
2.6. Statistical Analysis
The corrected mortality of T. dendrolimi was calculated using the Abbott (1925) [40] formula. The 24 h LC50 values and their 95% confidence intervals for the insecticide were determined through probit analysis using SPSS 21.0 (IBM Corp., New York, NY, USA). Data normality and homogeneity of variances were verified by Shapiro–Wilk test and Levene’s test, respectively. Sublethal effect data (parasitism, emergence rate, etc.) were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s HSD post hoc test for multiple comparisons (p < 0.05). All percentage data were arcsine square-root transformed prior to analysis to meet ANOVA assumptions. Results are presented as mean ± standard error (SE). The NOEC was calculated via Dunnett’s multiple comparisons test. Data from the sublethal effect experiments were analyzed using one-way analysis of variance (ANOVA) to assess statistical significance (p < 0.05). Significant differences between treatment groups were identified using the Tukey–Kramer honestly significant difference test at p < 0.05 level.
3. Results
3.1. Acute Contact Toxicity and Risk Assessment
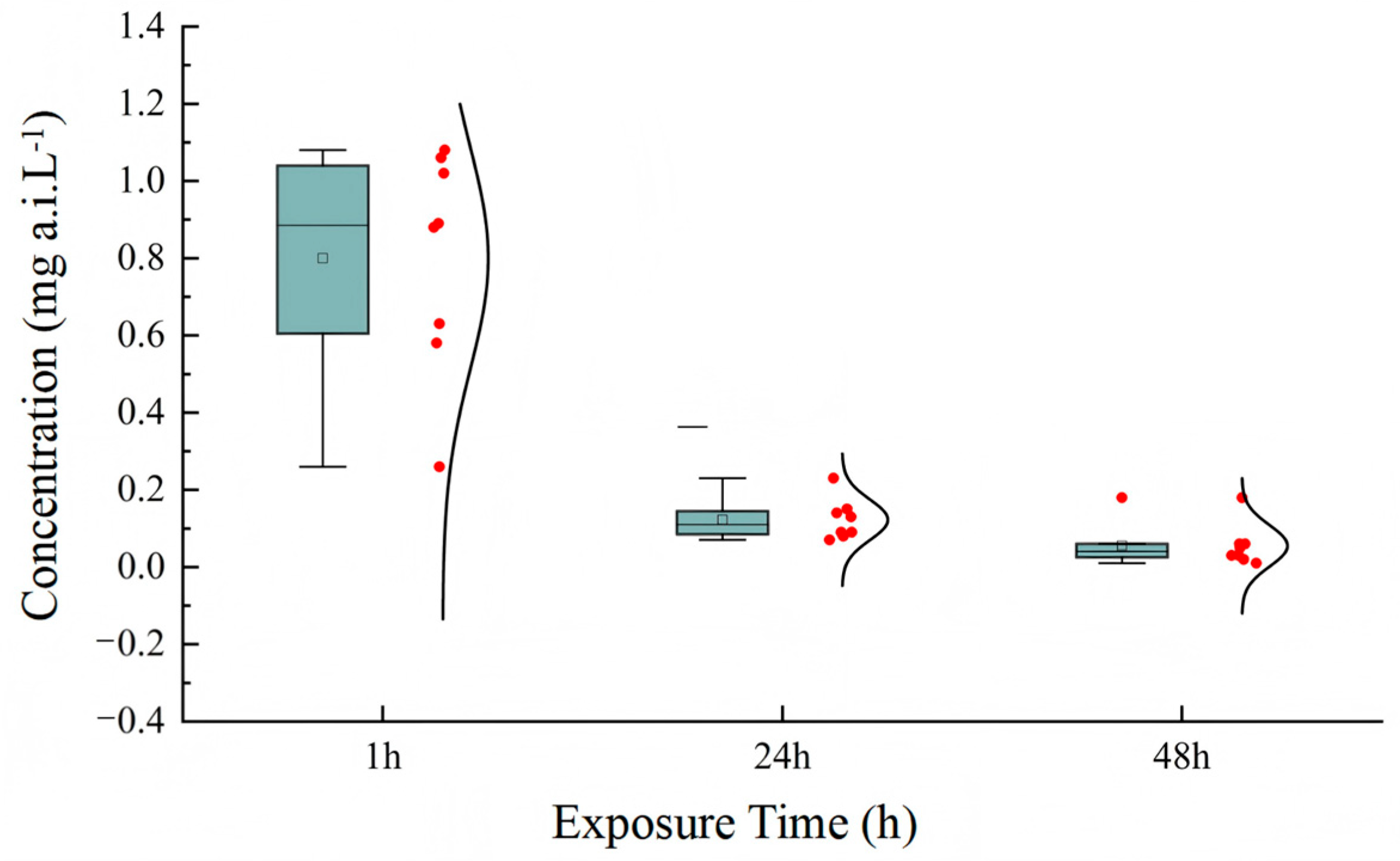
In the acute toxicity tests, LC50 values for acetamiprid in active ingredient were calculated separately for each of the eight tests performed. The results are presented as box plots in Figure 1. The complete statistical parameters for all toxicity tests (LC50 values with 95% CIs, slopes ± SE, and χ2 values) are provided in Supplementary Table S1. The mortality in both solvent and blank control did not exceed 15%. The LC50 values of acetamiprid for T. dendrolimi were 0.80, 0.12, and 0.06 mg a.i. L−1 after different exposure times. The results also indicate that the endpoint of toxicity decreased with the increase in exposure time. The risk assessment results showed that acetamiprid was slight to moderately toxic to T. dendrolimi.
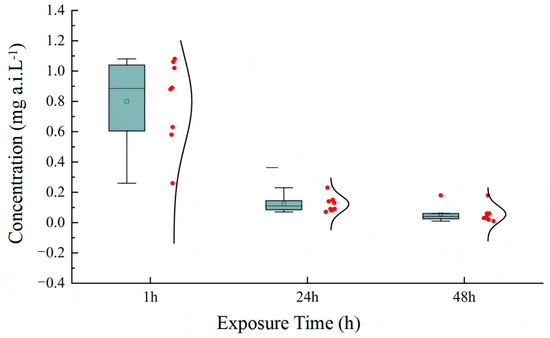
Figure 1.
Box plots representing the LC50 mean values for acetamiprid as calculated for T. dendrolimi in the tests with the active ingredient. Eight independent tests (each with 7 concentrations of acetamiprid) were performed to determine LC50 values. Box plots show LC50 values at three exposure times (24 h, 48 h, 72 h; n = 8 tests total).
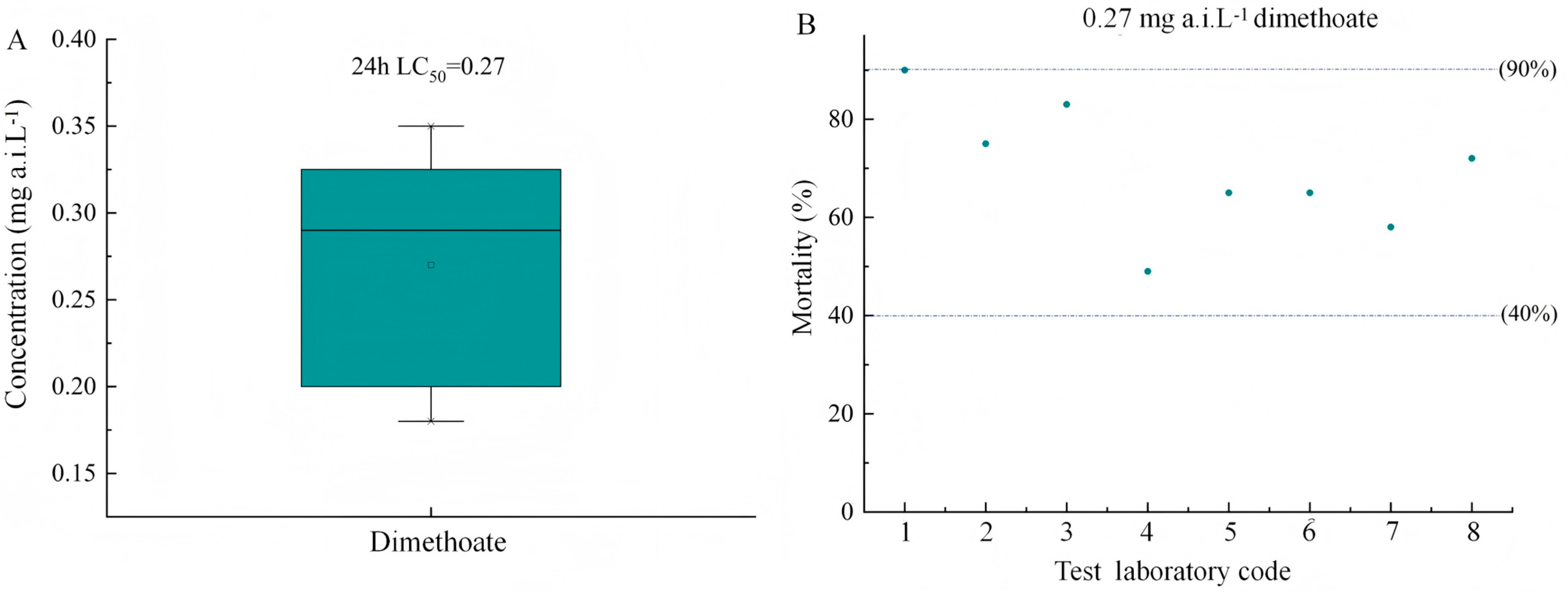
The dimethoate constantly produces 40–90% cumulative mean adult mortality at the concentration of 0.27 mg a.i. L−1 (Figure 2), establishing baseline sensitivity thresholds. Time-dependent assays were not conducted for dimethoate, as its role was to validate assay sensitivity at 24 h (standard duration for reference insecticides). The histogram data is based on 8 experiments with different T. dendrolimi strains. These results confirm methodological consistency with established organophosphate toxicity profiles for parasitoids.
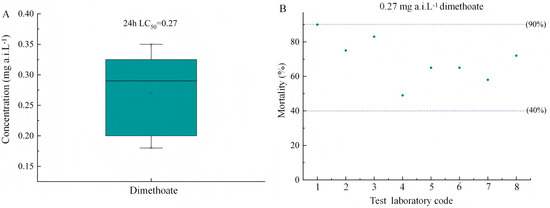
Figure 2.
The toxicity of reference item dimethoate to T. dendrolimi: (A) the 24 h LC50 mean values of dimethoate after the egg parasitoid was exposed to dimethoate; (B) mean mortality (±SE) of T. dendrolimi adults exposed to dimethoate at 0.27 mg a.i. L−1 (n = 8 replicates). The number on the x-axis refers to eight different toxicity tests conducted on Trichogramma dendrolimi using dimethoate as a reference insecticide.
3.2. The Effect of Acetamiprid on the Development of T. dendrolimi
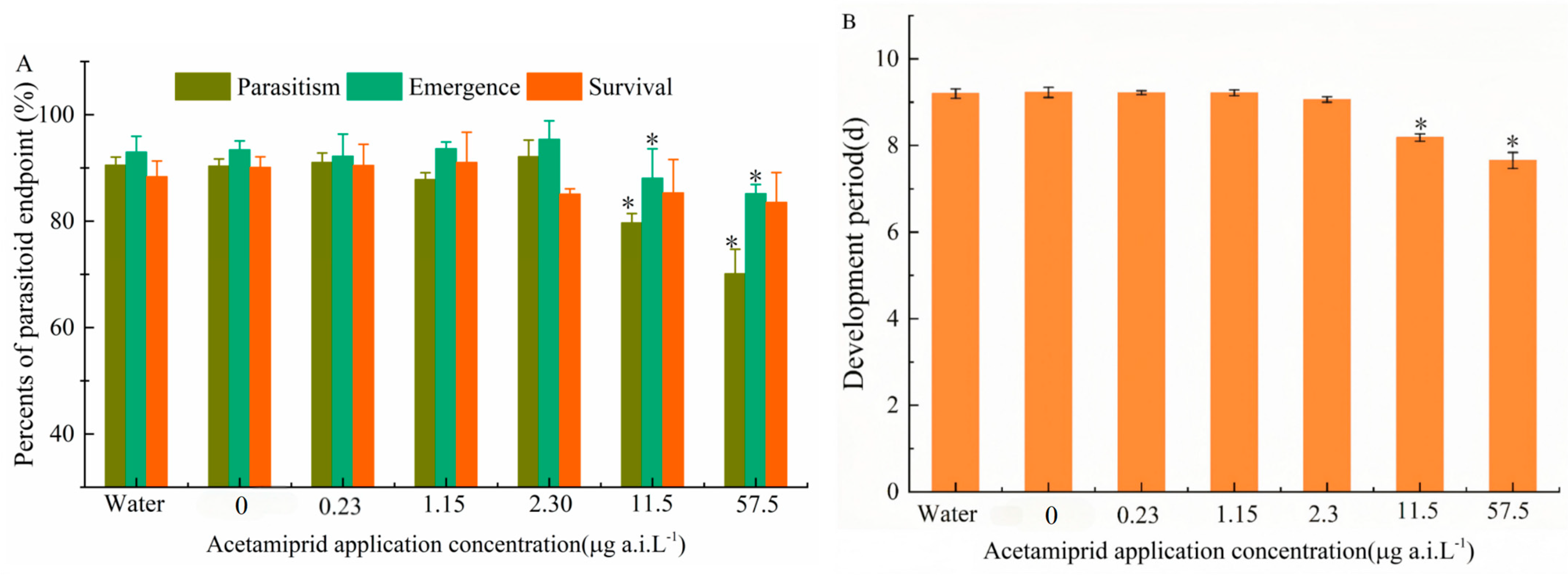
The sublethal effects of a single application of acetamiprid on parasitism, emergence rate, developmental period, and survival were evaluated. Significant differences were identified by Tukey’s HSD test (p < 0.05). No significant differences were observed between the solvent control and blank control groups. However, parasitism rate significantly decreased at 11.5 and 57.5 μg a.i. L−1 (F5,24 = 8.41; p < 0.001). Similarly, emergence rate showed similar trends (F5,24 = 7.92; p < 0.001) (Figure 3A). Additionally, the Developmental period was reduced at 57.5 μg a.i. L−1 (F5,24 = 5.63; p < 0.001) (Figure 3B). In contrast, none of the tested concentrations had a significant effect on survival. The lowest observed effect concentration (LOEC) for parasitism and emergence rate was determined to be 11.5 μg a.i. L−1, while the no-observed-effect concentration (NOEC) was established at 2.3 μg a.i. L−1.
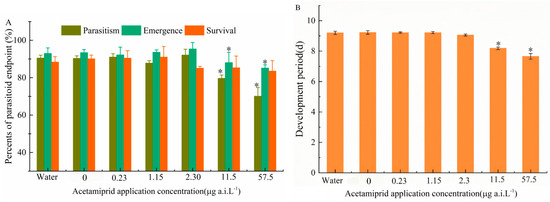
Figure 3.
(A) The effect of different acetamiprid concentrations on the parasitism rate, emergence rate, and next-generation survival rates; (B) the development period of T. dendrolimi. The asterisks ‘*’ indicate significant differences between the treatment and control samples at p < 0.05 (ANOVA, Tukey’s HSD).
3.3. Comparison of the Sensitivity to Acetamiprid Between T. dendrolimi and Other Parasitoid Wasps
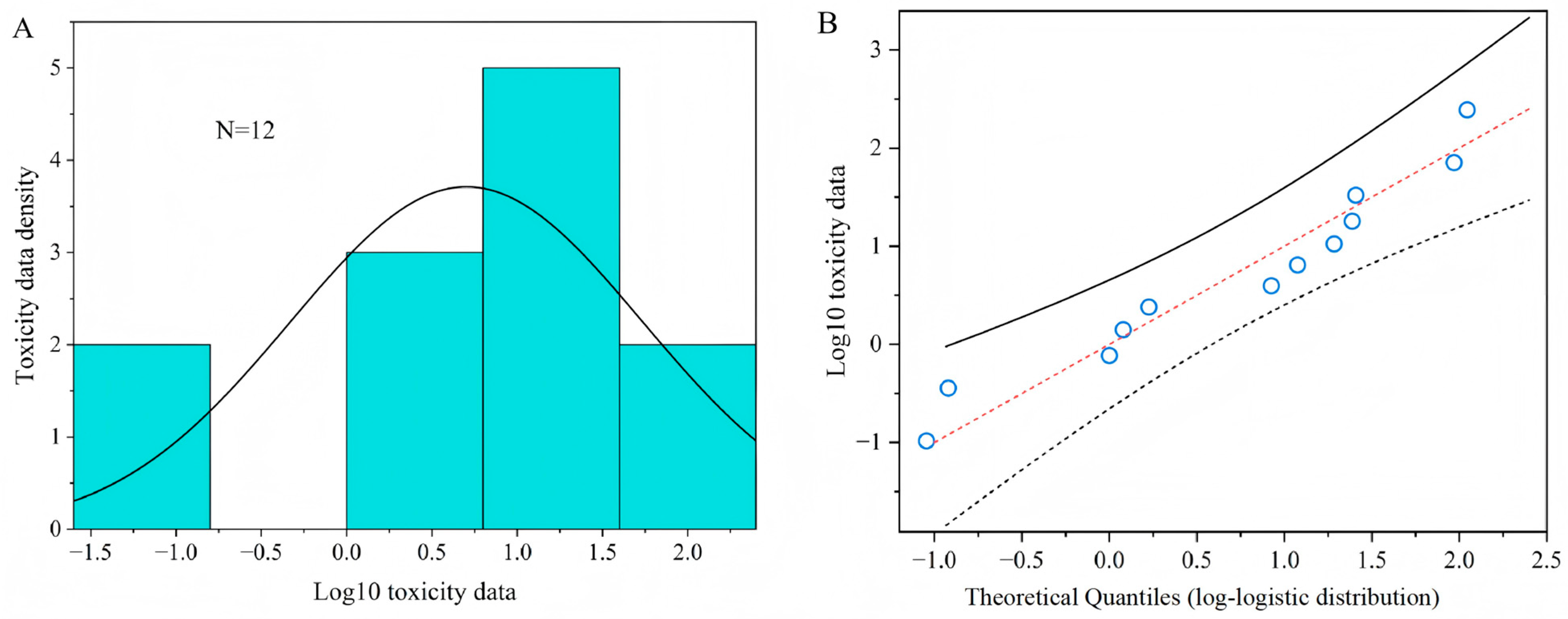
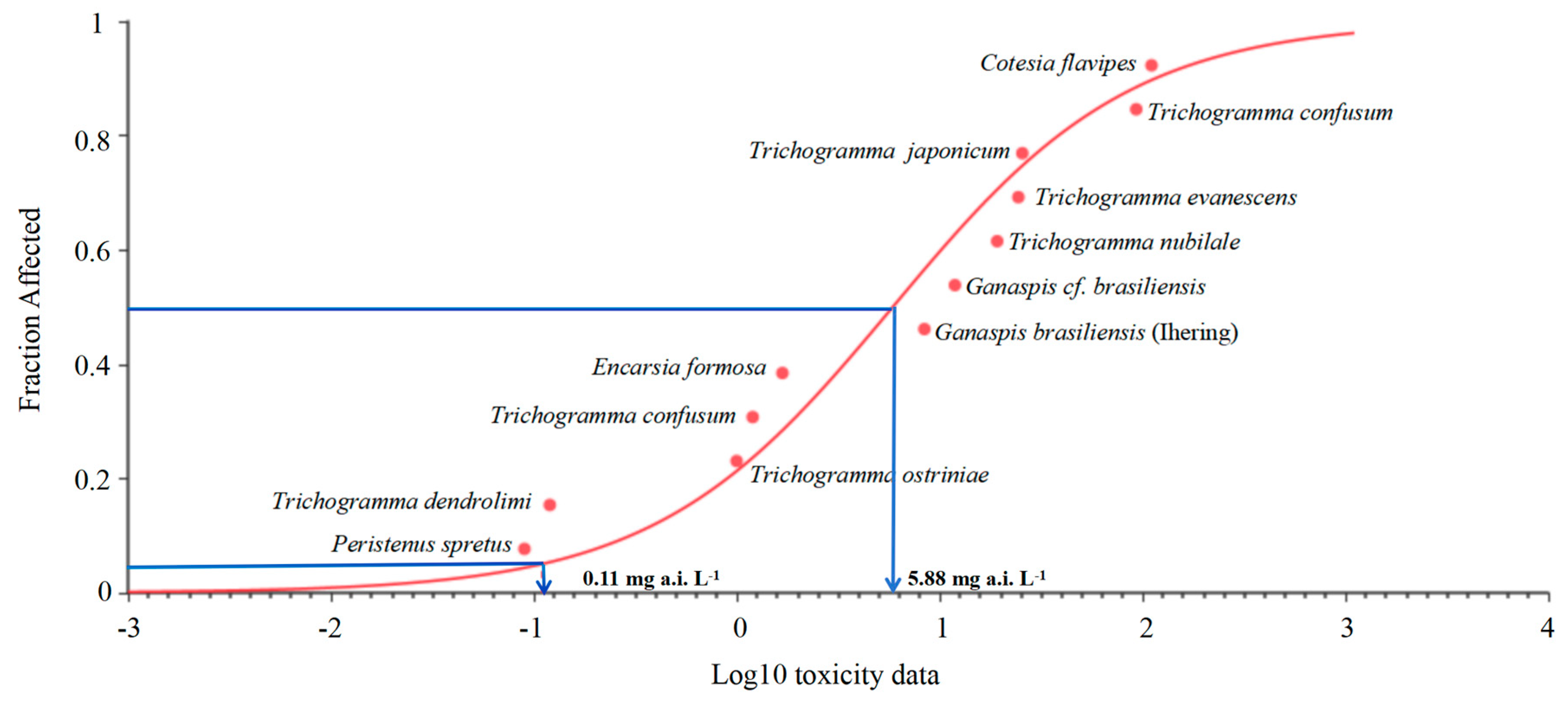
The HC5 and HC50 values (0.11 mg a.i. L−1 and 5.88 mg a.i. L−1 ) with their corresponding 95% confidence interval, obtained from a log-logistic distribution model based on the median lethal concentration (LC50) of twelve parasitoids to acetamiprid, are presented in Table 1. The goodness of fit of toxic data was accepted by the Kolmogorov–Smirnov test (p = 0.895), Cramer von Mises test (p = 0.126), and Anderson–Darling test (p = 0.752), at the 5% significance level for the SSD curves. Figure 4A shows the probability density diagram and histogram of acute toxicity data of acetamiprid to parasitoids after logarithmic transformation. The logarithmic value of the acute toxicity data of the acetamiprid presents a bell-shaped distribution, which accords with the logarithmic-logistic distribution model. Figure 4B is the Q-Q diagrams of logarithm values of acute toxicity data. It can also be seen intuitively from the diagrams that the logarithm values of acute toxicity data are all near the theoretical distribution straight line, indicating that they conform to log-logistic distribution. The results show that the log-logistic distribution model can well reflect the sensitivity distribution of parasitoids to acetamiprid.

Table 1.
HC5, HC50, and PAF values of acetamiprid to parasitic species.
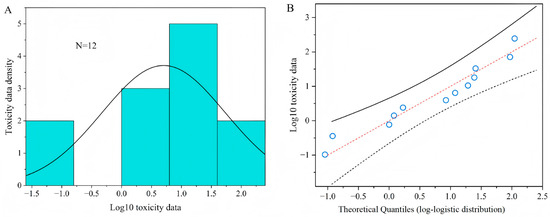
Figure 4.
Distribution and goodness of fit test of toxicity data. (A) A histogram and fitted density plot of toxicity data of acetamiprid; (B) a Q-Q plot of toxicity data versus fitted log-logistic distribution of acetamiprid.
In addition, according to the maximum exposure concentration (100 mg a.i. L−1), the potentially affected fraction (PAF) of species exposed to acetamiprid was 76.8%, which means that at this concentration, 76.8% of the parasitoids will be affected and the result indicated a certain risk to the ecosystem. By analyzing the SSD curves constructed for acetamiprid (Figure 5), the Peristenus spretus [41] appears to be among the most sensitive parasitic species for which toxicity data were available, followed by Trichogramma ostriniae [42], T. dendrolimi (this paper), Trichogramma confusum [42], Encarsia formosa [43], Ganaspis brasiliensis [36], Ganaspis cf. Brasiliensis [37], T. nubilale [44], T. evanescens [44], T. japonicum [45], T. ostriniae [46], T. confusum [47] and Cotesia flavipes [35].
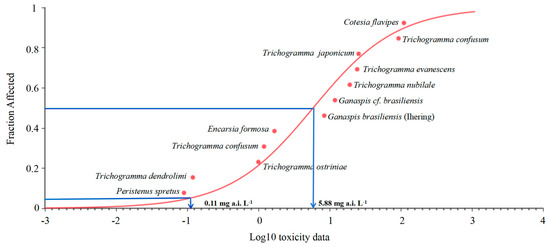
Figure 5.
Species sensitivity distribution (SSD) constructed based on LC50 values for acetamiprid obtained in this study for T. dendrolimi and from literature for other parasitoid.
4. Discussion
The documented toxicity of neonicotinoids to Trichogramma parasitoids has been well established through multiple studies, including acetamiprid’s effects on T. chilonis and T. brasiliensis [48] and thiamethoxam’s toxicity to T. platneri [49] and T. chilonis [50]. Our research significantly expands this understanding by conducting a systematic evaluation of both acute toxicity and sublethal impacts of acetamiprid on T. dendrolimi, a crucial biocontrol agent in northeastern China’s agricultural ecosystems. Of particular concern, the field-recommended concentrations (100 mg a.i. L−1) exceeded the LC50 by 830-fold, indicating high risk of population suppression. The no-observed-effect concentration (NOEC) concept, defined as the highest exposure level for which no (adverse) effects were observed can also be considered for risk assessment, especially about long-term studies [51]. The NOEC values of imidacloprid and hexaflumuron on the effects on reproduction and growth of Coccinella septempunctata were found to be 3.42 g a.i. ha−1 and 1.52 g a.i. ha−1, respectively [52]. The NOEC for Trichogramma brassicae exposed to thiamethoxam was 0.05 μg a.i. L−1 [53], while for T. evanescens, it was 1.2 μg a.i. L−1 [54].
Concentrations below HC5 could align with IPM if sublethal effects are minimized. Therefore, it is necessary to evaluate the compound’s sublethal effects of other species to find the NOEC values. Because there are few tests on NOEC in the database, we only used acute toxicity data for sensitivity analysis. In the present study, we evaluated the effects of the pesticide acetamiprid on T. dendrolimi using four toxicity biological parameters: parasitism percentage, emergence percentage, survival rate, and development period. Our 21-day chronic exposure study established a NOEC of 2.3 μg a.i. L−1 for both parasitism and emergence rates in T. dendrolimi, representing an important addition to the limited existing database on parasitoid NOECs. Sublethal exposure effects manifested primarily as reduced parasitism rates (≥2.3 μg a.i. L−1) and altered developmental dynamics (11.5–57.5 μg a.i. L−1), despite no adverse effects on survival rates within 14 days post-emergence. This pattern of sublethal impacts, including reduced reproductive capacity without affecting survival, has been documented across multiple parasitoid systems [55,56,57,58], suggesting common mechanisms of action. Sublethal effects on parasitoids include reduced fecundity in T. brassicae [52] and altered host-searching behavior in Aphidius ervi [59]. The observed reduction in parasitism and emergence rates without effects on developmental duration or offspring survival suggests specific sublethal mechanisms of action. Behavioral disruption appears predominant, with acetamiprid’s known neurotoxic effects on hymenopteran chemoreceptions [60], potentially impairing host location and oviposition activities. This aligns with findings in T. chilonis where neonicotinoids reduced foraging efficiency by 40–60% [48]. At the physiological level, acetamiprid’s action as a nicotinic acetylcholine receptor antagonist [19] may specifically affect adult nervous system functions required for parasitism, while sparing developmental processes in offspring. This explains the dichotomy between reduced parasitism rates but unaffected developmental duration, a pattern also observed in T. brassicae exposed to imidacloprid [61].
The ecological ramifications of these findings are profound. The SSD curves in Figure 5 allowed a comparison of the relative acute toxicity of T. dendrolimi to acetamiprid with other parasitic species reported in the open literature [36,37]. Notably, T. dendrolimi exhibited greater susceptibility to acetamiprid than six commonly deployed biocontrol agents: G. cf. brasiliensis, T. nubilale, T. evanescens, T. japonicum, T. ostriniae, and T. confusum. These interspecific comparisons employed standardized exposure protocols (equivalent routes and durations), ensuring methodological consistency and data reliability. This pronounced sensitivity gradient among parasitoid species carries significant implications for integrated pest management (IPM) systems where multiple wasp species provide complementary pest control services [62]. Quantitative risk assessment revealed alarming exposure scenarios: field-recommended concentrations (100 mg a.i. L−1), our SSD analysis predicts 76.8–97.9% population impacts, which could severely compromise biological control services. The HC5 value (0.11 mg a.i. L−1) was 48-fold higher than the NOEC (2.3 μg a.i. L−1), which underscores the importance of sublethal assessments, as standard acute toxicity tests may underestimate ecological risks [55]. A compound that kills 50% of the beneficial arthropods can be more acceptable than the one that decreases their fecundity and parasitism potential and makes the surviving individuals malformed. This challenges current risk assessment paradigms—while IOBC classifies insecticides with LC50 < 0.01 mg a.i. L−1 as ‘harmful’ [63], our SSD analysis suggests HC5 (0.11 mg a.i. L−1) may better predict field-level risk.
Formulation-dependent toxicity represents another critical consideration. Comparative studies demonstrate commercial acetamiprid formulations as exhibiting enhanced toxicity relative to pure compounds at equivalent active ingredient concentrations [64,65]. Commercial products exhibit higher acute toxicity to Hymenopteran species at equivalent active ingredient concentrations, with surfactant-enhanced bioavailability identified as a primary mechanism [64,65]. The adjuvants in these formulations induce prolonged sublethal behavioral alterations, including >48 h locomotor inhibition and 40–60% reduction in host-seeking efficiency [64]. These effects correlate with observations where inert ingredients in agricultural formulations amplify pharmacological impacts through improved cuticular penetration [64,65]. Field-realistic concentrations (1.25 g·L−1) significantly impair reproductive functions, with 25–35% egg-laying reduction persisting at 0.1× field doses [64,66]. The degradation dynamics of commercial formulations in soil systems further complicate risk assessments, as organic matter content modulates their persistence [65]. These formulation effects necessitate urgent revision of regulatory protocols that currently evaluate only pure compounds, systematically underestimating ecological risks [64,66].
A limitation of our study was that we only used an inertial substrate glass tube to establish the exposure–response relationships of acetamiprid on T. dendrolimi. Future studies should investigate the effects of acetamiprid under more realistic field conditions, considering factors such as sunlight, wind, and rain that may alter compound residues and toxicity. However, under the open-field condition, the compound residues are produced due to multiple degrading factors, such as sunlight, wind, and rain [67], and may not exert high toxicity on the parasitic wasps as described in this study. Therefore, further studies need to be conducted to identify the compounds that can be used safely under field scenarios without breaking the balance of the agroecosystem. In addition, in the sublethal toxicity test, the NOEC values have their drawbacks when used to evaluate the risk of pesticides. Because in actual conditions, the recommended maximum field application rates of pesticides will often be higher than the NOEC values. Evaluative indicators with more different values should be considered to assess the effects of the sublethal doses of pesticides. For example, 50% of the affected Trichogramma wasps are used to ensure the recovery of non-target arthropod populations. In addition, our sublethal tests focused solely on T. dendrolimi; future work should evaluate NOECs for other parasitoids.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects16070698/s1. Supplementary Table S1 provides complete virulence analysis data.
Author Contributions
Conceptualization, Y.Z., J.R. and S.C.; methodology, S.C.; formal analysis, S.C.; investigation, Y.Z., J.R. and S.C.; data curation, S.C.; writing—original draft preparation, Y.Z. and S.C.; writing—review and editing, Y.Z. and S.C.; supervision, Y.Z. and S.C.; funding acquisition, Y.Z. and S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from the National Natural Science Foundation of China (No. 32202341), and the Basic Research Program (Free Exploration Category) of Shanxi Provincial Department of Science and Technology (Grant No. 202403021222343).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The test samples used in this experiment were from the Beijing Academy of Agricultural and Forestry Science (BAAFS). We would like to thank all the reviewers who participated in the review.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jacquet, F.; Jeuffroy, M.-H.; Jouan, J.; Le Cadre, E.; Litrico, I.; Malausa, T.; Reboud, X.; Huyghe, C. Pesticide-free agriculture as a new paradigm for research. Agron. Sustain. Dev. 2022, 42, 8. [Google Scholar] [CrossRef]
- Papari, S.; Dousti, A.; Fallahzadeh, M.; Haddi, K.; Desneux, N.; Saghaei, N. Side effects of insecticides used for management of Tuta absoluta Meyrick (Lepidoptera: Gelechidae) on the biocontrol agent Trichogramma brassicae Bezdenko (Hymenoptera: Trichogrammatidae). CABI Agric. Biosci. 2024, 5, 101. [Google Scholar] [CrossRef]
- Cheng, S.; Lin, R.; Wang, L.; Qiu, Q.; Qu, M.; Ren, X.; Zong, F.; Jiang, H.; Yu, C. Comparative susceptibility of thirteen selected pesticides to three different insect egg parasitoid Trichogramma species. Ecotoxicol. Environ. Saf. 2018, 166, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Dourado, L.R.; Leite, G.L.D.; Lemes, P.G.; Tuffi Santos, L.D.; dos Santos, J.B.; Silva, L.F.; Teixeira, G.L.; Guanabens, R.E.M.; Zanuncio, J.C.; Soares, M.A. Nicosulfurons selectivity on Trichogrammatidae (Hymenoptera) in free-choice tests. Braz. J. Biol. 2023, 83, e267402. [Google Scholar] [CrossRef]
- Cordeiro, J.M.B.; Leite, G.L.D.; Santos, L.D.T.; Tavares, W.S.T.; Caldeira, Z.V.; Silva, R.S.; Santos, J.B.; Zanuncio, J.C.; Soares, M.A. Toxicity of herbicides on biological parameters of Trichogrammatidae. J. Plant Dis. Prot. 2025, 132, 4. [Google Scholar] [CrossRef]
- Milonas, P.G.; Partsinevelos, G.; Kapranas, A. Susceptibility of different developmental stages of Trichogramma parasitoids to insecticides commonly used in the Mediterranean olive agroecosystem. Bull. Entomol. Res. 2020, 111, 301–306. [Google Scholar] [CrossRef]
- Zang, L.S.; Wang, S.; Zhang, F.; Desneux, N. Biological Control with Trichogramma in China: History, Present Status and Perspectives. Annu. Rev. Entomol. 2021, 66, 463–484. [Google Scholar] [CrossRef]
- Cluever, J.D.; Beiermann, C.W.; Lawrence, N.C.; Bradshaw, J.D. Assessing the toxicity of selected pesticides to Trichogramma ostriniae (Hymenoptera: Trichogrammatidae) pupae as a first step in the development of a potential novel deployment programme. Biocontrol Sci. Technol. 2023, 33, 1065–1084. [Google Scholar] [CrossRef]
- Rakes, M.; Pasini, R.A.; Morais, M.C.; Araújo, M.B.; de Bastos Pazini, J.; Seidel, E.J.; Bernardi, D.; Grützmacher, A.D. Pesticide selectivity to the parasitoid Trichogramma pretiosum: A pattern 10-year database and its implications for integrated pest management. Ecotoxicol. Environ. Saf. 2021, 208, 111504. [Google Scholar] [CrossRef]
- Raven, C.; Nahrung, H.F. Trichogramma spp. as potential augmentative biocontrol agents of Poinciana looper, Pericyma cruegeri (Butler) (Lepidoptera: Noctuidae). Urban For. Urban Green. 2020, 50, 126656. [Google Scholar] [CrossRef]
- Kumaraswamy, S.; Sindhura, K.A.; Radhika, D.H.; Damodaram, K.J.P. Parasitoids as biocontrol agents in India. Curr. Opin. Insect Sci. 2024, 66, 101282. [Google Scholar] [CrossRef] [PubMed]
- Santana, E.D.R.; Thiesen, L.V.; Ribeiro, L.D.P.; Takahashi, T.A.; Parra, J.R.P.; Yamamoto, P.T. Harmful to Parents, Harmless to Offspring: Lethal and Transgenerational Effects of Botanical and Synthetic Insecticides on the Egg Parasitoid Trichogramma atopovirilia. Insects 2025, 16, 493. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; He, K.L.; Zhang, F.; Lu, X.; Babendreier, D. Mass rearing and release of Trichogramma for biological control of insect pests of corn in China. Biol. Control 2014, 68, 136–144. [Google Scholar] [CrossRef]
- Iqbal, A.; Chen, Y.M.; Hou, Y.Y.; Ruan, C.C.; Desneux, N.; Khan, M.Q. Rearing Trichogramma ostriniae on the factitious host Antheraea pernyi via multiparasitism with Trichogramma chilonis facilitates enhanced biocontrol potential against Ostrinia furnacalis. Biol. Control 2021, 156, 104556. [Google Scholar] [CrossRef]
- Babendreier, D.; Tang, R.; Horgan, F.G. Prospects for Integrating Augmentative and Conservation Biological Control of Leaffolders and Stemborers in Rice. Agronomy 2022, 12, 2958. [Google Scholar] [CrossRef]
- Pinto, J.D. A review of the New World genera of Trichogrammatidae (Hymenoptera). J. Hymenopt. Res. 2006, 15, 38–163. [Google Scholar]
- Preetha, G.; Stanley, J.; Suresh, S.; Samiyappan, R. Risk assessment of insecticides used in rice on miridbug, Cyrtorhinus lividipennis Reuter, the important predator of brown planthopper, Nilaparvata lugens (Stal.). Chemosphere 2010, 80, 498–503. [Google Scholar] [CrossRef]
- Li, X.; Zhu, J.H.; Ye, Z.P.; Chen, J.Y.; Fu, Y.G.; Zhang, F.P. The control efficiency of Scutellista caerulea Fonscolombe on Parasaissetia nigra Nietner. Acta Ecol. Sin. 2022, 42, 8483–8491. [Google Scholar]
- Matsuda, K.; Kanaoka, S.; Akamatsu, M.; Sattelle, D.B. Diverse actions and target-site selectivity of neonicotinoids: Structural insights. Mol. Pharmacol. 2009, 76, 1–10. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Pei, X.G.; Yu, Z.T.; Gao, Y.; Wang, L.X.; Zhang, N.; Song, X.; Wu, S.; Gao, C. Effects of nicotinic acetylcholine receptor subunit deletion mutants on insecticide susceptibility and fitness in Drosophila melanogaster. Pest Manag. Sci. 2022, 78, 3519–3527. [Google Scholar] [CrossRef]
- Chen, X.; Song, M.; Qi, S.; Wang, C. Safety evaluation of eleven insecticides to Trichogramma nubilale (Hymenoptera: Trichogrammatidae). J. Econ. Entomol. 2013, 106, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Ruberson, J.R. Lethal effects of selected novel pesticides on immature stages of Trichogramma pretiosum (Hymenoptera: Trichogrammatidae). Pest Manag. Sci. 2017, 73, 2465–2472. [Google Scholar] [CrossRef] [PubMed]
- Araújo, K.R.S.; Rodrigues, R.H.F.; Fernandes, A.K.M.; Almeida, V.D.L.; Godoy, M.S.D.; Pastori, P.L. Compatibility of mixtures of phytosanitary products recommended for melon and their selectivity for Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae). Rev. Ceres 2025, 72, 1–12. [Google Scholar] [CrossRef]
- Saber, M. Acute and population level toxicity of imidacloprid and fenpyroximate on an important egg parasitoid, Trichogramma cacoeciae (Hymenoptera: Trichogrammatidae). Ecotoxicology 2011, 20, 1476–1484. [Google Scholar] [CrossRef]
- Bengochea, P.; Amor, F.; Saelices, R.; Hernando, S.; Budia, F.; Adán, A.; Medina, P. Kaolin and copper-based products applications: Ecotoxicology on four natural enemies. Chemosphere 2013, 91, 1189–1195. [Google Scholar] [CrossRef]
- Poorjavad, N.; Goldansaz, S.H.; Dadpour, H.; Khajehali, J. Effect of Ferula assafoetida essential oil on some biological and behavioral traits of Trichogramma embryophagum and T. evanescens. Biocontrol 2014, 59, 403–413. [Google Scholar] [CrossRef]
- Luo, Z.Y.; Gao, L.P.; Li, W.J.; Chen, J.H.; Ali, M.Y.; Zhang, F.; Li, F.Q.; Wang, X.P.; Zhang, J.P. Assessing the lethal effects of pesticide residue exposure on beneficial parasitoids and their host, Halyomorpha halys (Stål) (Hemiptera: Pentatomidae). J. Econ. Entomol. 2025, 118, 242–252. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment China. Guidelines on Environmental Safety Assessment for Chemical Pesticides (HJ/T 88-2016); China Environment Science Press: Beijing, China, 2016.
- Rodrigues, A.C.; Jesus, F.T.; Fernandes, M.A.; Morgado, F.; Soares, A.M.; Abreu, S.N. Mercury toxicity to freshwater organisms: Extrapolation using species sensitivity distribution. Bull. Environ. Contam. Toxicol. 2013, 91, 191–196. [Google Scholar] [CrossRef]
- Wang, X.N.; Liu, Z.T.; Yan, Z.G.; Zhang, C.; Wang, W.L.; Zhou, J.L.; Pei, S.W. Development of aquatic life criteria for triclosan and comparison of the sensitivity between native and non-native species. J. Hazard. Mater. 2013, 260, 1017–1022. [Google Scholar] [CrossRef]
- Giddings, J.M.; Wirtz, J.; Campana, D.; Dobbs, M. Derivation of combined species sensitivity distributions for acute toxicity of pyrethroids to aquatic animals. Ecotoxicology 2019, 28, 242–250. [Google Scholar] [CrossRef]
- Thunnissen, N.W.; Lautz, L.S.; van Schaik, T.W.G.; Hendriks, A.J. Ecological risks of imidacloprid to aquatic species in the Netherlands: Measured and estimated concentrations compared to species sensitivity distributions. Chemosphere 2020, 254, 126604. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Pan, X.L.; Wu, X.H.; Xu, J.; Dong, F.S.; Zheng, Y.Q. Predicting and assessing the toxicity and ecological risk of seven widely used neonicotinoid insecticides and their aerobic transformation products to aquatic organisms. Sci. Total Environ. 2022, 84, 157670. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.H.; Fu, M.R.; Lin, R.H.; Zhang, Y.; Liu, Y.Q.; Jiang, H.; Brock, T.C.M. Toxic effects of hexaflumuron on the development of Coccinella septempunctata. Environ. Sci. Pollut. Res. 2014, 21, 1418–1424. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, Z.R.; Tariq, K.; Handler, A.M.; Ali, A.; Ullah, F.; Ali, F.; Zang, L.S.; Gulzar, A.; Ali, S. Toxicological risk assessment of some commonly used insecticides on Cotesia flavipes, a larval parasitoid of the spotted stem borer Chilo partellus. Ecotoxicology 2021, 30, 448–458. [Google Scholar] [CrossRef]
- Fellin, L.; Dal Zotto, G.; Lisi, F.; Chiesa, S.G.; Saddi, A.; Fusillo, M.; Anfora, G.; Biondi, A.; Mori, N.; Rossi Stacconi, M.V. Assessment of non-target toxicity of insecticides on Ganaspis brasiliensis (Ihering) in laboratory and field conditions. Pest Manag. Sci. 2024, 80, 5421–5431. [Google Scholar] [CrossRef]
- Lisi, F.; Cavallaro, C.; Fellin, L.; Gugliuzzo, A.; Desneux, N.; Anfora, G.; Rossi-Stacconi, M.V.; Biondi, A. Non-target effects of neurotoxic insecticides on Ganaspis cf. brasiliensis, a classical biological control agent of the spotted wing drosophila. CABI Agric. Biosci. 2024, 5, 48. [Google Scholar]
- Van Vlaardingen, P.; Traas, T.P.; Wintersen, A.M.; Aldenberg, T. ETX 2.0. A Program to Calculate Hazardous Concentrations and Fraction Affected, Based on Normally Distributed Toxicity Data (RIVM Report No. 601501028/2004); National Institute of Public Health and the Environment (RIVM): Bilthoven, The Netherlands, 2004. [Google Scholar]
- Aldenberg, T.; Jaworska, J.S. Uncertainty of the hazardous concentration and fraction affected for normal species sensitivity distributions. Ecotoxicol. Environ. Saf. 2000, 46, 1–18. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 8, 265–267. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Abid, B.; Ali, A.; Luo, S.P.; Lu, Y.H. Insecticide toxicity to Adelphocoris lineolatus (Hemiptera: Miridae) and its nymphal parasitoid Peristenus spretus (Hymenoptera: Braconidae). J. Econ. Entomol. 2015, 108, 1779–1785. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, X.; Huang, X.; Yu, X.; Zhang, W.; Zhang, X. Comparative ecotoxicity of neonicotinoid insecticides to three species of Trichogramma parasitoid wasps (Hymenoptera: Trichogrammatidae). Ecotoxicol. Environ. Saf. 2019, 183, 109587. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, P.; Yang, X.B.; Ruan, C.C.; Biondi, A. Selectivity of novel and traditional insecticides used for management of whiteflies on the parasitoid Encarsia formosa. Pest Manag. Sci. 2019, 75, 2716–2724. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, R.; Zhao, X.; Chen, L.; Wu, C.; Cang, T.; Wang, Q. Susceptibility of adult Trichogramma nubilale (Hymenoptera: Trichogrammatidae) to selected insecticides with different modes of action. Crop Prot. 2012, 34, 76–82. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, C.; Wang, Y.; Cang, T.; Chen, L.; Yu, R. Assessment of toxicity risk of insecticides used in rice ecosystem on Trichogramma japonicum, an egg parasitoid of rice lepidopterans. J. Econ. Entomol. 2012, 105, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, L.; Yu, R.; Zhao, X.; Wu, C.; Cang, T.; Wang, Q. Insecticide toxic effects on Trichogramma ostriniae (Hymenoptera: Trichogrammatidae). Pest Manag. Sci. 2012, 68, 1564–1571. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; An, X.; Jiang, J.; Wang, Q.; Cai, L.; Zhao, X. Susceptibility to selected insecticides and risk assessment in the insect egg parasitoid Trichogramma confusum (Hymenoptera: Trichogrammatidae). J. Econ. Entomol. 2013, 106, 142–149. [Google Scholar] [CrossRef]
- Shankarganesh, K.; Paul, B.; Gautam, R.D. Studies on ecological safety of insecticides to egg parasitoids, Trichogramma chilonis Ishii and Trichogramma brasiliensis (Ashmead). Natl. Acad. Sci. Lett. 2013, 36, 581–585. [Google Scholar] [CrossRef]
- Brunner, J.F.; Dunley, J.E.; Doerr, M.D.; Beers, E.H. Effect of pesticides on Colpoclypeus florus (Hymenoptera: Eulophidae) and Trichogramma platneri (Hymenoptera: Trichogrammatidae), parasitoids of leafrollers in Washington. J. Econ. Entomol. 2001, 94, 1075–1084. [Google Scholar] [CrossRef]
- Preetha, G.; Stanley, J.; Suresh, S.; Kuttalam, S.; Samiyappan, R. Toxicity of selected insecticides to Trichogramma chilonis: Assessing their safety in the rice ecosystem. Phytoparasitica 2009, 37, 209–215. [Google Scholar]
- Poquet, Y.; Bodin, L.; Tchamitchian, M.; Fusellier, M.; Giroud, B.; Lafay, F.; Buleté, A.; Tchamitchian, S.; Cousin, M.; Pélissier, M.; et al. A pragmatic approach to assess the exposure of the honey bee (Apis mellifera) when subjected to pesticide spray. PLoS ONE 2014, 9, e113728. [Google Scholar] [CrossRef]
- Yu, C.H.; Lin, R.H.; Fu, M.R.; Zhou, Y.M.; Zong, F.L.; Jiang, H.; Lv, N.; Piao, X.; Zhang, J.; Liu, Y.; et al. Impact of imidacloprid on life-cycle development of Coccinella septempunctata in laboratory microcosms. Ecotoxicol. Environ. Saf. 2014, 110, 168–173. [Google Scholar] [CrossRef]
- Parsaeyan, E.; Safavi, S.A.; Saber, M.; Poorjavad, N. Effects of emamectin benzoate and cypermethrin on the demography of Trichogramma brassicae Bezdenko. Crop Prot. 2018, 110, 269–274. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, C.; Cang, T.; Yang, L.; Yu, W.; Zhao, X.; Wang, Q.; Cai, L. Toxicity risk of insecticides to the insect egg parasitoid Trichogramma evanescens Westwood (Hymenoptera: Trichogrammatidae). Pest Manag. Sci. 2014, 70, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhao, J.; Zheng, Y.; Desneux, N.; Wu, K. Lethal effect of imidacloprid on the coccinellid predator Serangium japonicum and sublethal effects on predator voracity and on functional response to the whitefly Bemisia tabaci. Ecotoxicology 2012, 21, 1291–1300. [Google Scholar] [CrossRef]
- Gholamzadeh Chitgar, M.; Hajizadeh, J.; Ghadamyari, M.; Karimi-Malati, A.; Hoda, H. Sublethal effects of diazinon, fenitrothion and chlorpyrifos on the functional response of predatory bug, Andrallus spinidens Fabricius (Hemiptera: Pentatomidae) in the laboratory conditions. J. King Saud Univ. Sci. 2014, 26, 113–118. [Google Scholar] [CrossRef]
- Ajudia, D.; Siddhapara, M.; Chaudhari, L. Relative toxicity of various insecticides against egg parasitoid, Trichogramma chilonis Ishii under laboratory condition. Int. J. Trop. Insect Sci. 2025, 45, 375–382. [Google Scholar] [CrossRef]
- Desneux, N.; Pham-Delègue, M.H.; Kaiser, L. Effects of sub-lethal and lethal doses of lambda-cyhalothrin on oviposition experience and host-searching behaviour of a parasitic wasp, Aphidius ervi. Pest Manag. Sci. 2004, 60, 472–480. [Google Scholar] [CrossRef]
- Aliouane, Y.; El Hassani, A.K.; Gary, V.; Armengaud, C.; Lambin, M.; Gauthier, M. Subchronic exposure of honeybees to sublethal doses of pesticides: Effects on behavior. Environ. Toxicol. Chem. 2009, 28, 113–122. [Google Scholar] [CrossRef]
- Saber, M.; Hejazi, M.J.; Kamali, K.; Moharramipour, S. Lethal and sublethal effects of fenitrothion and deltamethrin residues on the egg parasitoid Trissolcus grandis (Hymenoptera: Scelionidae). J. Econ. Entomol. 2013, 98, 35–40. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Zhu, Y.C.; Ma, C.; Huang, Y.; Shen, J. Sublethal effects of spinetoram on the two-spotted spider mite, Tetranychus urticae (Acari: Tetranychidae). Pestic. Biochem. Physiol. 2020, 163, 94–101. [Google Scholar] [CrossRef]
- International Organization for Biological Control [IOBC]. Guidelines for pesticide selectivity. IOBC-WPRS Bull. 2023, 156, 1–12. [Google Scholar]
- Nagy, K.; Duca, R.C.; Lovas, S.; Creta, M.; Scheepers, P.T.J.; Godderis, L.; Ádám, B. Systematic review of comparative studies assessing the toxicity of pesticide active ingredients and their product formulations. Environ. Res. 2020, 181, 108926. [Google Scholar] [CrossRef] [PubMed]
- Potts, J.; Jones, D.L.; Macdonald, A.; Ma, Q.; Cross, P. Acetamiprid fate in a sandy loam with contrasting soil organic matter contents: A comparison of the degradation, sorption and leaching of commercial neonicotinoid formulations. Sci. Total Environ. 2022, 842, 156711. [Google Scholar] [CrossRef]
- Li, C.; Zhang, S.; Han, C.; Han, X.; Song, J.; Ju, J.; Zhu, H. Acetamiprid in Rizhao green tea: Residue dynamics, degradation pathways, and ecological risks via integrated experimental and computational approaches. J. Hazard. Mater. 2025, 495, 138786. [Google Scholar] [CrossRef]
- Hewa-Kapuge, S.; McDougall, S.; Hoffmann, A.A. Effects of methoxyfenozide, indoxacarb, and other insecticides on the beneficial egg parasitoid Trichogramma nr. brassicae (Hymenoptera: Trichogrammatidae) under laboratory and field conditions. J. Econ. Entomol. 2003, 96, 1083–1090. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).