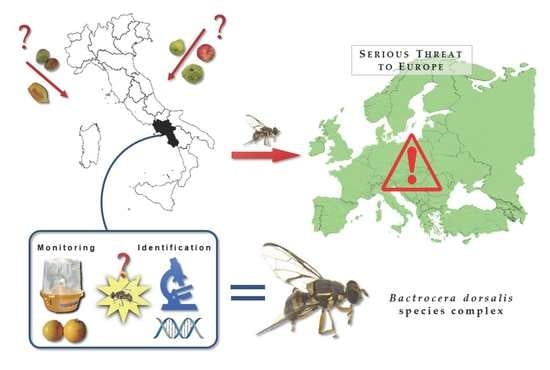
First Record of an Invasive Fruit Fly Belonging to Bactrocera dorsalis Complex (Diptera: Tephritidae) in Europe
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruit Fly Trapping
2.2. Morphological Identification of Fruit Fly
2.3. Molecular Characterization of Fruit Fly
2.4. Morphological Re-Examination
3. Results
3.1. Fruit Fly Trapping
3.2. Morphological Identification of Fruit Fly
3.3. Molecular Characterization of Fruit Fly
3.4. Morphological Re-Examination
- (a)
- (b)
- (c)
- (d)
- The abdominal terga III–V have a moderately broad medial longitudinal dark band and broad lateral longitudinal dark bands [20].
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aluja, M.; Norrbom, A. Fruit Flies (Tephritidae): Phylogeny and Evolution of Behavior; CRC Press: Boca Raton, FL, USA, 1999; pp. 16–944. [Google Scholar]
- White, I.M.; Elson-Harris, M.M. Fruit Flies of Economic Significance: Their Identification and Bionomics; CAB International: Wallingford, UK, 1992; p. 601. ISBN 0-85198-790-7. [Google Scholar]
- Pape, T.; Bickel, D.J.; Meier, R. Diptera Diversity: Status, Challenges and Tools; Brill Academic Publishers, Incorporated: Leiden, The Netherlands, 2009; p. 459. [Google Scholar]
- Leblanc, L.; Hossain, M.A.; Khan, S.A.; San Jose, M.; Rubinoff, D. A preliminary survey of the fruit flies (Diptera: Tephritidae: Dacinae) of Bangladesh. Proc. Hawaii Entomol. Soc. 2013, 45, 51–58. [Google Scholar]
- San Jose, M.; Leblanc, L.; Geib, S.M.; Rubinoff, D. An evaluation of the species status of Bactrocera invadens and the systematics of the Bactrocera dorsalis (Diptera: Tephritidae) Complex. Ann. Entomol. Soc. Am. 2013, 106, 684–694. [Google Scholar] [CrossRef]
- Drew, R.A.I.; Hancock, D.L. The Bactrocera dorsalis complex of fruit flies (Diptera: Tephritidae: Dacinae) in Asia. Bull. Entomol. Res. 1994, 2, 1–68. [Google Scholar] [CrossRef]
- Stephens, A.E.A.; Kriticos, D.J.; Leriche, A. The current and future potential geographical distribution of the oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae). Bull. Entomol. Res. 2007, 97, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Barr, N.B.; Ledezma, L.A.; Leblanc, L.; San Jose, M.; Rubinoff, D.; Geib, S.M.; Fujita, B.; Bartels, D.W.; Garza, D.; Kerr, P.; et al. Genetic Diversity of Bactrocera dorsalis (Diptera: Tephritidae) on the Hawaiian Islands: Implications for an Introduction Pathway into California. J. Econ. Entomol. 2014, 107, 1946–1958. [Google Scholar] [CrossRef] [PubMed]
- Lux, S.A.; Copeland, R.S.; White, I.M.; Manrakhan, A.; Billah, M.K. A new invasive fruit fly species from the Bactrocera dorsalis (Hendel) group detected in East Africa. Int. J. Trop. Insect Sci. 2011, 23, 355–361. [Google Scholar] [CrossRef]
- Drew, R.A.I.; Tsuruta, K.; White, I.M. A new species of pest fruit fly (Diptera: Tephritidae: Dacinae) from Sri Lanka and Africa. Afr. Entomol. 2005, 13, 149–154. [Google Scholar]
- Schutze, M.K.; Aketarawong, N.; Amornsak, W.; Armstrong, K.F.; Augustinos, A.; Barr, N.; Bo, W.; Bourtzis, K.; Boykin, L.M.; Cáceres, C.; et al. Synonymization of key pest species within the Bactrocera dorsalis species complex (Diptera: Tephritidae): Taxonomic changes based on a review of 20 years of integrative morphological, molecular, cytogenetic, behavioural and chemoecological data. Syst. Entomol. 2014, 40, 456–471. [Google Scholar] [CrossRef]
- Schutze, M.K.; Bourtzis, K.; Cameron, S.L.; Clarke, A.R.; De Meyer, M.; Hee, A.K.W.; Hendrichs, J.; Krosch, M.N.; Mwatawala, M. Integrative taxonomy versus taxonomic authority without peer review: The case of the Oriental fruit fly, Bactrocera dorsalis (Tephritidae). Syst. Entomol. 2017, 47, 609–620. [Google Scholar] [CrossRef]
- Schutze, M.K.; Mahmood, K.; Pavasovic, A.; Bo, W.; Newman, J.; Clarke, A.R.; Krosch, M.N.; Cameron, S.L. One and the same: Integrative taxonomic evidence that Bactrocera invadens (Diptera: Tephritidae) is the same species as the Oriental fruit fly Bactrocera dorsalis. Syst. Entomol. 2015, 40, 472–486. [Google Scholar] [CrossRef] [Green Version]
- Leblanc, L.; San Jose, M.; Barr, N.; Rubinoff, D. A phylogenetic assessment of the polyphyletic nature and intraspecific color polymorphism in the Bactrocera dorsalis complex (Diptera, Tephritidae). ZooKeys 2015, 540, 339–367. [Google Scholar] [CrossRef] [PubMed]
- Elnagar, S.; El-Sheikh, M.; Hashem, A.; Afia, Y. Recent invasion by Bactrocera zonata (Saunders) as a new pest competing with Ceratitis capitata (Wiedemann) in attacking fruits in Egypt. Asp. Appl. Biol. 2010, 104, 97–102. [Google Scholar]
- Taher, M. Bactrocera zonata (Saunders) in Egypt: Disease and pest outbreaks. Arab Near East Plant Prot. Newslett. 1998, 27, 30. [Google Scholar]
- Vargas, R.I.; Mau, R.F.; Stark, J.D.; Piñero, J.C.; Leblanc, L.; Souder, S.K. Evaluation of methyl eugenol and cue-lure traps with solid lure and insecticide dispensers for fruit fly monitoring and male annihilation in the Hawaii area wide pest management program. J. Econ. Entomol. 2010, 103, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, K. Identification of pest species in oriental fruit fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae) species complex. Pakistan J. Zool. 2004, 36, 219–240. [Google Scholar]
- David, K.J.; Ramani, S. An illustrated key to fruit flies (Diptera: Tephritidae) from Peninsular India and the Andaman and Nicobar Islands. Zootaxa 2011, 3021, 1–31. [Google Scholar]
- Drew, R.A.; Romig, M.C. Keys to the Tropical Fruit Flies (Tephritidae: Dacinae) of South-East Asia: Indomalaya to North-West Australasia; CABI: Oxfordshire, UK, 2016. [Google Scholar]
- Plant Health Australia. The Australian Handbook for the Identification of Fruit Flies; Version 3.0; Plant Health Australia: Canberra, Australia, 2018. [Google Scholar]
- Virgilio, M.; White, I.; De Meyer, M. A set of multi-entry identification keys to African frugivorous flies (Diptera, Tephritidae). ZooKeys 2014, 428, 97–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebiola, M.; Bernardo, U.; Monti, M.M.; Navone, P.; Viggiani, G. Pnigalio agraules (Walker) and Pnigalio mediterraneus Ferriere and Delucchi (Hymenoptera: Eulophidae): Two closely related valid species. J. Nat. Hist. 2009, 43, 2465–2480. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Simon, C.; Frati, F.; Beckenbach, A.T.; Crespi, B.; Liu, H.; Flook, P. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 1994, 87, 651–701. [Google Scholar] [CrossRef]
- Nugnes, F.; Gebiola, M.; Monti, M.M.; Gualtieri, L.; Giorgini, M.; Wang, J.; Bernardo, U. Genetic diversity of the invasive gall wasp Leptocybe invasa (Hymenoptera: Eulophidae) and of its Rickettsia endosymbiont, and associated sex-ratio differences. Munderloh UG, editor. PLoS ONE 2015, 10, e0124660. [Google Scholar] [CrossRef] [PubMed]
- Boykin, L.M.; Schutze, M.K.; Krosch, M.N.; Chomič, A.; Chapman, T.A.; Englezou, A.; Armstrong, K.F.; Clarke, A.R.; Hailstones, D.; Cameron, S.L. Multi-gene phylogenetic analysis of south-east Asian pest members of the Bactrocera dorsalis species complex (Diptera: Tephritidae) does not support current taxonomy. J. Appl. Entomol. 2014, 138, 235–253. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef] [PubMed]
- San Jose, M.; Doorenweerd, C.; Leblanc, L.; Barr, N.; Geib, S.; Rubinoff, D. Incongruence between molecules and morphology: A seven-gene phylogeny of Dacini fruit flies paves the way for reclassification (Diptera: Tephritidae). Mol. Phylogenet. Evol. 2018, 121, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Posada, D. jModelTest: Phylogenetic model averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, U.; Sasso, R.; Gebiola, M.; Viggiani, G. First record of a walnut shield bearer Coptodisca (Lepidoptera: Heliozelidae) in Europe. J. Appl. Entomol. 2012, 136, 638–640. [Google Scholar] [CrossRef]
- Bernardo, U.; van Nieukerken, E.J.; Sasso, R.; Gebiola, M.; Gualtieri, L.; Viggiani, G. Characterization, distribution, biology and impact on Italian walnut orchards of the invasive North-American leafminer Coptodisca lucifluella (Lepidoptera: Heliozelidae). Bull. Entomol. Res. 2015, 105, 210–224. [Google Scholar] [CrossRef]
- Cioffi, M.; Cornara, D.; Corrado, I.; Jansen, M.G.M.; Porcelli, F. The status of Aleurocanthus spiniferus from its unwanted introduction in Italy to date. Bull. Insectol. 2013, 66, 273–281. [Google Scholar]
- Montecchio, L.; Faccoli, M. First record of thousand cankers disease Geosmithia morbida and walnut twig beetle Pityophthorus juglandis on Juglans nigra in Europe. Plant Dis. 2014, 98, 696. [Google Scholar] [CrossRef]
- Garonna, A.P.; Scarpato, S.; Vicinanza, F.; Espinosa, B. First report of Toumeyella parvicornis (Cockerell) in Europe (Hemiptera: Coccidae). Zootaxa 2015, 3949, 142–146. [Google Scholar] [CrossRef]
- Lupi, D.; Bernardo, U.; Bonsignore, C.P.; Colombo, M.; Dindo, M.L.; Faccoli, M.; Ferracini, C.; Gualtieri, L.; Marullo, R.; Mazzon, L.; et al. Insects and globalization: Sustainable control of exotic species in Italian agro-forestry ecosystems. Landsc. Manag. Funct. Biodivers. IOBC-WPRS Bull. 2014, 100, 87–90. [Google Scholar]
- Dowling, T.E.; Secor, C.L. The role of hybridization and introgression in the diversification of animals. Annu. Rev. Ecol. Evol. Syst. 1997, 28, 593–619. [Google Scholar] [CrossRef]
- Pieterse, W.; Benítez, H.A.; Addison, P. The use of geometric morphometric analysis to illustrate the shape change induced by different fruit hosts on the wing shape of Bactrocera dorsalis and Ceratitis capitata (Diptera: Tephritidae). Zool. Anz. A J. Comp. Zool. 2017, 269, 110–116. [Google Scholar] [CrossRef]
- EPPO. EPPO A1 List of Pests Recommended for Regulation as Quarantine Pests (Version 2018-09). European and Mediterranean Plant Protection Organisation. 2018. Available online: https://www.eppo.int/QUARANTINE/listA1.htm (accessed on 11 October 2018).
- Vargas, R.; Piñero, J.; Leblanc, L. An overview of pest species of Bactrocera fruit flies (Diptera: Tephritidae) and the integration of biopesticides with other biological approaches for their management with a focus on the Pacific region. Insects 2015, 6, 297–318. [Google Scholar] [CrossRef]
- Tsuruta, K.; White, I.M.; Bandara, H.M.J.; Rajapakse, H. A preliminary notes on the host-plants of fruit flies of the Tribe Dacini (Diptera, Tephritidae) in Sri Lanka. Esakia 1997, 37, 149–160. [Google Scholar]
- Clarke, A.R.; Armstrong, K.F.; Carmichael, A.E.; Milne, J.R.; Raghu, S.; Roderick, G.K.; Yeates, D.K. Invasive phytophagous pests arising through a recent tropical evolutionary radiation: The Bactrocera dorsalis complex of fruit flies. Annu. Rev. Entomol. 2005, 50, 293–319. [Google Scholar] [CrossRef] [Green Version]
- CABI. Bactrocera dorsalis (oriental fruit fly): Invasive Species Compendium Datasheets, Maps, Images, Abstracts and Full Text on Invasive Species of the World. 2018. Available online: http://www.cabi.org/isc/datasheet/17685 (accessed on 11 October 2018).
- Khamis, F.M.; Masiga, D.K.; Mohamed, S.A.; Salifu, D.; De Meyer, M.; Ekesi, S. Taxonomic identity of the invasive fruit fly pest, Bactrocera invadens: Concordance in morphometry and dna barcoding. PLoS ONE 2012, 7, e44862. [Google Scholar] [CrossRef]
- Iwahashi, O. Distinguishing between the two sympatric species Bactrocera carambolae and B. papayae (Diptera: Tephritidae) based on aedeagal length. Ann. Entomol. Soc. Am. 1999, 92, 639–643. [Google Scholar] [CrossRef]
- Drew, R.A.I.; Romig, M.C. Tropical Fruit Flies of South-East Asia: (Tephritidae: Dacinae); CABI International: Oxfordshire, UK, 2013; 856p. [Google Scholar]
- EPPO PQR—EPPO Database on Quarantine Pests. Available online: http://www.eppo.int (accessed on 19 October 2018).
- Egartner, A.; Lethmayer, C. Invasive fruit flies of economic importance in Austria—Monitoring activities. Integr. Prot. Fruit Crops IOBC-WPRS Bull. 2017, 123, 45–49. [Google Scholar]
- Egartner, A.; Lethmayer, C.A.R.; Blümel, S. Monitoring activities on invasive fruit flies (Tephritidae, Diptera) in Austria. In Proceedings of the XI European Congress of Entomology, Naples, Italy, 2–6 July 2018; p. 153, ISBN 978-88-9092-621-1. [Google Scholar]
- Qin, Y.-J.; Krosch, M.N.; Schutze, M.K.; Zhang, Y.; Wang, X.-X.; Prabhakar, C.S.; Susanto, A.; Hee, A.K.W.; Ekesi, S.; Badji, K.; et al. Population structure of a global agricultural invasive pest, Bactrocera dorsalis (Diptera: Tephritidae). Evol. Appl. 2018, 6, 1138. [Google Scholar] [CrossRef]
- De Villiers, M.; Hattingh, V.; Kriticos, D.J.; Brunel, S.; Vayssières, J.F.; Sinzogan, A.; Billah, M.K.; Mohamed, S.A.; Mwatawala, M.; Abdelgader, H.; et al. The potential distribution of Bactrocera dorsalis: Considering phenology and irrigation patterns. Bull. Entomol. Res. 2015, 106, 19–33. [Google Scholar] [CrossRef]
- Nugnes, F.; Russo, E.; Ucciero, E.; Minucci, E.; Porcelli, F.; Bernardo, U. The prevention of biological invasions: The crucial role of import checks at Border Inspection Posts (BIPs). Some case studies. In Proceedings of the XI European Congress of Entomology, Naples, Italy, 2–6 July 2018; p. 162, ISBN 978-88-9092-621-1. [Google Scholar]
- Epsky, N.D.; Kendra, P.E.; Schnell, E.Q. History and development of food-based attractants. In Trapping and the Detection, Control, and Regulation of Tephritid Fruit Flies; Shelly, T., Epsky, N., Jang, E.B., Reyes-Flores, J., Vargas, R., Eds.; Springer: Dordrecht, The Netherlands, 2014; p. 638. [Google Scholar]
- Khamis, F.; Karam, N.; Guglielmino, C.R.; Ekesi, S.; Masiga, D.; De Meyer, M.; Kenya, E.U.; Malacrida, A.R. Isolation and characterization of microsatellite markers in the newly discovered invasive fruit fly pest in Africa, Bactrocera invadens (Diptera: Tephritidae). Mol. Ecol. Resour. 2008, 8, 1509–1511. [Google Scholar] [CrossRef]



| Voucher | Localities | N. of Caught Flies | Province | Coordinates | Genbank Accession Code | ||
|---|---|---|---|---|---|---|---|
| LCO-HCO | 2183-3014 | ITS1 | |||||
| BD_1 | Nocera Inferiore | 6(2) | Salerno | 40°44′ N, 14°37′ E | MK106007 | MK106010 | MK158099 |
| BD_2 | MK106008 | MK106011 | MK158100 | ||||
| BD_3 | Palma Campania | 1(1) | Naples | 40°51′ N, 14°33′ E | MK106009 | MK106012 | MK158101 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nugnes, F.; Russo, E.; Viggiani, G.; Bernardo, U. First Record of an Invasive Fruit Fly Belonging to Bactrocera dorsalis Complex (Diptera: Tephritidae) in Europe. Insects 2018, 9, 182. https://doi.org/10.3390/insects9040182
Nugnes F, Russo E, Viggiani G, Bernardo U. First Record of an Invasive Fruit Fly Belonging to Bactrocera dorsalis Complex (Diptera: Tephritidae) in Europe. Insects. 2018; 9(4):182. https://doi.org/10.3390/insects9040182
Chicago/Turabian StyleNugnes, Francesco, Elia Russo, Gennaro Viggiani, and Umberto Bernardo. 2018. "First Record of an Invasive Fruit Fly Belonging to Bactrocera dorsalis Complex (Diptera: Tephritidae) in Europe" Insects 9, no. 4: 182. https://doi.org/10.3390/insects9040182
APA StyleNugnes, F., Russo, E., Viggiani, G., & Bernardo, U. (2018). First Record of an Invasive Fruit Fly Belonging to Bactrocera dorsalis Complex (Diptera: Tephritidae) in Europe. Insects, 9(4), 182. https://doi.org/10.3390/insects9040182