Sex Difference in Running Stability Analyzed Based on a Whole-Body Movement: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Secondary Data Analysis
2.2. Movement Synergy Extraction
2.3. PCA-Based Variable Computation
2.4. Statistical Analysis
3. Results
3.1. Movement Synergies
3.2. Sex Differences in Running Stability
4. Discussion
Limitations and Future Study
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Gent, R.N.; Siem, D.; Van Middelkoop, M.; Van Os, A.G.; Bierma-Zeinstra, S.M.A.; Koes, B.W. Incidence and Determinants of Lower Extremity Running Injuries in Long Distance Runners: A Systematic Review. Br. J. Sports Med. 2007, 41, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Taunton, J.E.; Ryan, M.B.; Clement, D.B.; McKenzie, D.C.; Lloyd-Smith, D.R.; Zumbo, B.D. A Retrospective Case-Control Analysis of 2002 Running Injuries. Br. J. Sports Med. 2002, 36, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Ristolainen, L.; Heinonen, A.; Waller, B.; Kujala, U.M.; Kettunen, J.A. Gender Differences in Sport Injury Risk and Types of Inju-Ries: A Retrospective Twelve-Month Study on Cross-Country Skiers, Swimmers, Long-Distance Runners and Soccer Players. J. Sports Sci. Med. 2009, 8, 443. [Google Scholar] [PubMed]
- Fredericson, M.; Moore, W.; Guillet, M.; Beaulieu, C. High Hamstring Tendinopathy in Runners Meeting the Challenges of Diagnosis, Treatment, and Rehabilitation. Phys. Sportsmed. 2005, 33, 32–43. [Google Scholar] [CrossRef]
- Hallam, L.C.; Amorim, F.T. Expanding the Gap: An Updated Look Into Sex Differences in Running Performance. Front. Physiol. 2022, 12, 2334. [Google Scholar] [CrossRef]
- Vickers, A.J.; Vertosick, E.A. An Empirical Study of Race Times in Recreational Endurance Runners. BMC Sports Sci. Med. Rehabil. 2016, 8, 26. [Google Scholar] [CrossRef]
- Joyner, M.J. Physiological Limits to Endurance Exercise Performance: Influence of Sex. J. Physiol. 2017, 595, 2949–2954. [Google Scholar] [CrossRef]
- Hewett, T.E. Neuromuscular and Hormonal Factors Associated with Knee Injuries in Female Athletes: Strategies for Intervention. Sport. Med. 2000, 29, 313–327. [Google Scholar] [CrossRef]
- Mohr, M.; Pieper, R.; Löffler, S.; Schmidt, A.R.; Federolf, P.A. Sex-Specific Hip Movement Is Correlated With Pelvis and Upper Body Rotation During Running. Front. Bioeng. Biotechnol. 2021, 9, 521. [Google Scholar] [CrossRef]
- Almonroeder, T.G.; Benson, L.C. Sex Differences in Lower Extremity Kinematics and Patellofemoral Kinetics during Running. J. Sports Sci. 2017, 35, 1575–1581. [Google Scholar] [CrossRef]
- Bruening, D.A.; Frimenko, R.E.; Goodyear, C.D.; Bowden, D.R.; Fullenkamp, A.M. Sex Differences in Whole Body Gait Kinematics at Preferred Speeds. Gait Posture 2015, 41, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Neal, B.S.; Barton, C.J.; Gallie, R.; O’Halloran, P.; Morrissey, D. Runners with Patellofemoral Pain Have Altered Biomechanics Which Targeted Interventions Can Modify: A Systematic Review and Meta-Analysis. Gait Posture 2016, 45, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Danielsson, A.; Horvath, A.; Senorski, C.; Alentorn-Geli, E.; Garrett, W.E.; Cugat, R.; Samuelsson, K.; Hamrin Senorski, E. The Mechanism of Hamstring Injuries—A Systematic Review. BMC Musculoskelet. Disord. 2020, 21, 641. [Google Scholar] [CrossRef] [PubMed]
- Promsri, A.; Longo, A.; Haid, T.; Doix, A.-C.M.; Federolf, P. Leg Dominance as a Risk Factor for Lower-Limb Injuries in Downhill Skiers—A Pilot Study into Possible Mechanisms. Int. J. Environ. Res. Public Health 2019, 16, 3399. [Google Scholar] [CrossRef] [PubMed]
- Promsri, A.; Mohr, M.; Federolf, P. Principal Postural Acceleration and Myoelectric Activity: Interrelationship and Relevance for Characterizing Neuromuscular Function in Postural Control. Hum. Mov. Sci. 2021, 77, 102792. [Google Scholar] [CrossRef]
- Promsri, A.; Haid, T.; Federolf, P. Complexity, Composition, and Control of Bipedal Balancing Movements as the Postural Control System Adapts to Unstable Support Surfaces or Altered Feet Positions. Neuroscience 2020, 430, 113–124. [Google Scholar] [CrossRef]
- Haid, T.H.; Zago, M.; Promsri, A.; Doix, A.-C.M.; Federolf, P.A. PManalyzer: A Software Facilitating the Study of Sensorimotor Control of Whole-Body Movements. Front. Neuroinform. 2019, 13, 24. [Google Scholar] [CrossRef]
- Troje, N.F. Decomposing Biological Motion: A Framework for Analysis and Synthesis of Human Gait Patterns. J. Vis. 2002, 2, 371–387. [Google Scholar] [CrossRef]
- Federolf, P.A. A Novel Approach to Study Human Posture Control: “Principal Movements” Obtained from a Principal Component Analysis of Kinematic Marker Data. J. Biomech. 2016, 49, 364–370. [Google Scholar] [CrossRef]
- Dingwell, J.B.; Cusumano, J.P.; Cavanagh, P.R.; Sternad, D. Local Dynamic Stability Versus Kinematic Variability of Continuous Overground and Treadmill Walking. J. Biomech. Eng. 2001, 123, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Dingwell, J.B.; Marin, L.C. Kinematic Variability and Local Dynamic Stability of Upper Body Motions When Walking at Different Speeds. J. Biomech. 2006, 39, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Bruijn, S.M.; Meijer, O.G.; Beek, P.J.; Van Dieen, J.H. Assessing the Stability of Human Locomotion: A Review of Current Measures. J. R. Soc. Interface 2013, 10, 20120999. [Google Scholar] [CrossRef] [PubMed]
- England, S.A.; Granata, K.P. The Influence of Gait Speed on Local Dynamic Stability of Walking. Gait Posture 2007, 25, 172–178. [Google Scholar] [CrossRef]
- Hamacher, D.; Singh, N.B.; Van Dieën, J.H.; Heller, M.O.; Taylor, W.R. Kinematic Measures for Assessing Gait Stability in Elderly Individuals: A Systematic Review. J. R. Soc. Interface 2011, 8, 1682–1698. [Google Scholar] [CrossRef] [PubMed]
- Federolf, P.; Tecante, K.; Nigg, B. A Holistic Approach to Study the Temporal Variability in Gait. J. Biomech. 2012, 45, 1127–1132. [Google Scholar] [CrossRef]
- Matcuk, G.R.; Mahanty, S.R.; Skalski, M.R.; Patel, D.B.; White, E.A.; Gottsegen, C.J. Stress Fractures: Pathophysiology, Clinical Presentation, Imaging Features, and Treatment Options. Emerg. Radiol. 2016, 23, 365–375. [Google Scholar] [CrossRef]
- Hoenig, T.; Tenforde, A.S.; Strahl, A.; Rolvien, T.; Hollander, K. Does Magnetic Resonance Imaging Grading Correlate With Return to Sports After Bone Stress Injuries? A Systematic Review and Meta-Analysis. Am. J. Sports Med. 2022, 50, 834–844. [Google Scholar] [CrossRef]
- Mohr, M.; Pieper, R.; Löffler, S.; Schmidt, A.; Federolf, P. Sex-Specific Whole-Body Running Kinematics: Data, Code, and Supplemental Files. Mendeley Data 2021, 2, 521. [Google Scholar] [CrossRef]
- Malinzak, R.A.; Colby, S.M.; Kirkendall, D.T.; Yu, B.; Garrett, W.E. A Comparison of Knee Joint Motion Patterns between Men and Women in Selected Athletic Tasks. Clin. Biomech. 2001, 16, 438–445. [Google Scholar] [CrossRef]
- Ferber, R.; Davis, I.M.C.; Williams, D.S. Gender Differences in Lower Extremity Mechanics during Running. Clin. Biomech. 2003, 18, 350–357. [Google Scholar] [CrossRef]
- Chumanov, E.S.; Wall-Scheffler, C.; Heiderscheit, B.C. Gender Differences in Walking and Running on Level and Inclined Surfaces. Clin. Biomech. 2008, 23, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Maurer, C.; Federolf, P.; von Tscharner, V.; Stirling, L.; Nigg, B.M. Discrimination of Gender-, Speed-, and Shoe-Dependent Movement Patterns in Runners Using Full-Body Kinematics. Gait Posture 2012, 36, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Phinyomark, A.; Hettinga, B.A.; Osis, S.T.; Ferber, R. Gender and Age-Related Differences in Bilateral Lower Extremity Mechanics during Treadmill Running. PLoS ONE 2014, 9, e105246. [Google Scholar] [CrossRef]
- Phinyomark, A.; Osis, S.; Hettinga, B.A.; Leigh, R.; Ferber, R. Gender Differences in Gait Kinematics in Runners with Iliotibial Band Syndrome. Scand. J. Med. Sci. Sport. 2015, 25, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, M.; Ogawa, H.; Shimizu, N.; Kanehisa, H.; Yanai, T.; Kawakami, Y. Gender Differences in Hip and Ankle Joint Kinematics on Knee Abduction during Running. Eur. J. Sport Sci. 2014, 14, S302–S309. [Google Scholar] [CrossRef] [PubMed]
- Willson, J.D.; Loss, J.R.; Willy, R.W.; Meardon, S.A. Sex Differences in Running Mechanics and Patellofemoral Joint Kinetics Following an Exhaustive Run. J. Biomech. 2015, 48, 4155–4159. [Google Scholar] [CrossRef]
- Boyer, K.A.; Freedman Silvernail, J.; Hamill, J. Age and Sex Influences on Running Mechanics and Coordination Variability. J. Sports Sci. 2017, 35, 2225–2231. [Google Scholar] [CrossRef]
- de Leva, P. Adjustments to Zatsiorsky-Seluyanov’s Segment Inertia Parameters. J. Biomech. 1996, 29, 1223–1230. [Google Scholar] [CrossRef]
- Promsri, A.; Haid, T.; Federolf, P. How Does Lower Limb Dominance Influence Postural Control Movements during Single Leg Stance? Hum. Mov. Sci. 2018, 58, 165–174. [Google Scholar] [CrossRef]
- Promsri, A.; Haid, T.; Werner, I.; Federolf, P. Leg Dominance Effects on Postural Control When Performing Challenging Balance Exercises. Brain Sci. 2020, 10, 128. [Google Scholar] [CrossRef] [Green Version]
- Promsri, A.; Federolf, P. Analysis of Postural Control Using Principal Component Analysis: The Relevance of Postural Accelerations and of Their Frequency Dependency for Selecting the Number of Movement Components. Front. Bioeng. Biotechnol. 2020, 8, 480. [Google Scholar] [CrossRef] [PubMed]
- Ó’Reilly, D.; Federolf, P. Identifying Differences in Gait Adaptability across Various Speeds Using Movement Synergy Analysis. PLoS ONE 2021, 16, e0244582. [Google Scholar] [CrossRef] [PubMed]
- Longo, A.; Federolf, P.; Haid, T.; Meulenbroek, R. Effects of a Cognitive Dual Task on Variability and Local Dynamic Stability in Sustained Repetitive Arm Movements Using Principal Component Analysis: A Pilot Study. Exp. Brain Res. 2018, 236, 1611–1619. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.; Swift, J.B.; Swinney, H.L.; Vastano, J.A. Determining Lyapunov Exponents from a Time Series. Phys. D Nonlinear Phenom. 1985, 16, 285–317. [Google Scholar] [CrossRef]
- Kantz, H. A Robust Method to Estimate the Maximal Lyapunov Exponent of a Time Series. Phys. Lett. A 1994, 185, 77–87. [Google Scholar] [CrossRef]
- Cappellini, G.; Ivanenko, Y.P.; Poppele, R.E.; Lacquaniti, F. Motor Patterns in Human Walking and Running. J. Neurophysiol. 2006, 95, 3426–3437. [Google Scholar] [CrossRef]
- Legramandi, M.A.; Schepens, B.; Cavagna, G.A. Running Humans Attain Optimal Elastic Bounce in Their Teens. Sci. Rep. 2013, 3, 1310. [Google Scholar] [CrossRef]
- Koller, A.; Fuchs, B.; Leichtfried, V.; Schobersberger, W. Decrease in Eccentric Quadriceps and Hamstring Strength in Recreational Alpine Skiers after Prolonged Skiing. BMJ Open Sport Exerc. Med. 2015, 1, bmjsem-2015. [Google Scholar] [CrossRef]
- Mann, R.; Sprague, P. A Kinetic Analysis of the Ground Leg during Sprint Running. Res. Q. Exerc. Sport 1980, 51, 334–348. [Google Scholar] [CrossRef]
- Higashihara, A.; Nagano, Y.; Takahashi, K.; Fukubayashi, T. Effects of Forward Trunk Lean on Hamstring Muscle Kinematics during Sprinting. J. Sports Sci. 2015, 33, 1366–1375. [Google Scholar] [CrossRef]
- Harbo, T.; Brincks, J.; Andersen, H. Maximal Isokinetic and Isometric Muscle Strength of Major Muscle Groups Related to Age, Body Mass, Height, and Sex in 178 Healthy Subjects. Eur. J. Appl. Physiol. 2012, 112, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Federolf, P.; Angulo-Barroso, R.M.; Busquets, A.; Ferrer-Uris, B.; Gløersen, Ø.; Mohr, M.; Ó’Reilly, D.; Promsri, A.; van Andel, S.; Wachholz, F.; et al. Letter to the Editor Regarding “The Assessment of Center of Mass and Center of Pressure during Quiet Stance: Current Applications and Future Directions”. J. Biomech. 2021, 128, 110729. [Google Scholar] [CrossRef] [PubMed]
- Ivanenko, Y.P.; Poppele, R.E.; Lacquaniti, F. Motor Control Programs and Walking. Neuroscientist 2006, 12, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, C.E.; Beattie, P.F.; Sacko, R.S.; Hand, A. Risk Factors Associated with Non-Contact Anterior Cruciate Ligament Injury: A Systematic Review. Int. J. Sports Phys. Ther. 2018, 13, 575–587. [Google Scholar] [CrossRef]
| Total | Male (n = 10) | Female (n = 12) | |
|---|---|---|---|
| Age (yrs.) | 25.7 ± 3.3 | 26.8 ± 2.8 | 24.6 ± 3.5 |
| Weight (kg) | 67.9 ± 9.3 | 74.7 ± 6.8 | 61.2 ± 5.2 * |
| Height (cm) | 174.7 ± 8.5 | 180.3 ± 4.8 | 169.2 ± 7.1 * |
| Body mass index (kg/m2) | 22.3 ± 2.1 | 23.0 ± 2.2 | 21.4 ± 2.0 |
| Preferred running speed (km/h) | 10.4 ± 1.0 | 10.8 ± 1.1 | 10.1 ± 0.8 |
| Physical activity (h/week) | (%) | (%) | (%) |
| 10–20 | 34.8 | 60.0 | 15.4 |
| 5–10 | 52.2 | 30.0 | 69.2 |
| 1–5 | 13.0 | 10.0 | 15.4 |
| Running habits (h/week) | (%) | (%) | (%) |
| 5–10 | 13.6 | 0 | 25 |
| 1–5 | 72.7 | 80 | 66.7 |
| 0 | 13.6 | 20 | 8.3 |
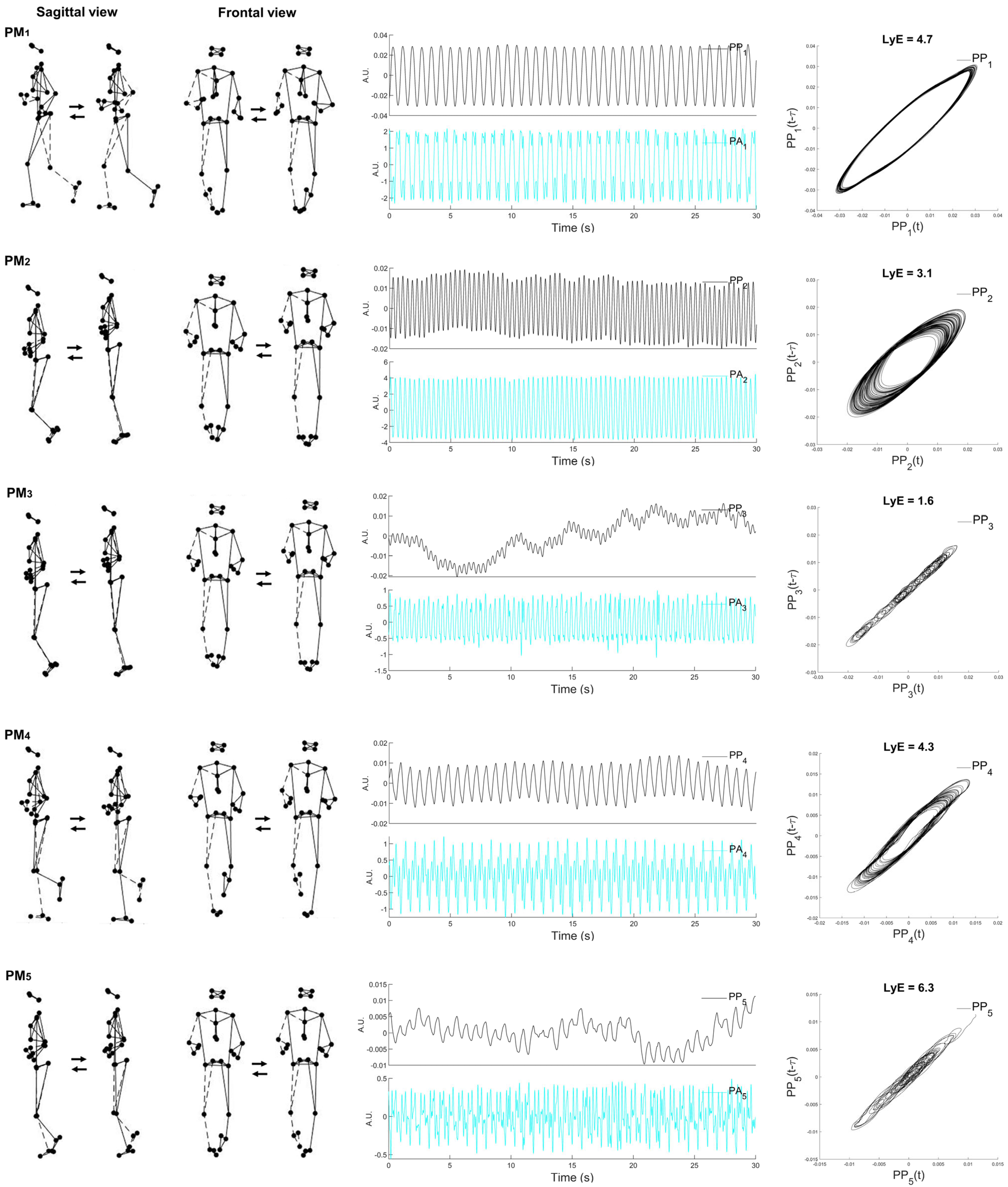
| PMk | Eigenvalues (%) | Descriptive Movements |
|---|---|---|
| 1 | 60.7 | The swing phase: anti-phase arm and leg movements in the sagittal plane combined with trunk rotation |
| 2 | 16.1 | Both hip and knee flexion and extension movements combined with whole-body movements in the vertical direction |
| 3 | 11.3 | Both knee flexion and extension combined with the anteroposterior sliding of the treadmill |
| 4 | 5.2 | The mid-stance phase: anti-phase arm and leg movements |
| 5 | 4.0 | The mid-stance phase: anti-phase arm and leg movements combined with the mediolateral sliding of the treadmill |
| PPk_LyE | Male | Female | p-Value | Effect Size | Observed Power |
|---|---|---|---|---|---|
| 1 | 5.1 ± 0.8 | 5.5 ± 0.9 | 0.312 | 0.470 | 0.613 |
| 2 | 3.8 ± 0.9 | 3.8 ± 0.5 | 0.913 | 0.000 | 0.500 |
| 3 | 2.2 ± 0.8 | 2.3 ± 0.4 | 0.642 | 0.158 | 0.514 |
| 4 | 3.5 ± 0.6 | 4.2 ± 0.7 | 0.015 * | 1.074 | 0.845 |
| 5 | 5.7 ± 2.0 | 7.5 ± 2.0 | 0.047 * | 0.900 | 0.790 |
| PPk_rSTD | Male | Female | p-Value | Effect Size | Observed Power |
| 1 | 26.9 ± 1.9 | 29.4 ± 1.9 | 0.005 * | 1.315 | 0.903 |
| 2 | 14.4 ± 1.3 | 14.6 ± 0.8 | 0.652 | 0.185 | 0.519 |
| 3 | 12.4 ± 3.4 | 11.4 ± 2.7 | 0.461 | 0.326 | 0.558 |
| 4 | 8.5 ± 0.6 | 8.0 ± 0.8 | 0.102 | 0.707 | 0.715 |
| 5 | 7.6 ± 1.3 | 6.6 ± 1.1 | 0.053 | 0.830 | 0.764 |
| PAk_RMS | Male | Female | p-Value | Effect Size | Observed Power |
| 1 | 1.7 ± 0.2 | 1.9 ± 0.1 | 0.016 * | 1.265 | 0.893 |
| 2 | 3.2 ± 0.2 | 3.3 ± 0.2 | 0.085 | 0.500 | 0.625 |
| 3 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.919 | 0.000 | 0.500 |
| 4 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.337 | 0.000 | 0.500 |
| 5 | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.115 | 1.000 | 0.824 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Promsri, A. Sex Difference in Running Stability Analyzed Based on a Whole-Body Movement: A Pilot Study. Sports 2022, 10, 138. https://doi.org/10.3390/sports10090138
Promsri A. Sex Difference in Running Stability Analyzed Based on a Whole-Body Movement: A Pilot Study. Sports. 2022; 10(9):138. https://doi.org/10.3390/sports10090138
Chicago/Turabian StylePromsri, Arunee. 2022. "Sex Difference in Running Stability Analyzed Based on a Whole-Body Movement: A Pilot Study" Sports 10, no. 9: 138. https://doi.org/10.3390/sports10090138
APA StylePromsri, A. (2022). Sex Difference in Running Stability Analyzed Based on a Whole-Body Movement: A Pilot Study. Sports, 10(9), 138. https://doi.org/10.3390/sports10090138






