Changes in the Geographic Distribution of the Diana Fritillary (Speyeria diana: Nymphalidae) under Forecasted Predictions of Climate Change
Abstract
1. Introduction
2. Methods
2.1. Study Species
2.2. Distributional Dataset
2.3. Species Distributional Modeling
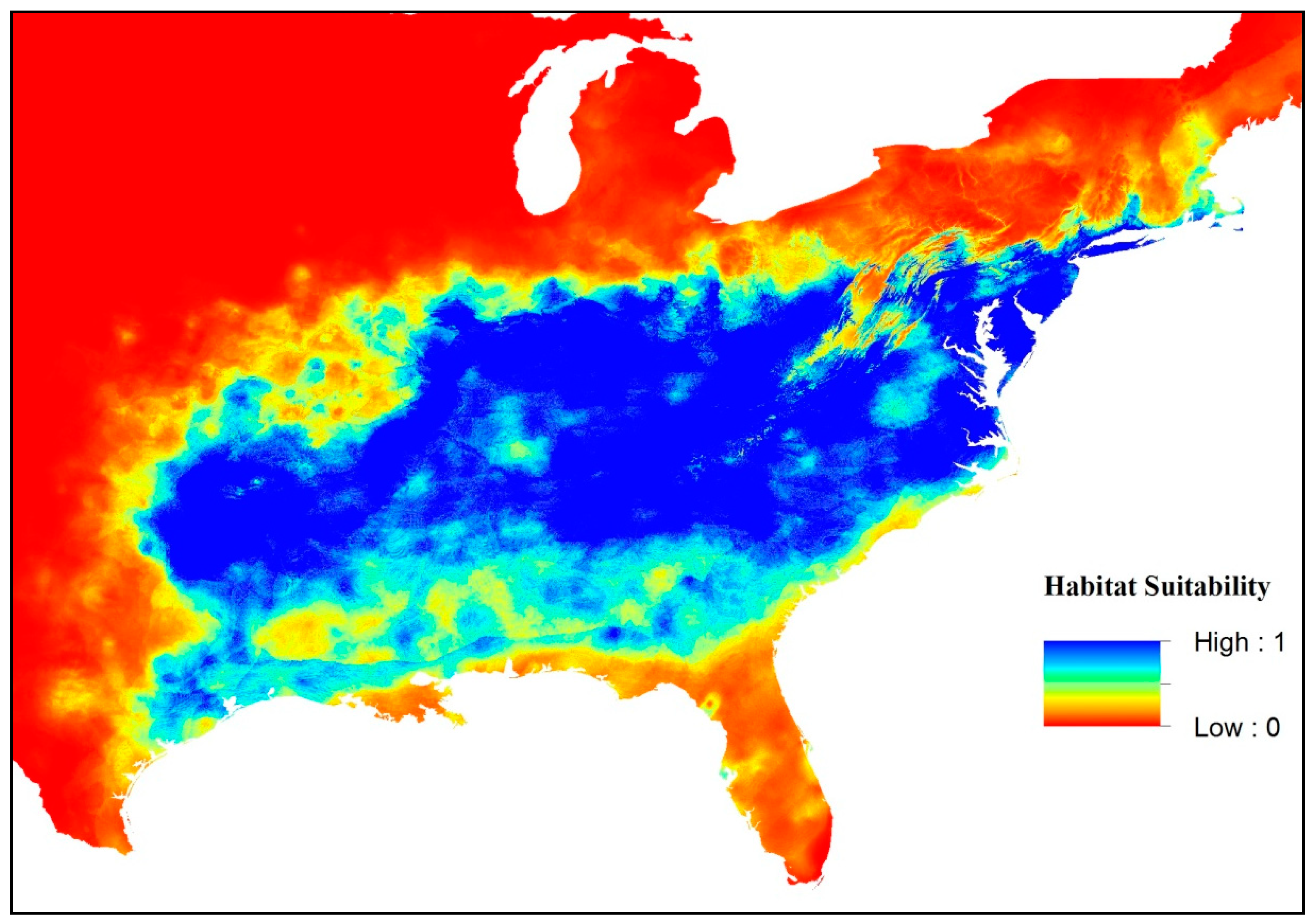
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Parmesan, C. Climate and species’ ranges. Nature 1996, 382, 765–766. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.D.; Franco, A.M.; Hill, J.K. Range retractions and extinction in the face of climate warming. Trends Ecol. Evol. 2004, 21, 415–416. [Google Scholar] [CrossRef] [PubMed]
- Crozier, L.; Dwyer, G. Combining population-dynamic and ecophysiological models to predict climate-induced insect range shifts. Am. Nat. 2006, 167, 853–866. [Google Scholar] [PubMed]
- Walther, G.R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.; Fromentin, J.M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological Responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosenzweig, C.; Pounds, J.A. Fingerprints of global warming on wild animals and plants. Nature 2003, 421, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Walther, G.R.; Beibner, S.; Conradin, A. Trends in the upward shift of alpine plants. J. Veg. Sci. 2005, 16, 541–548. [Google Scholar] [CrossRef]
- Cheung, W.W.L.; Lanm, W.Y.V.; Sarmiento, J.L.; Kearney, K.; Watson, R.; Pauly, D. Projecting global marine biodiversity impacts under climate change scenarios. Fish Fish. 2009, 10, 235–251. [Google Scholar] [CrossRef]
- Perry, A.L.; Low, P.J.; Ellis, J.R.; Reynolds, J.D. Climate change and distribution shifts in marine fishes. Science 2005, 308, 1912–1915. [Google Scholar] [CrossRef] [PubMed]
- Epstein, P.R.; Diaz, H.; Elias, F.S.; Grabherr, G.; Graham, N.E.; Martens, W.J.M.; Mosley-Thompson, E.; Susskind, E.J. Biological and physical signs of climate change: Focus on mosquito-borne disease. Bull. Am. Meteorol. Soc. 1998, 78, 409–417. [Google Scholar] [CrossRef]
- Thomas, C.D.; Lennon, J.J. Birds extend their ranges northwards. Nature 1999, 399, 213. [Google Scholar] [CrossRef]
- Hitch, A.T.; Leberg, P.L. Breeding distributions of North American bird species moving north as a result of climate change. Conserv. Biol. 2007, 21, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Parmesan, C.; Ryrholm, N.; Stefanescu, C.; Hill, J.; Thomas, C.; Descimon, H.; Huntley, B.; Kaila, L.; Kullberg, J.; Tammaru, T.; et al. Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature 1999, 399, 579–583. [Google Scholar] [CrossRef]
- Wilson, R.J.; Gutiérrez, D.; Gutiérrez, J.; Martinez, D.; Aguado, R.; Monserrat, V.J. Changes to the elevational limits and extent of species ranges associated with climate change. Ecol. Lett. 2005, 8, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.J.; Gutiérrez, D.; Gutiérrez, J.; Monserrat, V. An elevational shift in butterfly species richness and composition accompanying recent climate change. Glob. Chang. Biol. 2007, 13, 1873–1887. [Google Scholar] [CrossRef]
- Asher, J.; Fox, R.; Warren, M.S. British butterfly distributions and the 2010 target. J. Insect Conserv. 2011, 15, 291–299. [Google Scholar] [CrossRef]
- Wilson, R.J.; Maclean, I.M.D. Recent evidence for the climate threat to Lepidoptera and other insects. J. Insect Conserv. 2011, 15, 259–268. [Google Scholar] [CrossRef]
- Garcia, K.; Lasco, R.; Ines, A.; Lyon, B.; Pulhin, F. Predicting geographic distribution and habitat suitability due to climate change of selected threatened forest tree species in the Philippines. Appl. Geogr. 2013, 44, 12–22. [Google Scholar] [CrossRef]
- Pearson, R.G.; Dawson, T.P. Predicting the impacts of climate change on the distribution of species: Are bioclimate envelope models useful? Glob. Ecol. Biogeogr. 2003, 12, 361–371. [Google Scholar] [CrossRef]
- Peterson, A.T. Projected climate change effects on Rocky Mountain and Great Plain birds: Generalities on biodiversity consequences. Glob. Chang. Biol. 2003, 9, 647–655. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Fordham, D.A.; Resit, A.H.; Araújo, M.B.; Elith, J.; Keith, D.A.; Pearson, R.; Auld, T.D.; Mellin, C.; Morgan, J.W.; Regan, T.J.; et al. Plant extinction risk under climate change: Are forecast range shifts alone a good indicator of species vulnerability to global warming? Glob. Chang. Biol. 2012, 18, 1357–1371. [Google Scholar] [CrossRef]
- Thuiller, W.; Lavorel, S.; Araújo, M.B.; Sykes, M.T.; Prentice, I.C. Climate change threats to plant diversity in Europe. Proc. Natl. Acad. Sci. USA 2005, 102, 8245–8250. [Google Scholar] [CrossRef] [PubMed]
- Willis, K.J.; Araújo, M.B.; Bennett, K.D.; Figueroa-Range, B.; Froyd, C.A.; Myers, N. How can a knowledge of the past help to conserve the future? Biodiversity conservation and the relevance of long-term ecological studies. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2007, 362, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.J.; Anderson, R.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Weber, T.C. Maximum entropy modeling of mature hardwood forest distribution in four US states. For. Ecol. Manag. 2011, 261, 779–788. [Google Scholar] [CrossRef]
- Araújo, M.B.; Luoto, M. The importance of biotic interactions for modeling species distributions under climate change. Glob. Ecol. Biogeogr. 2007, 16, 743–753. [Google Scholar] [CrossRef]
- Araújo, M.B.; Pearson, R.G.; Thuiller, W.; Erhard, M. Validation of species-climate impact models under climate change. Glob. Chang. Biol. 2005, 11, 1504–1513. [Google Scholar] [CrossRef]
- Araújo, M.B.; Whittaker, R.J.; Ladle, R.J.; Erhard, M. Reducing uncertainty in projections of extinction risk from climate change. Glob. Ecol. Biogeogr. 2005, 14, 529–538. [Google Scholar] [CrossRef]
- Green, R.E.; Collingham, Y.C.; Willis, S.G.; Gregory, R.D.; Smith, K.W.; Huntley, B. Performance of climate envelope models in predicting recent changes in bird population size from observed climatic change. Biol. Lett. 2008, 4, 599–602. [Google Scholar] [CrossRef] [PubMed]
- Wells, C.N.; Tonkyn, D.W. Range collapse in the Diana fritillary, Speyeria diana (Nymphalidae). Insect Conserv. Divers. 2014, 7, 365–380. [Google Scholar] [CrossRef]
- Wells, C.N.; Marko, P.B.; Tonkyn, D.W. The phylogeographic history of the threatened Diana fritillary, Speyeria diana (Lepidoptera: Nymphalidae): With implications for conservation. Conserv. Genet. 2015, 16, 703–716. [Google Scholar] [CrossRef]
- Wells, C.N.; Munn, A.; Woodworth, C. Geomorphic Morphometric Differences between Populations of Speyeria diana (Lepidoptera: Nymphalidae). Fla. Entomol. 2018, 101, 195–202. [Google Scholar] [CrossRef]
- Opler, P.A.; Krizek, G. Butterflies East of the Great Plains; Johns Hopkins University Press: Baltimore, MD, USA, 1984; p. 294. ISBN 0801829380. [Google Scholar]
- Allen, T.J. The Butterflies of West Virginia and their Caterpillars; University of Pittsburgh Press: Pittsburgh, PA, USA, 1997; p. 388. ISBN 0822939738. [Google Scholar]
- Cech, R.; Tudor, G. Butterflies of the East Coast; Princeton University Press: Princeton, NJ, USA, 2005; p. 345. ISBN 069109055. [Google Scholar]
- Baltosser, W. Flitting with disaster: Humans and habitat are keys to our state butterfly’s future. Ark. Wildl. 2007, 38, 6–11. [Google Scholar]
- Ross, G.N. What’s for dinner? A new look at the role of phytochemicals in butterfly diets. News Lepidopterists’ Soc. 2003, 45, 83–89. [Google Scholar]
- Ross, G.N. Diana’s Mountain Retreat. Nat. Hist. 2008, 72, 24–28. [Google Scholar]
- Adams, J.K.; Finkelstein, I. Late season observations on female Diana fritillary (Speyeria diana) aggregating behavior. News Lepidopterists’ Soc. 2006, 48, 106–107. [Google Scholar]
- Araújo, M.B.; Peterson, A.T. Uses and misuses of bioclimatic envelope modeling. Ecology 2012, 93, 1527–1539. [Google Scholar] [CrossRef] [PubMed]
- Loiselle, B.A.; Jørgensen, P.M.; Consiglio, T.; Jiménez, I.; Blake, J.G.; Lohmann, L.G.; Montiel, O.M. Predicting species distributions from herbarium collections: Does climate bias in collection sampling influence model outcomes? J. Biogeogr. 2007, 35, 105–116. [Google Scholar] [CrossRef]
- Renner, I.W.; Warton, D.I. Equivalence of MAXENT and Poisson Point Process Models for Species Distribution Modeling in Ecology. Biometrics 2013, 69, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Gomes, V.H.F.; Jff, S.D.I.; Raes, N.; Amaral, I.L.; Salomão, R.P.; Coelho, L.d.; Matos, F.D.d.A.; Castilho, C.V.; Filho, D.d.L.; López, D.C.; et al. Species Distribution Modelling: Contrasting presence-only models with plot abundance data. Sci. Rep. 2018, 8, 1003. [Google Scholar] [CrossRef] [PubMed]
- Elith, J.; Graham, C.H. NCEAS Species Distribution Modelling Group, Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Elith, J.; Kearney, M.; Phillips, S. The art of modeling range-shifting species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M.; Elith, J.; Graham, C.H.; Lehmann, A.; Leathwick, J.; Ferrier, S. Sample selection bias and presence-only distribution models: Implications for background and pseudo-absence data. Ecol. Appl. 2009, 19, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, L.; Hughes, L.; Poulsen, M. Predicting species distributions: Use of climatic parameters in BIOCLIM and its impact on predictions of species’ current and future distributions. Ecol. Model. 2005, 186, 250–269. [Google Scholar] [CrossRef]
- Dennis, R.L.H. Butterflies and Climate Change; Manchester University Press: Manchester, UK, 1993. [Google Scholar]
- Hill, J.K.; Thomas, C.D.; Huntley, B. Modelling present and potential future ranges of European butterflies using climate response surfaces. In Butterflies: Ecology and Evolution Taking Flight; Boggs, C.L., Watt, W.B., Ehrlich, P.R., Eds.; University of Chicago Press: Chicago, IL, USA, 2003; pp. 149–167. [Google Scholar]
- Peterson, A.T.; Martínez-Meyer, E.; González-Salazar, C.; Hall, P.W. Modeled climate change effects on distributions of Canadian butterfly species. Can. J. Zool. 2004, 82, 851–858. [Google Scholar] [CrossRef]
- Mitikka, V.; Heikkinen, R.K.; Luoto, M.; Araújo, M.B.; Saarinen, K.; Pöyry, J.; Fronzek, S. Predicting range expansion of the map butterfly in Northern Europe using bioclimatic models. Biodivers. Conserv. 2008, 17, 623–641. [Google Scholar] [CrossRef]
- Filz, K.J.; Schmitt, T.; Engler, J.O. How fine is fine-scale? Questioning the use of fine-scale bioclimatic data in species distribution models used for forecasting abundance patterns in butterflies. Eur. J. Entomol. 2013, 110, 311–317. [Google Scholar] [CrossRef]
- Zinetti, F.; Dapporto, L.; Vovlas, A.; Chelazzi, G.; Bonelli, S.; Balletto, E.; Ciofi, C. When the rule becomes the exception: No evidence of gene flow between two Zerynthia cryptic butterflies suggests the emergence of a new model group. PLoS ONE 2013, 8, e65746. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.K.; Thomas, C.D.; Fox, R.; Telfer, M.G.; Willis, S.G.; Asher, J.; Huntley, B. Responses of butterflies to twentieth century climate warming: Implications for future ranges. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2002, 269, 2163–2171. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, B.; Van Dyck, H. Does habitat fragmentation affect temperature-related life-history traits? A laboratory test with a woodland butterfly. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2005, 272, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, M.; Van Dyck, H.; Karlsson, B. Reproductive plasticity, ovarian dynamics and maternal effects in response to temperature and flight in Pararge aegeria. J. Insect Physiol. 2010, 56, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Morecroft, M.D.; Bealey, C.E.; Howells, O.; Rennie, S.; Woiwod, I.P. Effects of drought on contrasting insect and plant species in the UK in the mid-1990s. Glob. Ecol. Biogeogr. 2002, 11, 7–22. [Google Scholar] [CrossRef]
- Kadmon, R.; Farbr, O.; Danin, A. Effect of roadside bias on the accuracy of predictive maps produced by bioclimatic models. Ecol. Appl. 2004, 14, 401–413. [Google Scholar] [CrossRef]
- Gent, P.R.; Danabasoglu, G.; Donner, L.J.; Holland, M.M.; Hunke, E.C.; Jayne, S.R.; Lawrence, D.M.; Neale, R.B.; Rasch, P.J.; Vertenstein, M.; et al. The community climate system model, Version 4. J. Clim. 2011, 24, 4973–4991. [Google Scholar] [CrossRef]
- Hasumi, H.; Emori, S. K-1 Coupled GCM (MIROC) Description; K-1 Tech. Rep. 1; Center for Climate Systems Research, University of Tokyo: Tokyo, Japan, 2004; p. 34. [Google Scholar]
- Nozawa, T.; Nagashima, T.; Shiogama, H.; Crooks, S.A. Detecting natural influence on surface air temperature change in the early twentieth century. Geophys. Res. Lett. 2005, 32, L20719. [Google Scholar] [CrossRef]
- Habel, J.C.; Rödder, D.; Scalercio, S.; Meyer, M.; Schmitt, T. Strong genetic cohesiveness between Italy and North Africa in four butterfly species. Biol. J. Linn. Soc. 2010, 99, 818–830. [Google Scholar] [CrossRef]
- Habel, J.C.; Husemann, M.; Schmitt, T.; Dapporto, L.; Vandewoestijne, S. A forest butterfly in Sahara desert oases: Isolation does not matter. J. Hered. 2013, 104, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Moss, R.H.; Edmonds, J.A.; Hibbard, K.A.; Manning, M.R.; Rose, S.K.; van Vuuren, D.P.; Carter, T.R.; Emori, S.; Kainuma, M.; Kram, T.; et al. The next generation of scenarios for climate change research and assessment. Nature 2010, 463, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Van Vuuren, D.P.; Edmonds, J.; Kainuma, M.; Riahi, K.; Thomson, A.; Hibbard, K.; Hurtt, G.C.; Kram, T.; Krey, V.; Lamarque, J.F.; et al. The representative concentration pathways: An overview. Clim. Chang. 2011, 109, 5–31. [Google Scholar] [CrossRef]
- Moss, R.H.; Babiker, M.; Brinkman, S.; Calvo, E.; Carter, T.; Edmonds, J.; Elgizouli, I.; Emori, S.; Erda, L.; Hibbard, K.; et al. Towards New Scenarios for Analysis of Emissions, Climate Change, Impacts, and Response Strategies; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2008; p. 132. [Google Scholar]
- Riahi, K.; Rao, S.; Krey, V.; Cho, C.; Chirkov, V.; Fischer, G.; Kindermann, G.; Nakicenovic, N.; Rafaj, P. RCP 8.5—A scenario of comparatively high greenhouse gas emissions. Clim. Chang. 2011, 109, 33–57. [Google Scholar] [CrossRef]
- Thomson, A.; Calvin, K.; Smith, S.; Kyle, P.; Volke, A.; Patel, P.; Delgado-Arias, S.; Bond-Lamberty, B.; Wise, M.; Clarke, L.; et al. RCP4.5: A pathway for stabilization of radiative forcing by 2100. Clim. Chang. 2011, 109, 77–94. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A. Insights into the area under the receiver operating characteristic curve (AUC) as a discrimination measure in species distribution modelling. Glob. Ecol. Biogeogr. 2012, 21, 498–507. [Google Scholar] [CrossRef]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Beaumont, L.J.; Hughes, L.; Pitman, A.J. Why is the choice of future climate scenarios for species distribution modelling important? Ecol. Lett. 2008, 11, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.C.; Hill, J.K.; Ohlemuller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- Fourcade, Y.; Besnard, A.G.; Secondi, J. Paintings predict the distribution of species, or the challenge of selecting environmental predictors and evaluation statistics. Glob. Ecol. Biogeogr. 2018, 27, 245–256. [Google Scholar] [CrossRef]

| National Museums (N. American) | Location | No. of S. diana | Range of Specimen Dates | No. of Counties |
|---|---|---|---|---|
| Carnegie Museum of Natural History | Pittsburgh, Pennsylvania | 142 | 1889–2000 | 26 |
| National Museum of Natural History | Washington, DC | 129 | 1907–2002 | 26 |
| American Museum of Natural History | New York, NY | 104 | 1921–1985 | 28 |
| The Field Museum | Chicago, IL | 98 | 1889–1995 | 23 |
| California Academy of Sciences | San Francisco, CA | 88 | 1886–2000 | 12 |
| Georgia Museum of Natural History | Athens, GA | 15 | 1935–1987 | 8 |
| Cleveland Museum of Natural History | Cleveland, Ohio | 6 | 1921–1965 | 6 |
| Denver Museum of Nature and Science | Denver, Colorado | 4 | 1939–1973 | 3 |
| Mount Magazine State Park | Paris, Arkansas | 4 | 1997 | 1 |
| National History Museums (European) | ||||
| British Natural History Museum | London, UK | 31 | 1777–1989 | 17 |
| Paris Muséum national d’Histoire naturelle | Paris, France | 8 | 1890 | 1 |
| Oxford Museum of Natural History | Oxford, UK | 4 | 1937–1971 | 4 |
| Zoölogisch Museum Amsterdam | Amsterdam, The Netherlands | 4 | 1884–1921 | 3 |
| Naturalis Biodiversity Center | Leiden, Netherlands | 4 | ||
| Royal Ontario Museum | Ontario, Canada | 3 | 1933–1968 | 3 |
| University Collections | ||||
| University of Florida | Gainesville, Florida | 409 | 1900–2007 | 43 |
| University of Michigan | East Lansing, Michigan | 66 | 1909–1985 | 13 |
| Clemson University | Clemson, South Carolina | 43 | 1926–1978 | 5 |
| Peabody, Yale University | New Haven, Connecticut | 29 | 1904–1961 | 8 |
| University of Missouri | Columbia, Missouri | 29 | 1886–1980 | 8 |
| University of Wyoming | Laramie, Wyoming | 13 | 1955–1979 | 4 |
| University of Arkansas, Little Rock | Little Rock, Arkansas | 12 | 2005–2007 | 5 |
| University of California, Berkley | Berkley, California | 12 | 1926–1981 | 6 |
| University of Nebraska | Lincoln, Nebraska | 14 | 1954–2003 | 7 |
| North Carolina State University | Raleigh, North Carolina | 10 | 1904–1964 | 9 |
| University of Arkansas, Fayetteville | Fayetteville, Arkansas | 10 | 1977–1994 | 5 |
| Virginia Polytechnic Inst | Blacksburg, Virginia | 8 | 1911–1977 | 1 |
| Louisiana State University | Baton Rouge, Louisiana | 7 | 1984–1988 | 1 |
| University of Wisconsin | Madison, WI | 5 | 1926–1951 | 2 |
| College of Charleston | Charleston, South Carolina | 4 | 2008 | 2 |
| West Virginia University | Morgontown, West Virginia | 3 | 1977–1995 | 2 |
| Furman University | Greenville, South Carolina | 3 | 1929–1990 | 3 |
| Dalton State College | Dalton, Georgia | 2 | 2001 | 1 |
| State Agencies, online databases, listserves, individuals, and organizations | ||||
| Field Surveys | 469 | 1995–2012 | 46 | |
| Butterflies and Moths of America (BAMONA) | 435 | 1938–2012 | 39 | |
| North Carolina 19th Approximation (http://149.168.1.196/nbnc/) | 276 | 1938–2011 | 31 | |
| West Virginia Divisions of Natural Resources (wvdnr.gov) | 204 | 1978–1999 | 11 | |
| Literature survey | 153 | 1818–2011 | 54 | |
| Kentucky Dept. of Fish and Wildlife Resources (fw.ky.gov) | 146 | 1936–2006 | 21 | |
| NABA annual count data (naba.org) | 103 | 1999–2010 | 27 | |
| Georgia Dept. of Natural Resources (gadnr.org) | 77 | 1994–2001 | 15 | |
| Global Biodiversity Information Facility (GBIF) | 75 | 1974–2004 | 49 | |
| North Carolina Natural Heritage Program (nchp.org) | 69 | 1989–2003 | 21 | |
| The Lepidopterists’ Society (lepsoc.org) | 50 | 1973–2008 | 25 | |
| All Taxa Biodiversity Inventory (ATBI) (dlia.org/atbi) | 46 | 1936–2007 | 4 | |
| Carolina Butterfly Society (CBS) | 44 | 2001–2009 | 5 | |
| Carolinaleps | 41 | 2007–2009 | 9 | |
| Washington Area Butterfly Club | 29 | 2007 | 1 | |
| Oklahoma Leps | 21 | 2005–2009 | 5 | |
| Insect.net | 21 | 2007–2009 | 9 | |
| Reference | Location | Date of Record(s) | Description |
|---|---|---|---|
| Cramer & Stoll 1775 | Jamestown, Virginia | 1775 | holotype; male described by Pieter Cramer |
| Blatchley 1859 | Vanderburgh County, Indiana | 1850s | first record from Indiana, most northern record |
| Edwards 1864 | Kanawha, West Virginia | 20–31 August 1864 | first description of female, took over 30 specimens |
| Edwards 1874 | Coalburgh, West Virginia | August, September 1873 | description of rearing Argynnis larvae |
| Aaron 1877 | Tennessee/North Carolina | 1877 | populations are ample along Blue Ridge |
| Kentucky | 1877 | locally abundant populations | |
| Strecker 1878 | 1878 | West Virginia, Georgia, Kentucky, Tennessee, Arkansas | |
| Thomas 1878 | Kentucky, Arkansas, southern Illinois | 1878 | common in Kentucky & Arkansas |
| Fisher 1881 | Illinois | 1880 | present in southern Illinois |
| Holland 1883 | Salem, North Carolina | 1858–1861 | described as “first pinned female specimen” |
| Edwards 1884 | southern Ohio | 1880s | first description in Ohio |
| Hulst 1885 | Waynesville, North Carolina | 1882 | locally abundant populations |
| Warren Springs, North Carolina | 1882 | very common along the French Broad River | |
| Blatchley 1886 | Evansville, Indiana | early 1900s | locally abundant populations |
| French 1886 | eastern United States | 1886 | W. Virginia to Georgia, Southern Ohio to Illinois, Kentucky, Tennessee, Arkansas |
| Hine 1887a, b | Medina County, Ohio | 9 August 1887 | single worn male, northernmost record in OH |
| Kingsley 1888 | Virginia | 1887 | Argynnis diana is described as the handsomest insect found in the United States |
| Scudder 1889 | southeast United States | 1880s | Semnopsyche diana; an inhabitant of hilly country of the south, 38th parallel of latitude, taken as far west as Missouri and “Arkansaw” |
| Skinner & Aaron 1889 | Pennsylvania | 1880s | stray individual found in Pennsylvania |
| Dixey 1890 | eastern United States | 1889 | description of Argynnis diana wing spot pattern |
| Blatchley 1891 | Illinois | 1890s | female specimen from northern Danville, IL |
| Skinner 1896 | southern Illinois | 1890s | Diana specimens from southern Illinois are larger than those further east |
| Holland 1898 | southern United States | 1890s | in two Virginias and Carolinas, northern Georgia, Tennessee, Kentucky, occasionally in southern Ohio and Indiana, and in Missouri and Arkansas; the most magnificent and splendid species of the genus |
| Snyder 1900 | Clay County, Illinois | 1900 | northern limit of S. diana in Illinois |
| Strecker 1900 | Missouri | 1853 | pair captured in copula, very early female |
| Maynard 1901 | habitat is West Virginia to Georgia, southern Ohio to Illinois, Tennessee, and Arkansas | ||
| Sell 1916 | Greene County, Missouri | 22 August 1900 | southeast of Springfield |
| Smyth 1916 | southeast United States | 1880–1916 | Asheville, Brevard, North Carolina, Caesar’s Head, South Carolina, Montgomery, Washington and Giles Counties, Virginia |
| Wood 1916 | Camp Craig, Virginia | August 1914 | describes female color variation |
| Murrill 1919 | Virginia | 1919 | Poverty Valley |
| Holland 1931 | 1930s | The Virginias and Carolinas, northern GA Tennessee, Kentucky, occasionally in southern OH, Indiana, and in Missouri and Arkansas | |
| Knobel 1931 | Hope, Arkansas | 1930 | from Mrs. Louise Knobel |
| Kite 1934 | Taney County, Missouri | 31 July 1925 | male and female reported |
| Clark 1937 | Virginia | 1930s | ranges from Bath County, Virginia to FL east almost to tidewater, and west to Illinois and Arkansas |
| Clark & Williams 1937 | Virginia | late 1800s–1935 | Bath, Alleghany, Giles, Bland, Dickenson, Smyth, Patrick, Montgomery & Washington Counties |
| Allen 1941 | West Virginia | 1940 | Pocahontas County, west to Kanawha and Lincoln Counties; abundant in Jefferson NF (Monroe County), Babcock State Park (Fayette County), and Fork Creek Wildlife Management Area (Boone County) |
| Chermock 1942 | Conestee Falls, North Carolina | summer 1941 | southern. Ohio and West Virginia, through the Appalachian mountains into Georgia and South Carolina, most abundant in mountains south of Great Smoky Mountains National Park |
| Bock 1949 | Cincinnati, Ohio | 1947 | author collects hundreds of specimens from North Carolina mountains; gone from Indiana and Ohio |
| Clark & Clark 1951 | Southern Illinois | early 1900s | |
| Chesterfield County, Virginia | 1930 | last known county record | |
| Northampton County, Virginia | 1930 | last known county record | |
| Klots 1951 | Brevard, North Carolina | 1950 | in large numbers along roadsides; Chiefly in mountains and piedmont, W. Virginia s. to Georgia, w. to southern Ohio, Indiana, Missouri, and Arkansas |
| Mather & Mather 1958 | Madison Parish, Louisiana | 1958 | record is a stray individual |
| Evans 1959 | Smoky Mountains of Tennessee | September 1957 | identification of an unknown S. diana larva |
| Curtis & Boscoe 1962 | Buncombe County, North Carolina | 27 June 1962 | collecting record near Asheville |
| Hovanitz 1963 | Salem, Roanoke County, Virginia | 13 June 1937 | comprehensive distribution data |
| Ross & Lambremont 1963 | Louisiana | 1950s | stray record from Mather & Mather 1958 |
| Masters 1968 | Newton County, Missouri | 1960s | locally very common |
| Masters & Masters 1969 | Perry County, Indiana | 15 July 1962 | last record known from Indiana |
| Shull & Badger 1971 | Indiana | 1971 | no longer resident in Indiana |
| Harris 1972 | Georgia | 1972 | summarizes historic reports from White, Union, Fannin, Habersham, Rabun Counties |
| Irwin & Downey 1973 | Vermilion County, Illinois | 20 August 1960 | female, last known Illinois record |
| Southern Illinois | 1880 | Illinois natural history survey | |
| Howe 1975 | 1950s | extirpated from type locality, Jamestown | |
| Kentucky, West Virginia | 1970s | species is scarce in Kentucky and West | |
| Virginia | |||
| Georgia | 1970s | not uncommon in northern Georgia | |
| Ceasar’s Head, South Carolina | 1970s | stable populations, not uncommon | |
| Nelson 1979 | Ozark plateau of Oklahoma | 1969 | only found in eastern counties |
| Schowalter & Drees 1980 | Poverty Hollow, Virginia | 1973, 1978 | field-captured and lab-reared S. diana gynandromorphs described in detail |
| Pyle 1981 | eastern United States | 1980s | has decreased its range because of forest loss, common in the Great Smoky Mountains |
| Hammond & McCorkle 1983 | Virginia & Tennessee | 1975–1978 | Appalachian populations are expanding |
| Opler 1983 | eastern United States | 1980s | some populations under decline |
| Opler & Krizek 1984 | 1950s | extirpated from Virginia Piedmont and coast | |
| 1800s | extirpated from Ohio River valley | ||
| Shuey et al. 1987 | Cincinnati, Ohio | 1900s–1930 | eliminated by deforestation by early 1900s |
| Shull 1987 | Indiana | late 1800s | occurs in mountains and piedmont of West Virginia south to Georgia, west to southern Ohio, Indiana, Missouri, and Arkansas |
| Watson & Hyatt 1988 | Tennessee | 1980s | resident species of northeastern Tennessee |
| Kohen 1989 | Cumberland, Kentucky | July 1984 | aberrant male on milkweed |
| Cohen & Cohen 1991 | Bath County, Virginia | 1990 | George Washington National Forest |
| Montgomery County, Virginia | 1990 | photograph of pair in copula | |
| Krizek 1991 | western Virginia | 11 July 1991 | males preferred nectar over horse manure |
| Adams 1992 | Fannin County, Georgia | 28 August 1992 | female netted by Irving Finkelstein |
| Opler & Malikul 1992 | eastern United States | 1992 | central Appalachians west to Ozarks, formerly Atlantic coastal plain of Va., NC, and Ohio River Valley, rich forested valleys |
| Skillman & Heppner 1992 | Coopers Creek WMA Georgia | 10 June 1988 | Gynandromorph specimen found in n. GA |
| Carlton & Nobles 1996 | Arkansas, Missouri, Oklahoma | 1819–1995 | survey of Interior Highlands |
| Allen 1997 | West Virginia | 1997 | ranges from Virginia and W. Virginia south to northern Georgia and Alabama. A small population persists in Ozark Mountains of Arkansas and Missouri |
| Ross 1997 | Coweeta Forest, North Carolina | 1990, 1996 | classified as uncommon, 2–5 individuals sighted |
| Ross 1998 | Mount Magazine, Arkansas | 30 June 1993 | photograph of male, locally abundant |
| Mount Magazine, Arkansas | 20 August 1992 | photograph of female, locally abundant | |
| Glassberg 1999 | eastern United States | 1999 | formerly throughout Ohio River Valley and southeastern Virginia and northwest N.C |
| Moran & Baldridge 2002 | Arkansas, Missouri, Oklahoma | 1997–1999 | 22 counties inhabited, Arkansas expanding |
| Scholtens 2004 | Oconee County, South Carolina | 2002 | present in Sumter National Forest |
| Cech & Tudor 2005 | 2000s | locally common in mountain colonies, s. W. Virginia to n. GA; also e. AL/KY, Ozarks | |
| Vaughan & Shepherd 2005 | Red List species profile | 2005 | core of species distribution is in the southern Appalachians from central Virgina and W. VA through the mountains to northern Georgia and Alabama. Also in Ozarks of Missouri, Arkansas, and eastern Oklahoma |
| Adams & Finkelstein 2006 | Fannin County, Georgia | 12 October 2006 | lots of aggregating females flying late |
| Rudolph et al., 2006 | Ouachita Mountains, Arkansas | 1999–2005 | feeding records by month sites |
| Spencer 2006 | Arkansas | 2006 | uncommon to locally common in colonies Scattered throughout the Interior Highlands Coastal Plain |
| Campbell et al., 2007 | North Carolina | 17 June 2004 | at least four males visiting flowering sourwood |
| Ross 2008 | Mount Magazine, Arkansas | 2008 | description of Mount Magazine State Park |
| Wells et al., 2010 | Mount Magazine, Arkansas | 2009 | copulating pair photographed |
| Wells et al., 2011 | Georgia, North Carolina, Tennessee | 2009 | females collected for rearing trial |
| State | County | Ecoregion | # S. diana (m/f) | Survey Dates |
|---|---|---|---|---|
| Arkansas | Benton | Ozark Plateau | 7 (7/1) | 12–14 June 2007, 22–23 June 2009 |
| Carroll | Ozark Plateau | 9 (7/2) | 15–16 June 2007, 23–24 June 2009 | |
| Boone | Ozark Plateau | 2 (2/0) | 16 June 2007 | |
| Faulkner | Arkansas River Valley | 5 (5/0) | 18–20 June 2006, 20 June 2007, 16 June 2008, 3–6 August 2009 | |
| Conway | Arkansas River Valley | 15 (11/4) | 22 June 2007, 26 June 2008, 5 August 2009 | |
| Pulaski | Arkansas River Valley | 4 (2/2) | 28 August 2009 | |
| Logan | Arkansas River Valley | 37 (29/8) | 20–24 June 2006, 21–24 June 2007, 1–3 August 2009 | |
| Montgomery | Ouachita Mountains | 12 (7/5) | 31 July 2008, 1–3 September 2009 | |
| Polk | Ouachita Mountains | 5 (1/4) | 1–3 September 2009 | |
| Saline | Ouachita Mountains | 8 (7/1) | 14 June 2008, 18 June 2009 | |
| Oklahoma | Leflore | Ouachita Mountains | 3 (0/3) | 30 August 2009 |
| Georgia | Fannin | Blue Ridge Mountains | 26 (17/9) | 12–13 July & 1 August 2006, 12 July 2007, 22 June & 20 July 2008 |
| Rabun | Blue Ridge Mountains | 8 (2/6) | 7 September 2008, 29 August 2009 | |
| Union | Blue Ridge Mountains | 14 (6/8) | 29 July 2007, 15 June & 5–7 August 2008, | |
| North Carolina | Ashe | Blue Ridge Mountains | 4 (4/0) | 22–23 June 2007 |
| Buncombe | Blue Ridge Mountains | 13 (8/5) | 27 July 2006, 30 July 2007, 9 August 2008 | |
| McDowell | Blue Ridge Mountains | 15 (10/5) | 9 September 2007, 24 June 2008, 30 June, 11 September 2009 | |
| Transylvania | Blue Ridge Mountains | 24 (19/5) | 5 June 2006, 16 July & 5 September 2007, 14 June 2008, 26 June 2009 | |
| Watauga | Blue Ridge Mountains | 7 (5/2) | 30 May & 9 June 2006, 25 July 2008, 19 September 2009 | |
| South Carolina | Greenville | Blue Ridge Escarpment | 12 (7/5) | 31 June 2006, 27–29 July 2007, 1 September 2008, 8–13 September 2009 |
| Tennessee | Blount | Great Smoky Mountains | 42 (33/9) | 1–26 June 2007, 1–28 June & 20–29 August 2008, 1–15 September 2009 |
| Sevier | Great Smoky Mountains | 33 (25/8) | 1–26 June 2007, 26–29 June 2008, 5 June-26 September 2009 | |
| Carter | Appalachian Mountains | 57 (35/22) | 5–9 June & 5–11 July 2006, 30–31 May 2007, 29–30 August 2008 | |
| Sullivan | Appalachian Mountains | 36 (25/11) | 13–16 July 2006, 20–22 July 2007, 5 August, 18–20 September 2009 | |
| Virginia | Montgomery | Appalachian Mountains | 21 (14/7) | 3–7 July 2007, 2–4 July 2008 |
| Bioclimate Variables | Abbreviation | % Contribution | |||||
|---|---|---|---|---|---|---|---|
| CCCM-45 | MIROC-45 | AVG | CCCM-85 | MIROC-85 | AVG | ||
| Annual Mean Temperature | Bio 1 | 4.4 | 0.7 | 2.5 | 0.5 | 1.4 | 0.96 |
| Max Temperature of Warmest Month | Bio 5 | 0.6 | 1.7 | 1.2 | 1.4 | 0.8 | 1.1 |
| Min Temperature of Coldest Month | Bio 6 | 3.9 | 36.3 | 20.1 | 2.6 | 3.3 | 10.4 |
| Mean Temperature of Wettest Quarter | Bio 8 | 14.1 | 10.2 | 12.2 | 4.0 | 16.8 | 2.6 |
| Mean Temperature of Driest Quarter | Bio 9 | 15.5 | 5.1 | 10.3 | 30.2 | 19.8 | 25.0 |
| Mean Temperature of Warmest Quarter | Bio 10 | 0.5 | 0.8 | 0.7 | 0.1 | 0.3 | 0.2 |
| Mean Temperature of Coldest Quarter | Bio 11 | 0.8 | 12.5 | 11.9 | 3.3 | 1.5 | 2.4 |
| Precipitation of Wettest Month | Bio 13 | 3.7 | 0.2 | 3.5 | 2.0 | 5.8 | 3.9 |
| Precipitation Seasonality | Bio 15 | 6.0 | 3.7 | 4.9 | 8.7 | 2.7 | 5.6 |
| Precipitation of Wettest Quarter | Bio 16 | 0.8 | 0.6 | 0.7 | 0.2 | 0.9 | 0.6 |
| Precipitation of Warmest Quarter | Bio 18 | 1.1 | 0.3 | 1.0 | 1.9 | 1.0 | 1.5 |
| Precipitation of Driest Month | Bio 14 | 0.9 | 1.6 | 1.4 | 2.7 | 8.0 | 5.4 |
| Precipitation of Driest Quarter | Bio 17 | 4.2 | 2.3 | 3.3 | 2.2 | 2.6 | 2.4 |
| Precipitation of Coldest Quarter | Bio 19 | 0.1 | 0.2 | 0.2 | 0.2 | 1.7 | 0.9 |
| Elevation | Elev | 2.0 | 1.0 | 1.5 | 4.9 | 2.0 | 3.5 |
| Isothermality (BIO 2/BIO 7) (*100) | Bio 3 | 11.0 | 3.5 | 7.3 | 8.5 | 6.6 | 7.6 |
| Temperature Seasonality (standard deviation *100) | Bio 4 | 6.4 | 1.0 | 3.7 | 0.0 | 4.2 | 2.1 |
| Mean Diurnal Range (Mean of monthly (max temp—min temp)) | Bio 2 | 0.6 | 3.0 | 1.8 | 2.0 | 3.6 | 2.8 |
| Temperature Annual Range (BIO 5–BIO 6) | Bio 7 | 1.2 | 1.9 | 1.6 | 1.5 | 1.0 | 1.3 |
| Annual Precipitation | Bio 12 | 22.3 | 13.4 | 17.9 | 22.9 | 15.9 | 19.4 |
| AUC | 0.86 | 0.96 | 0.91 | 0.87 | 0.86 | 0.87 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wells, C.N.; Tonkyn, D. Changes in the Geographic Distribution of the Diana Fritillary (Speyeria diana: Nymphalidae) under Forecasted Predictions of Climate Change. Insects 2018, 9, 94. https://doi.org/10.3390/insects9030094
Wells CN, Tonkyn D. Changes in the Geographic Distribution of the Diana Fritillary (Speyeria diana: Nymphalidae) under Forecasted Predictions of Climate Change. Insects. 2018; 9(3):94. https://doi.org/10.3390/insects9030094
Chicago/Turabian StyleWells, Carrie N., and David Tonkyn. 2018. "Changes in the Geographic Distribution of the Diana Fritillary (Speyeria diana: Nymphalidae) under Forecasted Predictions of Climate Change" Insects 9, no. 3: 94. https://doi.org/10.3390/insects9030094
APA StyleWells, C. N., & Tonkyn, D. (2018). Changes in the Geographic Distribution of the Diana Fritillary (Speyeria diana: Nymphalidae) under Forecasted Predictions of Climate Change. Insects, 9(3), 94. https://doi.org/10.3390/insects9030094




