3.1. Mycotretus tigrinus (Olivier, 1792)
Erotylus tigrinus Olivier 1792: 437 [
18]. Type locality: Suriname.
Mycotretus tigrinus (Olivier, 1792). Lacordaire 1842: 145 [
4] (new combination).
Mycotretus leopardus Crotch 1876 [
5]. Type locality: Peru. Crotch 1876: 451 (junior synonym).
Mycotretus multimaculatus Taschenberg 1870: 197 [
17]. Type locality: Colombia,
new synonym.
Mycotretus tigrinus pardalis Crotch 1876: 451 [
5] (as a variety). Type locality: Ecuador,
new synonym.
Diagnosis. Dorsal coloration with several circular and subcircular black spots, extremely variable in size and number and asymmetrically distributed. Penile flagellum slightly elongated (approximately 0.7 × the length of penis), slightly sinuous and with a membranous portion between its virga and head. Head of flagellum sclerotized and elongated, with an arcuate sclerotization posteriorly and an inflection at the basal half of the lateral edges. Inner contours slightly separated; anterior edge with outer sclerotization, more or less prominent and, sometimes, forming two outer, narrowed and lateral tips.
Redescription. Length (in mm) = 4.71–8.26 (6.87 ± 0.94,
n = 23). Body elongate, slightly oval, widest at the anterior third of elytra, TL/EW = 1.57–1.80 (1.70 ± 0.05), glabrous and glossy, dorsal and ventral coloration homogeneously yellowish or reddish-brown (
Figure 1A–L). Mouthparts with same background color as body, mandibles apex blackish and with two teeth; mentum plate pentagonal, with strongly sclerotized margin; antennae yellowish or reddish-brown, last antennomeres blackish. Scutellar shield yellowish, reddish-brown or blackish, glabrous and bearing few punctures. Dorsal coloration: head lacking or, usually, with one to four asymmetrical subcircular black spots (
Figure 1C,I, arrow); pronotum with several circular and subcircular black spots (
Figure 1A,C–L), extremely variable in size and number (usually more than ten), and asymmetrically distributed in all examined specimens (except for the specimen from Río Toro, Peru with more symmetrical pronotal spots). Elytral coloration similar to that of pronotum, with several circular and, usually, free and sparsely distributed spots (
Figure 1A,C–L).
Male terminalia. (
Figure 2A–F). Penis (
Figure 2A, pen) slightly elongate and curved; basal portion with a short sclerotized projection linked to apophyses; internal sac with well-developed and slightly elongated flagellum (
Figure 2A, fla), 0.7 × the length of penis (
n = 3), with slight sinuosity and a membranous portion between the virga and the head of the flagellum (
Figure 2B–D, mp), head of flagellum (
Figure 2B–D) sclerotized and elongated, with arcuate sclerotization posteriorly (
Figure 2B–D, black arrow) and inflection at the basal half of the lateral edges. Inner contours slightly separated; anterior edge with outer sclerotization, more or less prominent and, in some individuals, forming two outer, narrowed and lateral tips (
Figure 2C, red arrows). Apophyses (
Figure 2A, apo) 1.12 × as long as penis (
n = 2). Tegmen sclerotized (
Figure 2E); parameres reduced and sclerotized, with densely pubescent outgrowths, slightly dilated, narrowed and acute at apex (
Figure 2E, arrow). Tergite VIII, sclerotized with sparsely distributed bristles and sternite VIII slightly sclerotized. Laterotergite IX sclerotized, posteriorly elongated and pubescent, outer contours angulated (
Figure 2F, terg IX); anteroventral edge with paired and subparallel lateral struts, connected at its anterior tip by small transverse, slightly sclerotized sclerite (
Figure 2F, arrow). Posterior edge of sternite IX, sclerotized, undivided, outer contour rounded; anteriorly membranous. Tergite X, sclerotized, anterior edge with sparsely distributed bristles.
Female terminalia. (
Figure 2G–I). Gonostyli and gonocoxites strongly sclerotized (
Figure 2G, black and red arrows, respectively, under sternite VIII), baculi of paraprocts sclerotized and sinuous (
Figure 2H, arrows); spermatheca oval and sclerotized (
Figure 2I). Tergite VIII sclerotized and sternite VIII with a conspicuous median strut (
Figure 2G, sternite VIII, big black arrow).
Distribution. Northern to southern Neotropical region (
Figure 3).
Remarks. (1) As occurs for other Olivier primary types [
15,
19], the repository of the type of
M. tigrinus is unknown. We identified this species based on the original description, early redescriptions [
1,
20], a series of specimens from several museums, and images of two “topotypes” from the RBINS (
Figure 1D, dorsal view of one specimen). (2) Alvarenga [
2] stated that the repository of the type of
M. multimaculatus was unknown. However, according to Horn et al. [
21], Taschenberg specimens would be housed in the ZNS. The specimens are indeed in the ZNS and images were sent to us by the curator (
Figure 1H, lectotype). The Taschenberg specimen clearly shows the same color pattern of the other
M. tigrinus examined by us and we synonymize it with
M. tigrinus. (3) Another examined specimen from Peru, the “type of
M. leopardus”, is kept in the UMZC. The authorship of
M. leopardus had been attributed to Gorham [
1] by previous authors [
2,
8,
22], although Gorham attributed it to Kirsch (see Gorham [
1]). However, we verified that the first author to provide a diagnosis for
M. leopardus (and “in litteris” by Kirsh) was Crotch [
5], who also published the new name as a synonym of
M. tigrinus. In this case, according to the International Code of Zoological Nomenclature (ICZN, Article 50.7, p. 53 [
23]), the authorship of names first published as junior synonyms is attributed to “the person who published it as a synonym, even if some other originator is cited, and is not the person who subsequently adopted it as a valid name”. It is no coincidence that the type specimen of
M. leopardus is in UMZC, where the remaining Crotch types are kept. Therefore, here we attribute the name
M. leopardus to Crotch [
5] and confirm the synonymy of
M. leopardus with
M. tigrinus proposed by him. (4) We have not located the type specimen of
M. tigrinus pardalis in the UMZC, or in other European museums visited or consulted by us, and there is a great chance that the type is lost. Based on the original description and historical material examined, we conclude that
M. tigrinus pardalis, originally described as a variety, is merely an intraspecific variation of
M. tigrinus. (5) The type of
M. conspersus was also not located, but based on the historical material examined, especially on an old specimen from MNHN identified as
M. conspersus (see Material examined below), aside from the comments provided by Lacordaire [
4], we confirm the synonymy of
M. conspersus with
M. tigrinus. Material examined. Lectotype of M. multimaculatus Taschenberg, 1870, here designated (ZNS) “multimaculatus, Zeitschr. 1870. Colomb. Wallis [green label, handwritten, box label] \ LECTOTYPE Mycotretus multimaculatus Taschenberg, 1870 det. I. Pecci-Maddalena 2017 [red label, printed]”; 1 specimen (ZNS) “multimaculatus, Zeitschr. 1870. Colomb. Wallis [green label, handwritten, box label]”; 1 specimen (UMZC) “Cayen [green label, handwritten] \ TYPE [printed, crossed out] tigrinus, coll Reiche [handwritten]”; 1 specimen (UMZC) “So Pau [handwritten, São Paulo?]”; 2 specimens (UMZC) “Chevr. [printed]”; 2 specimens (UMZC) “green label \ Bates [printed]”; lectotype of M. leopardus Crotch, 1876, here designated (UMZC) “TYPE [blue label, printed] \ TYPE [printed] leopardus Peru [handwritten]”; 1 male (BMNH, dissected) “Ega [handwritten], 57, 125 [handwritten on label back] \ M. tigrinus Oliv. [handwritten]”; 1 specimen (BMNH) “Santarem [front] 53, 92 [back] [handwritten, disc-shaped label]”; 1 specimen (BMNH) “Demarara [handwritten, Demerara?] \ 1419 [printed] \ tigrinus Ol., 1419. S Am [handwritten]”; 1 specimen (BMNH) “W Burnett, Brasil [? handwritten] \ Ent. Club. 44-12. [printed]”; 1 specimen (BMNH) “Rio Grande, 84 a 8 [handwritten]”; 1 specimen (BMNH) “Rio Grande, 86-9. [handwritten]” 1 specimen (BMNH) “Cayenne [front] 58, 74 [back] [handwritten]”; 1 specimen (BMNH) “Para [handwritten, disc-shaped label] \ Mycotretus tigrinus Ol [handwritten] \ Pascoe Coll. 93–60 [printed]”; 1 specimen (BMNH) “Buenavista, BOLIVIA, II-IV, 1925 [printed] \ Provincia, d’SARA, 1700 ft. [printed] \ ex coll. F. Mason. B.M.1926–296. [printed]”; 1 specimen (MRSN) “latreille [handwritten]”; 1 specimen (MRSN) “Lacordaire [handwritten]”; 1 specimen (MRSN) “Brasilia Truqui [printed]”; 1 specimen (SDEI) “Chaucham [? Chauchamayo? handwritten] \ Schenkling det. [printed]”; 1 specimen (RBINS) “Mycotretus tigrinus, Surinam Oliv [handwritten] \ Coll. R. I. Sc. N. B., Surinam, Coll. Chapuis [printed]”; 1 specimen (RBINS) “det …., MYCOTRETUS [printed] tigrinus Ol. [handwritten] \ Coll. R. I. Sc. N. B., Surinam, Coll. Chapuis [printed]”; 1 specimen (MNHN) “4125, 33T [? disc-shaped label]”; 1 specimen (MNHN) “prov. De Sta Catherin, bords dela mer, 1820 [handwritten, disc-shaped label] \ Micotretus conspersus Ger [handwritten] \ tigrinus [handwritten]”; 1 specimen (MNHN) “7151, 34. [?, handwritten, disc-shaped label] \ 2192 [?, handwritten]”; 1 specimen (MNHN) “6546, 34. [handwritten, disc-shaped label] \ 1947 [?, handwritten]”; 1 specimen (MNHN) “tigrinus, Cayenne [handwritten]”; 1 specimen (MZSP) “III. 1930 [handwritten], Goyaz [printed], Viannopolis, Coll R Stitr [handwritten] \ Mycotretus tigrinus Ol. [handwritten]”; 1 male (MNRJ, dissected) “Coleção M. Alvarenga [printed] \ Peru Rio Toro [printed] \ Mycotretus multimaculatus Tasch., 1870 [handwritten] M. Alvarenga det. 1971 [printed]”; 1 female (DZUP, dissected) “Coleção M. Alvarenga [printed] \ Buenavista, Bolivia, iii/viii. 1950 [handwritten] \ DZUP 371251 [printed]”; 1 male (DZUP, dissected) “Coleção M. Alvarenga [printed] \ Buenavista, Bolivia, iii/viii. 1950 [handwritten] \ DZUP 371252 [printed]”; 1 male (DZUP, dissected) “Coleção M. Alvarenga [printed] \ Chapare 400 m, Bolivia [printed], viii. 1954 [handwritten], R. Zischka [printed] \ DZUP 371231”; 1 female (DZUP, dissected) “Coleção M. Alvarenga [printed] \ Chapare 400 m, Bolivia [printed], viii. 1954 [handwritten], R. Zischka [printed] \ DZUP 371232”; 1 male (DZUP, dissected) “Coleção M. Alvarenga [printed] \ Chapare 400 m, Bolivia [printed], viii. 1954 [handwritten], R. Zischka [printed] \ DZUP 371237”; 1 female (DZUP, dissected) “Coleção M. Alvarenga [printed] \ Jacaré P.N. Xingu, M. Grosso Brasil, XI-1961, Alvarenga e Werner [printed] \ DZUP 371247 [printed]”; 1 male (DZUP, dissected) “Coleção M. Alvarenga [printed] \ Parque Sooretama, LINHARES E. Santo, Brasil X-1962, M. Alvarenga leg. [printed] \ DZUP 371259 [printed]”; 1 female (DZUP, dissected) “Coleção M. Alvarenga [printed] \ Buenavista, Bolivia, 1956, A. Martinez [handwritten] \ DZUP 371254 [printed]”; 1 female (DZUP, dissected) “Coleção M. Alvarenga [printed] \ Jacaré P.N. Xingu, M. Grosso Brasil, XI-1961, Alvarenga e Werner [printed] \ DZUP 371246 [printed]”; 1 male (DZUP, dissected) “Coleção M. Alvarenga [printed] \ Parque Sooretama, LINHARES E. Santo, Brasil X-1962, M. Alvarenga leg. [printed] \ DZUP 371258 [printed]”; 1 male (DZUP, dissected) “Coleção M. Alvarenga [printed] \ COLEÇÃO CAMPOS SEABRA [printed] \ Mycotretus tigrinus Ol. [handwritten] J. Guerin det. 19[printed]53 [handwritten] \ CORUPA, Santa Catarina BRASIL [printed] X-1951 [handwritten] ANTON MALLER [printed] \ DZUP 371286 [printed]”; 1 female (DZUP, dissected) “Coleção M. Alvarenga [printed] \ COLEÇÃO CAMPOS SEABRA [printed] \ Mycotretus tigrinus Ol. [handwritten] J. Guerin det. 19[printed]53 [handwritten] \ CORUPA, S. Catarina BRASIL [printed] I [handwritten] A. MALLER [printed] \ DZUP 371285 [printed]”; 1 female (DZUP, dissected) “II. [handwritten] 196[printed]5[handwritten], Brasilien, Nova Teutonia, 27°11′B, 52°23′L, Fritz Plaumann, 300.500 m [printed] \ DZUP 125458 [printed]”; 1 female (CELC) “Viçosa/MG/Brasil, 21/06/99, M.D. MOREIRA [printed] \ Erotylidae [printed]”; 1 male (MCNZ, dissected) “Nova Hamburgo, RS, 28/VII/1986 [handwritten], C.J. Becker leg. [printed] \ Col. MCN 238428 [printed]”; 1 female (MCNZ, dissected) “Viamão, RS, Beco do Pesqueiro, AR-Mata Ripária, 30°09′S, 50°58′W, 31.V.2000, A. Bonaldo col. [printed] \ Col. MCN. [printed] 217998 [handwritten]”; 1 female (MCNZ, dissected) “S.F.de Paula, RS (B. dos Bugres), 14.XII.1999, Franceschini, Bonaldo & Silva [printed] \ Col. MCN [printed] 168.713 [handwritten] \ M. tigrinus [handwritten]”; 1 female (MCNZ, dissected) “Tapes, RS (Faz. São Miguel), 17.XII.2003, Equipe Probio col. [printed] \ Col. MCN 225615 [printed]”; 1 female (MCNZ, dissected) “Col. São Pedro de Alcantara, RS, 08/XI/1977 [handwritten], R. Balesteim [? handwritten] \ Col. MCN 238427 [printed]”; 1 female (DZUP, dissected) “Brasil–Paraná, Reserva, 23/III/2007 [printed] \ DZUP 469238 [printed]”; 1 male (MCNZ, dissected) “S.F.de Paula, RS (B. dos Bugres), 14.XII.1999, Franceschini, Bonaldo & Silva [printed] \ Col. MCN [printed] 168.714 [handwritten]”.
Doubtful identification. 1 female (MCNZ, dissected, pronotum not in good conditions) “Tapes, RS (Faz. São Miguel), 14. V.2003, R.S.de Araujo col. [printed] \ Col. MCN 221798 [printed]”.
3.2. Mycotretus centralis Arrow, 1909
Mycotretus centralis Arrow 1909: 196 [
9]. Type-locality: San Jerónimo, Guatemala.
Diagnosis. Pronotum with black, free and subcircular spots, symmetrically and transversely arranged. Penile flagellum well-developed and slightly elongated, approximately 0.95 × the length of the penis, with a shallow sinuosity and without a membranous portion between the virga and head; in dorsal view, flagellum medially enlarged and slightly sclerotized and, posteriorly, at flagellum head and virga connection, strongly sclerotized. Head of flagellum sclerotized, U-shaped, each branch ending in a blunt and narrowed tip, or forming a shallow, small and narrow denticle at the outer apical edge.
Redescription. Length (in mm) = 5.07–6.89 (6.13 ± 0.60,
n = 9). Body elongate, slightly oval, widest at anterior third of elytra, TL/EW = 1.60–1.77 (1.69 ± 0.06), glabrous and glossy, dorsal and ventral coloration homogeneously yellowish or reddish-brown (
Figure 4A–L). Mouthparts of same background color as body, mandible apices blackish and with two teeth; mentum plate pentagonal, with strongly sclerotized margin; antennae yellowish or reddish-brown, last antennomeres blackish. Scutellar shield yellowish, reddish-brown or blackish, glabrous and bearing few punctures. Dorsal coloration: head with one large and subcircular black spot on disc,
Figure 4C,G, arrow (specimens from Guatemala with one or two black spots, small, free and close to major spot on disc); pronotum with black, free and subcircular spots (
Figure 4) symmetrically and transversely arranged, as follows: three large basal spots (medial one resembling the fusion of the two smallest spots, e.g.,
Figure 4I, arrow) or with four apparent spots (in this case, medial spots not completely fused, e.g.,
Figure 4H, arrow); four spots on disc; two or four anterior spots (in the last case, two medial spots bigger than outer ones,
Figure 4C, white arrow). In some individuals, spots connected to lateral pronotal edges (
Figure 4E, arrow). Elytral coloration with several black, circular and free spots, sparsely distributed. In some individuals, there are elongated spots on each elytron, resembling the fusion of two or three spots, especially on humeral angles. On the disc, somewhat transverse spots can be present (
Figure 4L, arrow).
Male terminalia. (
Figure 5A–F). Penis (
Figure 5A, pen) slightly elongate and curved; basal portion with short sclerotized projection linked to apophyses; internal sac with a well-developed and slightly elongated flagellum (
Figure 5A, fla), 0.95 × the length of penis (
n = 3), with a shallow sinuosity and without a membranous portion between the virga and flagellum head; in dorsal view, flagellum medially enlarged and slightly sclerotized and, posteriorly, at flagellum head and virga connection, strongly sclerotized (
Figure 5B–D, black arrow), flagellum head sclerotized (
Figure 5B–D), U-shaped, with each branch ending in a blunt and narrowed tip (
Figure 5D, red arrow) or, forming a shallow, small and narrow denticle at the outer apical edge (
Figure 5C, red arrow). Apophyses (
Figure 5A, apo) 1.2 × as long as penis (
n = 3). Tegmen sclerotized (
Figure 5E); parameres reduced and sclerotized, with densely pubescent outgrowths, slightly dilated, narrowed and acute at apex (
Figure 5E, arrow). Tergite VIII, sclerotized with sparsely distributed bristles and sternite VIII, slightly sclerotized. Laterotergite IX sclerotized (
Figure 5F, terg IX), posteriorly elongated and pubescent, outer contours angulated; anteroventral edge with paired and subparallel lateral struts, connected at its anterior tip, by a small, transverse and slightly sclerotized sclerite (
Figure 5F, arrow). Posterior edge of sternite IX, sclerotized, undivided, outer contour rounded; anteriorly membranous. Tergite X, sclerotized, anterior edge with sparsely distributed bristles.
Female terminalia. (
Figure 5G–I). Gonostyli and gonocoxites strongly sclerotized (
Figure 5G, black and red arrows, respectively, under sternite VIII), baculi of paraprocts sclerotized and sinuous (
Figure 5H, arrows); spermatheca oval and sclerotized (
Figure 5I). Tergite VIII sclerotized and sternite VIII with conspicuous median strut (
Figure 5G, sternite VIII, big black arrow).
Distribution. North and Central America, South and Southeast Brazil (
Figure 6).
Remarks. (1)
Mycotretus centralis has a disjunct geographical distribution (
Figure 6). See Discussion. (2) The flagellum head of the dissected Brazilian
M. centralis (
Figure 5C–D) is more sclerotized and thicker than a specimen from Guatemala (
Figure 5B). (3) Interestingly, based on the pronotal color pattern of
M. centralis described here, the specimen figured by Gorham as an example of
M. tigrinus (see Gorham [
1], Tab. III. Figure 9) is probably
M. centralis. (4) A female from Derrubadas (RS, Brazil) seems to have the usual pronotal coloration of
M. centralis. However, as the specimen may be a teneral, we considered it a doubtful identification.
Material examined. Lectotype of M. centralis Arrow, 1909, here designated (BMNH) (
Figure 4C–D) “B.C.A., Col., VII. Mycotretus [printed] tigrinus, Oliv. [handwritten] \ Mycotretus centralis type arrow [handwritten] \ Sp. figured. [printed] \ S. Geronimo, Guatemala. Champion. [printed] \ Type [printed, disc-shaped label with red contour] \ LECTOTYPE
Mycotretus centralis Arrow, 1909 [printed, red label]”; 1 male (BMNH, dissected) “S. Geronimo, Guatemala. Champion. [printed] \ B.C.A.,Col.,VII. Mycotretus [printed] tigrinus, Oliv. [handwritten] \ PARALECTOTYPE
Mycotretus centralis Arrow, 1909 [printed, yellow label]”; 2 specimens (BMNH, on the same card) “S. Geronimo, Guatemala. Champion. [printed] \ B.C.A.,Col.,VII. Mycotretus [printed] tigrinus, Oliv. [handwritten] \ PARALECTOTYPE
Mycotretus centralis Arrow, 1909 [printed, yellow label]”; 1 specimen (BMNH) “Toxpam [printed] \ Mexico. Salle coll. [printed] \ 2398 [printed] \ B.C.A.,Col.,VII. Mycotretus [printed] tigrinus, Oliv. [handwritten] \ PARALECTOTYPE
Mycotretus centralis Arrow, 1909 [printed, yellow label]”; 1 female (DZUP, dissected) “Coleção M. Alvarenga [printed] \ REPRÊSA RIO GRANDE, Guanabara BRASIL [printed] IX. 1964 [handwritten], F.M. Oliveira [printed] \ DZUP 371269 [printed]”; 1 female (DZUP, dissected) “Coleção M. Alvarenga [printed] \ REPRÊSA RIO GRANDE, Guanabara BRASIL [printed] X. 1967 [handwritten], F.M. Oliveira [printed] \ DZUP 371268 [printed]”; 1 female (DZUP, dissected) “Coleção M. Alvarenga [printed] \ PEDRA AZUL, 700M, M. Gerais, Brasil, XI.1972, Seabra & Oliveira [printed] \ DZUP 371277 [printed]”; 1 female (DZUP, dissected) “4 X [handwritten] 194[printed] 7[handwritten], Brasilien, Nova Teutonia, 27°11′B, 52°23′L, Fritz Plaumann, 300 b[?] 500 m [printed] \ DZUP 125457 [printed]”; 1 male (MCNZ, dissected) “Campo Bom, RS, 29/ IV/ [handwritten] 19 [printed] 88 [handwritten], C.J. Becker leg. [printed] \ Col. MCN [printed] 150.874 [handwritten]”; 1 male (MCNZ, dissected) “Carazinha, RS, 10/XI/ [handwritten] 19 [printed] 79 [handwritten], A. Lise leg. [printed] \ Col. MCN 28.682 [handwritten]”; 1 female (DZUP, dissected) “PITANGA, 24°41, 51°46, 700 m [printed] \ MAERZ 1963, F. Plaumann [printed] \ DZUP 125578 [printed]”;
Doubtful identification. 1 female (MCNZ, dissected) “27°14′14.7″ S, 53°58′46.0″ W [printed] \ Derrubadas, RS (Pq. Est. Turvo), 30.X.2003, L. Heydrich col. [printed] \ Col. MCN 227465 [printed]”.
3.3. Mycotretus tigrinoides Mader, 1942
Mycotretus tigrinoides Mader 1842: 174, 196 [
10]. Type locality: Ocobamba, Peru.
Diagnosis. Pronotum with sparsely, black, free, subcircular elongated spots, asymmetrically arranged. In most specimens, there are three spots with no definite shape close to basal pronotal edge; lateral spots more elongated than inner ones. Elytral spots somewhat transverse, forming true transverse spots in some specimens. Penile flagellum well-developed, sclerotized and slightly elongated, approximately 0.97 × the length of the penis, shallowly sinuous, with prominent medial desclerotization (absent in M. tigripennis). Anterior tip of virga with prominent and strong sclerotization (absent in M. tigripennis). The flagellum of M. tigrinoides is narrower dorsally than that of M. tigripennis. Head of flagellum sclerotized and subpentagonal, outer anterior contours forming a right angle.
Redescription. Length (in mm) = 3.95–5.46 (4.85 ± 0.51,
n = 13). Very similar to
M. tigripennis. Body elongate, subparallel-sided, widest at the anterior third of elytra, TL/EW = 1.80–1.91 (1.86 ± 0.03), glabrous and glossy; dorsal and ventral coloration (
Figure 7A–F) reddish-brown, with mouthparts and first antennomeres yellowish to reddish-brown; legs yellowish to reddish-brown with coxae and tibiae partially blackish in some individuals; mandible apices and last antennomeres blackish; mentum plate pentagonal, with strongly sclerotized margin. Venter usually with black and subcircular spots on prosternum, meso- and meta-ventrite, abdomen and legs (
Figure 7B, arrow). Scutellar shield reddish-brown or blackish, glabrous and bearing few punctures. Dorsal coloration: head with one large and subcircular black spot on disc,
Figure 7A, arrow, (specimen from Brasilia, DF, Brazil with three black, small and free spots close to major spot on disc); pronotum with sparsely, black, free, subcircular and elongated spots, asymmetrically arranged. In most specimens, three spots with no definite shape close to basal edge, lateral spots more elongated (
Figure 7C, black arrows) than inner ones (
Figure 7C, white arrow). Elytral coloration with several black, free and subcircular spots, sparsely and asymmetrically distributed. Elytral spots somewhat transverse, forming true transverse spots in some specimens (
Figure 7C,E).
Male terminalia. (
Figure 8A–D). Penis (
Figure 8A, pen) slightly elongated and curved; basal portion with short sclerotized projection linked to apophyses; internal sac with well-developed, sclerotized and slightly elongated flagellum (
Figure 8A, fla), 0.97 × the length of penis (
n = 2), shallowly sinuous, with prominent medial desclerotization (
Figure 8A, arrow, absent in
M. tigripennis). Anterior tip of virga with prominent and strong sclerotization (
Figure 8B,C, arrow) absent in
M. tigripennis). Flagellum of
M. tigrinoides narrowed dorsally compared to that of
M. tigripennis (
Figure 8B,C). Head of flagellum (
Figure 8B,C) sclerotized and subpentagonal, outer anterior contours forming a right angle (
Figure 8B,C, small arrow) and anterior edge enlarged, compared to posterior one. Apophyses (
Figure 8A, apo) 1.58 × as long as penis (
n = 2). Tegmen sclerotized (
Figure 8A, teg); parameres reduced and sclerotized, with densely pubescent outgrowths, slightly dilated and acute at apex. Tergite VIII, sclerotized with sparsely distributed bristles. Sternite VIII slightly sclerotized. Laterotergite IX sclerotized (
Figure 8D, terg IX), posteriorly elongated and pubescent, outer contours angulated; anteroventral edge with paired and subparallel lateral struts, connected at its anterior tip by small, transverse, slightly sclerotized sclerite (
Figure 8D, arrow). Posterior edge of sternite IX sclerotized, undivided, outer contour rounded; anteriorly membranous. Tergite X sclerotized; anterior edge truncate, with sparsely distributed bristles.
Female terminalia. (
Figure 8E,F). Gonostyli and gonocoxites strongly sclerotized (
Figure 8F, black and red arrows, respectively), baculi of paraprocts sclerotized and slightly arcuate (
Figure 8F, black small arrows); spermatheca oval and sclerotized (
Figure 8E, black small arrow). Tergite VIII sclerotized and sternite VIII with conspicuous median strut (
Figure 8E, big arrow).
Distribution. Peru, North and Central Brazil (Figure 10, localities 1–5, black numerals).
Remarks. Both
M. tigrinoides and
M. tigripennis (redescribed below) were described based only on their primary types (
Figure 7C,D,I,J, respectively), which were not dissected here. However, based on the original descriptions and images of holotypes, we identified the specimens examined by us as intraspecific variations.
Material examined. 1 specimen (SDEI) “Holotypus [printed, Red label] \ Coll. Kraatz [printed] \ Ocobambe Peru [printed] \Mycotretus tigrinoides [handwritten] Ma. [?] det. Mader n.sp. [handwritten]”; 1 male (DZUP, dissected) “Coleção M. Alvarenga [printed] \ SINOP, M. Grosso, Brasil, X. 1974, M. Alvarenga [printed] \ Lat 12° 31′ S, Lon 55° 37′ W [printed] \ DZUP 126115 [printed]”; 1 female (CAMB, dissected) “Brasília, DF – BRASIL [printed] XI [handwritten] 2000, Col. N. Degallier [printed] \ Coleção A.M. BELLO [printed]”; 1 male (MNRJ, dissected) “Coleção M. Alvarenga [printed] \ Tucuruí, Pará, Brasil, XI-1985, N. Degallier [handwritten] \ AI [printed]”; 1 specimen (DZUP) “Coleção M. Alvarenga [printed] \ SINOP, M. Grosso, Brasil, X. 1974, M. Alvarenga [printed] \ Lat 12° 31′ S, Lon 55° 37′ W [printed] \ DZUP 126110 [printed]”; 1 specimen (DZUP) “Coleção M. Alvarenga [printed] \ SINOP, M. Grosso, Brasil, X. 1974, M. Alvarenga [printed] \ Lat 12° 31′ S, Lon 55° 37′ W [printed] \ DZUP 126111 [printed]”; 1 specimen (DZUP) “Coleção M. Alvarenga [printed] \ SINOP, M. Grosso, Brasil, X. 1974, M. Alvarenga [printed] \ Lat 12°31′ S, Lon 55° 37′ W [printed] \ DZUP 126112 [printed]”; 1 specimen (DZUP) “Coleção M. Alvarenga [printed] \ SINOP, M. Grosso, Brasil, X. 1974, M. Alvarenga [printed] \ Lat 12° 31′ S, Lon 55°37′ W [printed] \ DZUP 126113 [printed]”; 1 specimen (DZUP) “Coleção M. Alvarenga [printed] \ V. VERA, M. Grosso, Brasil, X. 1973, M. Alvarenga [printed] \ Lon 55°36′ W, Lat 12° 46′ S [printed] \ DZUP 126117 [printed]”; 1 specimen (DZUP) “Coleção M. Alvarenga [printed] \ SINOP, M. Grosso, Brasil, X. 1974, M. Alvarenga [printed] \ Lat 12°31’ S, Lon 55°37′ W [printed] \ DZUP 126114 [printed]”; 1 specimen (DZUP) “Coleção M. Alvarenga [printed] \ V. VERA, M. Grosso, Brasil, X. 1973, M. Alvarenga [printed] \ Lon 55°36′ W, Lat 12°46′ S [printed] \ DZUP 126118 [printed]”; 1 specimen (DZUP) “Coleção M. Alvarenga [printed] \ SINOP, M. Grosso, Brasil, X. 1974, M. Alvarenga [printed] \ Lat 12°31′ S, Lon 55°37′ W [printed] \ DZUP 126119 [printed]”.
3.4. Mycotretus tigripennis Mader, 1942
Mycotretus tigripennis Mader 1942: 174, 197 [
10]. Type locality: Santa Inéz, Ecuador.
Diagnosis. Posterior and anterior edge of pronotum with a black, shallow and transverse mark, from which two lateral tooth-like spots arise, sometimes with a medial elongated spot; subcircular pronotal spots can be present laterally or on the disc. Elytral coloration with several black, free, subcircular or longitudinal spots, sparsely distributed (some of which are apparently fused). Penile flagellum well-developed and slightly elongated, approximately 1.34 × the length of the penis, anteriorly arcuate and with a shallow desclerotization posteriorly (absent in M. tigrinoides). Flagellum of M. tigripennis dorsally broader than that of M. tigrinoides. Head of flagellum sclerotized and subpentagonal, with outer anterior contours forming an acute angle.
Redescription. Length (in mm) = 4.52–6.25 (5.52 ± 0.49, n = 17). Body elongate, widest at the anterior third of elytra, TL/EW = 1.76–1.86 (1.81 ± 0.02), glabrous and glossy; dorsal and ventral coloration (
Figure 7G–L) homogeneously yellowish or reddish-brown; mouthparts and first antennomeres yellowish to reddish-brown; legs yellowish to reddish-brown, with coxae and tibiae partially blackish in some individuals; mandible apices partially black and last antennomeres blackish; mentum plate pentagonal, with a strongly sclerotized margin. Venter usually black with subcircular spots. Scutellar shield blackish, glabrous and bearing few punctures. Dorsal coloration: head with no spots or with one large and subcircular black spot on the disc (
Figure 7I, arrow); posterior and anterior edge of pronotum with black, shallow and transverse marks, from which two lateral tooth-like spots arise (
Figure 7I,K, small arrows), sometimes with in between elongated and medial spots (
Figure 7G, arrow); sometimes with subcircular pronotal spots laterally or on the disc. Elytral coloration with several black, free, subcircular or longitudinal spots, sparsely distributed (some apparently fused).
Male terminalia. (
Figure 9A–D). Penis (
Figure 9A, pen) slightly elongated and curved; basal portion with short sclerotized projection linked to apophyses; internal sac with well-developed and slightly elongated flagellum (
Figure 9A, fla), 1.34 × the length of penis (
n = 2), anteriorly arcuate and with shallow desclerotization posteriorly (
Figure 9A, arrow, absent in
M. tigrinoides). Flagellum of
M. tigripennis dorsally broader than that of
M. tigrinoides (
Figure 9B,C). Head of flagellum (
Figure 9B,C) sclerotized and subpentagonal; outer anterior contours forming an acute angle (
Figure 9B,C, arrow), anterior edge enlarged compared to posterior edge. Apophyses (
Figure 9A, apo) 1.51 × as long as penis (
n = 2). Tegmen sclerotized (
Figure 9A, teg); parameres reduced and sclerotized, with densely pubescent outgrowths, slightly dilated, narrowed and acute at apex. Tergite VIII, sclerotized with sparsely distributed bristles. Sternite VIII slightly sclerotized. Laterotergite IX sclerotized (
Figure 9D, terg IX), posteriorly elongated and pubescent; outer contours angulated; anteroventral edge with paired and subparallel lateral struts, connected at its anterior tip by small, transverse, slightly sclerotized sclerite (
Figure 9D, arrow). Posterior edge of sternite IX sclerotized, undivided; outer contour rounded; anteriorly membranous. Tergite X sclerotized; anterior edge truncate, with sparsely distributed bristles.
Female terminalia. (
Figure 9E,F). Gonostyli and gonocoxites strongly sclerotized (
Figure 9E, black and red arrows, respectively, under sternite VIII); baculi of paraprocts sclerotized and arcuate (
Figure 9F, black small arrows); spermatheca oval and sclerotized (
Figure 9F, big black arrow). Tergite VIII sclerotized and sternite VIII with conspicuous median strut (
Figure 9E, big arrow).
Distribution. Ecuador (Santa Inéz) and Southeast Brazil (
Figure 10, localities 6–8, reddish numerals).
Remarks. See the above remarks of M. tigrinoides concerning the identification of examined specimens of M. tigripennis. The specimens identified here as M. tigripennis (from Rio de Janeiro, Brazil) are far away from the type locality (Santa Inés, Ecuador) and we thought that it would be a new species at first. Although that remains a possibility, until other populations are studied, we prefer to consider these Brazilian specimens as intraspecific variation of M. tigripennis.
Material examined. 1 specimen (SDEI) “Holotypus [printed, red label] \ Santa Jnéz (Ecuad.) R. Haensch S. [printed] \ Coll. Kraatz [printed] \ Mycotretus tigripennis [handwritten] Ma. [?] Holotypus det. Mader [handwritten]”; 1 male (DZUP, dissected) “REPRÊSA RIO GRANDE, Guanabara BRASIL [printed] XII. 1960 [handwritten], F.M. Oliveira [printed] \ DZUP 127806 [printed]”; 1 female (DZUP, dissected) “Coleção M. Alvarenga [printed] \ REPRÊSA RIO GRANDE, Guanabara BRASIL [printed] II. 1967 [handwritten], F.M. Oliveira [printed] \ DZUP 127795 [printed]”; 1 female (DZUP, dissected) “Coleção M. Alvarenga [printed] \ REPRÊSA RIO GRANDE, Guanabara BRASIL [printed] III. 1967 [handwritten], F.M. Oliveira [printed] \ DZUP 127804 [printed]”; 1 female (MNRJ, dissected) “Coleção M. Alvarenga [printed] \ REPRÊSA RIO GRANDE, Guanabara BRASIL [printed] III. 1964 [handwritten], F.M. Oliveira [printed]”; 1 female (DZUP, dissected) “Coleção M. Alvarenga [printed] \ REPRÊSA RIO GRANDE, Guanabara BRASIL [printed] XII. 1960 [handwritten], F.M. Oliveira [printed] \ DZUP 127808 [printed]”; 1 female (DZUP, dissected) “Coleção M. Alvarenga [printed] \ REPRÊSA RIO GRANDE, Guanabara BRASIL [printed] III. 1967 [handwritten], F.M. Oliveira [printed] \ DZUP 127789 [printed]”; 1 male (DZUP, dissected) “Coleção M. Alvarenga [printed] \ REPRÊSA RIO GRANDE, Guanabara BRASIL [printed] III. 1967 [handwritten], F.M. Oliveira [printed] \ DZUP 127805 [printed]”; 1 specimen (DZUP) “Coleção M. Alvarenga [printed] \ REPRÊSA RIO GRANDE, Guanabara BRASIL [printed] VIII. 1969 [handwritten], F.M. Oliveira [printed] \ DZUP 127824 [printed]”; 1 specimen (DZUP) “Coleção M. Alvarenga [printed] \ REPRÊSA RIO GRANDE, Guanabara BRASIL [printed] IX. 1964 [handwritten], F.M. Oliveira [printed] \ DZUP 127796 [printed]”; 1 specimen (DZUP) “REPRÊSA RIO GRANDE, Guanabara BRASIL [printed] II. 1967 [handwritten], F.M. Oliveira [printed] \ DZUP 127797 [printed]”; 1 specimen (DZUP) “Coleção M. Alvarenga [printed] \ REPRÊSA RIO GRANDE, Guanabara BRASIL [printed] III. 1968 [handwritten], F.M. Oliveira [printed] \ DZUP 127801 [printed]”; 1 specimen (DZUP) “Coleção M. Alvarenga [printed] \ REPRÊSA RIO GRANDE, Guanabara BRASIL [printed] II. 1967 [handwritten], F.M. Oliveira [printed] \ DZUP 127794 [printed]”; 1 specimen (DZUP) “Coleção M. Alvarenga [printed] \ REPRÊSA RIO GRANDE, Guanabara BRASIL [printed] XII. 1960 [handwritten], F.M. Oliveira [printed] \ DZUP 127809 [printed]”; 1 specimen (DZUP) “Coleção M. Alvarenga [printed] \ FLORESTA da TIJUCA, D. Federal, Brasil [printed] I. 1961 [handwritten], C.A.C. Seabra\ DZUP 127830 [printed]”; 1 specimen (DZUP) “Coleção M. Alvarenga [printed] \ FLORESTA da TIJUCA, D. Federal, Brasil [printed] I. 1961 [handwritten], C.A.C. Seabra\ DZUP 127828 [printed]”; 1 specimen (DZUP) “Coleção M. Alvarenga [printed] \ FLORESTA da TIJUCA, D. Federal, Brasil [printed] I. 1961 [handwritten], C.A.C. Seabra\ DZUP 127829 [printed]”; 1 specimen (DZUP) “Coleção M. Alvarenga [printed] \ REPRÊSA RIO GRANDE, Guanabara BRASIL [printed] IX. 1969 [handwritten], F.M. Oliveira [printed] \ DZUP 126096 [printed]”.
3.5. Mycotretus prioteloides Mader, 1942
Mycotretus prioteloides Mader 1942: 174, 196 [
10]. Type locality: Coroico, Bolivia.
Diagnosis. Pronotal edges black, with a shallow and transverse black mark at posterior or anterior edges; pronotal portion with two transverse or subcircular black spots on the disc. Outer edges of pronotum and mesal sutural edge of elytra with black outline. Elytral coloration with several black, subcircular and transverse free spots. Penile flagellum well-developed and slightly elongated, approximately 0.8 × the length of penis, slightly sinuous, with a membranous portion between its virga and head. Head of flagellum sclerotized, slightly elongated and, unlike in M. tigrinus, conspicuously convex anteriorly; inner outline somewhat more separated than in M. tigrinus.
Redescription. Length (in mm) = 5.75–7 (6.29 ± 0.63,
n = 3). Body elongate, widest at anterior third of elytra, TL/EW = 1.75–1.91 (1.83 ± 0.08) (measurements based on examined specimen and in Mader’s original description based on holotype and one paratype), glabrous and glossy, dorsal and ventral coloration homogeneously yellowish or reddish-brown (
Figure 11A,C,D). Mouthparts reddish-brown with outer contour of last maxillary and labial palpomeres yellowish; mentum plate pentagonal, with strongly sclerotized margin; mandible apices and antennomeres 2–11 blackish (scape reddish-brown). Trochanters, apical portion of femora, tibiae and dorsum of tarsi blackish; tarsi (ventrally) and claws yellowish to reddish-brown. Elytral epipleuron partially black and yellowish (
Figure 11D, arrows). Scutellar shield blackish, glabrous and bearing few punctures. Dorsal coloration: head without spots; pronotal edges black, with a shallow and transverse black mark on the anterior (
Figure 11A, arrow) and posterior edge (
Figure 11C, arrow); disc of pronotum with two transverse (
Figure 11A) or subcircular (
Figure 11C) black spots. Outer edges of pronotum and mesal sutural edge of elytra with black outline. Elytral coloration with several black, subcircular and transverse free spots.
Male terminalia. (
Figure 11E–G). Penis (
Figure 11E, pen) slightly elongated and curved; basal portion with short sclerotized projection linked to apophyses; internal sac with well-developed and slightly elongated flagellum (
Figure 11E, fla), 0.8 × the length of penis (
n = 1), slightly sinuous, with a membranous portion between the virga and head (
Figure 11F, arrow). Head of flagellum (
Figure 11F) sclerotized, slightly elongated, and unlike in
M. tigrinus, conspicuously convex anteriorly (
Figure 11E, small arrow); inner outline somewhat more separated than in
M. tigrinus. Apophyses (
Figure 11E, apo) 1.3 × as long as penis. Tegmen sclerotized (
Figure 11A, teg); parameres reduced and sclerotized, with densely pubescent outgrowths, slightly dilated, narrowed and acute at apex. Tergite VIII, sclerotized with sparsely distributed bristles. Sternite VIII slightly sclerotized. Laterotergite IX sclerotized (
Figure 11G, terg IX), posteriorly elongated and pubescent, outer contours angulated; anteroventral edge with paired and subparallel lateral struts connected at its anterior tip by small, transverse, slightly sclerotized sclerite (
Figure 11G, arrow). Posterior edge of sternite IX sclerotized, undivided; anteriorly membranous. Tergite X sclerotized, anterior edge with sparsely distributed bristles.
Female terminalia. Unknown.
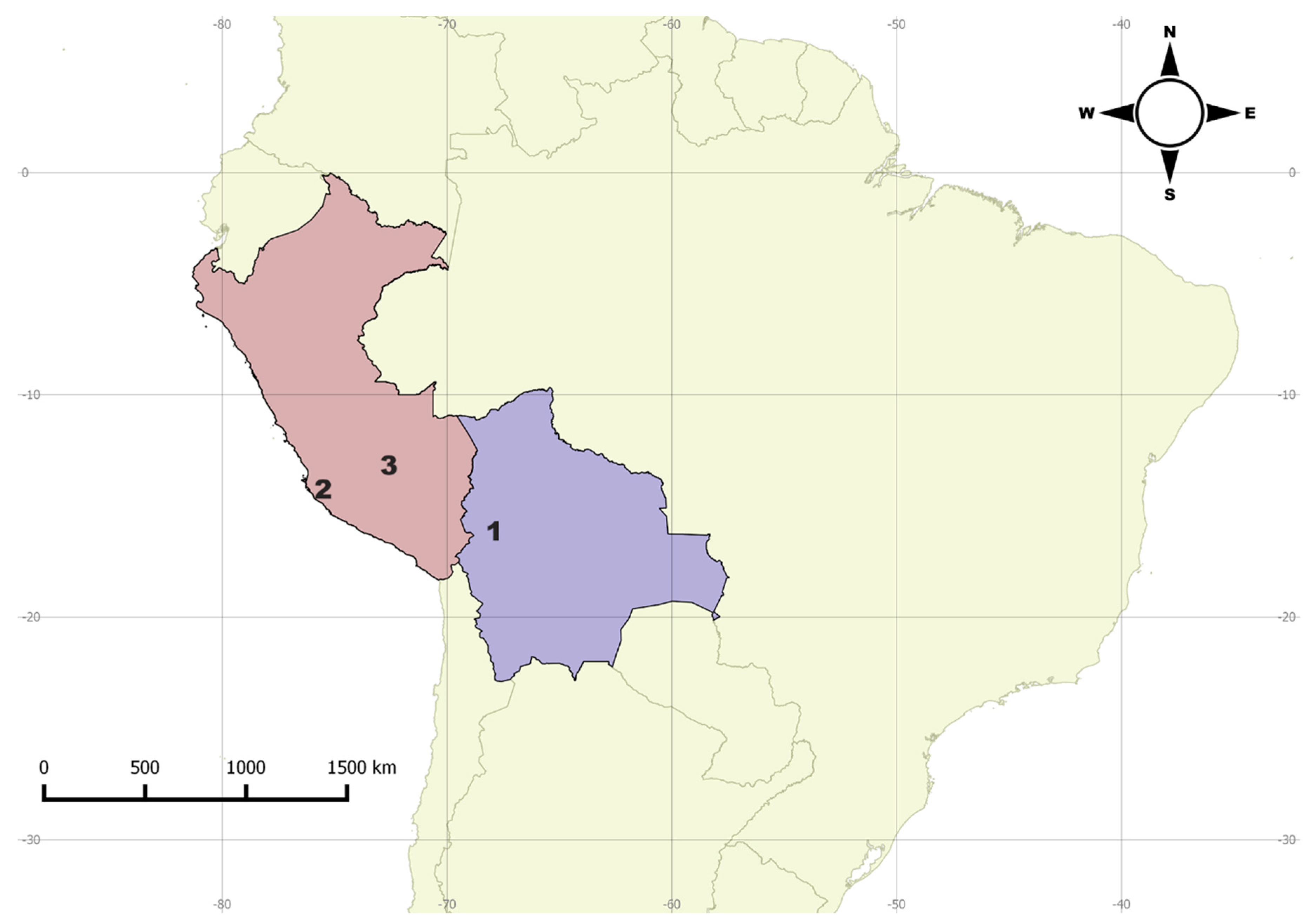
Distribution. Bolivia (Coroico), Peru (Calango, Machu Picchu) (
Figure 12).
Remarks. Although M. prioteloides is remarkably distinct from the other Mycotretus studied here, its male genitalia resembles that of M. tigrinus and, apparently, both species are closely related.
Material examined. 1 specimen (NMBS) “Coroico Bolivia [printed] \ prioteloides Mad. [handwritten] \ holo- [handwritten], TYPUS [printed], prioteloides [handwritten] [red label]”; 1 male (MNRJ, dissected) “Coleção M. Alvarenga [printed] \ Homeotipo [printed, red label] \ Torentoy Canyon (Base Machu – Pichu), 2000–2000 m––PERU, VI–VII. 964 B. Malkin [printed] \ Comparado com tipo [printed], Mycotretus prioteloides Mader, 1942 [handwritten], M. alvarenga det. 1971 [printed] \ 1747 [printed]”.