Effect of Aluminum on Microstructure and High-Temperature Oxidation Resistance of Austenitic Heat-Resistant Steel
Abstract
:1. Introduction
2. Experiment
3. Results and Discussion
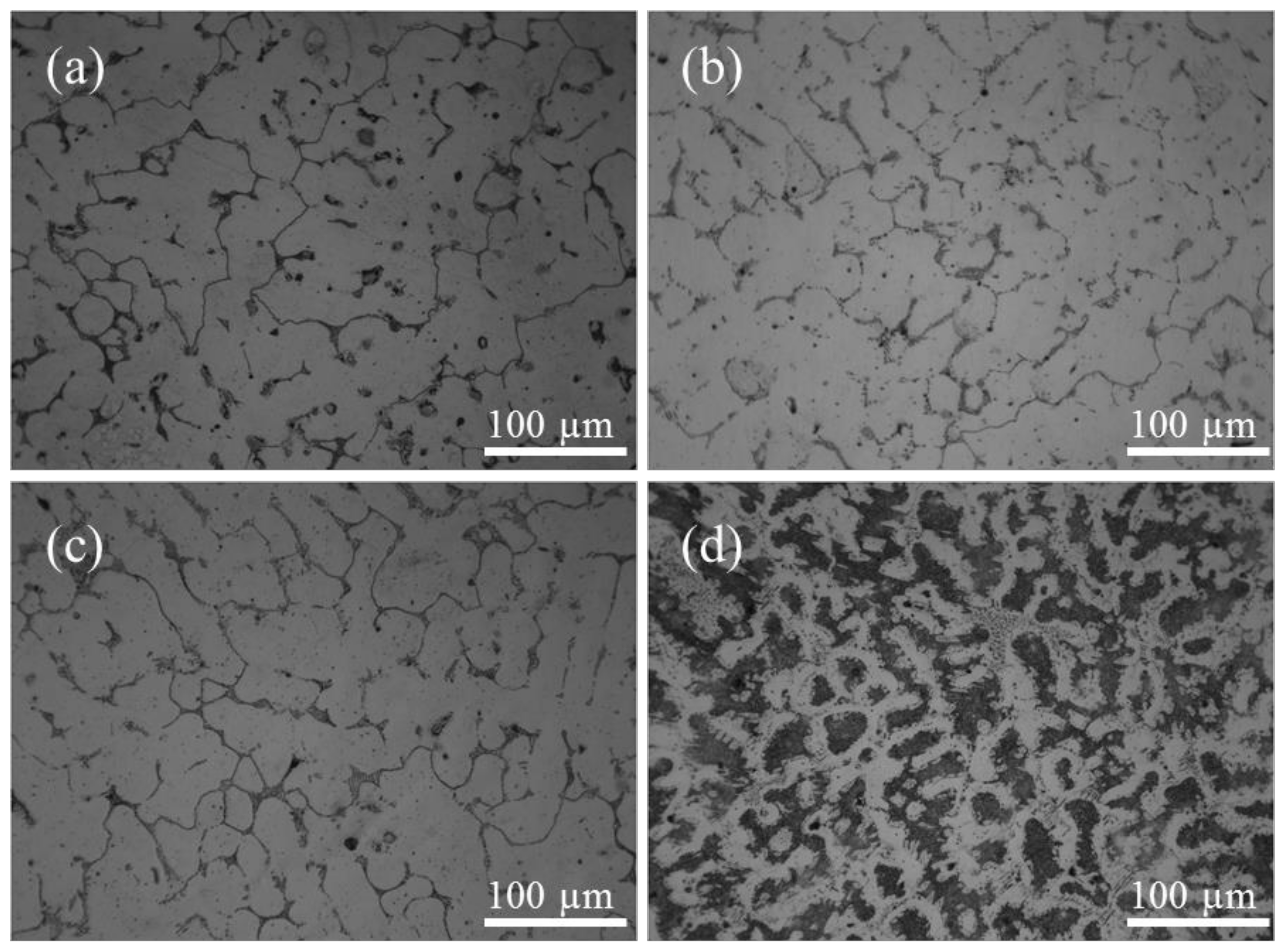
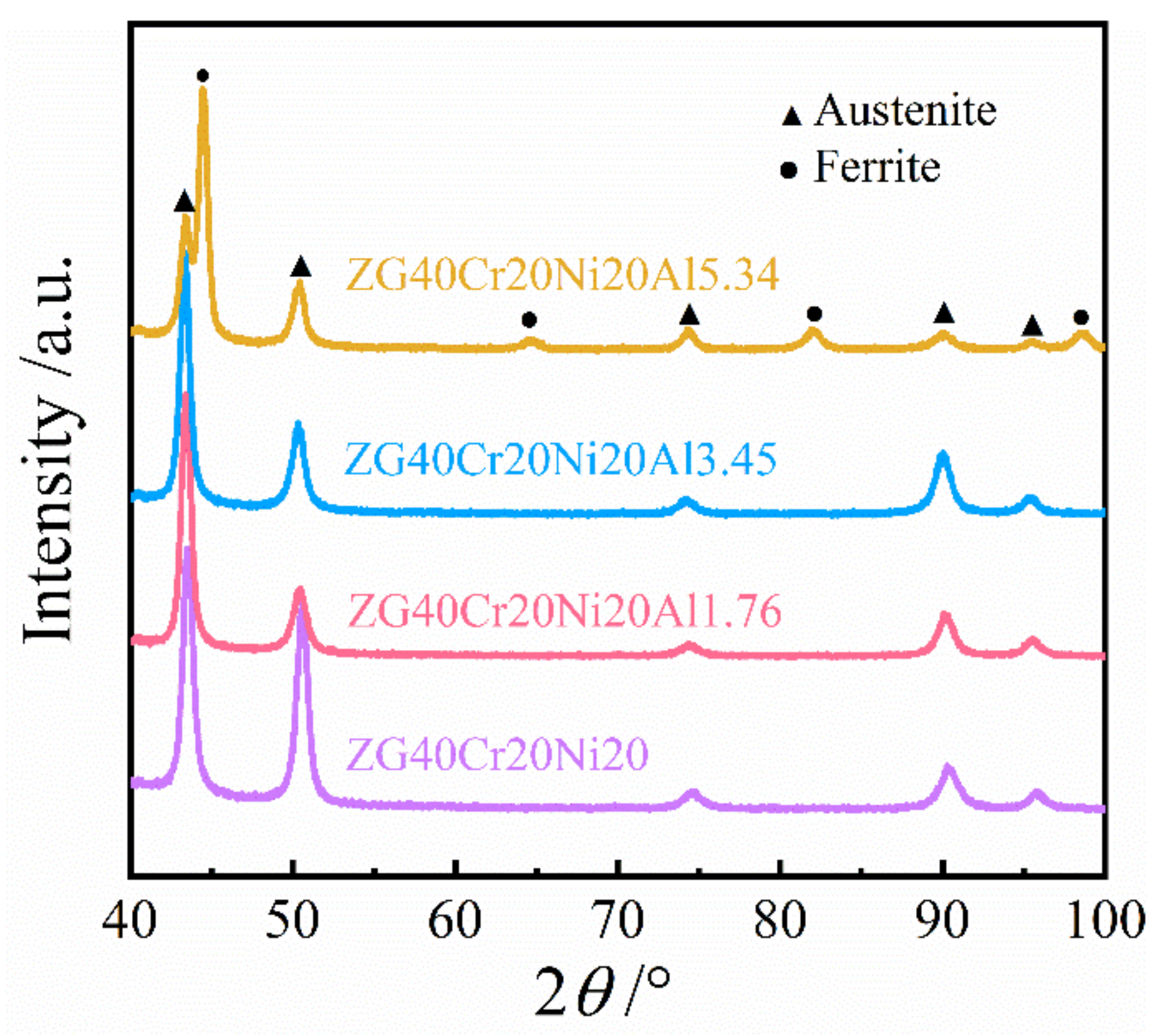
3.1. Microstructures of Steels
3.2. Oxidation Kinetic Analysis
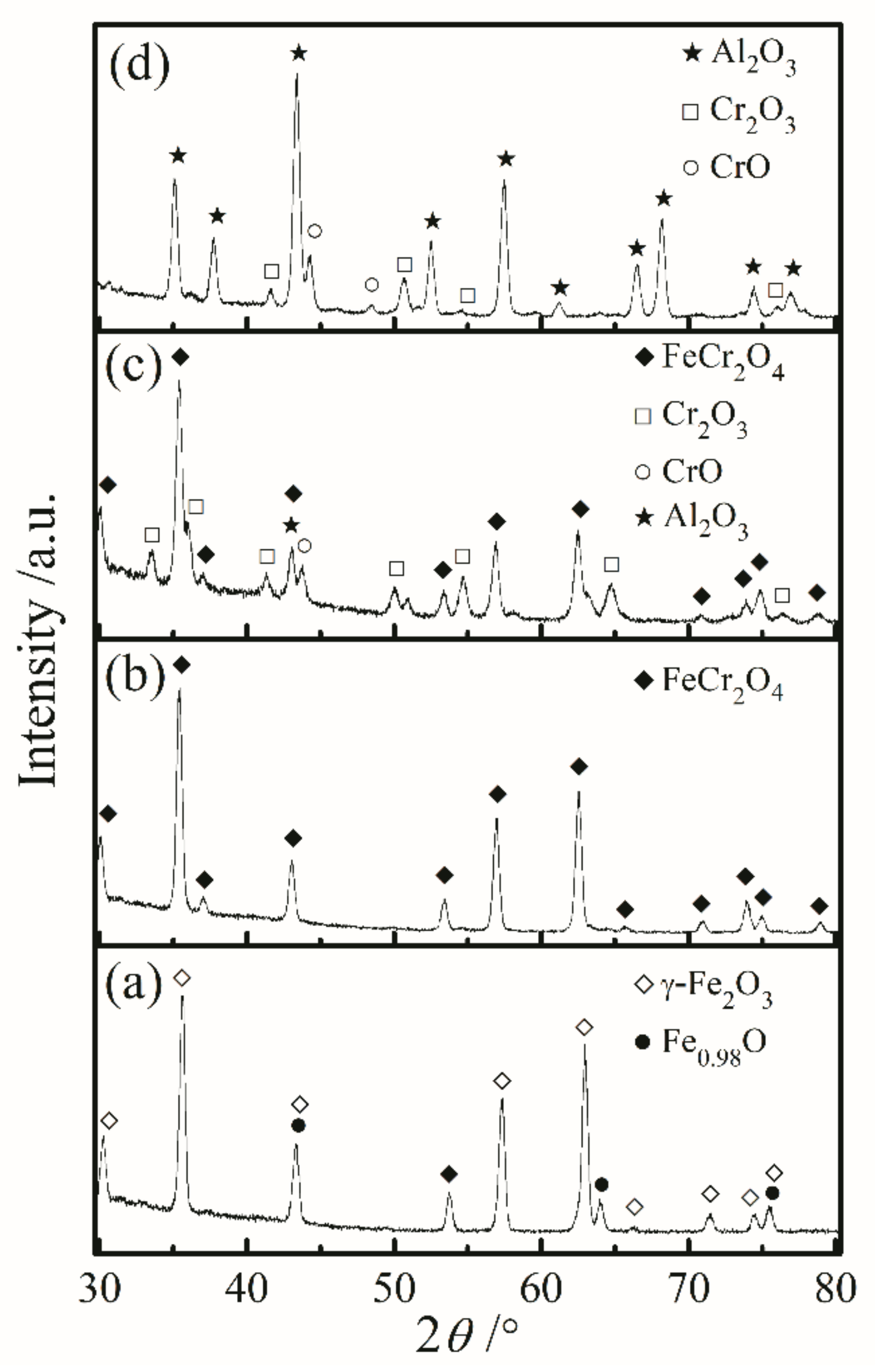
3.3. Surface Morphology and Phase Composition of Oxide
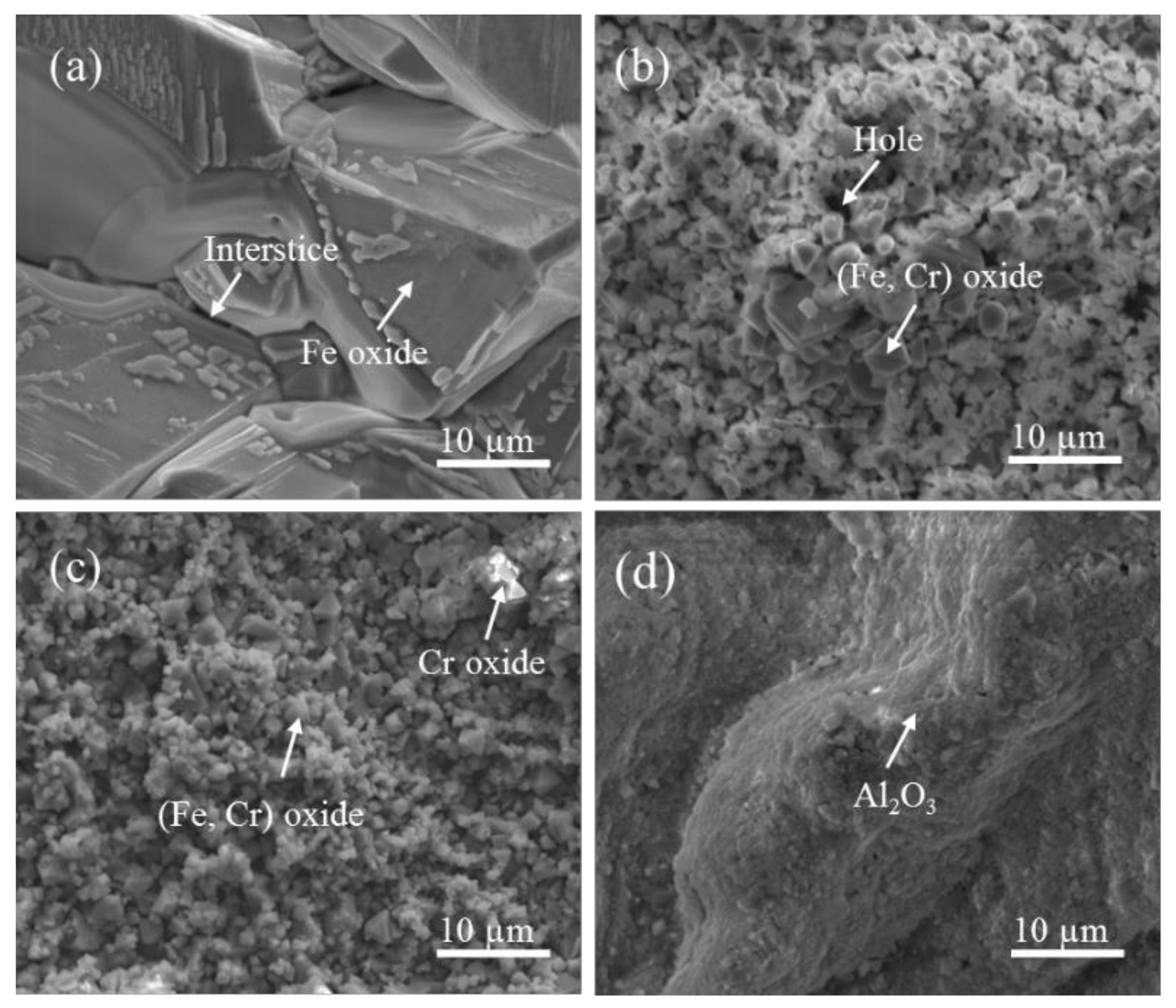
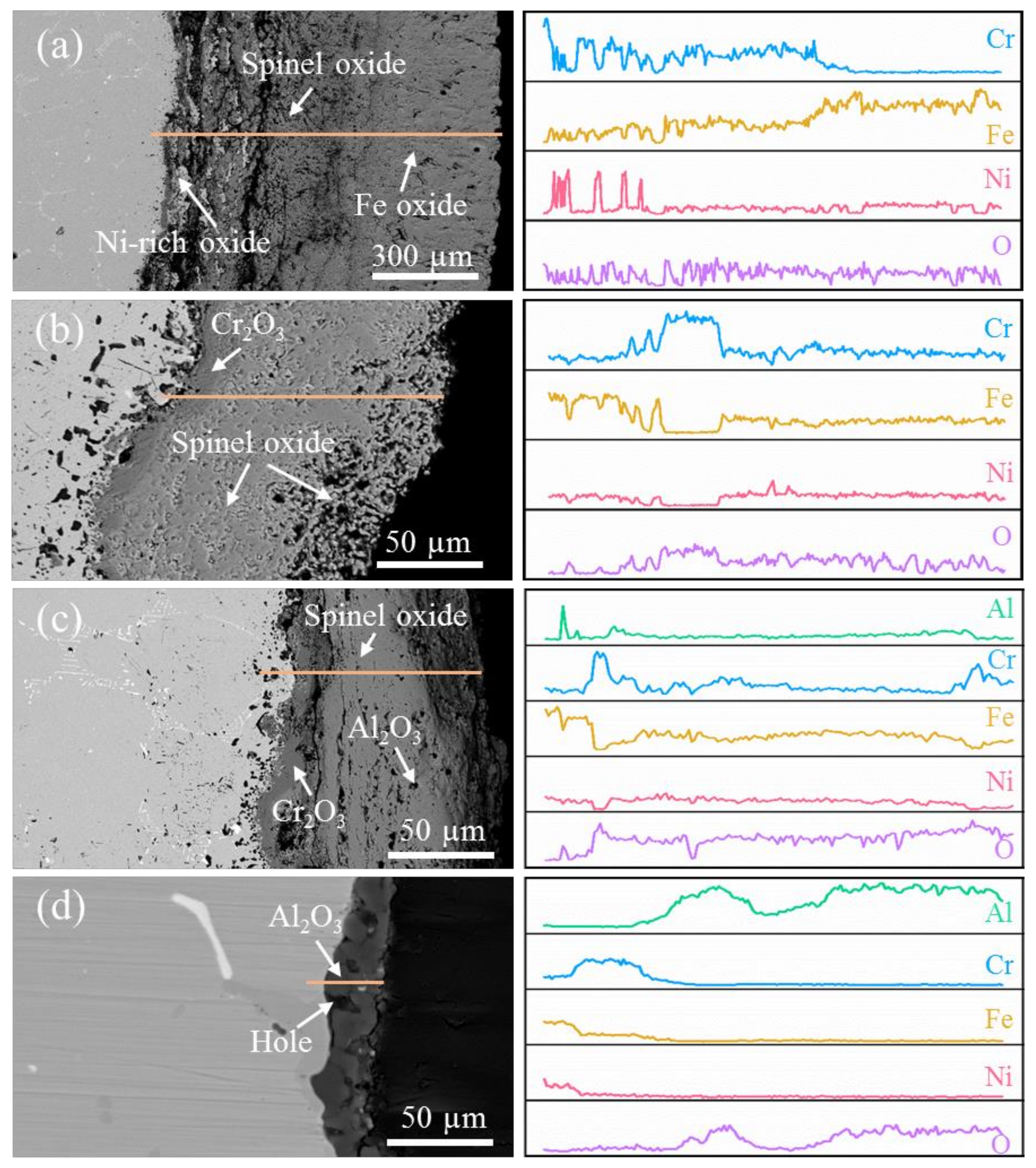
3.4. Examination of Cross Sections
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, J.; Jiao, D.; Luo, C. Microstructural evolution in austenitic heat-resistant cast steel 35Cr25Ni12NNbRE during long-term service. Mater. Sci. Eng. A 2010, 527, 2772–2779. [Google Scholar] [CrossRef]
- Lee, T.H.; Kim, S.; Jung, Y. Crystallographic details of precipitates in Fe-22Cr-21Ni-6Mo-(N) superaustenitic stainless steels aged at 900 °C. Metall. Mater. Trans. A 2000, 31, 1713–1723. [Google Scholar] [CrossRef]
- Whittaker, M.; Wilshire, B.; Brear, J. Creep fracture of the centrifugally-cast superaustenitic steels, HK40 and HP40. Mater. Sci. Eng. A 2013, 580, 391–396. [Google Scholar] [CrossRef] [Green Version]
- Kaya, A.A. Microstructure of HK40 alloy after high-temperature service in oxidizing/carburizing environment: II. Carburization and carbide transformations. Mater. Charact. 2002, 49, 23–34. [Google Scholar] [CrossRef]
- Saucedo-Muñoz, M.L.; Ortiz-Mariscal, A.; Lopez-Hirata, V.M.; Villegas-Cardenas, J.D.; Soriano-Vargas, O.; Avila-Davila, E.O. Precipitation analysis of as-cast HK40 steel after isothermal aging. Int. J. Miner. Metall. Mater. 2017, 24, 1125–1133. [Google Scholar] [CrossRef]
- Pint, B.A.; Peraldi, R.L.; Maziasz, P.J. The use of model alloys to develop corrosion-resistant stainless steels. Mater. Sci. Forum 2004, 461–464, 815–822. [Google Scholar] [CrossRef]
- Sharafi, S.; Farhang, M.R. Effect of aluminizing on surface microstructure of the HH309 stainless steel. Surf. Coat. Technol. 2006, 200, 5048–5051. [Google Scholar] [CrossRef]
- Opila, E.J. Volatility of common protective oxides in high-temperature water vapor: Current understanding and unanswered questions. Mater. Sci. Forum 2004, 461, 765–774. [Google Scholar] [CrossRef] [Green Version]
- Dudziak, T.; Łukaszewicz, M.; Simms, N.; Nicholls, J.R. Steam oxidation of TP347HFG, super 304H and HR3C—Analysis of significance of steam flowrate and specimen surface finish. Corros. Eng. Sci. Technol. 2015, 50, 272–282. [Google Scholar] [CrossRef]
- Saunders, S.R.J.; Monteiro, M.; Rizzo, F. The oxidation behaviour of metals and alloys at high temperatures in atmospheres containing water vapour: A review. Prog. Mater Sci. 2008, 53, 775–837. [Google Scholar] [CrossRef]
- Lu, Z.P.; Moro, K.L.; Yamamoto, Y.; Meyer, H.M.; Maziasz, P.J.; Payzank, E.A.; Pint, B.A.; Brady, M.P.; Liu, C.T. Creep-resistant, Al2O3-forming austenitic stainless steels. Science 2007, 316, 433–436. [Google Scholar]
- Yamamoto, Y.; Brady, M.P.; Lu, Z.P.; Liu, C.T.; Takeyama, M.; Maziasz, P.J.; Pint, B.A. Alumina-forming austenitic stainless steels strengthened by laves phase and MC carbide precipitates. Metall. Mater. Trans. A 2007, 38, 2737–2746. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Santella, M.L.; Brady, M.P.; Bei, H.; Maziasz, P.J. Effect of alloying additions on phase equilibria and creep resistance of alumina-forming austenitic stainless steels. Metall. Mater. Trans. A 2009, 40, 1868–1880. [Google Scholar] [CrossRef]
- Jussila, P.; Lahtonen, K.; Ki, M.L.; Ki, L.H.; Valden, M. Influence of minor alloying elements on the initial stages of oxidation of austenitic stainless steel materials. Surf. Interface Anal. 2010, 40, 1149–1156. [Google Scholar] [CrossRef]
- Brady, M.P.; Yamamoto, Y.; Santella, M.L.; Maziasz, P.J.; Pint, B.A.; Liu, C.T.; Lu, Z.P.; Bei, H. The development of alumina-forming austenitic stainless steels for high-temperature structural use. JOM 2008, 60, 12–18. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X.; Sun, X.; Lu, Z.P. Effects of silicon additions on the oxide scale formation of an alumina-forming austenitic alloy. Corros. Sci. 2012, 65, 317–321. [Google Scholar] [CrossRef]
- Brady, M.P.; Muralidharan, G.; Yamamoto, Y.; Pint, B.A. Development of 1100 °C capable alumina-forming austenitic alloys. Oxid. Met. 2017, 87, 1–10. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.F.; Zhao, J.Y.; Guan, S.K. Effects of Al on microstructure and high-temperature wear properties of austenitic heat-resistant steel. J. Iron. Steel Res. Int. 2012, 19, 62–66. [Google Scholar] [CrossRef]
- Sun, Y.F.; Lv, Y.Z.; Zhang, Y.; Zhao, J.Y.; Wu, Y. Microstructural and properties evolution of austenitic heat resistant steel after addition of aluminium. Mater. Sci. Technol. 2013, 29, 511–516. [Google Scholar] [CrossRef]
- Wang, H.T.; Wang, Y.Q.; Yu, H.S.; Min, G.H.; Wang, Z.F. Effects of composite scale on high temperature oxidation resistance of Fe-Cr-Ni heat resistant alloy. China Foundry 2009, 6, 109–114. [Google Scholar]


| Sample | C | Si | Mn | P | S | Cr | Ni | Nb | Al |
|---|---|---|---|---|---|---|---|---|---|
| 1# | 0.42 | 0.80 | 0.96 | 0.026 | 0.011 | 18.69 | 20.72 | 0.77 | 0 |
| 2# | 0.39 | 1.04 | 1.00 | 0.024 | 0.008 | 18.33 | 20.23 | 0.76 | 1.76 |
| 3# | 0.37 | 1.22 | 1.01 | 0.023 | 0.010 | 18.26 | 20.42 | 0.74 | 3.45 |
| 4# | 0.37 | 1.41 | 1.05 | 0.021 | 0.007 | 18.18 | 20.46 | 0.76 | 5.34 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, C.; Liu, R.; Wang, C.; Sun, Y.; Zhang, S. Effect of Aluminum on Microstructure and High-Temperature Oxidation Resistance of Austenitic Heat-Resistant Steel. Metals 2020, 10, 176. https://doi.org/10.3390/met10020176
Gu C, Liu R, Wang C, Sun Y, Zhang S. Effect of Aluminum on Microstructure and High-Temperature Oxidation Resistance of Austenitic Heat-Resistant Steel. Metals. 2020; 10(2):176. https://doi.org/10.3390/met10020176
Chicago/Turabian StyleGu, Chang, Ruizhuo Liu, Chengduo Wang, Yufu Sun, and Shaojun Zhang. 2020. "Effect of Aluminum on Microstructure and High-Temperature Oxidation Resistance of Austenitic Heat-Resistant Steel" Metals 10, no. 2: 176. https://doi.org/10.3390/met10020176
APA StyleGu, C., Liu, R., Wang, C., Sun, Y., & Zhang, S. (2020). Effect of Aluminum on Microstructure and High-Temperature Oxidation Resistance of Austenitic Heat-Resistant Steel. Metals, 10(2), 176. https://doi.org/10.3390/met10020176




