Recombinant Human Erythropoietin Effects on Well-Trained Athletes’ Endurance Performance: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Data Collection and Analysis
2.3. Inclusion and Exclusion Criteria
2.4. Assessment of Methodological Quality
3. Results
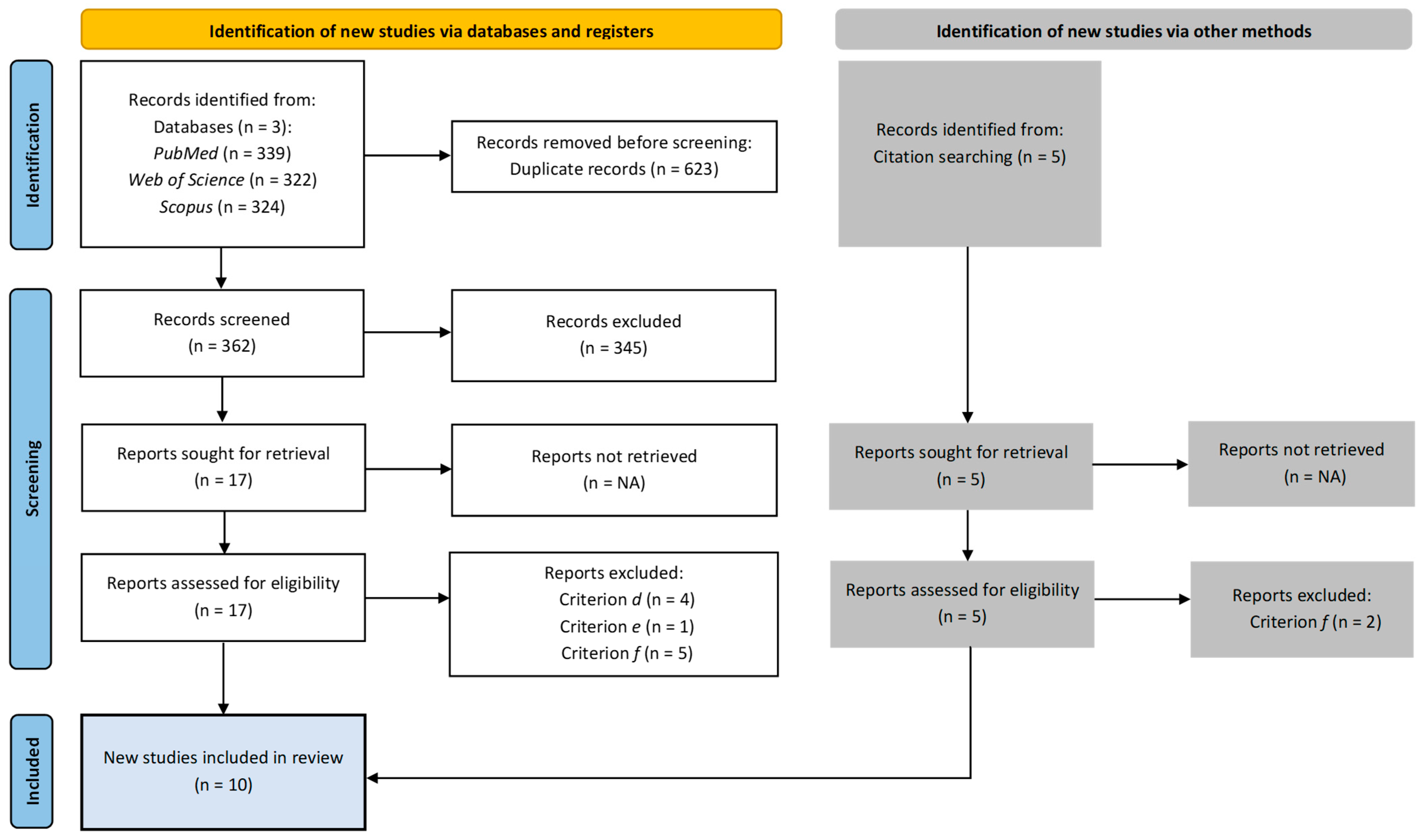
3.1. Study Selection
3.2. Methodological Quality and Level of Evidence
3.3. Tools and Protocols
3.4. Study Characteristics
3.5. Key Findings
4. Discussion
4.1. Interpretation of Findings
4.2. Limitations
4.3. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, T.Y.; Lai, Y.F.; Chen, Y.H.; Lu, D.W. The latest evidence of erythropoietin in the treatment of glaucoma. Int. J. Mol. Sci. 2022, 23, 16038. [Google Scholar] [CrossRef] [PubMed]
- Jelkmann, W. Recombinant human erythropoietin and its analogues. In Introduction to Biological and Small Molecule Drug Research and Development, 1st ed.; Ganellin, R., Roberts, S., Jefferis, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 1, pp. 307–326. [Google Scholar]
- Yasuoka, Y.; Izumi, Y.; Fukuyama, T.; Inoue, H.; Oshima, T.; Yamazaki, T.; Uematsu, T.; Kobayashi, N.; Shimada, Y.; Nagaba, Y.; et al. Effects of angiotensin II on erythropoietin production in the kidney and liver. Molecules 2021, 26, 5399. [Google Scholar] [CrossRef]
- Jelkmann, W. Regulation of erythropoietin production. J. Physiol. 2011, 589, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Bahlmann, F.H.; Fliser, D. Erythropoietin and renoprotection. Curr. Opin. Nephrol. Hypertens. 2009, 18, 15–20. [Google Scholar] [CrossRef]
- Mujika, I.; Padilla, S. Physiological and performance characteristics of male professional road cyclists. Sports Med. 2001, 31, 479–487. [Google Scholar] [CrossRef]
- Joyner, M.J.; Coyle, E.F. Endurance exercise performance: The physiology of champions. J. Physiol 2008, 586, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, M.N.; Raven, P.B.; Snell, P.G.; Stray-Gundersen, J.; Levine, B.D. Maximal oxygen uptake as a parametric measure of cardiorespiratory capacity. Med. Sci. Sports Exerc. 2007, 39, 103–107. [Google Scholar]
- Peyré-Tartaruga, L.A.; Machado, E.; Guimarães, P.; Borba, E.; Tartaruga, M.P.; Buzzachera, C.F.; Correale, L.; Lanferdini, F.J.; da Silva, E.S. Biomechanical, physiological and anthropometrical predictors of performance in recreational runners. PeerJ 2024, 12, e16940. [Google Scholar] [CrossRef]
- Durussel, J.; Daskalaki, E.; Anderson, M.; Chatterji, T.; Wondimu, D.H.; Padmanabhan, N.; Patel, R.K.; McClure, J.D.; Pitsiladis, Y.P. Haemoglobin mass and running time trial performance after recombinant human erythropoietin administration in trained men. PLoS ONE 2013, 8, e56151. [Google Scholar] [CrossRef]
- Annaheim, S.; Jacob, M.; Krafft, A.; Breymann, C.; Rehm, M.; Boutellier, U. RhEPO improves time to exhaustion by non-hematopoietic factors in humans. Eur. J. Appl. Physiol. 2016, 116, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Haile, D.W.; Durussel, J.; Mekonen, W.; Ongaro, N.; Anjila, E.; Mooses, M.; Daskalaki, E.; Mooses, K.; Mcclure, J.D.; Sutehall, S.; et al. Effects of EPO on blood parameters and running performance in Kenyan athletes. Med. Sci. Sports Exerc. 2019, 51, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.B.; Graae, J.; Bejder, J.; Bonne, T.C.; Seier, S.; Debertin, M.; Eibye, K.; Hostrup, M.; Nordsborg, N.B. Micro-doses of recombinant human erythropoietin enhance time trial performance in trained males and females. Med. Sci. Sports Exerc. 2023, 55, 311–321. [Google Scholar] [CrossRef]
- Perrey, S. Do we perform better when we increase red blood cells? Lancet Haematol. 2017, 4, e344–e345. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Konstantinopoulos, P.A.; Papailiou, J.; Kandarakis, S.A.; Andreopoulos, A.; Sykiotis, G.P. Erythropoietin abuse and erythropoietin gene doping: Detection strategies in the genomic era. Sports Med. 2005, 35, 831–840. [Google Scholar] [CrossRef]
- Robinson, N.; Giraud, S.; Saudan, C.; Baume, N.; Avois, L.; Mangin, P.; Saugy, M. Erythropoietin and blood doping. Br. J. Sports Med. 2006, 40, i30–i34. [Google Scholar] [CrossRef] [PubMed]
- Heuberger, J.A.; Cohen Tervaert, J.M.; Schepers, F.M.; Vliegenthart, A.D.; Rotmans, J.I.; Daniels, J.M.; Burggraaf, J.; Cohen, A.F. Erythropoietin doping in cycling: Lack of evidence for efficacy and a negative risk-benefit. Br. J. Clin. Pharmacol. 2013, 75, 1406–1421. [Google Scholar] [CrossRef]
- Trinh, K.V.; Diep, D.; Chen, K.J.Q.; Huang, L.; Gulenko, O. Effect of erythropoietin on athletic performance: A systematic review and meta-analysis. BMJ Open Sport Exerc. Med. 2020, 6, e000716. [Google Scholar] [CrossRef]
- Bird, S.R.; Goebel, C.; Burke, L.M.; Greaves, R.F. Doping in sport and exercise: Anabolic, ergogenic, health and clinical issues. Ann. Clin. Biochem. 2016, 53, 196–221. [Google Scholar] [CrossRef]
- Sgrò, P.; Sansone, M.; Sansone, A.; Romanelli, F.; Di Luigi, L. Effects of erythropoietin abuse on exercise performance. Phys. Sportsmed. 2018, 46, 105–115. [Google Scholar] [CrossRef]
- Johnson, N.; Phillips, M. Rayyan for systematic reviews. J. Electron. Resour. Libr. 2018, 30, 46–48. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Edvardsen, E.; Hansen, B.H.; Holme, I.M.; Dyrstad, S.M.; Anderssen, S.A. Reference values for cardiorespiratory response and fitness on the treadmill in a 20-to 85-year-old population. Chest 2013, 144, 241–248. [Google Scholar] [CrossRef]
- Howick, J.; Chalmers, I.; Glasziou, P.; Greenhalgh, T.; Heneghan, C.; Liberati, A.; Moschetti, I.; Phillips, B.; Thornton, H. The 2011 Oxford CEBM Level of Evidence. Available online: http://www.cebm.net/index.aspx?o=5653 (accessed on 16 January 2025).
- De Morton, N.A. The PEDro scale is a valid measure of the methodological quality of clinical trials: A demographic study. Aust. J. Physiother. 2009, 55, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Baz-Valle, E.; Fontes-Villalba, M.; Santos-Concejero, J. Total number of sets as a training volume quantification method for muscle hypertrophy: A systematic review. J. Strength Cond. Res. 2021, 35, 870–878. [Google Scholar] [CrossRef]
- Caillaud, C.; Connes, P.; Ben Saad, H.; Mercier, J. Erythropoietin enhances whole body lipid oxidation during prolonged exercise in humans. J. Physiol. Biochem. 2015, 71, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.; Woolford, S.M.; Eastwood, A.; Sharpe, K.; Barnes, P.G.; Gore, C.J. Temporal changes in physiology and haematology in response to high-and micro-doses of recombinant human erythropoietin. Drug Test. Anal. 2017, 9, 1561–1571. [Google Scholar] [CrossRef] [PubMed]
- Guadalupe-Grau, A.; Plenge, U.; Helbo, S.; Kristensen, M.; Andersen, P.R.; Fago, A.; Belhage, B.; Dela, F.; Helge, J.W. Effects of an 8-weeks erythropoietin treatment on mitochondrial and whole body fat oxidation capacity during exercise in healthy males. J. Sports Sci. 2015, 33, 570–578. [Google Scholar] [CrossRef]
- Haider, T.; Diaz, V.; Albert, J.; Alvarez-Sanchez, M.; Thiersch, M.; Maggiorini, M.; Hilty, M.P.; Spengler, C.M.; Gassmann, M. A single 60.000 IU dose of erythropoietin does not improve short-term aerobic exercise performance in healthy subjects: A randomized, double-blind, placebo-controlled crossover trial. Front. Physiol. 2020, 11, 537389. [Google Scholar] [CrossRef]
- Heuberger, J.A.; Rotmans, J.I.; Gal, P.; Stuurman, F.E.; van’t Westende, J.; Post, T.E.; Daniels, J.M.; Moerland, M.; van Veldhoven, P.L.; de Kam, M.L.; et al. Effects of erythropoietin on cycling performance of well trained cyclists: A double-blind, randomised, placebo-controlled trial. Lancet Haematol. 2017, 4, e374–e386. [Google Scholar] [CrossRef]
- Sutehall, S.; Martin-Rincon, M.; Wang, G.; Shurlock, J.; Durussel, J.; Mooses, M.; Wang, J.; Pitsiladis, Y.P. The performance effects of microdose recombinant human erythropoietin administration and carbon monoxide rebreathing. Curr. Sports Med. Rep. 2018, 17, 457–466. [Google Scholar] [CrossRef]
- Larsen, M.S.; Vissing, K.; Thams, L.; Sieljacks, P.; Dalgas, U.; Nellemann, B.; Christensen, B. Erythropoietin administration alone or in combination with endurance training affects neither skeletal muscle morphology nor angiogenesis in healthy young men. Exp. Physiol. 2014, 99, 1409–1420. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, T.S.; Kahn, M.J. Blood doping: Then and now. A narrative review of the history, science and efficacy of blood doping in elite sport. Blood Rev. 2020, 39, 100632. [Google Scholar] [CrossRef]
- Totonchi, Z.; Noohi, F.; Futuhi, F.; Azarfarin, R.; Radbin, P. Effects of recombinant erythropoietin on hemoglobin levels and blood transfusion needs in patients with preoperative anemia undergoing cardiac surgery. Ann. Card. Anaesth. 2022, 25, 466–471. [Google Scholar] [CrossRef]
- Kasiak, P.; Kowalski, T.; Faiss, R.; Malczewska-Lenczowska, J. Hemoglobin mass is accurately predicted in endurance athletes. J. Sports Sci. 2025. ahead-of-print. [Google Scholar]
- Schmidt, W.; Prommer, N. Impact of alterations in total hemoglobin mass on VO2max. Exerc. Sport Sci. Rev. 2010, 38, 68–75. [Google Scholar] [CrossRef]
- Lundby, C.; Achman-Andersen, N.J.; Thomsen, J.J.; Norgaard, A.M.; Robach, P. Testing for recombinant human erythropoietin in urine: Problems associated with current anti-doping testing. J. Appl. Physiol. 2008, 105, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Durussel, J.; Haile, D.W.; Mooses, K.; Daskalaki, E.; Beattie, W.; Mooses, M.; Mekonen, W.; Ongaro, N.; Anjila, E.; Patel, R.K.; et al. Blood transcriptional signature of recombinant human erythropoietin administration and implications for antidoping strategies. Physiol. Genom. 2016, 48, 202–209. [Google Scholar] [CrossRef]
- Athanasiadou, I.; Dokoumetzidis, A.; Voss, S.C.; El Saftawy, W.; Al-Maadheed, M.; Valsami, G.; Georgakopoulos, C. Hyperhydration effect on pharmacokinetic parameters and detection sensitivity of recombinant human erythropoietin in urine and serum doping control analysis of males. J. Pharm. Sci. 2019, 108, 2162–2172. [Google Scholar] [CrossRef] [PubMed]
- Ashenden, M.; Varlet-Marie, E.; Lasne, F.; Audran, M. The effects of microdose recombinant human erythropoietin regimens in athletes. Haematologica 2006, 91, 1143–1144. [Google Scholar]
- Ashenden, M.; Gough, C.E.; Garnham, A.; Gore, C.J.; Sharpe, K. Current markers of the Athlete Blood Passport do not flag microdose EPO doping. Eur. J. Appl. Physiol. 2011, 111, 2307–2314. [Google Scholar] [CrossRef]
- Agoston, R.; Izake, E.L.; Sivanesan, A.; Lott, W.B.; Sillence, M.; Steel, R. Rapid isolation and detection of erythropoietin in blood plasma by magnetic core gold nanoparticles and portable Raman spectroscopy. Nanomedicine 2016, 12, 633–641. [Google Scholar] [CrossRef]
| Line | Keywords | Operators | |
|---|---|---|---|
| Reference to erythropoietin | Erythropoietin, EPO | OR | |
| Reference to performance | Performance, endurance | OR | AND |
| Reference to subjects | Athletes | OR | |
| Author (Year) | Items in the PEDro Scale | Evidence Level | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Total | Rating | ||
| Andersen et al. (2023) [13] | Yes | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 9 | Excellent | 1b |
| Annaheim et al. (2016) [11] | Yes | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 9 | Excellent | 1b |
| Caillaud et al. (2015) [27] | Yes | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 6 | Good | 2b |
| Clark et al. (2017) [28] | Yes | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 9 | Excellent | 1b |
| Durussel et al. (2013) [10] | Yes | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 4 | Fair | 2b |
| Guadalupe-Grau et al. (2015) [29] | Yes | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 3 | Poor | 2b |
| Haider et al. (2020) [30] | Yes | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 9 | Excellent | 1b |
| Haile et al. (2019) [12] | Yes | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 5 | Fair | 2b |
| Heuberger et al. (2017) [31] | Yes | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 9 | Excellent | 1b |
| Sutehall et al. (2018) [32] | Yes | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 9 | Excellent | 1b |
| TOTAL | 7.2 | Good | 6-1b/4-2b | |||||||||||
| Author (Year), Country | Sample Size (Sex), Groups | Age (Years), Participants | Intervention (Duration and Dose of rHuEPO) | Outcomes |
|---|---|---|---|---|
| Andersen et al. (2023) [13], Denmark | n = 48 (M/F) rHuEPO, n = 24 Placebo, n = 24 | rHuEPO: 27.0 ± 6.0 Placebo: 26.0 ± 4.0 Recreational to well-trained athletes |
| tHb: +6.7% Hct: +2.6% VO2peak: +4.2% Ppeak: +2.9% MPO: +4.1% tlim: +4.3% |
| Annaheim et al. (2016) [11], Switzerland | n = 40 (M) H, n = 10 MED, n = 10 L, n = 10 Placebo, n = 10 | H: 35.7 ± 8.0 MED: 35.7 ± 5.4 L: 31.4 ± 7.7 Placebo: 31.2 ± 6.0 Endurance-trained athletes |
| Hb: L +8.5%, MED +8.3%, and H +14% Hct: L +11.8%, MED +11.4%, and H +18.8% VO2max: L +2.6%, MED +5.7%, and H +5.6% tlim: L +16.7% (no sig.), MED +44.8%, and H +69.7% Pmax: L +1.9% (no sig.), MED +2.7% (sig. only with respect to baseline values), and H +5.8% RPE in Pmax: L unchanged, MED +3.5%, and H +5.4% (no sig. in all groups) |
| Caillaud et al. (2015) [27], France | n = 12 (M) rHuEPO, n = 6 Placebo, n = 6 | rHuEPO: 26.8 ± 4.1 Placebo: 26.8 ± 6.0 Healthy aerobically trained athletes |
| Hb: +9.5% Hct: +12.3% VO2max: +6.3% MPO: +8.9% (no sig.) RER: −3.3% Carbohydrate oxidation rate: −18.3% |
| Clark et al. (2017) [28], Australia | n = 24 (M/F) H, n = 8 HP, n = 4 C, n = 8 CP, n = 4 | H: 28.1 ± 4.6 HP: 34.2 ± 13.4 C: 36.3 ± 8.5 CP: 33.5 ± 11.4 Healthy recreational athletes |
| tHb: H and C +18.4% (week 2), remains elevated at weeks 5 and 6, respectively Hct: H and C +7.9% (week 2), remains elevated at weeks 5 and 6, respectively VO2peak: H +6.2% and C +6.1% (week 2), remains elevated (+5.9%) in week 5 in group C TTE: H and C +28 s (week 2) and also increases by 28 s at week 5 in group C (both results not sig., but with a tendency to increase) [La−]: H and C −2.2 mmol·L−1 (week 2) and remains at −2.3 mmol·L−1 at week 6 in group H (sig. in both cases only with respect to baseline values) |
| Durussel et al. (2013) [10], UK | n = 19 (M) Runners, n = 10 Other activities, n = 9 | Runners and other activities: 26.0 ± 4.5 Endurance-trained athletes |
| tHb: +19.7% (week 5) and +7.9% (week 8) VO2max: +8.4% (week 5) and +3.6% (week 8) Time in 3000 m:
|
| Guadalupe-Grau et al. (2015) [29], Denmark | n = 6 (M) | 21.0 ± 2.0 Healthy athletes |
| Hb: +12.4% VO2max: +8.2% Fatmax: +8.3% Mb: +9.4% CS activity: +7.8% Capillaries per area: +13.7% |
| Haider et al. (2020) [30], Switzerland | n = 29 (M/F) 4 groups: rHuEPO males and females (rHuEPOm and rHuEPOf) and placebo males and females Cross-over study | 25.0 ± 3.0 Healthy athletes |
| Hb: Placebo 142 g·L−1 vs. rHuEPO 144 g·L−1 Hct: Placebo 41.8% vs. rHuEPO 42.4% VO2peak: Placebo 45.1 mL·kg−1·min−1 vs. rHuEPO 46.1 mL·kg−1·min−1 Ppeak: Placebo 3.5 W·kg−1 vs. rHuEPO 3.5 W·kg−1 Total distance covered: Placebo 7.4 km vs. rHuEPO 7.4 km MPO: Placebo 175 W vs. rHuEPO 175 W Note: No significant differences between groups in all parameters analyzed |
| Haile et al. (2019) [12], UK | n = 39 (M) Kenyan, n = 20 Caucasian, n = 19 | Kenyan: 26.4 ± 4.1 Caucasian: 26.0 ± 4.5 Endurance runners |
| Hb: Kenyan +10.1% (week 4) and +5.9% (sig. only with respect to baseline values) (week 8), Caucasian +16.2% (week 4) and +8.2% (week 8) Hct: Kenyan +12.1% (week 4) and +5.1% (week 8), Caucasian +18.6% (week 4) and +8.7% (week 8) VO2max: Kenyan +5.8% (week 4) and +2.6% (no sig.) (week 8), Caucasian +8.1% (week 4) and +4.4% (week 8) (Kenyan week 4 and Caucasian weeks 4 and 8, sig. only with respect to baseline values) Time in 3000 m: Kenyan −4.6% (week 4) and −3.3% (week 8), Caucasian −5.7% (week 4) and −3.4% (week 8) (all values analyzed sig. only with respect to baseline values) |
| Heuberger et al. (2017) [31], Netherlands | n = 48 (M) rHuEPO, n = 24 Placebo, n = 24 | rHuEPO: 33.5 [22.0–48.0] Placebo: 33.5 [20.0–50.0] Healthy, well-trained but non-professional cyclists |
| Hb: +12% Hct: +16% VO2max: Placebo +3.8% and rHuEPO +10% Pmax: Placebo +1.8% and rHuEPO +5.4% Gross efficiency: Placebo −0.5% and rHuEPO −0.9% MPO: Placebo +4.6% and rHuEPO +5.4% Mont Ventoux climbing time: Placebo 1 h 40 min 15 s and rHuEPO 1 h 40 min 32 s Note: Significant differences between groups only exist in Hb, Hct, VO2max, and Pmax |
| Sutehall et al. (2018) [32], UK | n = 14 (M) rHuEPO, n = 7 Placebo, n = 7 | rHuEPO and placebo: 30.0 ± 4.0 Endurance-trained athletes |
| tHb: +11.3% (day 38 post first rHuEPO injection) and +2.3% (day 21 post last rHuEPO injection) VO2max: +3.9% VT: No significant results RSA: No significant results in anaerobic parameters, the only exception being the 7 sprint, where the time to reach maximum power was significantly lower in rHuEPO group (−0.67 s) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alberdi-Garciandia, A.; Santos-Concejero, J. Recombinant Human Erythropoietin Effects on Well-Trained Athletes’ Endurance Performance: A Systematic Review. Sports 2025, 13, 78. https://doi.org/10.3390/sports13030078
Alberdi-Garciandia A, Santos-Concejero J. Recombinant Human Erythropoietin Effects on Well-Trained Athletes’ Endurance Performance: A Systematic Review. Sports. 2025; 13(3):78. https://doi.org/10.3390/sports13030078
Chicago/Turabian StyleAlberdi-Garciandia, Aitor, and Jordan Santos-Concejero. 2025. "Recombinant Human Erythropoietin Effects on Well-Trained Athletes’ Endurance Performance: A Systematic Review" Sports 13, no. 3: 78. https://doi.org/10.3390/sports13030078
APA StyleAlberdi-Garciandia, A., & Santos-Concejero, J. (2025). Recombinant Human Erythropoietin Effects on Well-Trained Athletes’ Endurance Performance: A Systematic Review. Sports, 13(3), 78. https://doi.org/10.3390/sports13030078







